Abstract
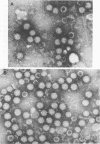
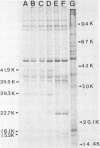
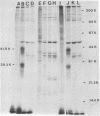
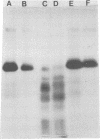
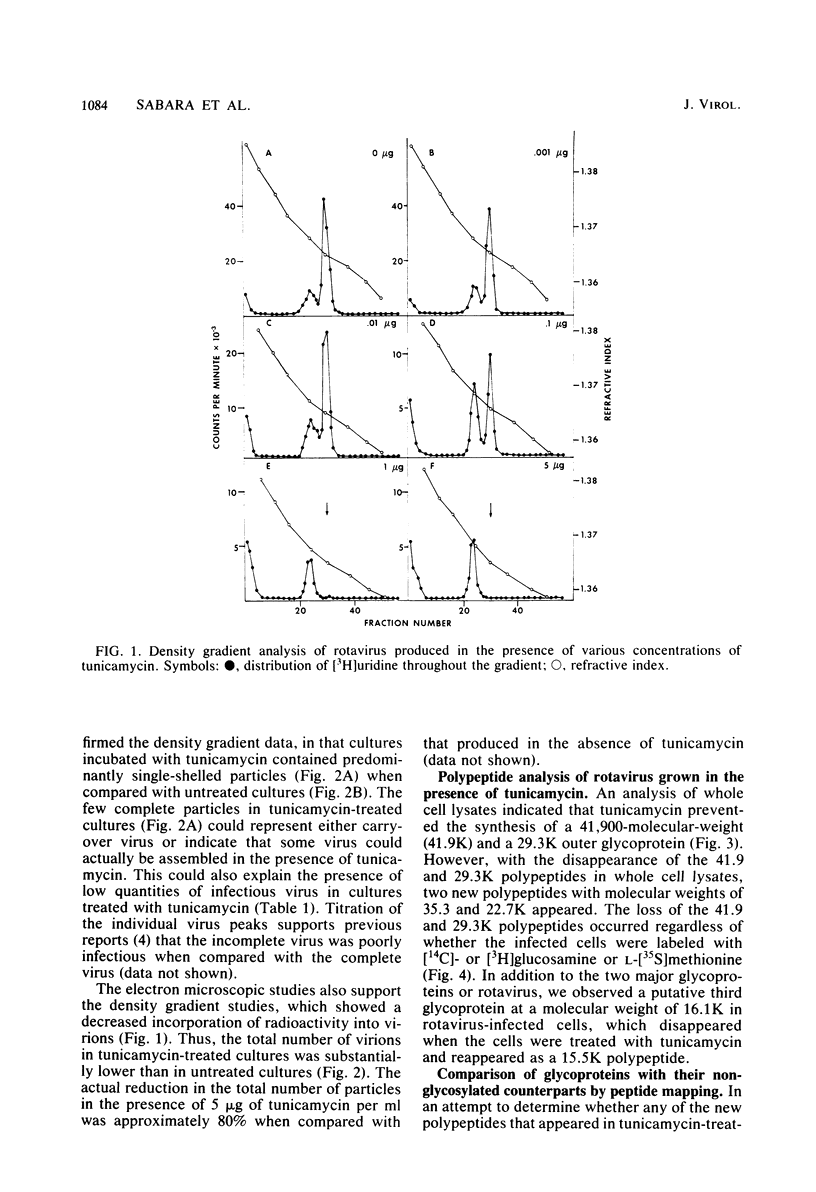
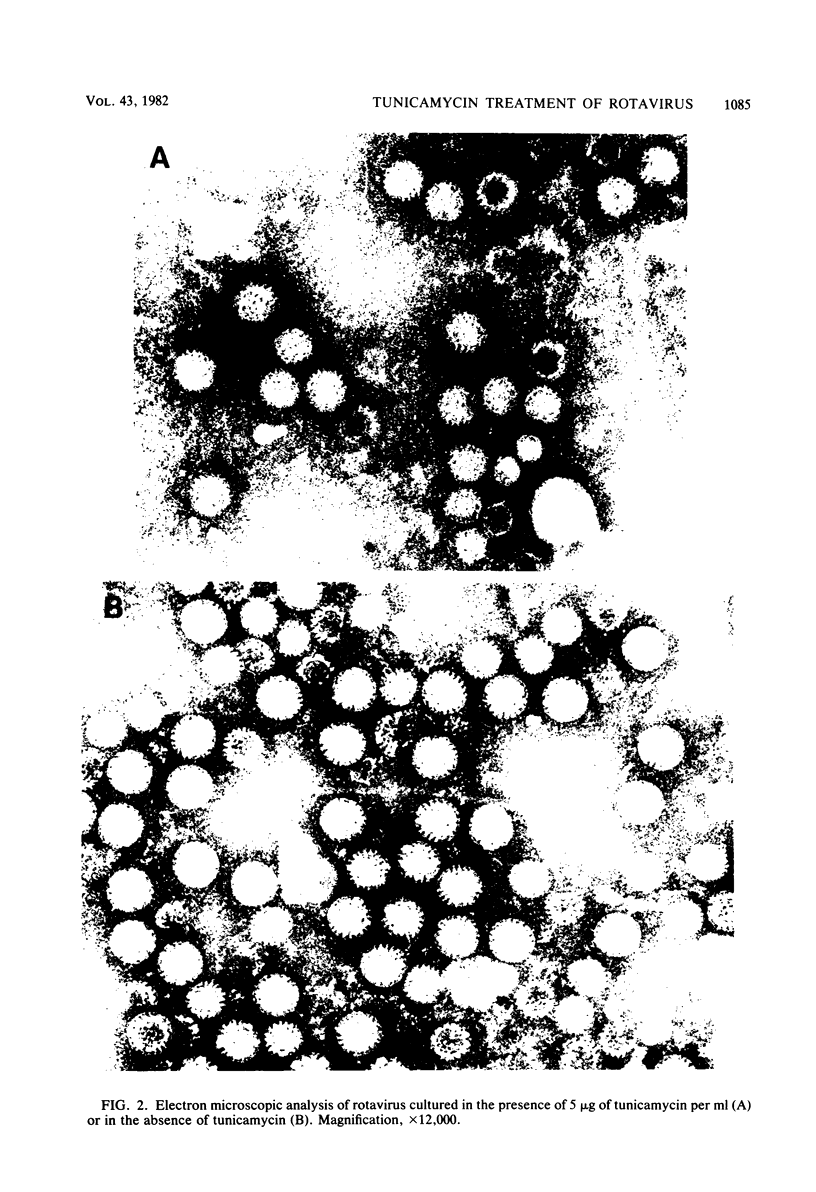
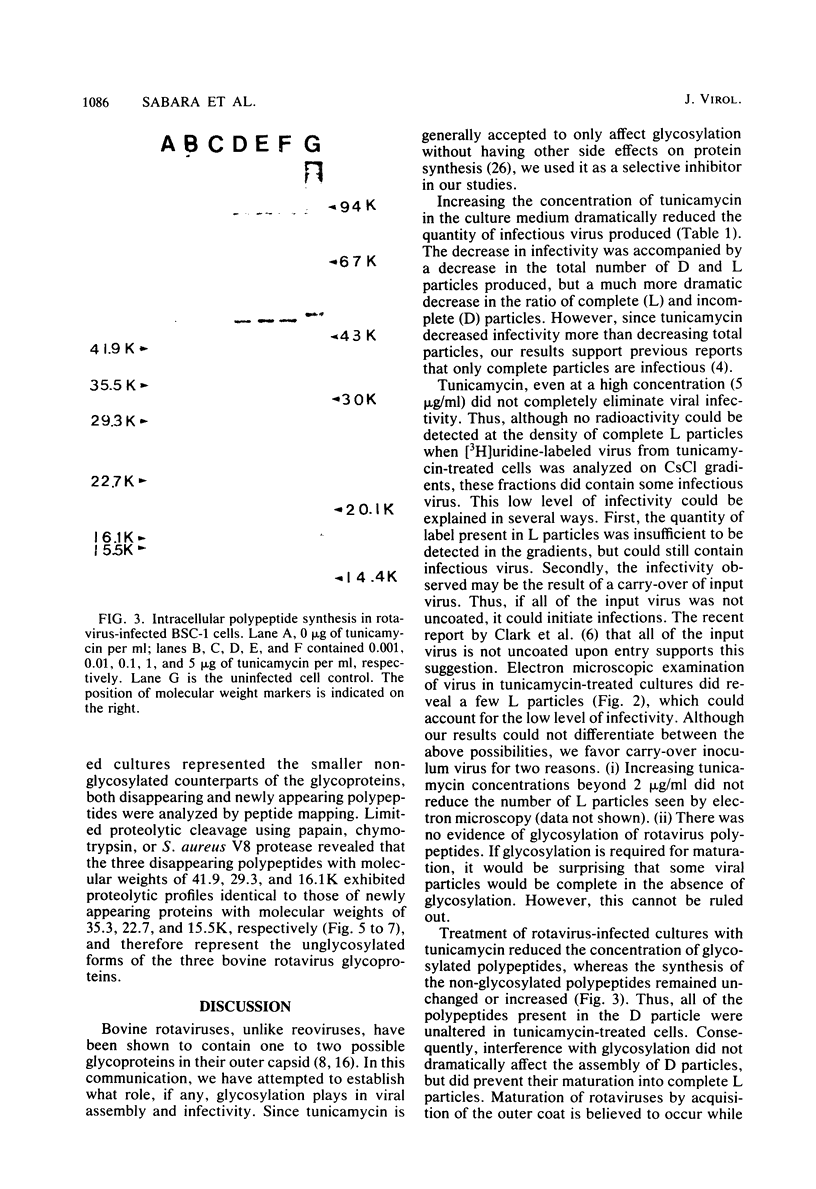
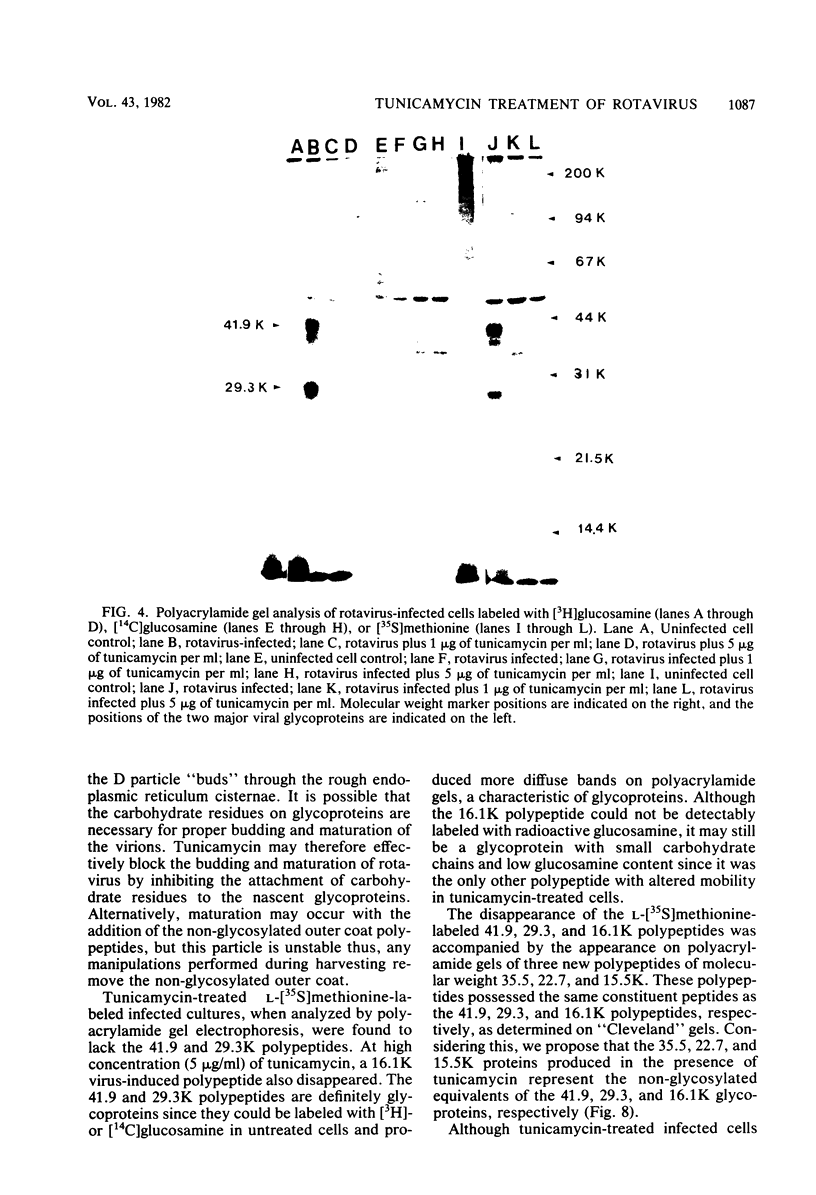
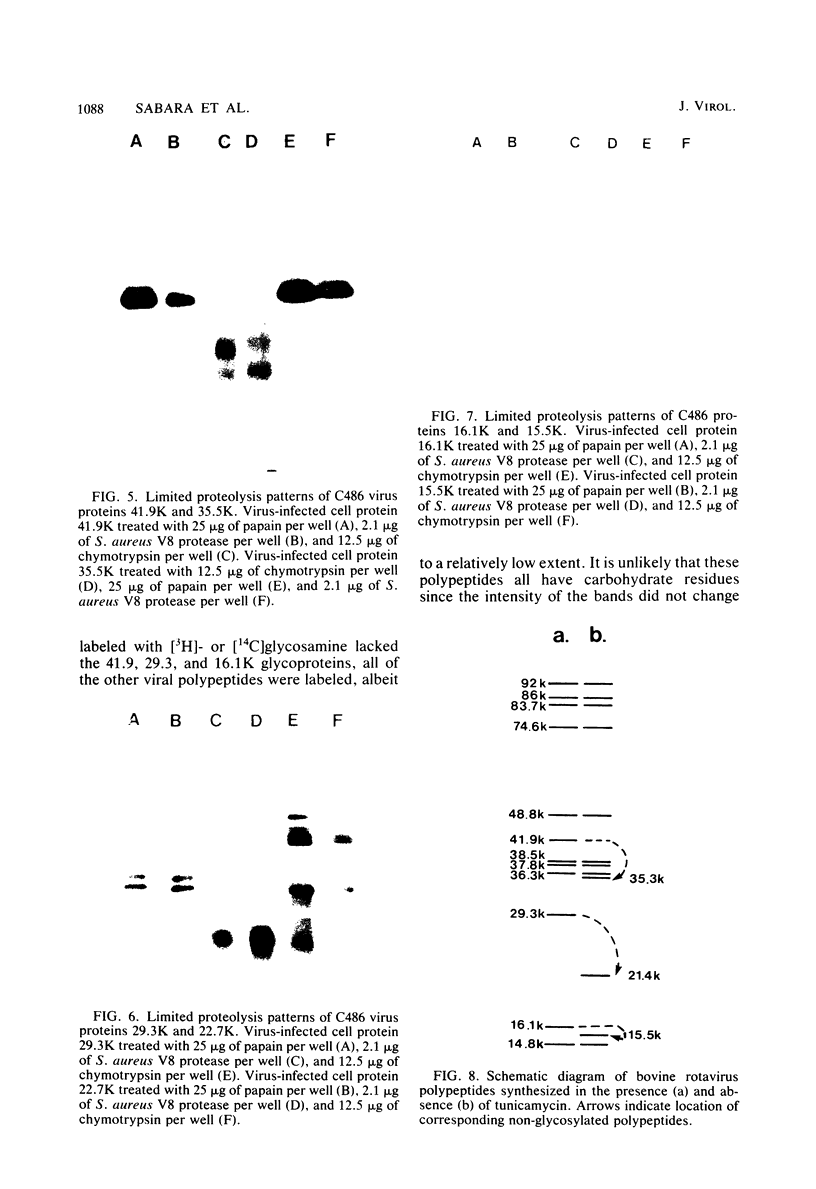
Bovine rotavirus grown in the presence or absence of tunicamycin was analyzed with respect to yield of infectious virus, the ratio of complete to incomplete particles, and polypeptide composition. Tunicamycin at a concentration of 1 microgram/ml reduced virus yields by 4 logs and completely prevented the incorporation of [3H]uridine into complete rotavirus particles, as determined by cesium chloride gradient analysis. Concomitant with a reduction in complete particles, three rotavirus polypeptides shifted in their relative position on polyacrylamide gels from 41,900-molecular-weight position (41.9K), 29.3K, and 16.1K to migrate at 35.5K, 22.7K, and 15.5K, respectively. Limited proteolysis indicated that the lower-molecular-weight polypeptides possessed the same constituent peptides as the larger polypeptides, suggesting that they represented the unglycosylated