Abstract
The lipid mediator sphingosine 1-phosphate (S1P) and its type 1 G protein-coupled receptor (S1P1) affect mammalian immunity through alterations in thymocyte emigration, differentiation of T cell subsets, lymphocyte trafficking in lymphoid organs and other tissues, T cell-dendritic cell and T cell-B cell interactions, and cytokine generation. Recent attention to effects of the S1P-S1P1 axis on non-migration functions of lymphocytes include delineation of a role in terminal differentiation and survival of Th17 effector cells and adaptive Treg cells of the CD4 T cell constellation, and a greater understanding of interactions of the S1P-S1P1 axis with immune cytokines in lymphocyte survival and activities. This breadth of involvement of the S1P-S1P1 axis in immune responses that often are altered in immunological diseases has provided many opportunities for novel therapeutic interventions. A spectrum of pharmacological and immunochemical agents is available that alter immunity by affecting either tissue and fluid concentrations of S1P or levels of expression and signaling activities of S1P1. Such agents have so far been beneficial in the settings of autoimmunity and rejection of transplanted organs, and are likely to become valuable constituents of combined drug programs.
1. Introduction
Sphingosine 1-phosphate (S1P), that is produced principally by stimulated mast cells and mononuclear phagocytes in tissues and by erythrocytes in blood [1, 2], is a major mediator of T cell lymphoid traffic, tissue migration, proliferation and cytokine secretion [3–6]. Of the family of G protein-coupled receptors (GPCRs) with high specificity for S1P, the type 1 receptor (S1P1) predominates in its level of expression and transduction of diverse T cell functional responses [7, 8]. Signals from the S1P-S1P1 axis inhibit T cell return from tissues to afferent lymphatics, T cell influx into lymph nodes, and marginal zone B cell entry into the T cell area of splenic follicles, in response to chemokines [6, 9, 10]. Positive S1P-S1P1 chemotactic signals are required for thymocyte emigration and for T cell efflux from lymph nodes and some other tissues to the higher concentrations of S1P in blood and lymph than tissue extracellular fluids. Lymphocyte proliferation is affected variably by the S1P-S1P1 axis, depending on the basal level of activity.
Analyses of the effects of S1P on non-migration functions of T cells initially focused on cytokine generation by the subsets of CD4 (helper/inducer) T cells termed Th1, that are central to classical cell-mediated immunity, and type Th2, that mediate inflammation and allergy. The first results suggested induction by the S1P-S1P1 axis of a moderate shift from Th1 to Th2, as there was very significant suppression of production of IFN-γ along with slight enhancement of production of IL-4 [3]. These observations all relied on stimulating the S1P-S1P1 axis by increasing the fluid-phase concentration of S1P. More recently, it was demonstrated that upregulation of expression of S1P1 selectively on CD4 T cells in a transgenic (TG) mouse model, which persisted despite the down-regulating effect of T cell activation, was alone sufficient to enhance T cell generation of IL-4 very significantly and augment IgE antibody responses leading to increased immediate-type hypersensitivity in tissues [5, 11]. Increased IL-4 production in this setting was at a transcriptional level and was mediated by concerted effects of higher levels of active transcription factors c-Maf, Jun B, and Gata3. As this increase in IL-4 augmented IgE antibody responses, it was postulated that such physiological elevations of IL-4 also might be capable of stimulating greater production of adaptive regulatory T cells (Treg cells) through STAT6 and/or crossed STAT5 mechanisms. The results of these investigations were the first revelation of our most recent appreciation that the S1P-S1P1 axis has major effects on terminal differentiation of subsets of CD4+ helper/inducer T cells, including both adaptive Treg cells and effector Th17 cells.
