SUMMARY
We have identified two genes, Smed-PTEN-1 and Smed-PTEN-2, capable of regulating stem cell function in the planarian Schmidtea mediterranea. Both genes encode proteins homologous to the mammalian tumor suppressor, phosphatase and tensin homolog deleted on chromosome 10 (PTEN). Inactivation of Smed-PTEN-1 and -2 by RNA interference (RNAi) in planarians disrupts regeneration, and leads to abnormal outgrowths in both cut and uncut animals followed soon after by death (lysis). The resulting phenotype is characterized by hyperproliferation of neoblasts (planarian stem cells), tissue disorganization and a significant accumulation of postmitotic cells with impaired differentiation capacity. Further analyses revealed that rapamycin selectively prevented such accumulation without affecting the normal neoblast proliferation associated with physiological turnover and regeneration. In animals in which PTEN function is abrogated, we also detected a significant increase in the number of cells expressing the planarian Akt gene homolog (Smed-Akt). However, functional abrogation of Smed-Akt in Smed-PTEN RNAi-treated animals does not prevent cell overproliferation and lethality, indicating that functional abrogation of Smed-PTEN is sufficient to induce abnormal outgrowths. Altogether, our data reveal roles for PTEN in the regulation of planarian stem cells that are strikingly conserved to mammalian models. In addition, our results implicate this protein in the control of stem cell maintenance during the regeneration of complex structures in planarians.
INTRODUCTION
The replacement of cells that are lost to normal physiological turnover relies, in part, on mechanisms that control stem cell number and the efficient, functional integration of their progeny into differentiated tissues. Examples of such tissue homeostasis are provided by the mammalian hematopoietic and digestive systems, in which millions of differentiated blood and epithelial cells expire daily, only to be replaced by the fresh division progeny of resident stem cells (Clarke and Fuller, 2006; Reya and Clevers, 2005). Failure in these processes can lead to a range of dysfunctions, including malignant transformation.
One of the most frequently mutated genes in human cancers is the gene encoding the phosphatase and tensin homolog deleted on chromosome 10 (PTEN) protein (Sansal and Sellers, 2004; Stiles et al., 2004; Sulis and Parsons, 2003). PTEN negatively regulates the phosphatidylinositol-3 kinase (PI3K)-AKT signaling, known to play crucial roles in cellular proliferation, differentiation and migration in both vertebrates and invertebrates (Sulis and Parsons, 2003). In mammals, when PTEN activity is lost in organs displaying high rates of cell turnover, primary and metastatic cancers are detected. Germline mutations of PTEN are found in both Cowden and Bannayan-Riley-Ruvalcaba syndromes (Liaw et al., 1997; Marsh et al., 1997; Marsh et al., 1999; Nelen et al., 1997), and somatic mutations have been associated with Non-Hodgkin’s lymphoma (Nakahara et al., 1998), breast, and prostate cancers (Li et al., 1997). More recently, activation of PI3K and AKT owing to PTEN loss of function has been reported to occur not only in breast, but also in some types of pancreatic, cancers (Asano et al., 2004; Kirkegaard et al., 2005). Thus, a rapidly growing body of evidence suggests a central role for PTEN in the regulation and misregulation of tissue homeostasis in mammals (Marx, 2007).
In invertebrates such as Drosophila melanogaster and Caenorhabditis elegans, PTEN appears to mainly control cell size and cell cycle during development (Hafen, 2004; Stiles et al., 2004). In C. elegans, PTEN (daf-18) has been associated with regulation of metabolic rates, longevity and dauer formation (Gil et al., 1999; Mihaylova et al., 1999; Ogg and Ruvkun, 1998). In Drosophila, PTEN plays critical roles during development, affecting cell proliferation, and cell and organ size (Gao et al., 2000; Goberdhan et al., 1999; Huang et al., 1999; Oldham et al., 2002). Although studies of PTEN in invertebrates have been highly informative, the study of PTEN in adult tissue homeostasis remains unexplored. As such, models are not yet available to dissect the mechanisms by which human diseases involving PTEN mutations are manifested.
In contrast to flies and nematodes, the adult freshwater planarian Schmidtea mediterranea possesses an abundant population of undifferentiated cells, many of which are continuously undergoing cell division. The dividing, undifferentiated cells are known as neoblasts, and the animal derives its robust tissue homeostasis and regenerative properties from both regulating the proliferation of these cells and by determining their division progeny (Newmark and Sánchez Alvarado, 2000). Neoblasts are considered the adult stem cells in planarians because they constantly self-renew and produce daughter cells that will go on to differentiate into all known cell types found in the animal, including the germline (Newmark and Sánchez Alvarado, 2002). In fact, neoblasts are the only known proliferative cell in the flatworm, and their division progeny is instrumental in supporting, and maintaining, differentiated tissues during physiological cell turnover (Reddien and Sánchez Alvarado, 2004; Sánchez Alvarado, 2006). After amputation, planarians develop a specialized structure at the cut surface, known as a regeneration blastema, which is the result of extensive proliferation by neoblasts.
Here, we report the identification of two PTEN orthologs in planarians (Smed-PTEN-1 and Smed-PTEN-2). RNA interference (RNAi) of these genes is lethal; the phenotype is characterized by an inhibition of tissue regeneration, abnormal outgrowths associated with a neoblast-hyperproliferative response, and a lack of cell differentiation and tissue maintenance. Strikingly, we also observed that the abnormalities and lethality detected in worms with RNAi of both Smed-PTEN genes [Smed-PTEN-1, -2(RNAi) worms] could be prevented by pharmacological treatment with rapamycin (an inhibitor of the protein kinase, target of rapamycin [TOR]), without affecting physiological neoblast proliferation. Because specific upregulation of the planarian Akt-like gene (Smed-Akt) is detected in RNAi-treated animals and prevented by rapamycin treatment, it is likely that an involvement of the AKT pathway is necessary for the Smed-PTEN genes to exert their function. Together, these observations support recent evidence demonstrating that PTEN activity can help distinguish differences between normal and abnormal self-renewing mechanisms in stem cells during malignant transformation in mammals (Yilmaz et al., 2006; Zhang et al., 2006). We propose that, in planarians, PTEN modulates AKT function and that the abnormalities observed in the Smed-PTEN phenotype are due to both autonomous and non-autonomous stem cell roles of PTEN. Additionally, our data revealed that AKT is required for rapamycin to compensate for PTEN loss of function. Thus, we propose that planarians may serve as a powerful model system in which to dissect the roles of tumor suppressor activities in regulating stem cell functions.
RESULTS
Three PTEN homologs exist in S. mediterranea
All known PTEN homologs share conserved phosphatase and tensin domains (Lee et al., 1999). Initially, we identified potential homologs by performing BLAST searches against the planarian genome with both the fly and human PTEN genes. We then took the predicted planarian sequences, which contained both of the conserved domains, and carried out reciprocal BLAST searches against the human and fly genomes. This returned a list of three potential orthologs of PTEN.
In addition to PTEN, humans possess two other PTEN-like genes that contain phosphatase and tensin domains, TPIP and TPTE (Sulis and Parsons, 2003). To clearly define the orthology between planarian PTEN-like genes and the other human homologs, phylogenetic analyses were carried out (see Methods). The results indicate that planaria have two paralogous bona fide PTEN orthologs and a gene orthologous to the human TPTE and TPIP genes (Fig. 1A). cDNAs for both PTEN genes had been previously cloned as part of a cDNA sequencing project (Sánchez Alvarado et al., 2002). We will refer to these genes as Smed-PTEN-1 and Smed-PTEN-2.
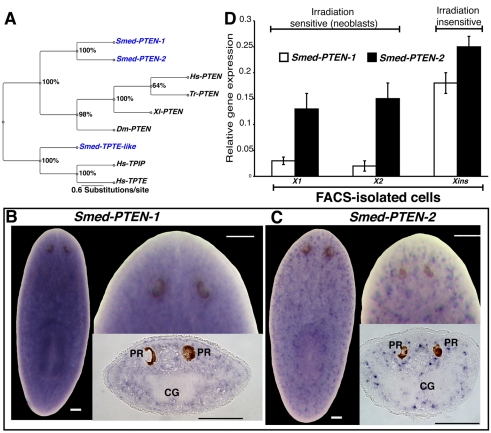
Fig. 1. Smed-PTEN phylogenetics and expression studies.
(A) Bayesian analysis of PTEN family members shown as an unrooted tree with relative branch lengths. Bootstrap support is shown for each node. Species used: Smed (Schmidtea mediterranea), Hs (Homo sapiens), Tr (Takifugu rubripes), Xl (Xenopus laevis), Dm (Drosophila melanogaster). (B, C) Representative whole-mount ISH in intact worms showing the widely distributed expression patterns (purple precipitation) for both Smed-PTEN-1 and Smed-PTEN-2, respectively. In both cases anterior is at the top; bars, 100 μm. A more detailed view of the expression patterns, including area in front of the photoreceptors, can be visualized at higher magnifications for both genes, as well as from tissue sections. PR: photoreceptors; CG: cephalic ganglia. (D) Smed-PTEN-1 and Smed-PTEN-2 qRT-PCR from different cell populations isolated by flow cytometry. Note that both genes are expressed in irradiation sensitive (X1 and X2) and insensitive (Xins) cells, consistent with the wide distribution observed in whole-mount ISH results. Expression levels are relative to the standard internal control clone (H.55.12e). Numbers are expressed as the average of triplicate experiments (± s.d.) from two different preparations.
