Abstract
It has long been known that hepatocytes possess the potential to replicate through many cell generations because regeneration can be achieved in rodents after serial two-thirds hepatectomy. It has taken considerable time and effort to harness this potential, with liver regeneration models involving hepatocyte transplantation developing over the past 15 years. This review will describe the experiments that have established the models and methodology for liver repopulation, and the use of cells other than adult hepatocytes in liver repopulation, including hepatic cell lines and hematopoietic, cord blood, hepatic and embryonic stem cells. Emphasis will be placed on the characteristics of the models and how they can influence the outcome of the experiments. Finally, an account of the development of murine models that are competent to accept human hepatocytes is provided. In these models, liver deficiencies are induced in immunodeficient mice, where healthy human cells have a selective advantage. These mice with humanized livers provide a powerful new experimental tool for the study of human hepatotropic pathogens.
Introduction
As practicing biologists, we are aware of the potential applications of the work we do. For many years, liver biologists were limited to in vitro work on liver cells, or in vivo studies using targeted mutations, induced carcinogenesis and liver regeneration. For a long time, liver cell transplantation was just a dream. As we will relate below, it has become an experimental reality in the laboratory over the past 15 years. However, liver cell transplantation remains distant from the majority of potential clinical applications. Experimental biology is an area where you can keep working on the model until you obtain optimal results; this never applies in the clinic. For fundamental liver biologists, cell transplantation using in vivo models provides an optimal test of the differentiation potential and tissue repair activity of specific cell types. For those oriented towards the study of immediate problems of human health, liver cell transplantation is providing us with a new generation of models that will, for the first time, permit manipulations of hepatotropic pathogens of man, including hepatitis B virus (HBV), hepatitis C virus (HCV) and Plasmodium falciparum. This review will discuss the evolution and utility of these model systems.
Initial discoveries from liver regeneration models
Transplanted hepatic cells can replace a diseased liver: the proliferative potential of adult hepatoctyes
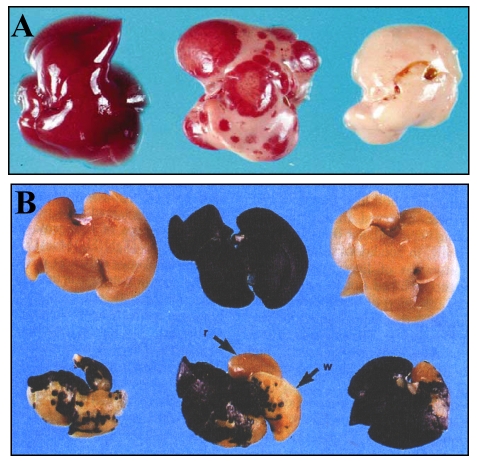
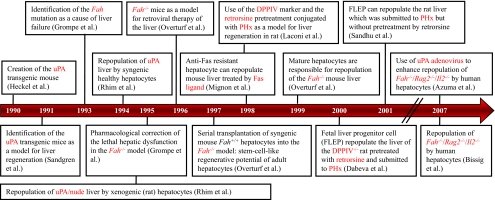
The initial observation that opened up the field of liver transplantation was serendipitous. With the goal of establishing an in vivo system to analyze the coagulation systems, Heckel et al. produced transgenic mice expressing uPA (urokinase-type plasminogen activator) from a chimeric murine uPA gene under the control of promoter sequences from the albumin enhancer/promoter to restrict expression to the liver (Alb-uPA) (Heckel et al., 1990). The resulting transgenic mice frequently died from neonatal bleeding. Sandgren et al. reported on the properties of transgenic lines, characterized by minimal expression of uPA with normalization of liver function over the first weeks (Sandgren et al., 1991). They observed that something happened to the livers of transgenic mice, which were white and fatty, whereas normalized juvenile animals had white livers with red spots, and older mice had entirely red livers (Fig. 1A).
Fig. 1. Morphology of Alb-uPA transgenic mouse livers.
(A) Gross appearance of Alb-uPA transgenic mouse liver from 5-week-old males. Left, non-transgenic control; center, hemizygous transgenic liver filled with regeneration nodules; right, homozyous transgenic liver, displaying a uniformly white color. Figure reprinted with permission from Elsevier (Sandgren et al., 1991). (B) Livers from an experiment using β-gal-positive donor hepatocytes and Alb-uPA recipients, all stained for β-gal activity. Top row, left, non-transgenic control; middle lacZ-positive control; right, control mouse transplanted with lacZ hepatocytes. Bottom, livers from Alb-uPA transgenic mice transplanted with lacZ hepatocytes. In addition to stained blue areas, white areas (w) and endogenous red regeneration nodules (r) are observed in the liver in the middle. Figure reprinted with permission from AAAS (Rhim et al., 1994).
Sandgren et al. demonstrated that the transgene was under-represented in DNA from red liver nodules (Sandgren et al., 1991). They concluded that transgene expression was toxic to hepatocytes, and deletion of the transgene was followed by clonal expansion of the normalized cells. The existence of only a few ‘cured’ cells was sufficient to ensure replacement of the diseased liver, revealing an unexpectedly high proliferative potential of adult liver cells. They then demonstrated, using genetically marked lacZ-expressing donor cells, that hepatic cell transplantation in young Alb-uPA transgenic mice resulted in replacement of the diseased liver tissue (Fig. 1B) (Rhim et al., 1994). They calculated that donor hepatocytes achieved 12–16 rounds of doubling during liver replacement. The transplanted liver cells underwent expansion only in the Alb-uPA transgenic mice and not in normal livers. It was concluded that a regenerative stimulus was necessary to obtain clonal expansion of the transplanted cells. Finally, Rhim et al. introduced the nude gene into the Alb-uPA trangenics to demonstrate that xenogenic hepatocytes from donor rats could reconstitute diseased livers in mice (Rhim et al., 1995).
These studies laid the foundations for liver cell transplantation. They described the application of a genetic-based animal model for competitive liver regeneration, in which exogenous cells introduced by transplantation, or endogenous hepatocytes lacking a deleterious transgene, have a selective advantage and can replace the diseased tissue. They defined the window where endogenous regeneration is on-going, and showed that immune deficiency genes permit xenotransplantation, leading Rhim et al. to speculate that Alb-uPA mice with ‘humanized’ livers, possessing human hepatocytes, could be produced to provide models for human disease (Rhim et al., 1995).
A second mouse system for liver cell transplantation: pharmacological attenuation of a lethal gene deficiency
Deficiency of a liver-specific enzyme, fumarylacetoacetate hydrolase (FAH) causes the human disease, hereditary tyrosinaemia type I. The same deficiency in the mouse is lethal to neonates (Grompe et al., 1993). As the last enzyme in the tyrosine degradation pathway, FAH deficiency leads to the accumulation of toxic metabolites in the liver. Blocking the first steps of the pathway should interfere with generation of the toxic metabolites, and the appropriate inhibitor, 2-(2-nitro-4-trifluoro-methylbenzyol)-1,3 cyclohexanedione (NTBC) was tried on a few human patients (Lindstedt et al., 1992). Grompe et al. described that the same drug, initiated in utero and maintained thereafter, permitted survival of FAH-deficient animals and normalized their liver function (Grompe et al., 1995). Withdrawal of the drug resulted in weight loss and death after two months. With the introduction of NTBC treatment, FAH deficiency in mice had been converted from a lethal disorder in neonates to a conditional lethal disorder.
Overturf et al. reported that transplantation of freshly isolated hepatocytes from wild-type congenic animals (differing from mutants in only one genetic locus) permitted survival of mutant Fah animals after drug withdrawal at the time of transplantation. DNA analysis of the liver revealed the presence of a wild-type Fah gene in animals in which NTBC treatment had been discontinued. Again, a selective advantage of transplanted cells was necessary to obtain repopulation (Overturf et al., 1996). As few as 1000 transplanted hepatocytes were sufficient to rescue the majority of animals
Serial and competitive repopulation by adult hepatocytes
Overturf et al. demonstrated that mouse hepatocytes can be used for up to six rounds of serial transplantation of FAH-deficient mice, amounting to 69 rounds of cell doubling (Overturf et al., 1997). To investigate whether all hepatocytes from perfused liver possess equal proliferative potential, competitive repopulation experiments were performed using DNA markers to track cells (Overturf et al., 1999). Centrifugal elutriation was employed to separate the donor hepatocytes into peaks corresponding to large, medium and small hepatocytes. For competitive repopulation, known numbers of isolated cell populations were mixed with known numbers of unfractionated populations. The medium hepatocytes competed best, whereas small hepatocytes competed least well, the latter being the most likely pool for containing potential liver stem cells. Similar experiments revealed that hepatocytes that had already repopulated a liver failed to demonstrate a selective advantage or disadvantage. Taken together, these experiments indicate that mature hepatocytes, and not liver ‘stem cells’, can repopulate the FAH-deficient liver.
A model for visual assessment of liver repopulation: the DPPIV-deficient Fischer rat
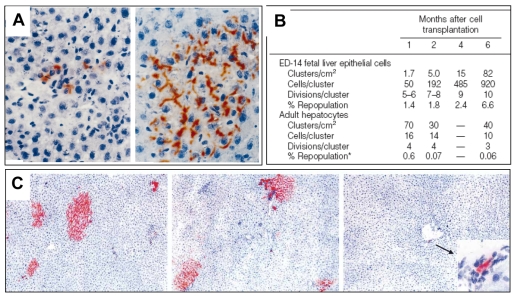
As an alternative to using genetic markers that confer liver toxicity, partial hepatectomy (PH) encourages proliferation of transplanted cells. Laconi et al. combined pretreatment with retrorsine, a pyrrolizidine alkaloid that causes persistent inhibition of hepatocyte division, with PH performed simultaneously with cell transplantation (Laconi et al., 1998). In addition, they used syngenic rats of the Fischer 344 line, of which one sub-strain, deficient in enzyme dipeptidyl peptidase IV (DPPIV) activity, serves as a recipient for wild-type donor cells (Gupta et al., 1995). A histochemical stain imparts localized red color to DPPIV-positive cells.
Adult hepatocytes were infused into the portal vein of retrorsine-treated hepatectomized recipients. A few days after transplantation, isolated cells or tiny clusters of DPPIV-positive cells were visualized; after a month, clusters were composed of 100 or more cells (Fig. 3A); and after 6 months, clusters had enlarged and coalesced to constitute a near total replacement of the liver. Transplanted hepatocytes were morphologically normal and functional, as revealed by immunohistochemical staining of several hepatocyte-specific enzymes. Significant growth of donor cells was not observed in rats that did not receive retrorsine or PH, again providing evidence that a proliferative stimulus is necessary.
Fig. 3. DPPIV-positive E14 embryonic liver cells transplanted into DPPIV mutant rats.
(A) Left, one week after transplantation, scattered cells diffusely stained red. Right, one month after transplantation, large clusters of fully differentiated hepatocytes showing bile canalicular staining of DPPIV. Reprinted with permission from the American Society for Investigative Pathology (Dabeva et al., 2000). (B) Table comparing hepatocyte clusters at different times after transplantation of either cells from E14 fetal livers or adult hepatocytes. Reprinted with permission from the American Society for Investigative Pathology (Sandhu et al., 2001). (C) Illustration of the effects of PH timing on the presence of E14 rat liver cells in DPPIV mutant rats. Left to right: PH was performed 1 day before (left), at the same time as (middle), or 1 day after (right) cell transplantation (Oertel et al., 2006).
Bone marrow- and cord blood-derived stem cells for liver repopulation
Much excitement was generated by reports that hematopoietic stem cells (HSCs) differentiated into hepatocytes in the liver (Theise et al., 2000). Indeed, reports of bone-marrow-derived cells participating in the generation of tissues of mesodermal (Ferrari et al., 1998), ectodermal (Korbling and Estrov, 2003), and endodermal (Petersen et al., 1999) origin all contributed to hopes for universal stem cell therapy.
The model can drive the biology: cell fusion mediated rescue of a gene deficiency disease
The FAH-deficient mouse was employed to test the ability of stem cells to repopulate the liver (Lagasse et al., 2000). Mutant females were subjected to lethal irradiation, and their bone marrow was reconstituted with cells from male Rosa26 mice ubiquitously expressing β-galactosidase (β-gal). The NTBC was withdrawn to see whether circulating donor cells could rescue the diseased liver. After 7 months, surviving animals were sacrificed and their livers were found to contain nodules of β-gal-expressing cells from donor animals. Further, when HSCs (c-kithi, Thylo, Lin−, Sca+) cells were purified using fluorescence-activated cell sorting (FACS) and transplanted into mice for bone marrow reconstitution, together with on/off NTBC drug cycling to improve survival, the regeneration nodules were again of donor origin. In addition, in all animals where the hematopoietic engraftment was successful, donor-derived ‘hepatocytes’ were present.
More information about how the differentiation plasticity of HSCs occurs was offered by a kinetic analysis revealing that the transition of bone marrow cells to hepatocytes is a slow process (Wang et al., 2002). Under optimum conditions, small clusters of donor hepatocytes were present at 7 weeks following transplantation, and by 22 weeks the clusters had become confluent. The authors estimated a total of around 170 repopulation nodules per liver (composed of 5×107 hepatocytes); these numbers did not increase when purified HSCs were used. In contrast, the numbers of repopulating clusters were several hundred-fold higher after transplantation of adult hepatocytes compared with bone marrow transplants, and nodule expansion was much more rapid with 50% repopulation within one month. Finally, in the absence of total body irradiation, which is presumably required for bone marrow replacement by cells carrying a wild-type Fah allele, no cell replacement was observed even with NTBC drug cycling.