equivalents. These results suggest that interference with glycosylation prevents proper assembly of the outer coat proteins in bovine rotavirus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babiuk L. A., Acres S. D., Rouse B. T. Solid-phase radioimmunoassay for detecting bovine (neonatal calf diarrhea) rotavirus antibody. J Clin Microbiol. 1977 Jul;6(1):10–15. doi: 10.1128/jcm.6.1.10-15.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiuk L. A., Mohammed K., Spence L., Fauvel M., Petro R. Rotavirus isolation and cultivation in the presence of trypsin. J Clin Microbiol. 1977 Dec;6(6):610–617. doi: 10.1128/jcm.6.6.610-617.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdwell C. R., Strauss J. H. Replication of Sindbis virus. IV. Electron microscope study of the insertion of viral glycoproteins into the surface of infected chick cells. J Virol. 1974 Aug;14(2):366–374. doi: 10.1128/jvi.14.2.366-374.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridger J. C., Woode G. N. Characterization of two particle types of calf rotavirus. J Gen Virol. 1976 May;31(2):245–250. doi: 10.1099/0022-1317-31-2-245. [DOI] [PubMed] [Google Scholar]
- Carpio M. M., Babiuk L. A., Misra V., Blumenthal R. M. Bovine rotavirus-cell interactions: effect of virus infection on cellular integrity and macromolecular synthesis. Virology. 1981 Oct 15;114(1):86–97. doi: 10.1016/0042-6822(81)90255-5. [DOI] [PubMed] [Google Scholar]
- Clark S. M., Roth J. R., Clark M. L., Barnett B. B., Spendlove R. S. Trypsin enhancement of rotavirus infectivity: mechanism of enhancement. J Virol. 1981 Sep;39(3):816–822. doi: 10.1128/jvi.39.3.816-822.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cohen J., Laporte J., Charpilienne A., Scherrer R. Activation of rotavirus RNA polymerase by calcium chelation. Arch Virol. 1979;60(3-4):177–186. doi: 10.1007/BF01317489. [DOI] [PubMed] [Google Scholar]
- Compans R. W. Location of the glycoprotein in the membrane of Sindbis virus. Nat New Biol. 1971 Jan 27;229(4):114–116. doi: 10.1038/newbio229114a0. [DOI] [PubMed] [Google Scholar]
- Espejo R. T., López S., Arias C. Structural polypeptides of simian rotavirus SA11 and the effect of trypsin. J Virol. 1981 Jan;37(1):156–160. doi: 10.1128/jvi.37.1.156-160.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauvel M., Spence L., Babiuk L. A., Petro R., Bloch S. Hemagglutination and hemagglutination-inhibition studies with a strain of Nebraska calf diarrhea virus (bovine rotavirus). Intervirology. 1978;9(2):95–105. doi: 10.1159/000148927. [DOI] [PubMed] [Google Scholar]