Smed-PTEN genes are expressed in neoblasts and differentiated cells
The general expression patterns of Smed-PTEN-1 and Smed-PTEN-2 were defined using whole-mount in situ hybridizations (ISH). Expression for both genes (Fig. 1B,C) is detected throughout mesenchymal tissues (dorsal/ventral and anterior/posterior), including postmitotic areas (i.e. the area in front of the photoreceptors and the pharynx). No obvious restriction to a particular organ system was observed; however, spatial expression patterns differ in that the Smed-PTEN-1 signal is dispersed, whereas Smed-PTEN-2 expression is detected in defined clusters throughout the mesenchyme (Fig. 1B,C). Expression patterns were confirmed as specific by performing several optimization rounds of the ISH protocol and standardizing the results with control probes of known expression patterns. Additionally, ISH with sense probes in wild-type worms and antisense probes in RNAi worms, for each Smed-PTEN gene confirmed the detected expression patterns (supplementary material Fig. S1 and data not shown).
Because the spatial signal distribution precluded us from defining the cell types expressing Smed-PTEN-1 and Smed-PTEN-2, we performed quantitative real-time PCR (qRT-PCR) analyses on diverse cell populations isolated by fluorescence activated cell sorting (FACS) (Fig. 1D). We tested the irradiation-sensitive cells comprised by the X1 and X2 populations, and the irradiation-insensitive cell types represented by the Xins area of the FACS profile (Hayashi et al., 2006; Reddien et al., 2005b). These experiments revealed that expression of both Smed-PTEN genes is detectable in all three cell subpopulations (Fig. 1D). These data suggest that the expression of Smed-PTEN-1 and Smed-PTEN-2 is widespread and includes neoblasts.
Silencing of Smed-PTEN-1 and Smed-PTEN-2 results in abnormal tissue growth and lethality
RNAi experiments were performed to abolish the expression of each Smed-PTEN gene individually or simultaneously. We designed and optimized a double-stranded RNA (dsRNA) microinjection schedule that consistently reproduces a particular phenotype (see below). This procedure requires a total of five microinjections distributed through 11 days (Fig. 2A). The dsRNA was synthesized in vitro according to standard protocols (Sánchez Alvarado and Newmark, 1999) and injected at different intervals during the schedule (Fig. 2A). Throughout the experiment, all groups were extensively evaluated under the microscope and scored for anatomical and/or behavioral changes (Reddien et al., 2005a). Additionally, abrogation of gene expression was confirmed by RT-PCR and whole-mount ISH (supplementary material Fig. S1 and data not shown).
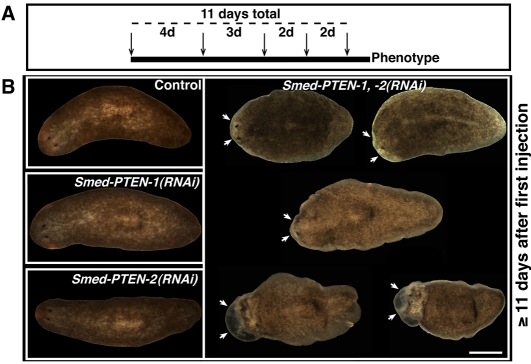
Fig. 2. Microinjection schedule and its effect in intact planarians.
(A) Microinjection schedule in intact planarians. A total of five injections (arrows) were performed during 11 days (dashed line). Injections were administered at different intervals (days), illustrated by numbers between arrows. (B) Representative images from experimental groups following 11 or more days after the first injection. Conditions shown: control (water-injected), Smed-PTEN-1(RNAi), Smed-PTEN-2(RNAi) and simultaneous Smed-PTEN-1, -2(RNAi). We did not observe any external differences between the first three conditions (left column), while in simultaneous RNAi animals important behavioral and macroscopic abnormalities were noted. Initial signs of the phenotypes (illustrated from top left to bottom, white arrows) began with lethargic movements and tissue regression in front of the photoreceptors, followed by development of external outgrowths and lysis. In all cases anterior end is to the left. Bar, 500 μm.
Representative results from different groups of animals, 12 days after the first microinjection, are shown in Fig. 2B. Planarians injected with water (control), dsRNA-Smed-PTEN-1 or dsRNA-Smed-PTEN-2 were indistinguishable from each other (Fig. 2B left panels). In contrast, all Smed-PTEN-1, -2(RNAi) worms (n>100) displayed behavioral and anatomical changes followed by death (100% penetrance). Signs of the phenotype began with a slowing of movements and a lack of normal photophobic response, followed by reabsorbtion of the most anterior area in front of the photoreceptors (head regression). Head regression in planarians is associated with impaired neoblast function (Guo et al., 2006; Reddien et al., 2005a; Reddien et al., 2005b); however, this phenotype is normally accompanied by a characteristic ventral curling, which did not occur at any time in Smed-PTEN-1, -2(RNAi) worms. Instead, as the phenotype progressed, abnormal growths became evident in different body parts, but tended to be more severe at anterior ends (Fig. 2B, arrows). These abnormal growths increased rapidly in size and number, and worms eventually began to lyse and die three to four days after the first obvious signs of the phenotype (supplementary material Table S1). In the majority of cases, these changes were most evident after the final injection (11±2.2 days after the first injection, average ± standard deviation [s.d.]); representative images of the phenotype (12 days after first injection) with different degrees of severity are shown in Fig. 2B (right panel). These data indicate that Smed-PTEN-1 and Smed-PTEN-2 functionally compensate each other, and that these genes play a role in regulating proper neoblast functions that may differ from those observed following either irradiation or silencing of smedwi-2 (a gene involved in the regulation of planarian stem cells) (Reddien et al., 2005b).
Smed-PTEN-1 and Smed-PTEN-2 are required for regeneration
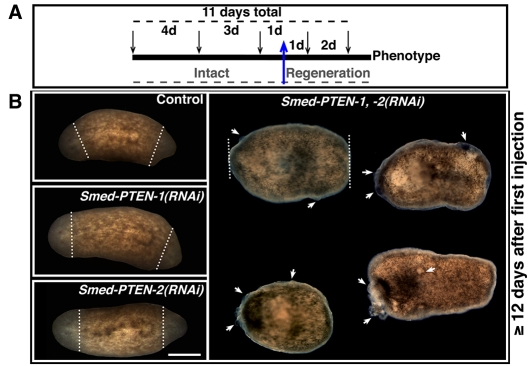
To determine whether Smed-PTEN genes play a role during regeneration, we performed an RNAi time-course strategy similar to that carried out in intact worms, except that animals were amputated at pre/postpharyngeal levels one day after the third injection (Fig. 3A). Trunk fragments resulting from these amputations received two additional injections (days 1 and 3 post-amputation); subsequent behavioral and anatomical changes were evaluated (Reddien et al., 2005a). Animals subjected to either Smed-PTEN-1(RNAi) or Smed-PTEN-2(RNAi) developed anterior and posterior regeneration blastemas, with macroscopic regenerative dynamics that were indistinguishable from those observed in control animals (i.e. normal wound healing, blastema formation, photoreceptor pigmentation, photophobic response, etc.; Fig. 3B, left panels). In contrast, the Smed-PTEN-1, -2(RNAi) worms showed normal wound healing, but failed to form blastemas and by approximately the second day post-amputation (10±0.8 days after the first injection) signs of the phenotype (no blastema formation, scattered outgrowths) were evident in all animals (n=47/47). These abnormal growths multiplied and within one week after amputation (6.25±3.3 days) all animals were dead (Fig. 3B, right panel). These results are consistent with the effect observed for Smed-PTEN-1, -2(RNAi) in intact worms (supplementary material Table S1). Furthermore, in both uncut and regenerating animals, overt signs of the phenotype were noted at similar times after the first injection (~11 days; Student’s t-test, P=0.25), suggesting that under these conditions amputation does not influence phenotype dynamics. These results support our previous conclusion that Smed-PTEN-1 and Smed-PTEN-2 are required for proper neoblast function and indicate that the planarian PTEN orthologs are necessary for blastema formation.
Fig. 3. Microinjection schedule and its effects during tissue regeneration.
(A) Microinjection schedule. A total of five injections (arrows) were performed during 11 days (top dashed line). Injections were administered at different intervals (numbers between arrows). The blue arrow depicts the stage at which bipolar amputation was performed and the dashed line (gray) at the bottom highlights the experimentation period during which animals were either intact or under regeneration. (B) Representative images of experimental groups between three and eight days post-amputation (the amputation level is represented by the white dashed line). Conditions shown: control (water-injected), Smed-PTEN-1(RNAi), Smed-PTEN-2(RNAi), and simultaneous Smed-PTEN-1, -2(RNAi). No external differences were observed during anterior or posterior blastema formation (unpigmented tissue formed after amputation) between the first three conditions (left column), whereas in simultaneous RNAi fragments, important behavioral and macroscopic abnormalities were noted (n=47/47 worms, similar numbers for each group were run in parallel). Abnormal outgrowths (white arrows) were evidenced in different parts of the animal (including the tissue surrounding the amputation area) followed by lysis shortly after visualization of the first sign of the phenotype. In all cases anterior is to the left. Bar, 500 μm.