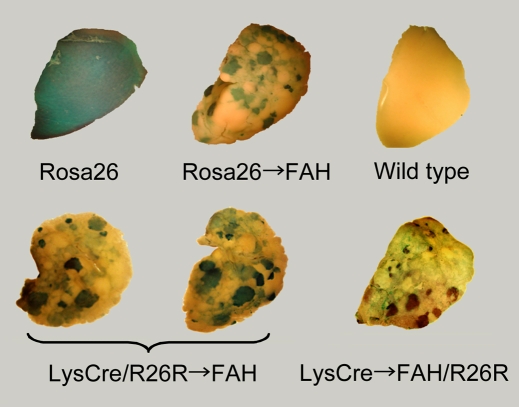
Wang et al. reported experiments designed to test the hypothesis that bone-marrow-derived hepatocytes were in fact the products of cell fusion (Wang et al., 2003b). However, two reports demonstrated that, upon cell fusion, the resulting hybrid cells could perfectly mimic the phenotype of one of the parents (Terada et al., 2002; Ying et al., 2002), as a result of re-programming of the gene expression pattern. Wang et al. tested the hypothesis that bone-marrow-derived hepatocytes were the result of cell fusion, using mice with reconstituted bone marrow and mouse strains with multiple DNA markers. Analysis of repopulated livers revealed patterns and relative concentrations of DNA markers that were consistent with cell fusion. A direct demonstration was obtained by cytogenetic analysis (Wang et al., 2003b) (Box 1). In addition, Camargo et al. and Willenbring et al. demonstrated that myelomonocytic cells are partners in the cell fusion events in the liver (Camargo et al., 2004; Willenbring et al., 2004) (Fig. 2). Finally, Vassilopoulos et al. employed HSCs expressing green fluorescent protein (GFP) in similar experiments (Vassilopoulos et al., 2003) (Table 1).
Box 1. Methods for demonstration of cell fusion-mediated rescue of Fah-deficient bone marrow chimeras.
Wang et al. used Southern blots to detect genotypic markers from three strains of mice (Wang et al., 2003b). Mice were subjected to lethal irradiation before receiving a bone marrow transplant from donor mice [genotype A, hereafter referred to as (A)]. NTBC was withdrawn in the primaryFah−/−recipient mice [genotype B, hereafter (B)]. Liver cells from these mice were then transplanted into Fah−/− recipients to obtain secondary transplants, before tertiary transplantation into another group of Fah−/− recipients (genotype C). In these tertiary recipients, the relative contributions of the donor (A) and the Fah mutant (B) markers were equivalent and under-represented compared with the level of repopulation. This is because the bone-marrow-derived hepatocytes carry alleles from both donor (genotype A) and recipient (genotype B) genotypes, as a result of cell fusion, which creates cells that are twice as large as normal hepatocytes (so that 50% repopulation calculated by area can be equivalent to 25% repopulation by cell number). The contribution of the marker allele C from the tertiary recipient was approximately reciprocal to the corrected degree of repopulation by bone marrow hepatocytes (taking into account that 30% of liver cells are non-hepatocytic). This clarifies that cell fusion events only occurred in the primary recipients and were not continuously generated.
A direct demonstration of cell fusion was obtained by cytogenetic analysis of bone marrow transplants from female Fah-positive Rosa26 donor mice into irradiated male Fah−/− recipients, in which NTBC treatment had been withdrawn. Chromosome counts from primary hepatocyte cultures were compatible with the hypothesis that cell fusion occurs when bone-marrow-derived hepatocytes are formed: significant numbers of hepatocytes contained 80 chromosomes including three X and one Y chromosome (80XXXY). The interpretation of these experiments is complicated by the fact that a large fraction of hepatocytes in adult rodents are tetraploid to begin with, so cells containing either 40 or 80 chromosomes, and two or four sex chromosomes, must be taken into account when calculating the karyotypes that are anticipated when cell fusion is, and is not, involved. Furthermore, metaphases have a tendency to break when chromosome spreads are air-dried, so a cell with 80 or 120 chromosomes may have lost variable numbers of its chromosomes.
Additional experiments, such as immunocytochemistry, were carried out to demonstrate that most of the hepatocytes in primary cultures were Fah-positive and contained a Y chromosome. In a few cases, FAH staining was follow by in situ hybridization to show that enzyme activity and Y chromosomes were present in the same cells.
Fig. 2. Livers stained for β-gal activity.
The Rosa26 liver constitutively expresses β-gal in all cells. Rosa26→FAH shows β-gal-expressing nodules after transplantation of Rosa26 hepatocytes into Fah−/− mice. LysM-Cre refers to a mouse line in which Cre recombinase, under the control of the LysM (lysozyme M) promoter, is expressed is macrophages. If the same mouse also has a Rosa26R gene (R=reporter: the gene contains a stop codon, flanked by loxP sites, upstream of the lacZ gene), when Cre recombinase is expressed in macrophages, all descendants of that cell will express β-gal. For the two images here, this proves that macrophages have fused with hepatocytes to create blue nodules. Finally, the last image is of LysM-Cre bone marrow injected into Fah−/− mice which also possess a Rosa26R sequence. The presence of blue nodules in this liver directly demonstrates that a macrophage has fused with a Rosa26R-containing hepatocyte to generate β-gal-positive hybrid cells. Figure reproduced with permission from the American Society for Clinical Investigation (Camargo et al., 2004).
Table 1.
Liver rescue in the FAH-deficient mouse following hematopoietic reconstitution with wild-type bone marrow
| Cell type | Route of transplantation | Recipient; liver damage | Comments | Reference |
|---|---|---|---|---|
| Unsorted mouse BM cells; Tg for lacZ gene | Retro-orbital (1–10×106 cells) | Fah−/− mice Serial NTBC stop from three weeks after Tx | 30–50% lacZ+ cells in the liver 7 months after Tx; Alb+, DPPIV+ and E-cadherin+; liver injury needed for expansion of BM-derived hepatocytes; restoration of liver function | (Lagasse et al., 2000; Wang et al., 2002) |
| Sorted HSC from mice: c-kithighThylowLin−Sca-1+; Tg for lacZ gene | Retro-orbital (10 to 1×103 cells) | Fah−/− mice Serial NTBC stop | lacZ+ nodules (50 to 1×105 cells) 6 months after Tx; HSC did not enhance the degree of liver engraftment; detection of Y chromosome; Alb+, DPPIV+ and E-cadherin+ | (Lagasse et al., 2000; Wang et al., 2002) |
| Isolated HSC cells from mice; GFP-transduced cells | Unspecified | Fah−/− mice Serial NTBC stop | Regenerative liver with FAH+ nodules; restoration of liver function; cell fusion associated with a more hepatocyte-like morphology | (Vassilopoulos et al., 2003) |
| Unsorted mouse cells | Retro-orbital (1×106 cells) | Fah−/− mice Serial NTBC stop from three weeks after Tx | 20–30% of transplanted cells in the liver 5 months after Tx; restoration of liver function; hepatocytes derived from BM arise from cell fusion | (Wang et al., 2003b) |
| Isolated mouse HSC CD45+/ Sca-1+; unsorted cells from Rag2−/− γ C−/−mice; unsorted cells from LysM-Cre mice (see legend of Fig. 2) | Retro-orbital (1×106 cells) | Fah−/− or Fah−/−/Rosa26R mice Serial NTBC stop | The different hematopoietic lineages contribute to the generation of FAH+ nodules; HSC-derived hepatocytes are primarily derived from mature myelomonocytic cells by fusion with host hepatocytes | (Camargo et al., 2004) |
Tg, transgenic;
Tx, transplantation;
AFP, alpha-fetoprotein.
What are the general conclusions to be drawn from this series of papers? FAH deficiency is a recessive mutation. Any cell providing a wild-type Fah allele can theoretically rescue such a deficiency, provided that Fah expression can be activated by reprogramming in the hybrid cells. Furthermore, in all of the experiments cited here (Lagasse et al., 2000; Wang et al., 2002; Vassilopoulos et al., 2003; Wang et al., 2003b; Willenbring et al., 2004; Camargo et al., 2004), Fah mutant mice were initially subjected to lethal irradiation and transplanted with bone marrow cells carrying a wild-type Fah allele; therefore, the animals were already heavily chimeric before NTBC withdrawal.
Direct transplantation of bone marrow or cells derived from bone marrow
Studies by Moran-Jimenez et al. made use of a potentially interesting class of recipient animals. They employed mice with a targeted mutation in the Hfe gene, an established model of hemochromatosis (Moran-Jimenez et al., 2008), which in humans is a disease involving increased absorption of iron and its deposition in parenchymal organs, leading to fibrinogenesis and in some cases diabetes. This recessive mutation does not cause lethality. The transplanted mice showed a slightly reduced iron concentration in the liver and a measurable increase in the hepatic Hfe mRNA concentration. (The mechanism underlying this correction is not clear and could be fusion and selective expansion of hybrid cells, or cell fusion where the cells remain as heterokaryons, or transdifferentiation.) The use of similar models, where a mutation compromises liver function without causing death, should permit meaningful comparisons of the relative selective advantages in the liver of donor cells of different origins.
Table 2 summarizes a number of other papers where either bone marrow or bone-marrow-derived mesenchymal stem cells (MSCs) were transplanted into mice or rats to analyze their potential for liver engraftment after induced liver damage. However, it is difficult to compare one experiment with another because: (1) in some cases, the transplanted cells were of human origin; (2) different cell numbers and different transplantation routes were employed; (3) recipients were irradiated and some were immunodeficient; (4) liver damage was induced by a number of different means; and (5) some investigators checked for cell fusion, whereas others did not. In all cases, successful liver engraftment with donor cells was low. With the exception of a study by Oh et al. (Oh et al., 2007), none of the experiments used any of the robust models for liver repopulation presented above.
Table 2.
Experimental transplantation of bone marrow into other liver injury models
| Cell type | Route of transplantation | Recipient; liver damage* | Comments | Reference |
|---|---|---|---|---|
| Isolated CD34+ or CD34+, CD38−, CD7− hHSC | Tail vein (2000 or 1×105 cells) | NOD/SCID (+/− β2M-null) mice Irradiation; CCl4 after Tx,(+/− HGF injection) | hAlb+ at 5 and 30 days, and only after liver injury; increase in HGF-treated mice | (Wang et al., 2003c) |
| Isolated CD3+ cells from βactin-Cre GFP mice | Intravenous (1.5×106 cells) | Cre reporter mice Irradiation | 0.1% of hepatocytes derived from BM; no cell fusion | (Harris et al., 2004) |
| Isolated HSC from mice (Fr25lin−); homed HSC (Fr25lin− PKH+) | Tail vein (1×105 cells) | C57BL/6/NCR mice Irradiation (+/−); CCl4 after Tx (+/−) | Liver injury induces HSC cell conversion independently of cell fusion; 7 days after Tx, 7.6% of transplanted cells found in the liver of injured mice (E-cadherin+, Alb+ and male chromosome) and restoration of liver function; homing increases cell conversion | (Jang et al., 2004) |
| Isolated MSC Flk1+ from mice | Tail vein (1×106 cells) | BALB/c mice Tx at the time of, or after, CCl4 treatment | Tx of mouse MSC Flk1+ cells immediately after CCl4 challenge protects liver from injury | (Fang et al., 2004) |
| hMSC, CD34+ and non- MSC/CD34− cells; expanded or non-expanded cultures | Intrahepatic (1×106 cells) | Sprague-Dawley rat allyl alcohol before and after Tx; cyclosporin A | hAFP+, hAlb+, hCK18/19+ and asialoglycoprotein receptor positive in MSC Tx; a maximum of 0.5% of MSC undergo hepatocyte-like differentiation; no cell fusion | (Sato et al., 2005) |
| Adherent proliferating rat MSC | Intravenous (3×106 cells) | Wistar rat CCl4 or DMN treatment before transplantation | Reduced mortality rate in both liver injury models | (Zhao et al., 2005) |
| Adherent proliferating hMSC preconditioned with EGF and HGF | Intrasplenic (1×106 cells) | Pfp−/−/Rag2−/− mice PH and propranolol hydrochloride treatment before Tx | HepPar1+, hAlb+, PCK1+ and CX32+; preferential engraftment in periportal area; no cell fusion | (Aurich et al., 2007) |
| Adherent proliferating rat MSC | Intravenous (3×106 cells) | Albino rat CCl4 treatment before Tx | MSC Tx have potential therapeutic effect against fibrotic process | (Abdel Aziz et al., 2007) |
| Expansion of rat MSC for hepatocyte-like differentiation; GFP transduction | Intrasplenic (7×107 cells) | Sprague-Dawley rat Tx 1 week before 90% hepatectomy | Concomitant immunostaining of Alb and GFP; persistence of transplanted cells 100 days after Tx; prevention of fatal liver failure in 30% of animals | (Miyazaki et al., 2007) |
| Unsorted DPPIV+ rat cells | Tail vein (5×107 cells) | DPPIV− rat Monocrotaline treatment; irradiation at time of Tx; AAF and 70% PH after Tx | 20% of DPPIV+ transplanted cells were AFP+; secondary transplantation of DPPIV+ hepatic oval cells; no cell fusion | (Oh et al., 2007) |
| Isolated mononuclear cells of C57BL/6J mice | Tail vein (1×107 cells) | Hfe knockout hemochromatotic mice Irradiation | 11% of transplanted cells in the liver (also duodenum); reduction of iron overload | (Moran-Jimenez et al., 2008) |
| Culture expansion of human MSC | Tail vein (1×106 cells) | NOD/SCID mice Irradiation; single or chronic CCl4 treatment | Chronic injury increased hMSCs transplantation, but differentiation into hepatocyte-like cells is a rare event | (di Bonzo et al., 2008) |
| Culture expansion of hMSC or hepatocyte- like differentiated cells (MDH) | Tail vein or intrasplenic (1.4–4.2×107 cells/kg body weight) | NOD/SCID mice CCl4 treatment before Tx | MSC and MDH differentiated into hepatocytes and rescued liver failure; intravenous Tx more effective in rescuing liver failure; MSC transplanted animals more resistant to oxidative stress; MSC promote proliferation of endogenous hepatocytes, suggesting possible paracrine effects | (Kuo et al., 2008) |
For irradiation a sub-lethal dosage was used.
h, human;
Tx, transplantation;
AFP, α-fetoprotein;
DMN, dimethylnitrosamine;
CK, cytokeratin;
EGF, epithelial growth factor;
HGF, hepatocyte growth factor;
MDH, mesenchymal differentiated hepatocyte.