- Kelley J. M., Emerson S. U., Wagner R. R. The glycoprotein of vesicular stomatitis virus is the antigen that gives rise to and reacts with neutralizing antibody. J Virol. 1972 Dec;10(6):1231–1235. doi: 10.1128/jvi.10.6.1231-1235.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal G., Perrault J., Graham A. F. Evidence for a glycoprotein in reovirus. Virology. 1976 Jul 15;72(2):308–321. doi: 10.1016/0042-6822(76)90160-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leavitt R., Schlesinger S., Kornfeld S. Tunicamycin inhibits glycosylation and multiplication of Sindbis and vesicular stomatitis viruses. J Virol. 1977 Jan;21(1):375–385. doi: 10.1128/jvi.21.1.375-385.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno S., Mukoyama A. Polypeptides of bovine rotavirus. J Gen Virol. 1979 May;43(2):309–316. doi: 10.1099/0022-1317-43-2-309. [DOI] [PubMed] [Google Scholar]
- Nagai Y., Ogura H., Klenk H. Studies on the assembly of the envelope of Newcastle disease virus. Virology. 1976 Feb;69(2):523–538. doi: 10.1016/0042-6822(76)90482-7. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Compans R. W. Effects of glucosamine, 2-deoxyglucose, and tunicamycin on glycosylation, sulfation, and assembly of influenza viral proteins. Virology. 1978 Feb;84(2):303–319. doi: 10.1016/0042-6822(78)90250-7. [DOI] [PubMed] [Google Scholar]