Disorganization of differentiated tissues and cellular infiltration occur in the absence of PTEN function
We sought to better understand the cellular basis for the observed phenotype in Smed-PTEN-1, -2(RNAi) animals. We first performed fluorescent immunohistochemistry with antibodies specific to differentiated tissues, specifically neurons, muscle and cilia (Cebrià et al., 1996; Robb and Sánchez Alvarado, 2002; Sakai et al., 2000). Results from these experiments revealed unexpected and severe disruptions to the anatomical and functional integrity of these tissues (Fig. 4). Photoreceptor neurons were found in disarray, and were often dispersed and lacking optic chiasmatas (Fig. 4A); body wall musculature displayed areas devoid of myosin positive cells (Fig. 4B); and the surface area of dorsal cilia fields was reduced (Fig. 4C). Such defects are consistent with the idea that tissue maintenance by neoblasts and their progeny are compromised after silencing of Smed-PTEN-1 and Smed-PTEN-2.
Fig. 4. The Smed-PTEN phenotype is characterized by a lack of tissue maintenance, tissue architecture disorganization and neoblast hyperproliferation.
(A–C) Representative confocal images (anterior, dorsal views) from control (water-injected) worms (left column in A–C) and Smed-PTEN-1, -2(RNAi) worms (right column), respectively. Immunostaining was performed by using different tissue markers: neuronal marker (anti-arrestin, VC-1) (A); muscle (anti-myosin heavy chain, TMUS-13) (B); and epithelium (anti-α acetylated tubulin) (C) antibodies, respectively. White arrows are used to indicate morphological disruptions. Bar, 100 μm. (D, E) Tissue disorganization and cellular infiltration in the Smed-PTEN phenotype. Representative images of paraffin-tissue sections stained with hematoxylin and eosin from control (D) and Smed-PTEN-1, -2(RNAi) (E) worms showing similar anatomical areas, illustrating dorsal epithelium (prepharyngeal area) and pharynx. Green arrowheads in the top panel of (D) indicate the columnar epithelium and basement membrane of control animals. The green arrowheads in the top panel of (E) denote the multiple cell layers in the epithelium and the disruption of basement membrane in Smed-PTEN-1, -2(RNAi) animals. The bottom panels illustrate cross-sections of the dorsal epithelium of the pharyngeal area, along with part of the pharynx (the large ovoid-shaped structure). Note the cellular infiltration in the pharynx of Smed-PTEN-1, -2(RNAi) worms, with cells possessing morphology similar to that described for neoblasts (i.e. rounded cells with large nuclei and scant cytoplasm), consistent with a neoblastoma (Best and Morita, 1982). Bars, 20 μm. (F) Cross sections (10 μm thickness) of the anterior end, with nuclei counterstaining (Sytox green) in both control worms (top) and Smed-PTEN-1, -2(RNAi) worms >12 days after the first dsRNA injection (bottom). Notice the anatomical disruption (white arrow) and the multiple cell layers of the epithelium (insets). Numbers represent the total count from nuclei counterstaining in control (n=730) and Smed-PTEN-1, -2(RNAi) worms (n=902), after a pool of worms (n=10) were dissociated (average of cells from three experiments ± s.d.). Dorsal side is to the top. CG: cephalic ganglia; GVS: gastrovascular system. Bar, 0.2 mm. (G) Representative images of whole-mount immunostaining using α-phosphorylated histone H3 (H3P) antibody in intact worms (12 days after first injection). Control (water-injected) and Smed-PTEN-1, -2(RNAi) worms are shown. (G′) H3P-signal quantification from both control and simultaneous Smed-PTEN-1, -2(RNAi) worms. A significant increase (P<0.0001) in mitotic activity was observed in RNAi treated animals. Mitotic activity in control worms and following individual Smed-PTEN RNAi treatments was qualitatively and quantitatively indistinguishable between the groups (data not shown). Results represent average ± s.d. from n≥10 worms in each condition.
Histological analyses of RNAi-treated animals not only confirmed the tissue disorganization detected by immunohistology, but also uncovered discontinuities in the basement membrane (BM) and the presence of abnormal invasive cells (Fig. 4D,E). The BM (extracellular matrix underneath the epithelial cells; Fig. 4D, lower arrows) separates the epithelium from the underlying mesenchyme (Nelson and Bissell, 2006). In the Smed-PTEN-1, -2(RNAi) animals, the integrity of the BM is markedly disrupted, and in some areas a physical barrier separating epithelial cells and mesenchymal tissues cannot be detected (Fig. 4E, top right). Such direct contact between epithelium and mesenchyme is also observed in planarians during normal wound healing after amputation and is known to be involved in the proliferation of nearby neoblasts (Hori, 1979a; Hori, 1979b; Hori, 1980). This may explain, in part, the overproliferation of cells detected in Smed-PTEN-1, -2(RNAi) animals.
The abnormal invasiveness of some cells in Smed-PTEN-1, -2(RNAi) animals was readily apparent in the pharynx (Fig. 4E, bottom right). Normally, postmitotic progeny of neoblasts migrate to the epithelium and pharynx to replace cells lost during cellular turnover and tissue regeneration (Newmark and Sánchez Alvarado, 2000). Although, in the Smed-PTEN phenotype no ectopic mitotic activity was noted (i.e. no mitotic figures were detected in front of the photoreceptors or pharynx; see Fig. 4G), the cellular infiltration (i.e. increase in cellularity in different organs) observed in the epithelium and pharynx (Fig. 4E) may be due to the migration of neoblast progeny. Bromodeoxyuridine (BrdU) experiments confirmed that the migratory ability of the neoblast progeny was not lost after abrogation of Smed-PTEN genes (data not shown). Additionally, these cellular infiltrations were characterized by the presence of basophilic cells (Fig. 4E).
Smed-PTEN-1 and Smed-PTEN-2 affect neoblast proliferation and cell number
To further characterize the Smed-PTEN-1, -2(RNAi) phenotype, we examined whether cell number and the anatomical composition of different tissue types were affected. First, we dissociated control and Smed-PTEN-1, -2(RNAi) planarians (n=10 for each group) into cell suspensions of equal volume. The cells were spotted onto slides and the nuclei counterstained with propidium iodide (PI) (see experimental procedures for details). PI signal quantification revealed an increase of ~20% in cell number in Smed-PTEN-1, -2(RNAi) worms; however, this increase in cell number was not associated with increases in animal length or cell size (Fig. 4F and data not shown). Qualitative anatomical analyses (visual inspection of nuclei counterstaining using Sytox green, Molecular Probes) were also performed in cross-sections of different areas (anterior, posterior) and revealed that the increase in cell number/density in the Smed-PTEN-1, -2(RNAi) phenotype was scattered rather than restricted to a specific tissue or organ (data not shown). However, in animals with a severe phenotype (n=5), we observed evidence for the occurrence of hyperplasia, in which the normally monolayered epidermis became multilayered in Smed-PTEN-1, -2(RNAi) animals (Fig. 4F). This finding is consistent with previous chemically-induced epithelial hyperplasia observed in planarian neoplasms (Foster, 1963).
Because neoblasts are the only known proliferative cell type in asexual planarians, we sought to evaluate whether cell proliferation was involved in the increase in cell number observed in Smed-PTEN-1, -2(RNAi) animals. We assayed whole animals with an anti-phosphorylated histone-3 (H3P) antibody that specifically labels mitotic neoblasts (Newmark and Sánchez Alvarado, 2000). When signs of the Smed-PTEN phenotype became visible (12 days after the first injection, as described above), mitotic activity was quantified (Reddien et al., 2005a; Reddien et al., 2005b) in four different groups: 1) control; 2) Smed-PTEN-1(RNAi); 3) Smed-PTEN-2 (RNAi) and 4) Smed-PTEN-1, -2(RNAi). We found no differences in mitotic activity between control animals and animals in which the Smed-PTEN genes were individually silenced (data not shown). However, a significant increase in mitotic cells (Student’s t-test, P<0.001) was observed in Smed-PTEN-1, -2(RNAi) animals (Fig. 4G). These data suggest that loss of PTEN activity in planarians leads to hyperproliferation and may be responsible for the observed hyperplasia (Fig. 4F).
Smed-PTEN-1 and Smed-PTEN-2 regulate neoblast differentiation
To better understand the nature of the effects exerted by PTEN on neoblasts, we tested several markers associated with neoblast function. First, we carried out a quantitative analysis based on ISH, performed in dissociated control and Smed-PTEN-1, -2(RNAi) animals, which revealed that there was an important increase in cells expressing neoblast markers after abrogation of both Smed-PTEN genes (supplementary material Fig. S2). This suggests that, in addition to an increase in mitotic activity, the number of cells expressing neoblast-specific markers increased in the Smed-PTEN phenotype. In contrast, a marked reduction in the expression of markers for differentiated cells such as neuronal (Smed-SYT), excretory (Smed-CA) and digestive (smedinx-9) cells was observed in Smed-PTEN-1, -2(RNAi) animals (Fig. 5A). We independently confirmed these results by qRT-PCR (supplementary material Table S2). The measured increment in the numbers of both proliferative neoblasts and their postmitotic progeny is consistent with the increase in cell numbers detected histologically (Fig. 4F and supplementary material Fig. S2) in Smed-PTEN-1, -2(RNAi) worms. Taken together, the data indicate that loss of Smed-PTEN function is characterized by an increase in neoblast numbers, but also a decrease in differentiated tissues – probably owing to an inability of the neoblast progeny to properly differentiate.