Human umbilical cord blood (UCB) stem cells
Recent studies provide evidence that UCB stem cells not only participate in the development of hematopoietic progenitors, but can also differentiate in vitro into adipocytes, osteocytes, chondrocytes, cardiomyocytes, neurons and hepatocytes (van de Ven et al., 2007). Thus, there is much excitement about use of the easy-to-obtain UCB stem cells for the treatment of both hematopoietic and non-hematopoietic diseases. In addition, many groups have reported the successful generation of hepatocyte-like cells from UCB using in vitro assays.
Liver regeneration models have been used to test entire mononuclear cell preparations, or cells selected for progenitor markers, by injecting them into immunocompromised mice (Tables 3 and 4). Many papers report that UCB cells can develop in the liver as hepatocyte-like cells. However, even when liver injury is induced by carbon tetrachloride (CCl4), 2-acetylaminofluorene (AAF) and the Fas ligand, the frequency of human cell engraftment in the liver is extremely low. Despite the low level of liver repopulation, two studies do report that human UCB cell transplantation significantly reduces the mortality caused by induced liver injury (Di Campli et al., 2005; Nonome et al., 2005).
Table 3.
Experimental transplantation of human UCB stem cells into the liver of adult animals
| Cell type | Route of transplantation | Recipient; liver damage* | Comments** | Reference |
|---|---|---|---|---|
| Adherent proliferating cells: hAlb+, vital staining | Intraliver (2×105 cells) | SCID mice With or without PH | 7 and 21 days after Tx: hAlb+, hAFP−, hGATA4−, downregulation of hβ2-microglobulin; frequency: not given | (Beerheide et al., 2002) |
| Isolated Lin−, CD38−, CD34−, ClqRp and Lin−, CD38−, CD34+, ClqRp cells | Tail vein (500 to 7×104 cells) | NOD/SCID mice Irradiation | HepPar1+, h c-Met8+ and hAlb+ 10 weeks after Tx; frequency: 0.05–0.1% | (Danet et al., 2002) |
| Unsorted mononuclear cell preparation | Tail vein (5×107 cells) | NOD/SCID mice Irradiation | HepPar1+ in livers 4, 6 and 16 weeks after Tx, no evidence for cell fusion; frequency: 0.008–0.03% | (Newsome et al., 2003) |
| Isolated CD34+ or CD34+, CD38−, CD7− cells | Tail vein (2000 or 1×105 cells) | NOD/SCID (+/−β2M-null) mice Irradiation; CCl 4 after Tx (+/− HGF injection) | hAlb+ cells at days 5 and 30, only in injured liver; more hAlb in HGF-treated mice; frequency: not given | (Wang et al., 2003c) |
| Adherent proliferating cells: hAlb+ | Portal vein (1×107 cells) | SCID mice AAF injection then PH | hAlb+ and HepPar1+ cells from 4–55 weeks; frequency: 0.1–1% | (Kakinuma et al., 2003) |
| Isolated CD34+/−, CD38+/−, c-kit+/− cells | Portal vein (1.5×104, 4×106cells) | NOD/SCID mice Retrorsine; CCl4 after Tx | Higher expression of hAlb in CD34+ transplanted cells; frequency: 1.2–1.7%; cell fusion in 77% of cells | (Tanabe et al., 2004) |
| Isolated CD34+, AC133+, c-kit+ cells | Intraperitoneally (4×105 cells) | NOD/SCID mice Allyl alcohol before Tx | 0.2% of engrafted cells are AFP+; in transplanted mice, decrease of mortality from 70 to 20% | (Di Campli et al., 2005) |
| Adherent proliferating cells | Tail vein (5×105 cells) | NOD/SCID mice Fas ligand or irradiation | hAlb+, HepPar1+, hAFP+ cells, positive for glutamine synthetase and transferrin; in transplanted mice treated with Fas ligand, decrease of mortality from 70 to 0% | (Nonome et al., 2005) |
| Unsorted mononuclear cells | Tail vein (1×106 cells) | NOD/SCID mice Irradiation; CCl4 after Tx | hAlb+, hCK18− cells; no protection from liver damage | (Sharma et al., 2005) |
| Unsorted mononuclear cells or isolated CD34+ cells; GFP-transduced cells | Tail vein (1–3×105 cells) | NOD/SCID mice Irradiation; CCl4 before Tx | Less than of 1% of GFP-positive cells in liver; cell fusion demonstrated for hepatocyte-like cells | (Kashofer et al., 2006) |
| Adherent proliferating cells | Intraliver or intrasplenic (2×105 cells) | NOD/SCID mice, uPA/Rag2−/− mice | Two types of hAlb+ cells: (1) human nucleus: no hepatocyte-like morphology; (2) only mouse nucleus detected: hepatocyte-like morphology (postulated gene transfer) | (Brulport et al., 2007) |
| Isolated CD34+ cells | Tail vein (2×104 to 1×107cells) | NOD/SCID/γC mice Irradiation | Expression of hAlb; frequency: 3.4% of human hepatocytes 6 months after Tx; cell fusion demonstrated. | (Fujino et al., 2007) |
| Unsorted mononuclear cells | Intrasplenic (2×106 cells) | NOD/SCID mice AAF and allyl alcohol before Tx; AAF for 7 days at day 11 post-Tx | hAlb+; rapid human cell repopulation in week 1; no difference in mortality rate (14.7%); frequency: 0.51% of human hepatocytes 6 months after Tx | (Shyu et al., 2007) |
| Adherent proliferating cells | Intrasplenic (1×106 cells) | SCID mice PH before transplantation | hAlb+ and AFP+ until 6 weeks; Alu+ hybridization and human mitochondria | (Campard et al., 2008) |
| Adherent proliferating CD34+ cells treated with SCF or HGF 24 hours before Tx | Tail vein (2×105 cells) | NOD/SCID mice Irradiation | SCF reduced long-term cell engraftment in liver of NOD/SCID mice; frequency: 0.3% of human cells 56 days after Tx | (Wulf-Goldenberg et al., 2008) |
For irradiation, a sub-lethal dosage was used;
Frequency signifies ‘frequency of transplanted cell in the liver’.
h, human;
HGF, hepatocyte growth factor;
SCF, stem cell factor;
Tx, transplantation;
CK, cytokeratin.
Table 4.
Experimental transplantation of human UCB stem cells in utero
| Cell type | Route of transplantation | Recipient;liver damage | Comments | Reference |
|---|---|---|---|---|
| Isolated CD34+/−, Lin−, CD38− cells | Intrafetal (2×104 cells) | Sheep | hAlb+, human hepatocyte Ag+; 1–2% of human hepatocytes at 2 months; in one animal, around 17% of human hepatocytes at 11 months of age | (Almeida-Porada et al., 2004) |
| Isolated CD34+ cells | Intrafetal (3–5×105 cells) intra-blastocyst (15–20 cells) | CD1 or C57BL/6 mice | hAlb+, HepPar1+ and α-antitrypsin 1+ 4 weeks after birth; HepPar1+ clusters around major blood vessels | (Turrini et al., 2005) |
| Isolated CD34+, Lin− cells | Intrafetal (1×105 cells) | Goat | hAlb+ and hHNF-3β+ mRNA | (Zeng et al., 2005) |
| Isolated CD34+, Lin− cells transduced with GFP | Intrafetal | Goat | hAlb+, human hepatocyte Ag+ and PCNA+. Around 30% of GFP+ cells in the liver after 3 months or 2 years; microarrays for human gene expression; no cell fusion | (Zeng et al., 2006) |
| Unsorted mononuclear cells; isolated CD34+, Lin− cells | Intrafetal (1×106, 1×104 cells) | Kun Ming Bai mice CCl4 6 months after birth | hAlb+, HNF-4+, human hepatocyte Ag+ and hAFP+; protection from CCl4 damage; most positive clusters were located in the vicinity of vascular structure | (Qian et al., 2006) |
| Unsorted mononuclear cells | Intrafetal (5×106 cells) | Rat | hAlb+, CK19+ and CK18+ | (Sun et al., 2007) |
h, human;
HNF, hepatocyte nuclear factor;
CK, cytokeratin.
A particularly interesting set of experiments involved intra-fetal injections of human UCB cells into mice, rats, sheep and goats (Almeida-Porada et al., 2004; Turrini et al., 2005; Zeng et al., 2005; Qian et al., 2006; Zeng et al., 2006; Sun et al., 2007) (Table 4). Cell engraftment into fetal liver would be expected to preclude the necessity of suppression of the immune response and of induced liver damage to obtain an environment appropriate for cell proliferation. In these models, high levels of human cells were detected in the livers of animals born from injected fetuses for up to two years after birth (Almeida-Porada et al., 2004; Qian et al., 2006; Zeng et al., 2006).
Whereas the published studies provide data on the ability of UCB stem cells to differentiate as non-hematopoietic cells, and in particular as liver cells, additional research is required to clarify the potential usefulness of, and the appropriate moment to use, UCB stem cells for tissue repair and in particular for the restoration of liver function.
Hepatic stem cells from fetal or adult liver
Intrinsic, long-term proliferative potential of E14 rat fetal liver epithelial progenitor (FLEP) cells
Dabeva et al. used DPPIV-negative rat recipients, subjected to retrorsine treatment and PH or PH alone, to reveal the robust repopulation potential of fetal liver progenitor cells (Dabeva et al., 2000). Sandhu et al. carried out a long-term quantitative study of the repopulation activity of FLEP cells compared with adult hepatocytes (AH), using rats that were stimulated only by PH (Sandhu et al., 2001). Fig. 3B reveals important differences between FLEP cells and AHs that only became clear after long-term observations. Oertel et al. demonstrated that PH must precede, or be simultaneous with, cell infusion for effective growth stimulation of donor cells to occur (Fig. 3C) (Oertel et al., 2006).
It is usually assumed that the environment of growth stimulation triggered by PH lasts for only about 1 month. Unexpectedly – and in the absence of any obvious selective advantage or ongoing liver degenerative process – FLEP cells, but not AHs, outstripped the endogenous cells of the liver, gradually replacing many of them over the long-term. This property reinforces the idea that early fetal liver cells are genuine stem cells. These important observations have been re-investigated and found to result from cell-cell competition (Oertel et al., 2006).
Oval cells for liver replacement
Hepatic stem cells of the adult liver have been recognized for decades and are characterized by their bipotentiality and capacity for self-renewal (Evarts et al., 1987; Fausto, 2004; Shafritz et al., 2006). In adult rodents, hepatic progenitor cells, so-called ‘oval cells’, are activated to proliferate when replication of hepatocytes is blocked. A few studies have now demonstrated the capacity of these progenitor cells to repopulate the liver. In competitive repopulation experiments, Wang et al. showed that freshly derived oval cells (obtained by treating mice with 3,5-diethylcarbonyl-1,4-dihydrocollidine) are at least as efficient as mature hepatocytes in repopulating FAH-deficient mice (Wang et al., 2003a). Song et al. used GFP-expressing liver progenitor cells from mice that were transduced with human α1-antitrypsin (Song et al., 2004). For transplantation, they treated congenic recipients with monocrotaline and subjected them to PH. Approximately 40–50% of the regenerated liver was GFP positive. In addition, 5–10% of the repopulating cells expressed human α1-antitrypsin.
Cell lines for liver regeneration
The establishment of progenitor cell lines derived from embryonic and adult liver, showing bipotential capacity to differentiate into hepatocytes and bile duct cells in vitro as well as in vivo, has been documented (Strick-Marchand et al., 2004). The advantages of fetal liver as a source of cells with liver repopulation potential are its capacity to proliferate without selective pressure and to retain bipotentiality (Sandhu et al., 2001; Strick-Marchand et al., 2004; Suzuki et al., 2002). More recently, Oertel et al. transplanted embryonic (day 14) fetal liver cells that had proliferated continuously for 6 months and found that they differentiated into mature hepatocytes and bile ducts, and replaced 23.5% of the total liver mass (Oertel et al., 2006). In addition, stem cell lines participated in liver regeneration of Alb-uPA/severe combined immunodeficiency (SCID) transgenic mice to levels approaching 5% of the tissue, and with no indication of cell fusion (Strick-Marchand et al., 2004).
Using culture selection, Herrera et al. isolated and characterized progenitor cells from normal adult human liver (Herrera et al., 2006). These pluripotent cells do not express oval cell markers (defined in the rodent) and can undergo mesenchymal differentiation in culture. In addition, the cells engrafted the liver in SCID mice with acute liver injury (Herrera et al., 2006).
Hepatic progenitor cell lines have also been isolated from human fetal livers (Dan et al., 2006). The clonal lines, designated hFLMPC (for human fetal liver multipotent progenitor cells), appear to represent a mesenchymal-epithelial transition cell, probably derived from mesoendoderm. The cells were shown to engraft the livers of immunodeficient mice with induced liver damage. In these mice, human albumin could be measured in the serum and visualized on liver sections, indicating hepatocyte functional activity of the human cells.
Hepatic stem cells identified by patterns of marker expression
One approach that has been vigorously pursued to identify and select progenitor cells from embryonic livers involves the analysis of marker expression in freshly isolated cells, and the determination of their differentiation potential in culture. The pattern of surface marker gene expression can then be used to purify desired cell populations by FACS or by immunoselection with magnetic beads, resulting in pure populations of stem cells for therapeutic purposes as well as biological studies.
Schmelzer et al. characterized two types of pluripotent hepatic progenitors, hepatic stem cells and hepatoblasts, which can be distinguished by differential expression of CK19 and surface markers such as epithelial cell adhesion molecule (EpCAM). The hepatic stem cells are located in both fetal and adult liver (Schmelzer et al., 2006; Schmelzer et al., 2007), and are thought to repopulate animal models of liver injury, but marker analysis of the engrafted cells is currently limited to immunostaining for albumin (Schmelzer et al., 2007).