- Newman J. F., Brown F., Bridger J. C., Woode G. N. Characterisation of a rotavirus.20b. Nature. 1975 Dec 18;258(5536):631–633. doi: 10.1038/258631a0. [DOI] [PubMed] [Google Scholar]
- Payne L. G., Norrby E. Adsorption and penetration of enveloped and naked vaccinia virus particles. J Virol. 1978 Jul;27(1):19–27. doi: 10.1128/jvi.27.1.19-27.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizer L. I., Cohen G. H., Eisenberg R. J. Effect of tunicamycin on herpes simplex virus glycoproteins and infectious virus production. J Virol. 1980 Apr;34(1):142–153. doi: 10.1128/jvi.34.1.142-153.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger S. M., Schnagl R. D., Holmes I. H. Further biochemical characterization, including the detection of surface glycoproteins, of human, calf, and simian rotaviruses. J Virol. 1977 Oct;24(1):91–98. doi: 10.1128/jvi.24.1.91-98.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottier P. J., Horzinek M. C., van der Zeijst B. A. Viral protein synthesis in mouse hepatitis virus strain A59-infected cells: effect of tunicamycin. J Virol. 1981 Nov;40(2):350–357. doi: 10.1128/jvi.40.2.350-357.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz R. T., Rohrschneider J. M., Schmidt M. F. Suppression of glycoprotein formation of Semliki Forest, influenza, and avian sarcoma virus by tunicamycin. J Virol. 1976 Sep;19(3):782–791. doi: 10.1128/jvi.19.3.782-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Smith E. M., Estes M. K., Graham D. Y., Gerba C. P. A plaque assay for the simian rotavirus SAII. J Gen Virol. 1979 Jun;43(3):513–519. doi: 10.1099/0022-1317-43-3-513. [DOI] [PubMed] [Google Scholar]
- Smith J. F., Brown D. T. Envelopments of Sindbis virus: synthesis and organization of proteins in cells infected with wild type and maturation-defective mutants. J Virol. 1977 Jun;22(3):662–678. doi: 10.1128/jvi.22.3.662-678.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohrer R., Hunter E. Inhibition of Rous sarcoma virus replication by 2-deoxyglucose and tunicamycin: identification of an unglycosylated env gene product. J Virol. 1979 Nov;32(2):412–419. doi: 10.1128/jvi.32.2.412-419.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap K. L., Ada G. L. Cytotoxic T cells specific for influenza virus-infected target cells. Immunology. 1977 Feb;32(2):151–159. [PMC free article] [PubMed] [Google Scholar]