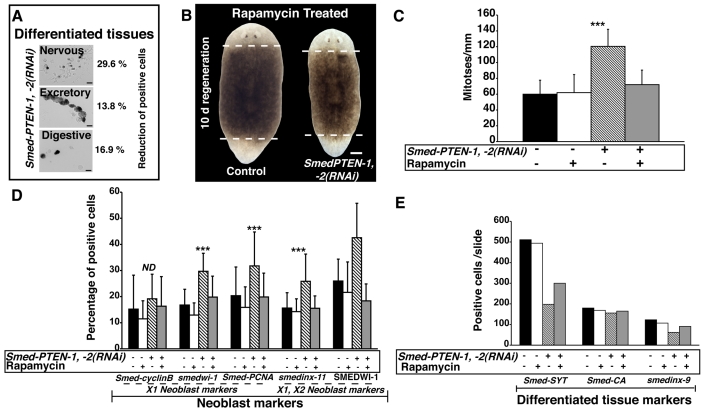
Fig. 5. PTEN-RNAi disrupts tissue maintenance and rapamycin treatment compensates for Smed-PTEN loss.
(A) Quantification of cells expressing markers of differentiated tissues. From a pool of dissociated animals, ISH was performed using probes specific to the nervous (synaptotagmin, Smed-Syt), excretory (carbonic anhydrase, Smed-CA) and digestive (smedinx-9) systems. Representative pictures of cells expressing each marker are shown (black precipitation). Cells expressing each marker were counted and the average of two independent experiments was recorded as the total number of positive cells per slide: Smed-Syt, n=608 and n=428; Smed-CA, n=109 and n=94; smedinx-9, n=142 and n=118 for control and Smed-PTEN-1, -2(RNAi) worms, respectively. Respective percentage reductions in the number of expressing cells after RNAi are shown. (B) Rapamycin treatment prevents abnormal outgrowths, lethality and rescues regenerative events in RNAi-treated animals. Worms subjected to Smed-PTEN-1, -2(RNAi) were unable to regenerate, external outgrowths were evident, and the worms failed to survive longer than 7 days post-amputation (n=27/27), whereas rapamycin-treated Smed-PTEN-1, -2(RNAi) or control worms developed anterior and posterior blastemas, and survived for more than one month after the first dsRNA injection (n=29/29). Representative images of rapamycin-treated control and Smed-PTEN-1, -2(RNAi) worms are shown. (C) Quantification of mitotic activity. Note that control and rapamycin-treated worms show similar levels of mitotic activity and that in Smed-PTEN-1, -2(RNAi) worms, proliferation was higher (***P< 0.01). However, simultaneous Smed-PTEN RNAi and rapamycin treatment prevent abnormal proliferation while keeping proliferative activity similar to control animals. Displayed values represent average ±s.d. of n≥8 worms per condition, for at least two independent replications. (D) Expression of neoblast markers in dissociated worms. An increase in the number of cells expressing each neoblast marker was always observed in Smed-PTEN-1, -2(RNAi) worms after the RNAi treatment, but was prevented with rapamycin treatment (Student’s t-test, ***P<0.001). (E) Expression of differentiated tissue markers in dissociated worms. The same pools of animals discussed in Fig. 5D were used to perform ISH with genes expressed in differentiated tissues (Smed-SYT, Smed-CA and smedinx-9). For panels (D) and (E), dissociations were performed from a pool of ten animals per group and cell numbers represent the average ± s.d. of 20 different fields per slide with an objective of 20× magnification. Total numbers of cells expressing each gene per slide are shown. ND: no difference.
Rapamycin prevents abnormalities produced by Smed-PTEN loss of function
PTEN loss of function is associated with activated PI3K-Akt signaling pathway in many malignancies related to cancer. The macrolide rapamycin operates through the multicomplex protein TOR, and has been shown to be effective in reversing the symptoms observed in PTEN loss of function models (Guertin and Sabatini, 2007; Sabatini, 2006; Yilmaz et al., 2006). We sought to test whether the effects of rapamycin in mammals could be recapitulated in planarians (see experimental procedure for details).
Planarians subjected to RNAi of both Smed-PTEN genes and, subsequently, to daily injections of rapamycin, failed to develop any macroscopic signs of the characteristic Smed-PTEN phenotype, either in the first two weeks (n=50/50), or after one month of treatment (n=19/20). However, exposure to rapamycin restored the ability of Smed-PTEN-1, -2(RNAi) animals to launch a regenerative response after amputation, i.e. rapamycin treatment restored neoblast proliferation in response to injury. RNAi-treated animals developed anterior and posterior blastemas with the same dynamics as controls (n=30/30), and survived for longer than two weeks after the first dsRNA injection (Fig. 5B). After suspending rapamycin treatment, only a fraction of worms developed signs of the Smed-PTEN phenotype within one month (n=7/19), indicating that the effect of rapamycin treatment perdures for several weeks. Moreover, similar levels of mitotic activity were observed among control worms, worms treated with rapamycin alone, and Smed-PTEN-1, -2(RNAi) worms receiving rapamycin, suggesting that rapamycin treatment does not affect basal neoblast proliferation, but specifically prevents abnormal proliferation in the absence of functional Smed-PTEN genes (Fig. 5C).
To further evaluate the effects of rapamycin in planarians, gene expression analyses were performed using markers of proliferative and postmitotic progeny of neoblasts (Fig. 5D). The results revealed that the percentage of cells expressing proliferative and postmitotic markers is similar between the control groups and rapamycin-treated Smed-PTEN-1, -2(RNAi) worms. However, the expression of differentiated tissue markers was lower in Smed-PTEN-1, -2(RNAi) worms treated with rapamycin than in control worms, or in worms receiving rapamycin alone (Fig. 5E), indicating that rapamycin may not fully rescue gene expression in differentiated tissues. Altogether, these results suggest that rapamycin treatment prevents the tissue homeostasis and regeneration defects observed in Smed-PTEN-1, -2(RNAi) animals.
Smed-Akt expression is upregulated in the Smed-PTEN phenotype
The oncoprotein kinase Akt is an important downstream target in the PI3K pathway that has central roles in PTEN loss of function (Guertin and Sabatini, 2007; Sabatini, 2006). Thus, we identified and cloned the planarian Akt ortholog (Smed-Akt). Unlike PTEN, there is a single Akt gene in S. mediterranea. Smed-Akt expression is widely distributed through the mesenchyme including postmitotic areas and in some cell clusters around the cephalic ganglia (supplementary material Fig. S3).
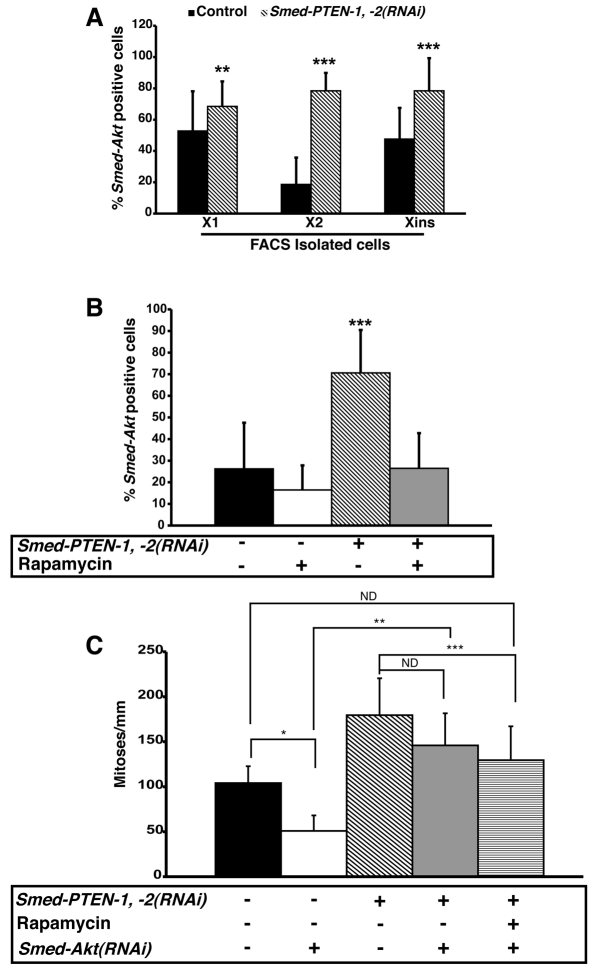
The wide distribution of the Smed-Akt signal was also confirmed by expression analyses of FACS-isolated cells revealing that Smed-Akt is expressed in approximately 50% of the cells in both the X1 (proliferating) and Xins (differentiated) fractions, whereas only approximately 20% of the cells in X2 express Smed-Akt (Fig. 6A). Strikingly, the distribution of Smed-Akt expression was dramatically altered after simultaneous abrogation of both Smed-PTEN genes (Fig. 6A), increasing significantly in the X1 and X2 compartments (1.3- and 4.2-fold, respectively), and in differentiated cells (Xins=1.6-fold). In fact, the increase in Smed-Akt positive cells was higher than that of any other neoblast marker (Fig. 5D), indicating that Smed-PTEN-1 and -2 play a role in regulating Smed-Akt expression in both neoblasts and cells of differentiated tissues. Furthermore, rapamycin treatment of Smed-PTEN-1, -2(RNAi) worms specifically prevents the abnormal increase of Smed-Akt signal, while maintaining its basal expression (Fig. 6B and C). Thus, rapamycin is not stochastically downregulating Smed-Akt expression, but it is effective in distinguishing between physiological levels of Smed-Akt and its abnormal expression after RNAi of Smed-PTEN-1 and Smed-PTEN-2.