Simper-Ronan et al. used monoclonal antibodies and micromagnetic immunobeads to isolate populations of cholangiocyte-marker-positive fetal rat liver progenitor epithelialcells (CMP-FLEC) for injection into DPPIV-negative, retrorsine-treated rats subjected to PH. These cells showed excellent repopulation capacity. Interestingly, if cell populations were depleted of CMP-FLEC, the repopulation potential was severely diminished (Simper-Ronan et al., 2006). Finally, studies have demonstrated that immuno-isolated delta-like-1-positive (Dlk-1+) rat fetal liver cells can account for the repopulation capacity of the FLEP (Oertel et al., 2008).
Liver repopulation by the progeny of embryonic stem (ES) cells
The culture and directed differentiation of both mouse and human ES cells (mES and hES cell lines, respectively) holds great promise for the future of cell-based therapy for tissue repair. Progress toward the goal of using such cells in liver repopulation models has been slow. A promising publication from Heo et al. described the isolation of mES cells with the potential to express GFP under control of the albumin promoter/enhancer. The cells were cultured as embryoid bodies, then re-plated and cultured in medium containing factors favorable for the emergence and proliferation of hepatocytes. As an original step, the authors used FACS for GFP expression in an effort to eliminate undifferentiated ES cells that could confer malignant growth. Using a second uPA transgenic mouse line [MUP-uPA, where the liver-specific promoter is from a major urinary protein (Mup) gene], they obtained clusters of GFP-positive hepatocytes that enlarged over 3 months and expressed a range of hepatocyte functions; the transplanted cells proliferated in harmony with host cells when the liver was injured (Heo et al., 2006). Sharma et al. used ES-HPC (mES-derived hepatic progenitor cells) for engraftment, after a similar FACS sorting operation, into Fah−/−/SCID mice. Although engrafted cells were observed throughout the liver, immunostaining for the FAH protein was negative. The authors concluded that the transplanted ES cells do not acquire a fully mature hepatocyte phenotype (Sharma et al., 2008).
It will take time to identify ES cell lines with sufficient propensity to differentiate into adult hepatocytes and the most favorable mouse model in which to optimize much-needed experimental tests for robust liver repopulation by ES cells.
Humanization of rodent models: a novel tool to study human pathogens
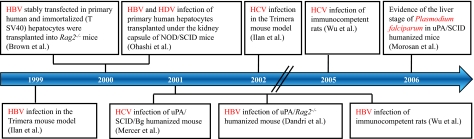
The evaluation of pharmacological compounds and their metabolism/detoxification must be carried out using human cells because of the evolutionary diversity of hepatic detoxification enzymes. Furthermore, the study of human-specific liver pathogens has been, until recently, severely hampered by the absence of appropriate cell culture systems and animal models (Box 2). Here, we focus on the development of humanized rodent models to study the three major hepatic pathogens (Fig. 4) HBV, HCV and P. falciparum, which are responsible for millions of deaths each year (Box 3).
Box 2. Limitations of available models for studies of hepatic pathogens.
The discovery of prophylactic and curative treatments for HBV, HCV and P. falciparum has been hampered by the lack of cell culture systems and small animal models. In vitro studies have shown that primary human hepatocytes (PHH) are susceptible to infection by HBV (Gripon et al., 1988), HCV (Fournier et al., 1998) and by sporozoites (the hepatic form of P. falciparum) (Mazier et al., 1985). However, these systems are limited because PHH rapidly revert to undifferentiated hepatocytes (the loss of differentiation leads to resistance to infection) and there are difficulties in obtaining fresh cells. HBV and HCV cell lines can be used in stable (Sells et al., 1987; Wakita et al., 2005) or transient transfection strategies, resulting in production and secretion of infectious virions, but cannot be used to study the entry steps of these viruses. In 2002, the human HepaRG cell line was described (Gripon et al., 2002) as a tool to study the entry steps of HBV infection. In vivo, the chimpanzee constitutes the best non-human primate for use in HBV, HCV and P. falciparum studies (Dandri et al., 2005b; Kremsdorf and Brezillon, 2007; Moreno et al., 2007). Except for this higher primate, the only non-human primate that is permissive for HCV infection is the marmoset (Kremsdorf and Brezillon, 2007), whereas the rhesus monkeys, cynomoglus monkeys, gibbons and orangutans are susceptible to HBV infection. In addition, Tupaia, a member of the tree shrew genus, can be infected with both HBV and HCV. However, these models are hampered by multiple drawbacks, including: (1) the inability to produce numerous progeny in a short time because of long gestation periods, (2) exorbitant housing and breeding costs, and (3) scarcity of the species threatened by extinction. HBV-and HCV-related viruses, such as GBV-B (GB virus B, a hepatotrophic virus of the Flaviviridae family) have been used to infect tamarins and marmosets to better characterize HCV replication and to test HCV antiviral drugs (Bright et al., 2004; Nam et al., 2004; Rijnbrand et al., 2005). For HBV, WM-HBV (woolly monkey HBV), WHBV (woodchuck HBV), GSHV (ground squirrel hepatitis virus), ASHV (artic squirrel hepatitis virus), DHBV (duck HBV) and HHBV (heron HBV) have all been used to improve understanding of the life cycle of the Hepadnaviridae family. Finally, transgenic mice have enabled numerous discoveries to be made in the field of HBV and HCV virology. However, these rodents cannot be used to study the entry step of HBV or HCV and are not suitable for evaluating the efficacy or toxicity of treatments.
Fig. 4. Steps in the creation of humanized rodent models for infection by liver pathogens.
The arrow represents a timeline. Each box represents an independent initial study describing the infection of a humanized model by a hepatotropic pathogen. Boxes connected to the timeline through the same line indicate that the studies were published in the same year.
Box 3. Epidemiology of three major hepatic pathogens.
Hepatitis B virus: around two billion people are infected worldwide, 350 million of whom are chronically infected (95% of infected adults resolve the infection whereas 95% of vertically-infected children became chronically infected), resulting in 0.5–1.2 million deaths/year due to chronic hepatitis, cirrhosis and hepatocellular carcinoma (HCC) (Lavanchy, 2004). HBV transmission results from exposure to infectious blood or body fluids – vertical transmission from chronically infected mother to child occurs in 20% of cases. An effective and safe prophylactic vaccine does exist.
Hepatitis C virus: around 130 million people are chronically infected. 60–85% of infected patients become chronically infected, and HCV accounts for an estimated 27% of cirrhosis and 25% of HCC worldwide (Alter, 2007). Transmission results from blood to blood contact. Vertical transmission and transmission during the birth process can occur rarely (in 6% of births) from women with viremia at the time of delivery. No vaccine is available.
Malaria (Plasmodium falciparum): around 500 million people worldwide are infected with malaria resulting in one million deaths/year from direct or indirect causes (Greenwood et al., 2008). The female Anopheles mosquito is the vector for human malaria. No vaccine is available.
Because of their short gestation period, their small size and their low maintenance costs, rodents would certainly be a desirable model for biological studies of human-specific pathogens. At least three interesting models to study HBV and HCV already exist: (1) the immunotolerized rat model; (2) the Trimera mouse model; and (3) the Alb-uPA mouse model. The Alb-uPA model has also been used to study the hepatic stage of P. falciparum. Moreover, the Fah−/−/Rag2−/−/Il2−/− model appears to be efficient for liver humanization and should provide an additional infection model for different classes of human pathogens (Table 5; Fig. 5).
Table 5.
Characteristics of HBV and HCV infection in three rodent models
| Rodent model | Humanization | Virus | Viremia | Duration of viral infection | Comments | Publications |
|---|---|---|---|---|---|---|
| Rat | Immunotolerization and transplantation of a human hepatoma cell line | HBV | Positive (non-quantitative PCR)
|
At least 60 days
|
Low viremia; transplantation of cells from a hepatoma line; immunocompetent rat | (Wu et al., 2003; Wu et al., 2001)
|
| HCV | 1–2×104 copies/ml | Minimum 4 months with peak viremia at 3 months | (Wu et al., 2005) | |||
| Trimera mice | Xenograft of human liver tissue | HBV | Up to 3×105 copies/ml
|
Around 1 month with peak viremia at 18 days
|
Low viremia; dificult to access human liver tissues; survival of xenografted tissue only in an heterotopic place; immunosuppressed mice | (Ilan et al., 1999)
|
| HCV | Around 7×104copies/ml | Around 1 month with peak viremia at 18 days | (Ilan et al., 2002; Eren et al., 2006) | |||
| Alb-uPA mice combined with either: SCID, SCID/Bg, Rag2−/−, or Rag2−/−/pfp−/− trait | Human hepatocyte transplantation | HBV | Up to 2×1010 copies/ml
|
At least 15 weeks
|
High viremia; dificult to access human liver tissues; real engraftment and proliferation of human cells in mouse liver; immunosuppressed mice | (Meuleman et al., 2005)
|
| HCV | 1×104 to 8 107 copies/ml | Up to 9 months with maximum viremia from 1 month | (Mercer et al., 2001; Meuleman et al., 2005; Kneteman et al., 2006) |
Bg, beige mutation.
Fig. 5. Steps in the creation of four humanized rodent models.
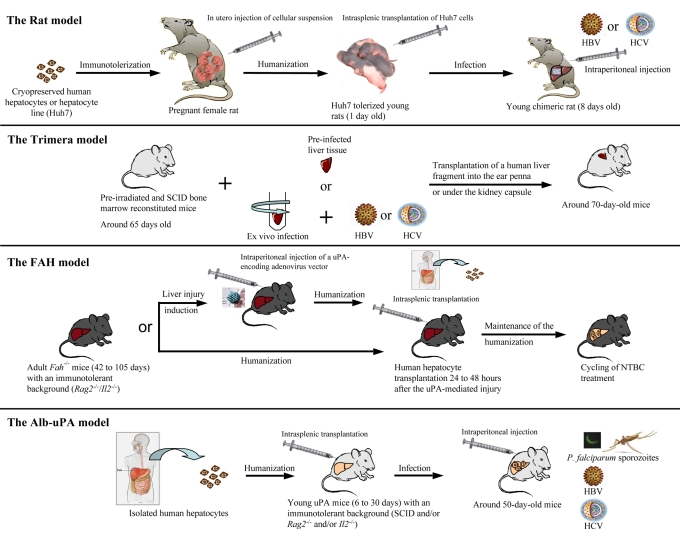
(A) The rat model: fetal rats are immunotolerized by in utero injection of human hepatocytes, new born rats receive human hepatocyte transplants and young chimeric rats are inoculated with HBV- or HCV-positive human serum. (B) The Trimera model: mice are irradiated and their bone marrow is reconstituted with immunodeficient cells, then they receive transplants with either HCV-infected or ex vivo-infected human liver fragments. (C) The FAH model: mice may or may not be injected with an adenoviral vector encoding the uPA protein. They all receive human hepatocyte transplants into the spleen and NTBC is withdrawn. (D) The Alb-uPA model: human hepatocytes are isolated, homozygous mice are then transplanted with human hepatocytes into the spleen and infected with HBV- or HCV-positive human serum.
Immunotolerization of fetal rats harboring transplanted human cells: a potential immunocompetent tool to evaluate vaccines
Taking advantage of the fact that the rat immune system does not develop until after 15–17 days of gestation, Ouyang et al. immunotolerized rat embryos to allow the transplantation and maintenance of a human hepatoma cell line (Huh7) or cryopreserved human hepatocytes (Ouyang et al., 2001). After birth, the treated animals were infected with either HBV or HCV particles (Wu et al., 2001; Wu et al., 2005). As is depicted in Fig. 5, fetal rats were tolerized, to accept the human cells by an intraperitoneal injection of cryopreserved human hepatocytes into pregnant females on the 17th day of gestation. Twenty-four hours after birth, the same cells were transplanted intrasplenically into the rats. After 14 days, the human hepatocytes represented around 6% of total hepatocytes. When the animals were inoculated with HCV-infected human serum, in the case of the Huh7 cell line, nearly 30% of the transplanted human cells (2% of total hepatocytes) were positive for the HCV core protein and subsequently developed biochemical (elevation of alanine aminotransferase concentration) and histological (presence of foci of mononuclear infiltrates) evidence of hepatitis, consistent with the human manifestations of HCV infection. Because of the immunocompetent background of the animals, this rat model is promising, especially for vaccine evaluation. However, the overall impact of the model is limited by the small percentage of infected human cells and because the viremia resulting from infection is weak (Table 5). Moreover, validation of these models, for example by confirmation of the antiviral effects of therapeutic molecules, remains necessary.
Human tissue xenotransplants in preconditioned mice to study HBV and HCV infection: the Trimera mouse model
The so-called ‘Trimera’ model involves the development of a chimeric mouse with three different tissue sources (Reisner and Dagan, 1998). Immunocompetent mice were pre-conditioned by total body irradiation before being reconstituted with SCID mouse bone marrow and then transplanted with human peripheral blood mononuclear cells (this final step is optional and is not used for evaluation of antiviral agents). Finally, these mice received transplants of either HBV- or HCV-infected liver fragments, taken directly from human patients, or ex vivo HBV- or HCV-infected liver fragments, into the ear pinna or under the kidney capsule (Fig. 5) (Galun et al., 1995; Ilan et al., 1999; Ilan et al., 2002; Eren et al., 2006). The transplants were maintained for several weeks, and HBV DNA and HCV RNA were detected in the serum for up to 1 month (Table 5).
This model can produce monoclonal antibodies (Ilan et al., 2002) and was validated as a tool for testing antiviral components. For HBV therapy, it has been used to study specific T-cell responses, vaccination strategies (Bocher et al., 2000; Bocher et al., 2001) and the therapeutic effect of monoclonal antibodies directed against HBV epitopes (Eren et al., 2000; Galun et al., 2002). In addition, concerning HCV, an inhibitor of the internal ribosomal entry site and an anti-HCV monoclonal antibody were demonstrated to act as HCV inhibitors (Ilan et al., 2002) using this model. More recently, two monoclonal antibodies directed against HCV envelope protein E2 were produced and characterized in this model. Following in vitro validation of the ability of these antibodies to immunoprecipitate HCV particles, an inhibitory effect on HCV infection was confirmed (Eren et al., 2006). Indeed, in the HCV-Trimera model, both monoclonal E2 antibodies were shown to inhibit ex vivo HCV infection of human liver fragments and both were effective for treating HCV-infected Trimera mice (Eren et al., 2006).
In conclusion, although the Trimera mouse model appears to be well-suited to produce antibodies, as well as to evaluate the inhibitory capacity of drugs, this approach does involve the use of heterotopic and xenogenic grafts, so the physiological relevance of observations need to be validated with other models. Moreover, this kind of transplantation does not allow the durable persistence of the human tissue, and gives rise to only low-level transient viremia.
Humanized FAH-deficient mice: a tool under development
As detailed above, the lethal condition of FAH deficiency can be alleviated by administering NTBC to the mice. Withdrawal of the drug after transplantation of FAH-expressing hepatocytes leads to efficient liver repopulation and counters the effects of FAH deficiency in endogenous cells. Until recently, the transplantation of human hepatocytes into this mouse was not successful: Fah−/−/nude, Fah−/−/non-obese diabetic (NOD)/SCID or Fah−/−/Rag1−/− mice did not permit persistence and repopulation by human hepatocytes (Azuma et al., 2007) (Box 4). Although nude mice lack T cells and Rag1−/− mice lack both B and T cells, both strains do still have some competent immune cells, leaving the potential for immune rejection of transplanted hepatocytes. Although Azuma et al. specified that human cell grafts do survive in the Fah−/−/NOD/SCID model, the efficiency is not great enough for the animals to tolerate withdrawal of NTBC treatment (Azuma et al., 2007).
Box 4. Genetic mutations conferring an immunotolerant background.
SCID mutation: this spontaneous mutation in the prkdc locus leads to recombination defects, resulting in a lack of B and T cells (Bosma et al., 1983).
Beige mutation: spontaneous mutation leading to an impairment of NK-cell function and defective cytotoxic-T cells and macrophages.
Rag2 knockout: targeted mutation disrupting the recombinase 2 gene (Rag2) and leading to a lack of mature T and B cells (Shinkai et al., 1992).
pfp knockout: targeted mutation disrupting the perforin 1 gene (Prf1, previously known as pore-forming protein) and leading to severe depletion of NK cells (Walsh et al., 1994).
Il2 (or γC) knockout: targeting mutation disrupting the Il2 receptor gamma chain gene, the knockout creates a lack of functional receptors for many cytokines (IL-2, IL-4, IL-7, IL-9 and IL-15) leading to impaired lymphocyte development and a lack of NK cells (DiSanto et al., 1995).
Recently two different groups have used a similar strategy to create a novel mouse model to study human liver cell transplantation. They backcrossed Rag2−/−/Il2−/− mice with Fah−/−mice to generate a new mouse model that could successfully receive transplants and be repopulated by human hepatocytes, presumably because the mice lacked not only B and T cells, but also natural killer (NK) cells (Azuma et al., 2007; Bissig et al., 2007). From these studies, differences have emerged in the methods used to obtain satisfactory repopulation. One group specified the necessity of treating the mice, by pre-injection, with an adenovirus encoding the uPA protein (Azuma et al., 2007) to allow engraftment of human cells, and found that additional treatment to control innate immunity was unnecessary. Another group did not use uPA treatment, but found that concomitant treatment of mice with the protease inhibitor nafamostat mesilate (to prevent human complement activation) and liposome molecules encapsulating a Kupffer cell toxin (clodronate) allowed for transplanted cell survival (Bissig et al., 2007). In both cases, the Fah−/−/Rag2−/−/Il2−/− mouse model was successfully transplanted and repopulated, with up to 90% of the mouse liver populated by human hepatocytes by 3 months post-transplantation. However, assuming that highly repopulated animals should possess at least 1 mg/ml of human albumin in their serum, only 16% of transplanted animals met this criterion, and this level of success was only found in the Azuma et al. study using uPA administration prior to transplantation. Interestingly, immunochemistry and histological staining provided evidence that human hepatocytes were interspersed among mouse hepatocytes and did not form individualized clones (Bissig et al., 2007). Moreover, highly humanized mice permitted long-term expansion and maintenance of human cells, and could be used to perform serial transplantation of human hepatocytes from one humanized mouse to a second generation, without requiring a new batch of human cells. Finally, the humanized livers of the mice expressed a broad range of human markers, including detoxification enzymes (Azuma et al., 2007), and thus should be useful for pharmacological studies.
The Alb-uPA immunodeficient mouse model: an efficient tool to study hepatotropic pathogens
Based on their proven utility as hosts for liver repopulation, Alb-uPA transgenic mice were backcrossed onto an immunodeficient background [SCID, Rag2−/− or Rag2−/−/Pfp−/− (perforin 1 gene knockout), see Box 4] to obtain a mouse model that tolerated the xenotransplantation of hepatocytes from humans, woodchucks and Tupaia belangeri (a tree shrew) (Dandri et al., 2001; Dandri et al., 2005a; Meuleman et al., 2005; Petersen et al., 1998; Rhim et al., 1994; Tateno et al., 2004). Because of the observed transgene deletion in mice heterozygous for the Alb-uPA transgene, optimum liver repopulation requires intrasplenic transplantation of high quality hepatocytes into immunodeficient 1–4-week-old mice that are homozygous for the Alb-uPA transgene (Mercer et al., 2001) (Table 5; Fig. 5). In these conditions, human hepatocytes engrafted and repopulated the mouse parenchyma. The resulting chimeric liver showed satisfactory hepatic architecture and intermingling of the mouse and human subcellular structures, indicating a physiological integration of transplanted cells (Meuleman et al., 2005; Tateno et al., 2004).
It was subsequently shown that this humanized mouse model could not only be used to recapitulate human infection, but also used to study various aspects of the viral life cycle of HBV and HCV. It is known that various genotypes of the HBV induce liver disease of differing severity (Schaefer, 2005). In order to improve the definition of virological differences among HBV genotypes, Sugiyama and colleagues used Alb-uPA/SCID mice as a tool to evaluate HBV DNA levels produced by different genotypes of virus, and succeeded in confirming their earlier in vitro results showing higher replication of the C versus Ae genotype (Sugiyama et al., 2006). A second group took advantage of immune suppression in the Alb-uPA/SCID mouse model to demonstrate that liver disease induced by HBV can be, at least in part, directly mediated by the virus, and is not solely the result of immune system activation (Meuleman et al., 2006). It should be noted that a drastic cytopathic effect was observed in this study since they used genotype E, which is a highly pathogenic strain of HBV, isolated from a patient with fulminant hepatitis. A third team demonstrated the ‘infectability’ of Alb-uPA/Rag2−/−/Pfp−/− mice, after either repopulation by Tupaia hepatocytes and infection with woolly monkey HBV, or repopulation by human hepatocytes and infection with human HBV. In both systems, the authors showed that treatment of repopulated mice with acylated HBV preS-derived lipopeptides prevented infection. From these results, they estimated that less than 100 μg/day of lipopeptides, administrated intracutanously, should be enough to treat patients (Petersen et al., 2008). This alternative approach could benefit patients undergoing liver transplantation to prevent vertical transmission as well as re-infection. It might also be effective in a post-exposure prophylactic strategy.
Studies in HCV-infected humanized mice demonstrated the antiviral activity of two compounds that had already been shown to be effective during clinical trials: interferon α2b (IFNα2b) and an anti-protease agent (BILN-2061) that significantly reduced HCV viremia. The antiviral effect depended on the viral genotype, but was independent of the source of human hepatocytes (Kneteman et al., 2006). Human hepatocytes, in the context of the Alb-uPA/SCID liver, maintain their ability to express numerous enzymes implicated in metabolic and detoxification (cytochrome p450 family) pathways (Katoh et al., 2007). Under these conditions the Alb-uPA model is well suited to evaluate both the antiviral potential of drugs and the potential hepato-toxicity of compounds. Recently, an independent group confirmed the antiviral properties of BILN-2061, but noted cardiotoxic side effects (Vanwolleghem et al., 2007).
As with HBV, it is clear that the immune response to viral infection plays a major role in the outcome of liver disease during HCV infection. To study the involvement of the innate immune system against viral infection, Walters et al. used the immunotolerant Alb-uPA/SCID mouse model to analyze transcriptome profiles of HCV-infected versus non-infected mice (Walters et al., 2006). HCV infection in the Alb-uPA/SCID mouse model activates the transcription of IFN-stimulated genes that are implicated in establishing the innate immune response, and thus active in the inhibition of HCV replication. As previously shown in HCV-infected patients and HCV transgenic mice, Walters et al. confirmed the relationship between severe HCV infection and perturbation of lipid metabolism in the liver of Alb-uPA/SCID mice. These observations indicate that the innate immune response plays a fundamental role in the pathogenesis of HBV and HCV infection, rather than liver disease being mediated exclusively by an HCV-specific adaptive immune response. Since we know that vertical transmission of viral infection occurs more frequently with HBV than HCV (Box 3), Alb-uPA/SCID mice should provide an adequate model to evaluate the role of the innate immune response against this mode of infection by both hepatitis viruses.
Infection by the P. falciparum parasite is restricted to humans and closely related species. Numerous studies have used models in which humanized mice carry human erythrocytes (for a review, see Moreno et al., 2007). Recently, sporozoites, which are the hepatic form of the parasite [for a review of the P. falciparum life cycle see Greenwood et al. (Greenwood et al., 2008)], were used to infect chimeric livers of humanized Alb-uPA/SCID mice. The reduction of the innate immune response by anti-macrophage and anti-NK cell treatments both enhanced the humanization of Alb-uPA/SCID mouse livers and allowed the infection of human hepatocytes by sporozoites, while promoting the maturation of the pathogen (Morosan et al., 2006). This new model should permit the evaluation of drugs specifically directed against the hepatic stage of the infection. Moreover, this model provides a starting point to create a future humanized model allowing study of the entire parasite cycle.
Overview of the information on liver replacement and rodent models for humanized livers
Requirement for on-going liver damage to stimulate cell proliferation
A common feature of all of these models (Table 6) is the failure of transplanted cells to proliferate in the absence of a proliferation stimulus. The necessary stimulus is most easily achieved by PH but is short-lived (2–4 weeks). In addition, the liver resection must occur simultaneously with, or precede, cell transplantation (Oertel et al., 2006). Alternatively, in mouse models created by FAH deficiency or in Alb-uPA transgenic mice, liver damage is continuous and does not require experimental intervention. The duration of liver damage in Alb-uPA animals is a function of the mouse genotype. Most investigators have worked with animals that are hemizygous for the transgene; in this case, rare hepatocytes delete the transgene and the resulting corrected cells are selected for in the diseased liver, repopulating the liver within 12 weeks and resulting in an environment of competitive regeneration. Corrected cells are more rare in animals that are homozygous for the transgene and essentially do not compete with transplanted cells. This effect has become important for successfully obtaining humanized livers. For homozygotes, the absence of transplanted liver cells is usually lethal and depends on the rare occurrence of transgene deletion/silencing (Table 6).
Table 6.
Characteristics of the models for liver regeneration/replacement
| Model and its nature | Proliferation induction (window for use) | Selective advantage for exogenous hepatocytes/cells | Advantages/caveats | Reference |
|---|---|---|---|---|
| Alb-uPA tg mice: intrinsic hepatocyte toxicity from Tg product | Tg hemizygous (2–6 weeks); Tg homozygous: (permanent until lethality) | Strong transitory; strong permanent | Competition with Tg-deleted endogenous hepatocytes; reproductive problems for homozygotes | (Rhim et al., 1994) |
| Fah−/− mice: conditionally lethal (NTBC blocks toxicity) | Permanent | Strong permanent | Recessive mutation: selects for products of cell fusion if wild- type Fah allele in transplanted cells | (Overturf et al., 1996) |
| DPPIV-deficient rats and mice (to receive DPPIV+ donor cells) | Must be provided | None | Powerful positive staining highlights even rare cells | (Gupta et al., 1995) |
| PH | ≤1 month (before Tx) | None | Can reveal long-term intrinsic selective cellular advantage | (Laconi et al., 1998) |
| PH + retrosine or AAF | ≤1 month + persistent inhibition of hepatocyte proliferation | Strong permanent | Host liver histology abnormal | (Laconi et al., 1998) |
| PH + CCl4 or allyl alcohol | ≤1 month + hepatocyte toxicity | Strong | Hepatocyte toxicity level can be controlled by dose; repeated treatments possible | |
| Recipient mouse sensitive to Jo2 MAB, donor resistant to apoptosis (Bcl2 overexpressed) | Within 24 hours of MAB administration | Strong | Variability of antibody activity | (Mignon et al., 1998) |
| Intrafetal injection | Intrinsic in fetus | None | Absence of immune response | (Almeida-Porada et al., 2004) |
Tg, transgene or transgenic;
Tx, transplantation;
MAB, monoclonal antibody.
One exception to the requirement for liver damage to stimulate donor hepatocyte proliferation has been observed with bone marrow to hepatocyte transition in FAH-deficient animals. However, the recipients’ hematopoietic system needed to be rescued after total body irradiation, and it was the progeny of these transferred cells that then conferred a wild-type Fah allele by cell fusion.
Stem cells versus adult hepatocytes or embryonic liver cells for liver repopulation
At first, it might seem that stem cells would be more appropriate to regenerate a liver than a distinct population of liver tissue cells. However, the pioneering work of Sandgren et al. and Rhim et al. demonstrated that some cells of the liver, presumably hepatocytes, possessed sufficient proliferative capacity to replace the liver tissue (Sandgren et al., 1991; Rhim et al., 1994). Judging from the numbers of regeneration nodules, it was calculated that 12–16 cell generations were required for replacement. This number of doubling events was significantly enhanced in serial repopulation experiments by Overturf et al., who estimated that a minimum of 69 rounds of hepatoctye doubling were required to repopulate a single liver through 6 serial transfers (Overturf et al., 1997). Thus, it is not an intrinsic limitation of replication capacity that limits the usefulness of hepatocytes for liver regeneration, but rather their restricted availability, especially with human hepatocytes.