Fig. 6. Smed-Akt expression analyses in Smed-PTEN phenotype and proliferative activity in triple RNAi worms.
(A) Smed-Akt expression in FACS-isolated cells from control and Smed-PTEN-1, -2(RNAi) worms at day 12 after the first injection (n=10 per group). ISH was performed in FACS-isolated cells from control and Smed-PTEN-1, -2(RNAi) worms; cells expressing Smed-Akt were quantified for each fraction (see Methods). In control animals, Smed-Akt expression levels are highest in fractions X1 and Xins and comparable to each other (Student’s t-test, **P=0.24), whereas Smed-Akt expression is lowest in the X2 fraction (Student’s t-test, P<0.0001). In animals treated with dsRNA against both Smed-PTEN paralogs, significant increases in Smed-Akt expressing cells are observed, relative to control worms, with the greatest increase observed in the X2 fraction (Student’s t-test, ***P<0.0001). (B) Smed-Akt expression in dissociated worms. Pools of animals (n=10 each) that had been treated with RNAi, rapamycin, or both, were dissociated and cells expressing Smed-Akt were quantified. Smed-PTEN-1, -2(RNAi) animals showed the highest number of Smed-Akt expressing cells (Student’s t-test, ***P<0.0001). Rapamycin treatment in Smed-PTEN-1, -2(RNAi) worms prevented the increase in Smed-Akt expressing cells, but maintained a similar number to control animals (Student’s t-test, P=0.5). (C) Mitotic activity in Smed-Akt(RNAi) worms, Smed-PTEN-1, -2(RNAi) worms or triple RNAi worms, with or without rapamycin treatment. Animals were processed for immunostaining 10 days after the first dsRNA-PTEN injection. Mitotic cells were detected in whole animals with H3P antibody and the signal quantified as described in the Methods (n>6 animals/condition). Smed-Akt(RNAi) worms showed a reduced number of mitotic cells relative to control worms (Student’s t-test, *P<0.002). Although, the hyperproliferative defects observed in the Smed-PTEN phenotype were not prevented in triple RNAi worms (Student’s t-test, **P=0.3), mitotic activity was significantly reduced to levels comparable with control animals (control vs. triple RNAi, rapamycin, P=0.5; Smed-PTEN-1, -2(RNAi) vs. triple RNAi, rapamycin, Student’s t-test, ***P=0.002). ND: no difference.
RNAi of Smed-Akt does not recapitulate the Smed-PTEN phenotype
To evaluate the function of Akt in the Smed-PTEN phenotype, RNAi experiments involving Smed-Akt and Smed-PTEN genes were carried out. At the gross morphological level, Smed-Akt(RNAi) animals displayed only locomotion defects (~12 days after first injection, n=40/40), and did not develop other external abnormalities or head regression even after more than one month (data not shown). Smed-Akt(RNAi) animals, however, displayed lower number of proliferative cells when compared with control animals (Student’s t-test, P<0.002), suggesting that Smed-Akt is necessary to maintain basal proliferation levels (Fig. 6C). Nevertheless, Smed-Akt(RNAi) animals are still capable of forming blastemas after amputation and regenerate with similar dynamics as control animals (data not shown, n=30/30). Together, the data indicate that Smed-Akt is not sufficient to affect either long-term homeostasis or tissue regeneration.
Worms subjected to triple RNAi [Smed-Akt(RNAi); Smed-PTEN-1, -2(RNAi)], with or without rapamycin treatment, failed to display head regression as observed in the Smed-PTEN phenotype, yet developed abnormal outgrowths and lethality with full penetrance (n=23/23, supplementary material Fig. 4). Moreover, the number of proliferating cells in triple RNAi worms and in Smed-PTEN-1, -2(RNAi) worms were not statistically different (Student’s t-test, P>0.3). These results suggest that abrogating Smed-Akt is not enough to prevent the abnormal hyperproliferation observed in the Smed-PTEN phenotype. Surprisingly, after rapamycin treatment, the number of proliferative cells in triple RNAi worms was the same as in the control group (Student’s t-test, P>0.4), although rapamycin treatment failed to prevent abnormal outgrowths (n=15/15, Fig. 6C and supplementary material Fig. S4). Altogether these data suggest that, irrespective of proliferative levels, functional abrogation of Smed-PTEN is sufficient to induce abnormal outgrowths. Additionally, functional disruption of both Smed-Akt and Smed-PTEN genes reduce the capacity of rapamycin to prevent the abnormalities observed in the Smed-PTEN phenotype, suggesting that to be fully effective, rapamycin treatment requires functional Akt.
DISCUSSION
The analyses described here reveal details of the striking evolutionary conservation of PTEN function in stem cell regulation. Our data confirm that S. mediterranea offers unique opportunities to dissect, on a molecular basis, conserved, complex signaling pathways affecting adult somatic stem cells during homeostasis, disease, and uniquely, regeneration. Moreover, the Smed-PTEN phenotype enables both the identification of factors controlling adult stem cells in tumor generation, and the opportunity to explore and test the mechanisms of action of therapeutic drugs that target elements of the PI3K-AKT-TOR pathway.
Multiple PTEN forms exist in S. mediterranea
Phylogenic analysis of the three PTEN-like genes in planaria has lead to two interesting conclusions. Firstly, the analysis shows that planaria have undergone a paralogous duplication of the ancestral, single PTEN gene. The presence of two PTEN genes has not been described in any other invertebrate. Consistent with a relatively recent duplication, is the fact that both paralogs must be inactivated by RNAi to produce a phenotype. Secondly, expansion of the vertebrate PTEN family into TPTE and TPIP was thought to be vertebrate-specific, because neither C. elegans nor Drosophila have a homolog to either of these genes. Surprisingly, planaria have a homolog that appears to be orthologous to both of these genes. These findings provide further evidence that S. mediterranea can be a useful invertebrate organism with which to functionally investigate questions involving genes that are present in vertebrates, but absent in Drosophila or C. elegans.
S. mediterranea: a model to study PTEN regulation of adult stem cells during tissue maintenance and regeneration
Although PTEN and its substrate are evolutionarily conserved, the phenotypes, and endpoints from experimental assays may differ among biological model systems, owing to the ubiquity, complexity, and multiple effectors of the highly branched PI3K pathway (Stiles et al., 2004). This is particularly illustrated by studies on PTEN modulation of chemotaxis in Dictyostelium, signaling pathways in yeast, regulation of metabolic variables, longevity and dauer formation in C. elegans, and growth and size determination at the cellular and organ levels in Drosophila (Hafen, 2004; Stiles et al., 2004; Sulis and Parsons, 2003). Nonetheless, in mammals, recent attention has been drawn to the subtle roles that PTEN signaling plays in abnormal stem cell proliferation and its participation in carcinogenesis (Janzen and Scadden, 2006; Polyak and Hahn, 2006; Rossi and Weissman, 2006; Yilmaz et al., 2006; Zhang et al., 2006).
In S. mediterranea, the evolutionary conservation of signaling pathways, the experimentally accessible adult stem cell population, and the robust regenerative capabilities of this organism provide a unique model with which to discover the mechanisms that control stem cells during homeostasis, tumorigenesis, and regeneration. Our data demonstrate that S. mediterranea provides a new invertebrate model system, in which the functions of different forms of PTEN can be abrogated in the entire adult animal, allowing for analysis of PTEN dysfunction in multiple organs, simultaneously – an experimental approach that is limited in mammalian models (Stiles et al., 2004; Sulis and Parsons, 2003). In addition, investigation of the possible roles of PTEN in the regeneration of complex structures is uniquely facilitated by the planarian model system. Understanding the in vivo function of PTEN in adult stem cell regulation is critical in order to design effective therapeutic strategies targeting components of the PI3K-Akt-TOR pathway.
Analysis of the stem cells and their microenvironment during malignant transformation can be performed in the Smed-PTEN phenotype
Unlike other invertebrate models (Gao et al., 2000; Goberdhan et al., 1999; Hafen, 2004; Huang et al., 1999; Mihaylova et al., 1999; Ogg and Ruvkun, 1998), abrogation of PTEN function in adult S. mediterranea consistently led to visible outgrowths. A central finding of our work is the overproliferation of neoblasts in animals in which both PTEN paralogs have been functionally abrogated. Because neoblasts are the only known dividing cells in planarians, and expression of both Smed-PTEN-1 and -2 were detected in these cells, it is reasonable to suggest that the observed hyperproliferation (Fig. 4G; Fig. 5C) may be due to autonomous cellular functions of PTEN in the planarian stem cells. Further, elimination of Smed-Akt and addition of rapamycin in Smed-PTEN-1, -2(RNAi) animals did not prevent migration of the non-mitotic division progeny of neoblasts; therefore, it is likely that other PTEN-regulated pathways may play a role in this particular aspect of the phenotype. One likely candidate is the integrin-mediated cell spreading and cell migration pathway, which is independent of mammalian TOR (mTOR) and is inhibited by PTEN (Gu et al., 1998). Hence, elimination of PTEN function in non-dividing planarian cells may enhance their migration and explain both tissue infiltration and the prevention of cephalic resorbption in rapamycin-treated Smed-Akt(RNAi); Smed-PTEN-1, -2(RNAi) animals. Further molecular analyses should help to formally test this possibility.
We also observed a marked disruption of the basement membrane, separating the epithelium from mesenchymal structures, which is accompanied by cellular infiltration and other morphological abnormalities (Fig. 4A–E). Interestingly, alterations of the epithelial-mesenchymal interactions, loss of tissue architecture, and the ensuing abnormal cell behaviors are considered to be both prerequisite and defining events in the progression of most cancers (Nelson and Bissell, 2006). In fact, anatomical alterations of epithelial-mesenchymal interactions represent a triggering signal in malignant transformation (Nelson and Bissell, 2006), as well as a proliferative response in neoblasts upon amputation (Hori, 1979b; Hori, 1980). Thus, we propose that the hyperproliferative response of neoblasts in the Smed-PTEN phenotype may be additionally influenced by the disruption of anatomical barriers, and as such could be considered as a non-autonomous cellular effect of PTEN on neoblasts.
In the past, planarians have been used as in vivo models for chemical-induced teratogenesis, and many of these studies led to the proposal that the hyperproliferative response of neoblasts to mutagenic compounds bears some resemblance to teratomas caused by embryonic stem cells, as well as benign and malignant tumors in mammals (Best and Morita, 1982; Foster, 1963; Foster, 1969; Schaeffer, 1993). Interestingly, the observation that neoblast numbers increase after abrogation of PTEN function in planarians, is accompanied by a generalized infiltration of neoblast-like cells in many different organs, which supports this possibility. It is also consistent with previous findings, showing that embryonic stem cells (PTEN−/−) possess an enhanced capability to produce teratomas, after subcutaneous injections in syngeneic mice with similar histological features (i.e. increased cellularity, and dysplasia mostly composed of small, round undifferentiated cells) (Di Cristofano et al., 1998). Taken together, our findings strongly indicate that the Smed-PTEN phenotype provides a novel invertebrate context, in which the autonomous and non-autonomous regulation of tumor suppressors on adult stem cells can be simultaneously analyzed in all the different organs of a whole organism.
Controlling abnormal stem cell proliferation by targeting components of the TOR pathway
The PI3K-Akt-TOR pathway is evolutionarily conserved and it has been shown that loss of PTEN function activates Akt. Because functional abrogation of the Smed-PTEN genes led to increases in Smed-Akt expression, and because the symptoms of the Smed-PTEN phenotype were prevented by rapamycin treatment, components of this pathway are likely to be conserved in S. mediterranea. The ability of rapamycin to regulate neoblast numbers, and their differentiation, during homeostasis and regeneration in the absence of functional Smed-PTEN genes, reveals a highly specific mechanism of action for rapamycin, and provides a unique opportunity to explore the mechanisms of action of this macrolide in vivo. Therefore, in planarians, it is possible to target abnormal proliferative cells while retaining an adequate neoblast response to meet physiological demands. This is consistent with recent findings demonstrating that rapamycin treatment compensates for PTEN loss, and is effective in preventing the development of leukemia-initiating cells, while maintaining normal hematopoietic function in mice (Yilmaz et al., 2006). However, the precise mechanism of action by which rapamycin distinguishes between abnormal and physiological stem cell proliferation is not completely understood and will require further analyses. Thus, the identification and characterization of additional, evolutionarily conserved components of the PI3K-Akt-TOR pathway, as well as possible targets of rapamycin, should be possible in planarians.
Even though an important consequence of abrogating Smed-PTEN genes was the generalized increase in Smed-Akt expression, disruption of Smed-Akt function did not prevent the hyperproliferation and visible outgrowths that have been previously reported for this pathway in mammals (Chen et al., 2006). In fact, the only aspect of the Smed-PTEN loss-of-function phenotype rescued by abrogation of Smed-Akt, is the regression of the area in front of the photoreceptor – a region of the animal devoid of proliferative activity, and which is maintained exclusively by the migration of postmitotic neoblast progeny (Newmark and Sánchez Alvarado, 2000; Reddien et al., 2005b). Interestingly, the highest proportion of Smed-Akt expression in Smed-PTEN-1, -2(RNAi) animals was observed in the X2 subpopulation of our FACS analyses, which includes migratory differentiating cells (Higuchi et al., 2007; Oviedo and Levin, 2007). Therefore, we propose that the abnormalities observed in the Smed-PTEN phenotype, such as head regression, may be associated with an abnormal increase in Smed-Akt expression, but others, such as hyperproliferation, lack of regeneration, and abnormal outgrowths are due specifically to the disruption of Smed-PTEN function. However, the partial preventative effect of rapamycin treatment in triple RNAi worms suggests that Akt is required to mediate rapamycin action in Smed-PTEN(RNAi) animals.
CONCLUSIONS
Taken together, our findings provide insights into the evolution of PTEN, and the regulatory mechanism operating in adult stem cells during homeostasis and regeneration. Moreover, similar to mammalian adult stem cells, planarian neoblasts exists in a tightly regulated environment that allows constant support of differentiated tissues, while keeping the required form and function throughout the life of the organism (Newmark and Sánchez Alvarado, 2002; Reddien and Sánchez Alvarado, 2004; Sánchez Alvarado, 2006). Thus, the Smed-PTEN phenotype revealed that components of the neoblast regulatory mechanisms used during proliferation, differentiation and regeneration are conserved between planarians and mammals. As such, it is now possible to use S. mediterranea as a model, in which the pathways controlling adult stem cell biology and cellular regulation can be studied during the regeneration of complex structures. Equally important is the fact that abnormal stem cell proliferation can be specifically induced in S. mediterranea, broadening the opportunity to understand the relationship between cancer and stem cells. Future work will exploit the biology of S. mediterranea to pursue pharmacological, genetic and physiological approaches to elucidate potential therapeutic targets that could resolve PI3K-Akt-TOR pathway alterations.
METHODS
Planarian culture
The clonal asexual strain CIW4 of Schmidtea mediterranea was used in all experiments. Animals were kept and maintained as previously described (Cebrià and Newmark, 2005; Sánchez Alvarado et al., 2002).
Smed-PTEN and Smed-Akt isolation
PTEN homologues Smed-PTEN-1 and -2 (accession numbers: AY068282 and AY066355, respectively) were identified in the Schmidtea mediterranea database SmedDb (Sánchez Alvarado et al., 2002). 5′ RACE was performed to isolate full lengths (Oviedo and Levin, 2007; Reddien et al., 2005b). Genomic sequences were obtained from the ongoing S. mediterranea Genome Project (Washington University Genome Sequence Center) to obtain Smed-Akt and Smed-PCNA sequences.
Phylogenetic analysis
PTEN sequences were aligned with the program T-Coffee (Notredame et al., 2000). Alignments were edited by hand and subject to Bayesian analysis through the freeware program Geneious (www.geneious.com) and the MrBayes plugin (Ronquist and Huelsenbeck, 2003). MrBayes was run with parameters of 1,000,000 generations, 4 chains, a subsample frequency of 500 and burnin of 100,000.
Gene expression studies
Whole-mount ISHs
Samples and probes for ISHs were processed as previously described (Oviedo and Levin, 2007; Reddien et al., 2005b). An optimized in situ protocol was also used for whole-mount analysis and is available upon request. Briefly, planaria are fixed with 4% formaldehyde in PBS, dehydrated and bleached in 6% H2O2 overnight, treated with 3 μg/ml ProteinaseK for 10 minutes, postfixed in 4% formaldehyde for 10 minutes and then subject to previously described hybridization and detection. All images are representative of at least three different experiments and were captured as previously described (Oviedo and Levin, 2007; Oviedo et al., 2003).
qRT-PCR
Use of total RNA isolation procedures and internal controls were as previously described (Oviedo and Levin, 2007; Reddien et al., 2005b).
ISH on dissociated cells and quantification of positive cells
Planarian dissociation (n=10 per condition) was performed as previously described (Oviedo and Levin, 2007; Reddien et al., 2005b) but without any filtration step. Briefly, dissociated cells were resuspended in equal volumes of calcium magnesium free media (CMF), spotted onto slides and fixed with 4% PFA. ISH was performed as previously described (Oviedo and Levin, 2007; Reddien et al., 2005b). Nuclear counterstaining was performed with propidium iodide (PI). Cells that were positive for PI and different probes (neoblasts and differentiated tissues) were quantified with a compound scope (20× objective) using IPLab software (Olympus). An average of total cell number was obtained from 20 different fields per slide (neoblast markers), or, using a 20× objective, the total number of positive cells per whole slide was recorded (differentiated tissue markers).