The surprising proliferative potential of rat E14 (FLEP) embryonic liver cells was brought to light by the work of Sandhu et al. (Sandhu et al., 2001). Using just PH as a proliferation stimulus, they compared the growth of E14 and adult liver cells. The differences were most apparent several months after hepatectomy, when the proliferative stimulus was no longer operative – adult hepatocytes had ceased growing, whereas embryonic cells continued to expand throughout the 6-month experiment, replacing healthy endogenous host hepatocytes. This intrinsic difference in growth potential between transplanted adult and fetal liver cells is of great interest, and the conservation of this phenomenon in human cells should be explored.
Liver-derived stem cell lines
Stem cell lines are beginning to be used with success for liver transplant experiments and it remains to be seen whether they will prove to be as robust as freshly isolated liver cells for long-term repopulation. For the preparation of humanized liver models, it would be a major advantage to be able to use cells from a tissue culture flask rather than await availability of fresh human hepatocytes. The emergence of malignant cells in stem cell lines is a disconcerting possibility but should not preclude their use for the development of experimental animals.
Differentiation plasticity and transdifferentiation of stem cells
There has been enormous enthusiasm about the possibility of using stem cells, particularly from humans, to promote tissue repair. The demand for donor liver greatly exceeds the supply. Alternatively, stem cells could be obtained from the afflicted individuals themselves, or from an immunotyped stem cell bank. The lesson obtained with the FAH model and liver replacement by HSCs has put us in a stronger position to devise therapeutic strategies in a rational manner. In particular, future experiments must be designed with appropriate genetic markers to address the question: transdifferentiation or cell fusion?
Although direct transplantation of bone marrow stem cells, cord blood cells and derivatives of ES cells, induced to undergo liver differentiation, is currently under investigation, it is too early to judge the effectiveness and future potential of these approaches. It will be particularly important to attempt such experiments using the best available models.
Advantages and drawbacks of humanized rodent models
The recent development of small animal models for experimental HBV, HCV or Plasmodium infection has opened new avenues for the evaluation of novel therapeutic and/or prophylactic compounds against these pathogens. Indeed, the rodent models are truly promising and each one has its own advantages and drawbacks. All are relatively complicated to use, but rodent models present the unquestionable advantage of being cheaper, and easier to maintain and breed than primates. The rat model may be the most accessible, notably because of their immunocompetency and the large number of reproducibly infected animals that could be obtained following transplantation of human cells from a cell line. The three mouse models described above are more physiologically relevant in that they are based on transplantation of freshly isolated human tissue or primary hepatocytes. The Alb-uPA and FAH models are certainly the most closely related, physiologically, to humans. Even if these models are developed in different immunotolerant settings, the humanized liver may contain up to 90% of human hepatocytes compared with just 6% of hepatoma cells in the rat (Table 5).
Until recently, the FAH model did not tolerate the transplantation of human cells. Backcrosses of the Fah−/− mouse onto the Rag2−/−/Il2−/− immunotolerant background resolved this problem (Fig. 6) and led to the emergence of a novel humanized mouse model for the study of pharmacological compounds. Several properties favor this new model over the Alb-uPA mouse. First, the transgenic Alb-uPA model can lose the transgene through DNA recombination, whereas in the Fah−/− model, exon 5 of the Fah gene is deleted, so the knockout mouse is not subject to spontaneous reversion. The Fah−/− mice can receive transplants at any time, whereas Alb-uPA mice must receive transplants during a narrow time window (between 4–30 days after birth). Another disadvantage of the Alb-uPA model is its poor breeding efficacy, although this can be overcome by conducting syngeneic wild-type hepatocyte transplants, thus correcting the hepatic defect of Alb-uPA mice (Brezillon et al., 2008).
Fig. 6. Steps in the creation of rodent models for liver cell transplantation.
The arrow represents a timeline. Each box represents an independent initial study describing a rodent model to study liver cell transplantation. Boxes connected to the timeline through the same line indicate that the studies were published in the same year.
In the FAH model, NTBC treatment is simple (it is added to drinking water), but experience is required for dealing with treatment cycles to ensure optimum repopulation; and to date, only a few laboratories have used this model. Above all, no HBV, HCV or P. falciparum infecitons has been reported in the Fah−/−/Rag2−/−/Il2−/− model. However, in the near future, we predict that this model should provide an additional tool for therapeutic studies. To date, HBV and HCV viremias clearly last longer and achieve higher levels in Alb-uPA/SCID mice than in other models (Table 5). Moreover, the tractability of this model for studying the hepatic stages of P. falciparum is clear. At present, all of these observations lead us to conclude that the Alb-uPA transgene, combined with the appropriate immunodeficiency genes, appears to constitute the most relevant humanized model for providing a bridge between in vitro research and clinical trials.
ACKNOWLEDGEMENTS
The authors acknowledge numerous fruitful discussions on liver repopulation with colleagues, in particular Hélène Gilgenkrantz, Jacques-Emmanuel Guidotti, Serban Morosan and Hélène Strick-Marchand.
Footnotes
COMPETING INTERESTS
The authors declare no competing financial interests.
REFERENCES
- Abdel Aziz MT, Atta HM, Mahfouz S, Fouad HH, Roshdy NK, Ahmed HH, Rashed LA, Sabry D, Hassouna AA, Hasan NM. (2007). Therapeutic potential of bone marrow-derived mesenchymal stem cells on experimental liver fibrosis. Clin. Biochem. 40, 893–899 [DOI] [PubMed] [Google Scholar]
- Almeida-Porada G, Porada CD, Chamberlain J, Torabi A, Zanjani ED. (2004). Formation of human hepatocytes by human hematopoietic stem cells in sheep. Blood 104, 2582–2590 [DOI] [PubMed] [Google Scholar]
- Alter MJ. (2007). Epidemiology of hepatitis C virus infection. World J. Gastroenterol. 13, 2436–2441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurich I, Mueller LP, Aurich H, Luetzkendorf J, Tisljar K, Dollinger MM, Schormann W, Walldorf J, Hengstler JG, Fleig WE, et al. (2007). Functional integration of hepatocytes derived from human mesenchymal stem cells into mouse livers. Gut 56, 405–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma H, Paulk N, Ranade A, Dorrell C, Al-Dhalimy M, Ellis E, Strom S, Kay MA, Finegold M, Grompe M. (2007). Robust expansion of human hepatocytes in Fah-/-/Rag2-/-/Il2rg-/-mice. Nat. Biotechnol. 25, 903–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beerheide W, von Mach MA, Ringel M, Fleckenstein C, Schumann S, Renzing N, Hildebrandt A, Brenner W, Jensen O, Gebhard S, et al. (2002). Downregulation of beta2-microglobulin in human cord blood somatic stem cells after transplantation into livers of SCID-mice: an escape mechanism of stem cells? Biochem. Biophys. Res. Commun. 294, 1052–1063 [DOI] [PubMed] [Google Scholar]
- Bissig KD, Le TT, Woods NB, Verma IM. (2007). Repopulation of adult and neonatal mice with human hepatocytes: a chimeric animal model. Proc. Natl. Acad. Sci. USA 104, 20507–20511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocher WO, Galun E, Marcus H, Daudi N, Terkieltaub D, Shouval D, Lohr HF, Reisner Y. (2000). Reduced hepatitis B virus surface antigen-specific Th1 helper cell frequency of chronic HBV carriers is associated with a failure to produce antigen-specific antibodies in the trimera mouse. Hepatology 31, 480–487 [DOI] [PubMed] [Google Scholar]
- Bocher WO, Dekel B, Schwerin W, Geissler M, Hoffmann S, Rohwer A, Arditti F, Cooper A, Bernhard H, Berrebi A, et al. (2001). Induction of strong hepatitis B virus (HBV) specific T helper cell and cytotoxic T lymphocyte responses by therapeutic vaccination in the trimera mouse model of chronic HBV infection. Eur. J. Immunol. 31, 2071–2079 [DOI] [PubMed] [Google Scholar]
- Bosma GC, Custer RP, Bosma MJ. (1983). A severe combined immunodeficiency mutation in the mouse. Nature 301, 527–530 [DOI] [PubMed] [Google Scholar]
- Brezillon NM, DaSilva L, L’Hôte D, Bernex F, Piquet J, Binart N, Morosan S, Kremsdorf D. (2008). Rescue of fertility in homozygous mice for the urokinase plasminogen activator transgene by the transplantation of mouse hepatocytes. Cell Transplant. (in press) [DOI] [PubMed] [Google Scholar]
- Bright H, Carroll AR, Watts PA, Fenton RJ. (2004). Development of a GB virus B marmoset model and its validation with a novel series of hepatitis C virus NS3 protease inhibitors. J. Virol. 78, 2062–2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JJ, Parashar B, Moshage H, Tanaka KE, Engelhardt D, Rabbani E, Roy-Chowdhury N, Roy-Chowdhury J. (2000). A long-term hepatitis B viremia model generated by transplanting nontumorigenic immortalized human hepatocytes in Rag-2-deficient mice. Hepatology 31, 173–181 [DOI] [PubMed] [Google Scholar]
- Brulport M, Schormann W, Bauer A, Hermes M, Elsner C, Hammersen FJ, Beerheide W, Spitkovsky D, Hartig W, Nussler A, et al. (2007). Fate of extrahepatic human stem and precursor cells after transplantation into mouse livers. Hepatology 46, 861–870 [DOI] [PubMed] [Google Scholar]
- Camargo FD, Finegold M, Goodell MA. (2004). Hematopoietic myelomonocytic cells are the major source of hepatocyte fusion partners. J. Clin. Invest. 113, 1266–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campard D, Lysy PA, Najimi M, Sokal EM. (2008). Native umbilical cord matrix stem cells express hepatic markers and differentiate into hepatocyte-like cells. Gastroenterology 134, 833–848 [DOI] [PubMed] [Google Scholar]
- Dabeva MD, Petkov PM, Sandhu J, Oren R, Laconi E, Hurston E, Shafritz DA. (2000). Proliferation and differentiation of fetal liver epithelial progenitor cells after transplantation into adult rat liver. Am. J. Pathol. 156, 2017–2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan YY, Riehle KJ, Lazaro C, Teoh N, Haque J, Campbell JS, Fausto N. (2006). Isolation of multipotent progenitor cells from human fetal liver capable of differentiating into liver and mesenchymal lineages. Proc. Natl. Acad. Sci. USA 103, 9912–9917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandri M, Burda MR, Torok E, Pollok JM, Iwanska A, Sommer G, Rogiers X, Rogler CE, Gupta S, Will H, et al. (2001). Repopulation of mouse liver with human hepatocytes and in vivo infection with hepatitis B virus. Hepatology 33, 981–988 [DOI] [PubMed] [Google Scholar]
- Dandri M, Burda MR, Zuckerman DM, Wursthorn K, Matschl U, Pollok JM, Rogiers X, Gocht A, Kock J, Blum HE, et al. (2005a). Chronic infection with hepatitis B viruses and antiviral drug evaluation in uPA mice after liver repopulation with tupaia hepatocytes. J. Hepatol. 42, 54–60 [DOI] [PubMed] [Google Scholar]
- Dandri M, Volz TK, Lutgehetmann M, Petersen J. (2005b). Animal models for the study of HBV replication and its variants. J. Clin. Virol. 34 Suppl. 1, S54–S62 [DOI] [PubMed] [Google Scholar]
- Danet GH, Luongo JL, Butler G, Lu MM, Tenner AJ, Simon MC, Bonnet DA. (2002). C1qRp defines a new human stem cell population with hematopoietic and hepatic potential. Proc. Natl. Acad. Sci. USA 99, 10441–10445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Bonzo LV, Ferrero I, Cravanzola C, Mareschi K, Rustichell D, Novo E, Sanavio F, Cannito S, Zamara E, Bertero M, et al. (2008). Human mesenchymal stem cells as a two-edged sword in hepatic regenerative medicine: engraftment and hepatocyte differentiation versus profibrogenic potential. Gut 57, 223–231 [DOI] [PubMed] [Google Scholar]
- Di Campli C, Piscaglia AC, Rutella S, Bonanno G, Vecchio FM, Zocco MA, Monego G, Michetti F, Mancuso S, Pola P, et al. (2005). Improvement of mortality rate and decrease in histologic hepatic injury after human cord blood stem cell infusion in a murine model of hepatotoxicity. Transplant. Proc. 37, 2707–2710 [DOI] [PubMed] [Google Scholar]