Planarian dissociation and FACS analyses
Intact animals (n~10 per condition) were dissociated as previously described (Oviedo and Levin, 2007; Reddien et al., 2005b). Either FACSVantage (Becton Dickinson) or MoFlo high-speed cell sorter (Dako) were used to isolate cells. Results were analyzed by using CELL Quest (Becton Dickinson) or Summit (Dako) software (Oviedo and Levin, 2007; Reddien et al., 2005b).
dsRNA synthesis and microinjections
dsRNA synthesis and microinjections were performed as previously described (Oviedo and Levin, 2007). Microinjection schedules were subjected to extensive rounds of optimization until reproducible results were obtained. Each injection was performed in the prepharyngeal area, consisting of three pulses of 32.2 nL each, at the time intervals depicted in Fig. 2A; Fig. 3A. Triple RNAi worms were injected on three consecutive days with dsRNA-Smed-Akt, followed by two days of rest, and then continued with the same schedule described for Smed-PTEN-1, -2(RNAi) worms. During the injection schedule, planarians were evaluated daily under the dissecting scope to observe behavioral reaction and external morphology. Signs of phenotypes were recorded when any of the following were first noticed: abnormal photophobic response, tip regression, external overgrowth, or epithelial damage. In most cases, the same number of animals for each condition (control, RNAi of an individual Smed-PTEN or simultaneous Smed-PTEN-RNAi) were fixed or processed for additional experiments when 50% of the group displayed signs of the phenotype.
Rapamycin treatment
Rapamycin was obtained from two sources: Calbiochem and Cell Signaling Technology. Concentrated stock was freshly diluted (20 nM) in planarian water before daily prepharyngeal injections (five pulses of 32 nL each per worm). Rapamycin injections were always scheduled for 2–3 hours before respective dsRNA injections.
Antibody labeling and image collection
Planarians were processed for immunostaining as previously described (Reddien et al., 2005a; Reddien et al., 2005b). Primary antibodies were diluted as follows: α-phosphorylated histone H3 (Upstate), 1:5000; α-arrestin (kind gift from K. Agata), 1:5000; anti-planarian myosin heavy chain monoclonal antibody TMUS-13 (kind gift from R. Romero), 1:100; α-acetylated tubulin monoclonal antibody (Sigma), 1:500; and anti-SMEDWI-1 antibody (kind gift from P. Newmark), 1:1000. Incubation with secondary antibody was performed at room temperature, overnight in goat anti-rabbit Alexa 568, 1:800, or goat anti-mouse Alexa 488, 1:400. At least seven or eight different whole body images were taken per condition to quantify the H3P signal as previously described (Reddien et al., 2005a; Reddien et al., 2005b). Representative images from each condition were additionally processed using an Olympus Fluoview-300 confocal microscope (Olympus), or as previously described (Oviedo and Levin, 2007).
Supplementary Material
ACKNOWLEDGEMENTS
We thank P. Newmark, K. Agata and R. Romero for reagents; J. LaVecchio, B. Girijesh and the Harvard Stem Cell Institute Flow Cytometry Cores for FACS analysis; P. Reddien for sharing protocols, reagents and valuable suggestions; S. Morrison for rapamycin advice; J. Dobeck for histological assistance; R. Kent for assistance with data analysis; J. Jenkin, K. Gallant, D. Qiu and J. Morokuma for lab assistance; and C.-B. Chien and C. Rodesh for advice with imaging. N.J.O. is an NIH fellow supported under Ruth L. Kirschstein National Research Service Award (F32 GM078774). N.J.O. also thanks the Oviedo-Pfister family for encouragement. This work was supported by NSF grant IBN#0347295, NHTSA grant DTNH22-06-G-00001, and NIH grant R21 HD055850 to M.L. and NIH-NIGMS RO-1 GM57260 to A.S.A. B.J.P. is a Damon Runyon Fellow (DRG#1888-05) and A.S.A. is a Howard Hughes Medical Institute Investigator. Part of this research was conducted in a Forsyth Institute facility renovated with support from Research Facilities Improvement Grant Number CO6RR11244 from the National Center for Research Resources, NIH.
Footnotes
COMPETING INTERESTS STATEMENT
The authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
N.J.O. performed and analyzed all of the experiments with the exception of those reported in Fig. 1A–C, which were executed and interpreted by B.J.P. B.J.P. also optimized the whole-mount in situ hybridization protocol. A.S.A. and N.J.O. conceived the experiments. A.S.A. and M.L. supervised N.J.O. M.L. contributed reagents and participated in manuscript preparation. A.S.A., N.J.O. and B.J.P. wrote the manuscript.
SUPPLEMENTARY MATERIAL
Supplementary material for this article is available at http://dmm.biologists.org/content/1/2-3/131/suppl/DC1
REFERENCES
- Asano T, Yao Y, Zhu J, Li D, Abbruzzese JL, Reddy SA. (2004). The PI 3-kinase/Akt signaling pathway is activated due to aberrant Pten expression and targets transcription factors NF-kappaB and c-Myc in pancreatic cancer cells. Oncogene 23, 8571–8580 [DOI] [PubMed] [Google Scholar]
- Best J, Morita M. (1982). Planarians as a model system for in vitro teratogenesis studies. Teratog. Carcinog. Mutagen. 2, 277–291 [DOI] [PubMed] [Google Scholar]
- Cebrià F, Newmark PA. (2005). Planarian homologs of netrin and netrin receptor are required for proper regeneration of the central nervous system and the maintenance of nervous system architecture. Development 132, 3691–3703 [DOI] [PubMed] [Google Scholar]
- Cebrià F, Vispo M, Bueno D, Carranza S, Newmark P, Romero R. (1996). Myosin heavy chain gene in Dugesia (G.) tigrina: a tool for studying muscle regeneration in planarians. Int. J. Dev. Biol. Suppl. 1, 177S–178S [PubMed] [Google Scholar]
- Chen ML, Xu PZ, Peng XD, Chen WS, Guzman G, Yang X, Di Cristofano A, Pandolfi PP, Hay N. (2006). The deficiency of Akt1 is sufficient to suppress tumor development in Pten+/– mice. Genes Dev. 20, 1569–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke MF, Fuller M. (2006). Stem cells and cancer: two faces of eve. Cell 124, 1111–1115 [DOI] [PubMed] [Google Scholar]
- Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP. (1998). Pten is essential for embryonic development and tumour suppression. Nat. Genet. 19, 348–355 [DOI] [PubMed] [Google Scholar]
- Foster JA. (1963). Induction of neoplasms in planarians with carcinogens. Cancer Res. 23, 300–303 [PubMed] [Google Scholar]
- Foster JA. (1969). Malformations and lethal growths in planaria treated with carcinogens. Natl. Cancer Inst. Monogr. 31, 683–691 [PubMed] [Google Scholar]
- Gao X, Neufeld TP, Pan D. (2000). Drosophila PTEN regulates cell growth and proliferation through PI3K-dependent and -independent pathways. Dev. Biol. 221, 404–418 [DOI] [PubMed] [Google Scholar]
- Gil EB, Malone Link E, Liu LX, Johnson CD, Lees JA. (1999). Regulation of the insulin-like developmental pathway of Caenorhabditis elegans by a homolog of the PTEN tumor suppressor gene. Proc. Natl. Acad. Sci. USA 96, 2925–2930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goberdhan DC, Paricio N, Goodman EC, Mlodzik M, Wilson C. (1999). Drosophila tumor suppressor PTEN controls cell size and number by antagonizing the Chico/PI3-kinase signaling pathway. Genes Dev. 13, 3244–3258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Tamura M, Yamada KM. (1998). Tumor Suppressor PTEN Inhibits Integrin- and Growth Factor-mediated Mitogen-activated Protein (MAP) Kinase Signaling Pathways. J. Cell Biol. 143, 1375–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertin DA, Sabatini DM. (2007). Defining the Role of mTOR in Cancer. Cancer Cell 12, 9–22 [DOI] [PubMed] [Google Scholar]
- Guo T, Peters AH, Newmark PA. (2006). A bruno-like Gene Is Required for Stem Cell Maintenance in Planarians. Dev. Cell 11, 159–169 [DOI] [PubMed] [Google Scholar]
- Hafen E. (2004). Cancer, type 2 diabetes, and ageing: news from flies and worms. Swiss Med. Wkly 134, 711–719 [DOI] [PubMed] [Google Scholar]
- Hayashi T, Asami M, Higuchi S, Shibata N, Agata K. (2006). Isolation of planarian X-ray-sensitive stem cells by fluorescence-activated cell sorting. Dev.Growth Differ. 48, 371–380 [DOI] [PubMed] [Google Scholar]
- Higuchi S, Hayashi T, Hori I, Shibata N, Sakamoto H, Agata K. (2007). Characterization and categorization of fluorescence activated cell sorted planarian stem cells by ultrastructural analysis. Dev. Growth Differ. 49, 571–581 [DOI] [PubMed] [Google Scholar]
- Hori I. (1979a). Regeneration of the epidermis and basement membrane of the planarian Dugesia japonica after total-body X irradiation. Radiat. Res. 77, 521–533 [PubMed] [Google Scholar]
- Hori I. (1979b). Structure and regeneration of the planarian basal lamina: an ultrastructural study. Tissue Cell 11, 611–621 [DOI] [PubMed] [Google Scholar]
- Hori I. (1980). Localization of newly synthesized precursors of basal lamina in the regenerating planarian as revealed by autoradiography. Tissue Cell 12, 513–521 [DOI] [PubMed] [Google Scholar]
- Huang H, Potter CJ, Tao W, Li DM, Brogiolo W, Hafen E, Sun H, Xu T. (1999). PTEN affects cell size, cell proliferation and apoptosis during Drosophila eye development. Development 126, 5365–5372 [DOI] [PubMed] [Google Scholar]
- Janzen V, Scadden DT. (2006). Stem cells: good, bad and reformable. Nature 441, 418–419 [DOI] [PubMed] [Google Scholar]
- Kirkegaard T, Witton CJ, McGlynn LM, Tovey SM, Dunne B, Lyon A, Bartlett JM. (2005). AKT activation predicts outcome in breast cancer patients treated with tamoxifen. J Pathol 207, 139–146 [DOI] [PubMed] [Google Scholar]
- Lee JO, Yang H, Georgescu MM, Di Cristofano A, Maehama T, Shi Y, Dixon JE, Pandolfi P, Pavletich NP. (1999). Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell 99, 323–334 [DOI] [PubMed] [Google Scholar]
- Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, et al. (1997). PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 275, 1943–1947 [DOI] [PubMed] [Google Scholar]
- Liaw D, Marsh DJ, Li J, Dahia PL, Wang SI, Zheng Z, Bose S, Call KM, Tsou HC, Peacocke M, et al. (1997). Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat. Genet. 16, 64–67 [DOI] [PubMed] [Google Scholar]
- Marsh DJ, Dahia PL, Zheng Z, Liaw D, Parsons R, Gorlin RJ, Eng C. (1997). Germline mutations in PTEN are present in Bannayan-Zonana syndrome. Nat. Genet. 16, 333–334 [DOI] [PubMed] [Google Scholar]
- Marsh DJ, Kum JB, Lunetta KL, Bennett MJ, Gorlin RJ, Ahmed SF, Bodurtha J, Crowe C, Curtis MA, Dasouki M, et al. (1999). PTEN mutation spectrum and genotype-phenotype correlations in Bannayan-Riley-Ruvalcaba syndrome suggest a single entity with Cowden syndrome. Hum. Mol. Genet. 8, 1461–1472 [DOI] [PubMed] [Google Scholar]
- Marx J. (2007). Molecular biology. Cancer’s perpetual source? Science 317, 1029–1031 [DOI] [PubMed] [Google Scholar]
- Mihaylova VT, Borland CZ, Manjarrez L, Stern MJ, Sun H. (1999). The PTEN tumor suppressor homolog in Caenorhabditis elegans regulates longevity and dauer formation in an insulin receptor-like signaling pathway. Proc. Natl. Acad. Sci. USA 96, 7427–7432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahara Y, Nagai H, Kinoshita T, Uchida T, Hatano S, Murate T, Saito H. (1998). Mutational analysis of the PTEN/MMAC1 gene in non-Hodgkin’s lymphoma. Leukemia 12, 1277–1280 [DOI] [PubMed] [Google Scholar]
- Nelen MR, van Staveren WC, Peeters EA, Hassel MB, Gorlin RJ, Hamm H, Lindboe CF, Fryns JP, Sijmons RH, Woods DG, et al. (1997). Germline mutations in the PTEN/MMAC1 gene in patients with Cowden disease. Hum. Mol. Genet. 6, 1383–1387 [DOI] [PubMed] [Google Scholar]
- Nelson CM, Bissell MJ. (2006). Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu. Rev. Cell Dev. Biol. 22, 287–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newmark P, Sánchez Alvarado A. (2000). Bromodeoxyuridine specifically labels the regenerative stem cells of planarians. Dev. Biol. 220, 142–153 [DOI] [PubMed] [Google Scholar]
- Newmark PA, Sánchez Alvarado A. (2002). Not your father’s planarian: a classic model enters the era of functional genomics. Nat. Rev. Genet. 3, 210–219 [DOI] [PubMed] [Google Scholar]
- Notredame C, Higgins DG, Heringa J. (2000). T-Coffee: A novel method for fast and accurate multiple sequence alignment. J. Mol. Biol. 302, 205–217 [DOI] [PubMed] [Google Scholar]
- Ogg S, Ruvkun G. (1998). The C. elegans PTEN homolog, DAF-18, acts in the insulin receptor-like metabolic signaling pathway. Mol. Cell 2, 887–893 [DOI] [PubMed] [Google Scholar]
- Oldham S, Stocker H, Laffargue M, Wittwer F, Wymann M, Hafen E. (2002). The Drosophila insulin/IGF receptor controls growth and size by modulating PtdInsP(3) levels. Development 129, 4103–4109 [DOI] [PubMed] [Google Scholar]
- Oviedo NJ, Levin M. (2007). smedinx-11 is a planarian stem cell gap junction gene required for regeneration and homeostasis. Development 134, 3121–3131 [DOI] [PubMed] [Google Scholar]
- Oviedo NJ, Newmark PA, Sánchez Alvarado A. (2003). Allometric scaling and proportion regulation in the freshwater planarian Schmidtea mediterranea. Dev. Dyn. 226, 326–333 [DOI] [PubMed] [Google Scholar]
- Polyak K, Hahn WC. (2006). Roots and stems: stem cells in cancer. Nat. Med. 12, 296–300 [DOI] [PubMed] [Google Scholar]
- Reddien PW, Bermange AL, Murfitt KJ, Jennings JR, Sánchez Alvarado A. (2005a). Identification of genes needed for regeneration, stem cell function, and tissue homeostasis by systematic gene perturbation in planaria. Dev. Cell 8, 635–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddien PW, Oviedo NJ, Jennings JR, Jenkin JC, Sánchez Alvarado A. (2005b). SMEDWI-2 is a PIWI-like protein that regulates planarian stem cells. Science 310, 1327–1330 [DOI] [PubMed] [Google Scholar]
- Reddien PW, Sánchez Alvarado A. (2004). Fundamentals of planarian regeneration. Annu. Rev. Cell Dev. Biol. 20, 725–757 [DOI] [PubMed] [Google Scholar]
- Reya T, Clevers H. (2005). Wnt signalling in stem cells and cancer. Nature 434, 843–850 [DOI] [PubMed] [Google Scholar]
- Robb SM, Sánchez Alvarado A. (2002). Identification of immunological reagents for use in the study of freshwater planarians by means of whole-mount immunofluorescence and confocal microscopy. Genesis 32, 293–298 [DOI] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP. (2003). MrBayes 3, Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574 [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Weissman IL. (2006). Pten, tumorigenesis, and stem cell self-renewal. Cell 125, 229–231 [DOI] [PubMed] [Google Scholar]
- Sabatini DM. (2006). mTOR and cancer: insights into a complex relationship. Nat. Rev. Cancer 6, 729–734 [DOI] [PubMed] [Google Scholar]
- Sakai F, Agata K, Orii H, Watanabe K. (2000). Organization and regeneration ability of spontaneous supernumerary eyes in planarians -eye regeneration field and pathway selection by optic nerves-. Zool. Sci. 17, 375–381 [DOI] [PubMed] [Google Scholar]
- Sánchez Alvarado A. (2006). Planarian regeneration: its end is its beginning. Cell 124, 241–245 [DOI] [PubMed] [Google Scholar]
- Sánchez Alvarado A, Newmark PA. (1999). Double-stranded RNA specifically disrupts gene expression during planarian regeneration. Proc. Natl. Acad. Sci. USA 96, 5049–5054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez Alvarado A, Newmark PA, Robb SM, Juste R. (2002). The Schmidtea mediterranea database as a molecular resource for studying platyhelminthes, stem cells and regeneration. Development 129, 5659–5665 [DOI] [PubMed] [Google Scholar]
- Sansal I, Sellers WR. (2004). The biology and clinical relevance of the PTEN tumor suppressor pathway. J. Clin. Oncol. 22, 2954–2963 [DOI] [PubMed] [Google Scholar]
- Schaeffer DJ. (1993). Planarians as a model system for in vivo tumorigenesis studies. Ecotoxicol. Environ. Saf. 25, 1–18 [DOI] [PubMed] [Google Scholar]
- Stiles B, Groszer M, Wang S, Jiao J, Wu H. (2004). PTENless means more. Dev. Biol. 273, 175–184 [DOI] [PubMed] [Google Scholar]
- Sulis ML, Parsons R. (2003). PTEN: from pathology to biology. Trends Cell Biol. 13, 478–483 [DOI] [PubMed] [Google Scholar]
- Yilmaz OH, Valdez R, Theisen BK, Guo W, Ferguson DO, Wu H, Morrison SJ. (2006). Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature 441, 475–482 [DOI] [PubMed] [Google Scholar]
- Zhang J, Grindley JC, Yin T, Jayasinghe S, He XC, Ross JT, Haug JS, Rupp D, Porter-Westpfahl KS, Wiedemann LM, et al. (2006). PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature 441, 518–522 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.