- DiSanto JP, Muller W, Guy-Grand D, Fischer A, Rajewsky K. (1995). Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor gamma chain. Proc. Natl. Acad. Sci. USA 92, 377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eren R, Ilan E, Nussbaum O, Lubin I, Terkieltaub D, Arazi Y, Ben-Moshe O, Kitchinzky A, Berr S, Gopher J, et al. (2000). Preclinical evaluation of two human anti-hepatitis B virus (HBV) monoclonal antibodies in the HBV-trimera mouse model and in HBV chronic carrier chimpanzees. Hepatology 32, 588–596 [DOI] [PubMed] [Google Scholar]
- Eren R, Landstein D, Terkieltaub D, Nussbaum O, Zauberman A, Ben-Porath J, Gopher J, Buchnick R, Kovjazin R, Rosenthal-Galili Z, et al. (2006). Preclinical evaluation of two neutralizing human monoclonal antibodies against hepatitis C virus (HCV): a potential treatment to prevent HCV reinfection in liver transplant patients. J. Virol. 80, 2654–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evarts RP, Nagy P, Marsden E, Thorgeirsson SS. (1987). A precursor-product relationship exists between oval cells and hepatocytes in rat liver. Carcinogenesis 8, 1737–1740 [DOI] [PubMed] [Google Scholar]
- Fang B, Shi M, Liao L, Yang S, Liu Y, Zhao RC. (2004). Systemic infusion of FLK1(+) mesenchymal stem cells ameliorate carbon tetrachloride-induced liver fibrosis in mice. Transplantation 78, 83–88 [DOI] [PubMed] [Google Scholar]
- Fausto N. (2004). Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology 39, 1477–1487 [DOI] [PubMed] [Google Scholar]
- Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F. (1998). Muscle regeneration by bone marrow-derived myogenic progenitors. Science 279, 1528–1530 [DOI] [PubMed] [Google Scholar]
- Fournier C, Sureau C, Coste J, Ducos J, Pageaux G, Larrey D, Domergue J, Maurel P. (1998). In vitro infection of adult normal human hepatocytes in primary culture by hepatitis C virus. J. Gen. Virol. 79 (Pt 10), 2367–2374 [DOI] [PubMed] [Google Scholar]
- Fujino H, Hiramatsu H, Tsuchiya A, Niwa A, Noma H, Shiota M, Umeda K, Yoshimoto M, Ito M, Heike T, et al. (2007). Human cord blood CD34+ cells develop into hepatocytes in the livers of NOD/SCID/gamma(c)null mice through cell fusion. FASEB J. 21, 3499–3510 [DOI] [PubMed] [Google Scholar]
- Galun E, Burakova T, Ketzinel M, Lubin I, Shezen E, Kahana Y, Eid A, Ilan Y, Rivkind A, Pizov G, et al. (1995). Hepatitis C virus viremia in SCID->BNX mouse chimera. J. Infect. Dis. 172, 25–30 [DOI] [PubMed] [Google Scholar]
- Galun E, Eren R, Safadi R, Ashour Y, Terrault N, Keeffe EB, Matot E, Mizrachi S, Terkieltaub D, Zohar M, et al. (2002). Clinical evaluation (phase I) of a combination of two human monoclonal antibodies to HBV: safety and antiviral properties. Hepatology 35, 673–679 [DOI] [PubMed] [Google Scholar]
- Greenwood BM, Fidock DA, Kyle DE, Kappe SH, Alonso PL, Collins FH, Duffy PE. (2008). Malaria: progress, perils, and prospects for eradication. J. Clin. Invest. 118, 1266–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gripon P, Diot C, Theze N, Fourel I, Loreal O, Brechot C, Guguen-Guillouzo C. (1988). Hepatitis B virus infection of adult human hepatocytes cultured in the presence of dimethyl sulfoxide. J. Virol. 62, 4136–4143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gripon P, Rumin S, Urban S, Le Seyec J, Glaise D, Cannie I, Guyomard C, Lucas J, Trepo C, Guguen-Guillouzo C. (2002). Infection of a human hepatoma cell line by hepatitis B virus. Proc. Natl. Acad. Sci. USA 99, 15655–15660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grompe M, al-Dhalimy M, Finegold M, Ou CN, Burlingame T, Kennaway NG, Soriano P. (1993). Loss of fumarylacetoacetate hydrolase is responsible for the neonatal hepatic dysfunction phenotype of lethal albino mice. Genes Dev. 7, 2298–2307 [DOI] [PubMed] [Google Scholar]
- Grompe M, Lindstedt S, al-Dhalimy M, Kennaway NG, Papaconstantinou J, Torres-Ramos CA, Ou CN, Finegold M. (1995). Pharmacological correction of neonatal lethal hepatic dysfunction in a murine model of hereditary tyrosinaemia type I. Nat. Genet. 10, 453–460 [DOI] [PubMed] [Google Scholar]
- Gupta S, Rajvanshi P, Lee CD. (1995). Integration of transplanted hepatocytes into host liver plates demonstrated with dipeptidyl peptidase IV-deficient rats. Proc. Natl. Acad. Sci. USA 92, 5860–5864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RG, Herzog EL, Bruscia EM, Grove JE, Van Arnam JS, Krause DS. (2004). Lack of a fusion requirement for development of bone marrow-derived epithelia. Science 305, 90–93 [DOI] [PubMed] [Google Scholar]
- Heckel JL, Sandgren EP, Degen JL, Palmiter RD, Brinster RL. (1990). Neonatal bleeding in transgenic mice expressing urokinase-type plasminogen activator. Cell 62, 447–456 [DOI] [PubMed] [Google Scholar]
- Heo J, Factor VM, Uren T, Takahama Y, Lee JS, Major M, Feinstone SM, Thorgeirsson SS. (2006). Hepatic precursors derived from murine embryonic stem cells contribute to regeneration of injured liver. Hepatology 44, 1478–1486 [DOI] [PubMed] [Google Scholar]
- Herrera MB, Bruno S, Buttiglieri S, Tetta C, Gatti S, Deregibus MC, Bussolati B, Camussi G. (2006). Isolation and characterization of a stem cell population from adult human liver. Stem Cells 24, 2840–2850 [DOI] [PubMed] [Google Scholar]
- Ilan E, Burakova T, Dagan S, Nussbaum O, Lubin I, Eren R, Ben-Moshe O, Arazi J, Berr S, Neville L, et al. (1999). The hepatitis B virus-trimera mouse: a model for human HBV infection and evaluation of anti-HBV therapeutic agents. Hepatology 29, 553–562 [DOI] [PubMed] [Google Scholar]
- Ilan E, Arazi J, Nussbaum O, Zauberman A, Eren R, Lubin I, Neville L, Ben-Moshe O, Kischitzky A, Litchi A, et al. (2002). The hepatitis C virus (HCV)-Trimera mouse: a model for evaluation of agents against HCV. J. Infect. Dis. 185, 153–161 [DOI] [PubMed] [Google Scholar]
- Jang YY, Collector MI, Baylin SB, Diehl AM, Sharkis SJ. (2004). Hematopoietic stem cells convert into liver cells within days without fusion. Nat. Cell Biol. 6, 532–539 [DOI] [PubMed] [Google Scholar]
- Kakinuma S, Tanaka Y, Chinzei R, Watanabe M, Shimizu-Saito K, Hara Y, Teramoto K, Arii S, Sato C, Takase K, et al. (2003). Human umbilical cord blood as a source of transplantable hepatic progenitor cells. Stem Cells 21, 217–227 [DOI] [PubMed] [Google Scholar]
- Kashofer K, Siapati EK, Bonnet D. (2006). In vivo formation of unstable heterokaryons after liver damage and hematopoietic stem cell/progenitor transplantation. Stem Cells 24, 1104–1112 [DOI] [PubMed] [Google Scholar]
- Katoh M, Sawada T, Soeno Y, Nakajima M, Tateno C, Yoshizato K, Yokoi T. (2007). In vivo drug metabolism model for human cytochrome P450 enzyme using chimeric mice with humanized liver. J. Pharm. Sci. 96, 428–437 [DOI] [PubMed] [Google Scholar]
- Kneteman NM, Weiner AJ, O’Connell J, Collett M, Gao T, Aukerman L, Kovelsky R, Ni ZJ, Hashash A, Kline J, et al. (2006). Anti-HCV therapies in chimeric scid-Alb/uPA mice parallel outcomes in human clinical application. Hepatology 43, 1346–1353 [DOI] [PubMed] [Google Scholar]
- Korbling M, Estrov Z. (2003). Adult stem cells for tissue repair-a new therapeutic concept? N. Engl. J. Med. 349, 570–582 [DOI] [PubMed] [Google Scholar]
- Kremsdorf D, Brezillon N. (2007). New animal models for hepatitis C viral infection and pathogenesis studies. World J. Gastroenterol. 13, 2427–2435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo TK, Hung SP, Chuang CH, Chen CT, Shih YR, Fang SC, Yang VW, Lee OK. (2008). Stem cell therapy for liver disease: parameters governing the success of using bone marrow mesenchymal stem cells. Gastroenterology 134, 2111–2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laconi E, Oren R, Mukhopadhyay DK, Hurston E, Laconi S, Pani P, Dabeva MD, Shafritz DA. (1998). Long-term, near-total liver replacement by transplantation of isolated hepatocytes in rats treated with retrorsine. Am. J. Pathol. 153, 319–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, Grompe M. (2000). Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat. Med. 6, 1229–1234 [DOI] [PubMed] [Google Scholar]
- Lavanchy D. (2004). Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J. Viral. Hepat. 11, 97–107 [DOI] [PubMed] [Google Scholar]
- Lindstedt S, Holme E, Lock EA, Hjalmarson O, Strandvik B. (1992). Treatment of hereditary tyrosinaemia type I by inhibition of 4-hydroxyphenylpyruvate dioxygenase. Lancet 340, 813–817 [DOI] [PubMed] [Google Scholar]
- Mazier D, Beaudoin RL, Mellouk S, Druilhe P, Texier B, Trosper J, Miltgen F, Landau I, Paul C, Brandicourt O, et al. (1985). Complete development of hepatic stages of Plasmodium falciparum in vitro. Science 227, 440–442 [DOI] [PubMed] [Google Scholar]
- Meuleman P, Libbrecht L, De Vos R, de Hemptinne B, Gevaert K, Vandekerckhove J, Roskams T, Leroux-Roels G. (2005). Morphological and biochemical characterization of a human liver in a uPA-SCID mouse chimera. Hepatology 41, 847–856 [DOI] [PubMed] [Google Scholar]
- Meuleman P, Libbrecht L, Wieland S, De Vos R, Habib N, Kramvis A, Roskams T, Leroux-Roels G. (2006). Immune suppression uncovers endogenous cytopathic effects of the hepatitis B virus. J. Virol. 80, 2797–2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer DF, Schiller DE, Elliott JF, Douglas DN, Hao C, Rinfret A, Addison WR, Fischer KP, Churchill TA, Lakey JR, et al. (2001). Hepatitis C virus replication in mice with chimeric human livers. Nat. Med. 7, 927–933 [DOI] [PubMed] [Google Scholar]
- Mignon A, Guidotti JE, Mitchell C, Fabre M, Wernet A, De La Coste A, Soubrane O, Gilgenkrantz H, Kahn A. (1998). Selective repopulation of normal mouse liver by Fas/CD95-resistant hepatocytes. Nat. Med. 4, 1185–1188 [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Hardjo M, Masaka T, Tomiyama K, Mahmut N, Medina RJ, Niida A, Sonegawa H, Du G, Yong R, et al. (2007). Isolation of a bone marrow-derived stem cell line with high proliferation potential and its application for preventing acute fatal liver failure. Stem Cells 25, 2855–2863 [DOI] [PubMed] [Google Scholar]
- Moran-Jimenez MJ, Garcia-Bravo M, Mendez M, Gutierrez-Vera I, Grau M, Navarro-Ordonez S, Fontanellas A, Enriquez-de-Salamanca R. (2008). Bone marrow transplantation into hemochromatotic mice decreases hepatic and duodenal iron overload. Int. J. Biochem. Cell Biol. 40, 135–146 [DOI] [PubMed] [Google Scholar]
- Moreno A, Perignon JL, Morosan S, Mazier D, Benito A. (2007). Plasmodium falciparum-infected mice: more than a tour de force. Trends Parasitol. 23, 254–259 [DOI] [PubMed] [Google Scholar]
- Morosan S, Hez-Deroubaix S, Lunel F, Renia L, Giannini C, Van Rooijen N, Battaglia S, Blanc C, Eling W, Sauerwein R, et al. (2006). Liver-stage development of Plasmodium falciparum, in a humanized mouse model. J. Infect. Dis. 193, 996–1004 [DOI] [PubMed] [Google Scholar]
- Nam JH, Faulk K, Engle RE, Govindarajan S, St Claire M, Bukh J. (2004). In vivo analysis of the 33 untranslated region of GB virus B after in vitro mutagenesis of an infectious cDNA clone: persistent infection in a transfected tamarin. J. Virol. 78, 9389–9399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome PN, Johannessen I, Boyle S, Dalakas E, McAulay KA, Samuel K, Rae F, Forrester L, Turner ML, Hayes PC, et al. (2003). Human cord blood-derived cells can differentiate into hepatocytes in the mouse liver with no evidence of cellular fusion. Gastroenterology 124, 1891–1900 [DOI] [PubMed] [Google Scholar]
- Nonome K, Li XK, Takahara T, Kitazawa Y, Funeshima N, Yata Y, Xue F, Kanayama M, Shinno E, Kuwae C, et al. (2005). Human umbilical cord blood-derived cells differentiate into hepatocyte-like cells in the Fas-mediated liver injury model. Am. J. Physiol. Gastrointest. Liver Physiol. 289, G1091–G1099 [DOI] [PubMed] [Google Scholar]
- Oertel M, Menthena A, Dabeva MD, Shafritz DA. (2006). Cell competition leads to a high level of normal liver reconstitution by transplanted fetal liver stem/progenitor cells. Gastroenterology 130, 507–520; quiz 590 [DOI] [PubMed] [Google Scholar]
- Oertel M, Menthena A, Chen YQ, Teisner B, Jensen CH, Shafritz DA. (2008). Purification of fetal liver stem/progenitor cells containing all the repopulation potential for normal adult rat liver. Gastroenterology 134, 823–832 [DOI] [PubMed] [Google Scholar]
- Oh SH, Witek RP, Bae SH, Zheng D, Jung Y, Piscaglia AC, Petersen BE. (2007). Bone marrow-derived hepatic oval cells differentiate into hepatocytes in 2-acetylaminofluorene/partial hepatectomy-induced liver regeneration. Gastroenterology 132, 1077–1087 [DOI] [PubMed] [Google Scholar]
- Ohashi K, Marion PL, Nakai H, Meuse L, Cullen JM, Bordier BB, Schwall R, Greenberg HB, Glenn JS, Kay MA. (2000). Sustained survival of human hepatocytes in mice: a model for in vivo infection with human hepatitis B and hepatitis delta viruses. Nat. Med. 6, 327–331 [DOI] [PubMed] [Google Scholar]
- Ouyang EC, Wu CH, Walton C, Promrat K, Wu GY. (2001). Transplantation of human hepatocytes into tolerized genetically immunocompetent rats. World J. Gastroenterol. 7, 324–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overturf K, Al-Dhalimy M, Tanguay R, Brantly M, Ou CN, Finegold M, Grompe M. (1996). Hepatocytes corrected by gene therapy are selected in vivo in a murine model of hereditary tyrosinaemia type I. Nat. Genet. 12, 266–273 [DOI] [PubMed] [Google Scholar]
- Overturf K, al-Dhalimy M, Ou CN, Finegold M, Grompe M. (1997). Serial transplantation reveals the stem-cell-like regenerative potential of adult mouse hepatocytes. Am. J. Pathol. 151, 1273–1280 [PMC free article] [PubMed] [Google Scholar]
- Overturf K, Al-Dhalimy M, Finegold M, Grompe M. (1999). The repopulation potential of hepatocyte populations differing in size and prior mitotic expansion. Am. J. Pathol. 155, 2135–2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. (1999). Bone marrow as a potential source of hepatic oval cells. Science 284, 1168–1170 [DOI] [PubMed] [Google Scholar]
- Petersen J, Dandri M, Gupta S, Rogler CE. (1998). Liver repopulation with xenogenic hepatocytes in B and T cell-deficient mice leads to chronic hepadnavirus infection and clonal growth of hepatocellular carcinoma. Proc. Natl. Acad. Sci. USA 95, 310–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen J, Dandri M, Mier W, Lutgehetmann M, Volz T, von Weizsacker F, Haberkorn U, Fischer L, Pollok JM, Erbes B, et al. (2008). Prevention of hepatitis B virus infection in vivo by entry inhibitors derived from the large envelope protein. Nat. Biotechnol. 26, 335–341 [DOI] [PubMed] [Google Scholar]
- Qian H, Wang J, Wang S, Gong Z, Chen M, Ren Z, Huang S. (2006). In utero transplantation of human hematopoietic stem/progenitor cells partially repairs injured liver in mice. Int. J. Mol. Med. 18, 633–642 [PubMed] [Google Scholar]
- Reisner Y, Dagan S. (1998). The Trimera mouse: generating human monoclonal antibodies and an animal model for human diseases. Trends Biotechnol. 16, 242–246 [DOI] [PubMed] [Google Scholar]
- Rhim JA, Sandgren EP, Degen JL, Palmiter RD, Brinster RL. (1994). Replacement of diseased mouse liver by hepatic cell transplantation. Science 263, 1149–1152 [DOI] [PubMed] [Google Scholar]
- Rhim JA, Sandgren EP, Palmiter RD, Brinster RL. (1995). Complete reconstitution of mouse liver with xenogeneic hepatocytes. Proc. Natl. Acad. Sci. USA 92, 4942–4946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijnbrand R, Yang Y, Beales L, Bodola F, Goettge K, Cohen L, Lanford RE, Lemon SM, Martin A. (2005). A chimeric GB virus B with 5′ nontranslated RNA sequence from hepatitis C virus causes hepatitis in tamarins. Hepatology 41, 986–994 [DOI] [PubMed] [Google Scholar]
- Sandgren EP, Palmiter RD, Heckel JL, Daugherty CC, Brinster RL, Degen JL. (1991). Complete hepatic regeneration after somatic deletion of an albumin-plasminogen activator transgene. Cell 66, 245–256 [DOI] [PubMed] [Google Scholar]
- Sandhu JS, Petkov PM, Dabeva MD, Shafritz DA. (2001). Stem cell properties and repopulation of the rat liver by fetal liver epithelial progenitor cells. Am. J. Pathol. 159, 1323–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Araki H, Kato J, Nakamura K, Kawano Y, Kobune M, Sato T, Miyanishi K, Takayama T, Takahashi M, et al. (2005). Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion. Blood 106, 756–763 [DOI] [PubMed] [Google Scholar]
- Schaefer S. (2005). Hepatitis B virus: significance of genotypes. J. Viral. Hepat. 12, 111–124 [DOI] [PubMed] [Google Scholar]
- Schmelzer E, Wauthier E, Reid LM. (2006). The phenotypes of pluripotent human hepatic progenitors. Stem Cells 24, 1852–1858 [DOI] [PubMed] [Google Scholar]
- Schmelzer E, Zhang L, Bruce A, Wauthier E, Ludlow J, Yao HL, Moss N, Melhem A, McClelland R, Turner W, et al. (2007). Human hepatic stem cells from fetal and postnatal donors. J. Exp. Med. 204, 1973–1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sells MA, Chen ML, Acs G. (1987). Production of hepatitis B virus particles in Hep G2 cells transfected with cloned hepatitis B virus DNA. Proc. Natl. Acad. Sci. USA 84, 1005–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafritz DA, Oertel M, Menthena A, Nierhoff D, Dabeva MD. (2006). Liver stem cells and prospects for liver reconstitution by transplanted cells. Hepatology 43, S89–S98 [DOI] [PubMed] [Google Scholar]
- Sharma AD, Cantz T, Richter R, Eckert K, Henschler R, Wilkens L, Jochheim-Richter A, Arseniev L, Ott M. (2005). Human cord blood stem cells generate human cytokeratin 18-negative hepatocyte-like cells in injured mouse liver. Am. J. Pathol. 167, 555–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma AD, Cantz T, Vogel A, Schambach A, Haridass D, Iken M, Bleidissel M, Manns MP, Scholer HR, Ott M. (2008). Murine embryonic stem cell-derived hepatic progenitor cells engraft in recipient livers with limited capacity of liver tissue formation. Cell Transplant. 17, 313–323 [DOI] [PubMed] [Google Scholar]
- Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM, et al. (1992). RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 68, 855–867 [DOI] [PubMed] [Google Scholar]
- Shyu MK, Yuan RH, Shih JC, Wu MZ, Chen HL, Kuo YC, Chien CL, Chow LP, Chen HL, Hsieh FJ. (2007). Kinetics and functional assay of liver repopulation after human cord blood transplantation. Dig. Liver Dis. 39, 455–465 [DOI] [PubMed] [Google Scholar]
- Simper-Ronan R, Brilliant K, Flanagan D, Carreiro M, Callanan H, Sabo E, Hixson DC. (2006). Cholangiocyte marker-positive and -negative fetal liver cells differ significantly in their ability to regenerate the livers of adult rats exposed to retrorsine. Development 133, 4269–4279 [DOI] [PubMed] [Google Scholar]
- Song S, Witek RP, Lu Y, Choi YK, Zheng D, Jorgensen M, Li C, Flotte TR, Petersen BE. (2004). Ex vivo transduced liver progenitor cells as a platform for gene therapy in mice. Hepatology 40, 918–924 [DOI] [PubMed] [Google Scholar]
- Strick-Marchand H, Morosan S, Charneau P, Kremsdorf D, Weiss MC. (2004). Bipotential mouse embryonic liver stem cell lines contribute to liver regeneration and differentiate as bile ducts and hepatocytes. Proc. Natl. Acad. Sci. USA 101, 8360–8365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama M, Tanaka Y, Kato T, Orito E, Ito K, Acharya SK, Gish RG, Kramvis A, Shimada T, Izumi N, et al. (2006). Influence of hepatitis B virus genotypes on the intra- and extracellular expression of viral DNA and antigens. Hepatology 44, 915–924 [DOI] [PubMed] [Google Scholar]
- Sun Y, Xiao D, Zhang RS, Cui GH, Wang XH, Chen XG. (2007). Formation of human hepatocyte-like cells with different cellular phenotypes by human umbilical cord blood-derived cells in the human-rat chimeras. Biochem. Biophys. Res. Commun. 357, 1160–1165 [DOI] [PubMed] [Google Scholar]
- Suzuki A, Zheng YW, Kaneko S, Onodera M, Fukao K, Nakauchi H, Taniguchi H. (2002). Clonal identification and characterization of self-renewing pluripotent stem cells in the developing liver. J. Cell Biol. 156, 173–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe Y, Tajima F, Nakamura Y, Shibasaki E, Wakejima M, Shimomura T, Murai R, Murawaki Y, Hashiguchi K, Kanbe T, et al. (2004). Analyses to clarify rich fractions in hepatic progenitor cells from human umbilical cord blood and cell fusion. Biochem. Biophys. Res. Commun. 324, 711–718 [DOI] [PubMed] [Google Scholar]
- Tateno C, Yoshizane Y, Saito N, Kataoka M, Utoh R, Yamasaki C, Tachibana A, Soeno Y, Asahina K, Hino H, et al. (2004). Near completely humanized liver in mice shows human-type metabolic responses to drugs. Am. J. Pathol. 165, 901–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, Meyer EM, Morel L, Petersen BE, Scott EW. (2002). Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature 416, 542–545 [DOI] [PubMed] [Google Scholar]
- Theise ND, Badve S, Saxena R, Henegariu O, Sell S, Crawford JM, Krause DS. (2000). Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology 31, 235–240 [DOI] [PubMed] [Google Scholar]
- Turrini P, Monego G, Gonzalez J, Cicuzza S, Bonanno G, Zelano G, Rosenthal N, Paonessa G, Laufer R, Padron J. (2005). Human hepatocytes in mice receiving pre-immune injection with human cord blood cells. Biochem. Biophys. Res. Commun. 326, 66–73 [DOI] [PubMed] [Google Scholar]
- van de Ven C, Collins D, Bradley MB, Morris E, Cairo MS. (2007). The potential of umbilical cord blood multipotent stem cells for nonhematopoietic tissue and cell regeneration. Exp. Hematol. 35, 1753–1765 [DOI] [PubMed] [Google Scholar]
- Vanwolleghem T, Meuleman P, Libbrecht L, Roskams T, De Vos R, Leroux-Roels G. (2007). Ultra-rapid cardiotoxicity of the hepatitis C virus protease inhibitor BILN 2061 in the urokinase-type plasminogen activator mouse. Gastroenterology 133, 1144–1155 [DOI] [PubMed] [Google Scholar]
- Vassilopoulos G, Wang PR, Russell DW. (2003). Transplanted bone marrow regenerates liver by cell fusion. Nature 422, 901–904 [DOI] [PubMed] [Google Scholar]
- Wakita T, Pietschmann T, Kato T, Date T, Miyamoto M, Zhao Z, Murthy K, Habermann A, Krausslich HG, Mizokami M, et al. (2005). Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat. Med. 11, 791–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh CM, Matloubian M, Liu CC, Ueda R, Kurahara CG, Christensen JL, Huang MT, Young JD, Ahmed R, Clark WR. (1994). Immune function in mice lacking the perforin gene. Proc. Natl. Acad. Sci. USA 91, 10854–10858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters KA, Joyce MA, Thompson JC, Proll S, Wallace J, Smith MW, Furlong J, Tyrrell DL, Katze MG. (2006). Application of functional genomics to the chimeric mouse model of HCV infection: optimization of microarray protocols and genomics analysis. Virol. J. 3, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Montini E, Al-Dhalimy M, Lagasse E, Finegold M, Grompe M. (2002). Kinetics of liver repopulation after bone marrow transplantation. Am. J. Pathol. 161, 565–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Grompe M. (2003a). The origin and liver repopulating capacity of murine oval cells. Proc. Natl. Acad. Sci. USA 100 Suppl. 1, 11881–11888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Olson S, Grompe M. (2003b). Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature 422, 897–901 [DOI] [PubMed] [Google Scholar]
- Wang X, Ge S, McNamara G, Hao QL, Crooks GM, Nolta JA. (2003c). Albumin-expressing hepatocyte-like cells develop in the livers of immune-deficient mice that received transplants of highly purified human hematopoietic stem cells. Blood 101, 4201–4208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willenbring H, Bailey AS, Foster M, Akkari Y, Dorrell C, Olson S, Finegold M, Fleming WH, Grompe M. (2004). Myelomonocytic cells are sufficient for therapeutic cell fusion in liver. Nat. Med. 10, 744–748 [DOI] [PubMed] [Google Scholar]
- Wu CH, Ouyang EC, Walton CM, Wu GY. (2001). Human hepatocytes transplanted into genetically immunocompetent rats are susceptible to infection by hepatitis B virus in situ. J. Viral. Hepat. 8, 111–119 [DOI] [PubMed] [Google Scholar]
- Wu CH, Ouyang EC, Walton C, Promrat K, Forouhar F, Wu GY. (2003). Hepatitis B virus infection of transplanted human hepatocytes causes a biochemical and histological hepatitis in immunocopetentent rats. World J. Gastroenterol. 9, 978–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GY, Konishi M, Walton CM, Olive D, Hayashi K, Wu CH. (2005). A novel immunocompetent rat model of HCV infection and hepatitis. Gastroenterology 128, 1416–1423 [DOI] [PubMed] [Google Scholar]
- Wulf-Goldenberg A, Eckert K, Fichtner I. (2008). Cytokine-pretreatment of CD34(+) cord blood stem cells in vitro reduces long-term cell engraftment in NOD/SCID mice. Eur. J. Cell Biol. 87, 69–80 [DOI] [PubMed] [Google Scholar]
- Ying QL, Nichols J, Evans EP, Smith AG. (2002). Changing potency by spontaneous fusion. Nature 416, 545–548 [DOI] [PubMed] [Google Scholar]
- Zeng F, Chen M, Katsumata M, Huang W, Gong Z, Hu W, Qian H, Xiao Y, Ren Z, Huang S. (2005). Identification and characterization of engrafted human cells in human/goat xenogeneic transplantation chimerism. DNA Cell Biol. 24, 403–409 [DOI] [PubMed] [Google Scholar]
- Zeng F, Chen MJ, Baldwin DA, Gong ZJ, Yan JB, Qian H, Wang J, Jiang X, Ren ZR, Sun D, et al. (2006). Multiorgan engraftment and differentiation of human cord blood CD34+ Lin- cells in goats assessed by gene expression profiling. Proc. Natl. Acad. Sci. USA 103, 7801–7806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao DC, Lei JX, Chen R, Yu WH, Zhang XM, Li SN, Xiang P. (2005). Bone marrow-derived mesenchymal stem cells protect against experimental liver fibrosis in rats. World J. Gastroenterol. 11, 3431–3440 [DOI] [PMC free article] [PubMed] [Google Scholar]