2. The S1P-S1P1 axis in differentiation of adaptive Treg cells
Approximately 5–10% of CD4 T cells are constitutive Treg cells that develop in a thymic pathway independently of antigen and of changes in concentrations of cytokines that affect differentiation of other T cell subsets [12, 13]. Adaptive Tregs, like some effector subsets of CD4 T cells, develop post-thymically in peripheral lymphoid tissues [14]. The discoveries that both adaptive Treg cells and the Th17 subset of effector cells, that are critical in mediation of many autoimmune reactions, develop in mice most efficiently in a lymphoid environment rich in beta-transforming growth factor (TGF-β) re-focused experimental attention on immune and pharmacological factors that separately, cooperatively, or oppositely influence differentiation of these respective T cell subsets. For example, IL-4 and its IL-4R are required for selective development of CD4+CD25+ Treg cells, that express high levels of the transcription factor FoxP3 which promotes their differentiation and functions [15, 16]. Much higher levels of IL-4 are generated by CD4 T cells of S1P1 TG mice than wild-type (WT) mice [5]. Thus, it was not surprising that conversion of CD4+CD25−FoxP3− splenic T cell precursors of adaptive Treg cells to fully differentiated CD4+CD25+FoxP3+ Treg cells is much greater in vitro and in vivo in S1P1 TG than WT C57BL/6 mice [17].
The possibility that enhanced signals from the S1P-S1P1 axis in S1P1 TG mice would increase peripheral differentiation of adaptive Treg cells was examined first using anti-T cell antigen receptor (TCR) antibodies and S1P to stimulate their splenic CD4+CD25−FoxP3− T cells, that had been purified to eliminate both constitutive Treg and preformed adaptive Treg cells. The conversion of FoxP3− T cells to FoxP3+ adaptive Treg cells was a mean of eight-fold higher for T cell-selective S1P1 TG mice than WT mice [17]. The same enhancement of generation of adaptive Treg cells by the S1P-S1P1 axis was found with antigen stimulation by contrasting results in OTII TCR TG mice, in which each transgenic TCR is specific for an ovalbumin peptide (OVA) antigen, with those in double transgenic (DTG) mice, derived by crossing S1P1 TG mice with OTII OVA-specific TCR single TG mice. Stimulation of CD4+CD25−FoxP3− T cells from these two types of mice with OVA and S1P in vitro induced far greater differentiation of DTG T cells than OTII T cells into adaptive FoxP3+ Treg cells [17]. Quantitative real-time PCR analyses of RNA isolated from replicate CD4+CD25− T cell suspensions in the same studies showed that expression of mRNA encoding FoxP3 was upregulated by OVA plus S1P to a level significantly higher than with OVA alone and was highest when the source was DTG mice as contrasted with OTII mice. In the absence of S1P, the level of FoxP3 mRNA in OVA-stimulated T cells of DTG mice was no higher than that in OVA-stimulated T cells of OTII mice.
The prevalence of adaptive Tregs rose to about 5% of total CD4 T cells after stimulation with S1P and anti-TCR antibodies for S1P1 TG mice, or with S1P and OVA for DTG mice [17]. This quantity approximated that of constitutive Tregs and thus doubled the frequency of the total effective Treg set. The functional suppressive activity of Tregs generated in vitro also was examined through quantification of their effects on proliferation of fresh WT C57BL/6 CD4+25−T cells stimulated by anti-CD3 plus anti-CD28 antibodies (a-TCR Abs) or by the mitogen PHA. When adaptive Tregs induced in an equal total number of preincubated CD4 T cells were introduced into fresh CD4+25− T cell suspensions, the suppression of proliferation was significantly greater for those from S1P1 TG mice than WT mice. When the levels of adaptive Treg cells in each suspension were equalized prior to addition to the responding CD4+25− T cells, T cell proliferation evoked by both stimuli was suppressed equivalently and up to a mean of 60% by adaptive Treg from S1P1 TG mice and WT mice. These results establish the important points that Treg cells generated from the CD4+25− T cells of WT and S1P1 TG mice in vitro have equal intrinsic suppressive functions and that the greater overall Treg activity of suspensions of T cells from S1P1 TG mice over those from WT mice, is attributable principally to their greater number rather than higher intrinsic activity.
IL-4-IL-4 receptor(R) requirements for S1P- S1P1 axis enhancement of generation of adaptive FoxP3+ Treg cells initially were demonstrated in vitro [17]. When CD4+CD25−FoxP3− T cells from WT mice and S1P1 TG mice were incubated on anti-TCR antibodies for up to seven days, concentrations of IL-4 were a mean of six-fold higher in the cultures of S1P1 TG mouse T cells early in the incubation with little effect of exogenous S1P and rose to a mean of 15-fold higher by the end of the week. Separate application of anti-IL4 R antibody and anti-IL-4 antibody demonstrated the capacity of each to significantly inhibit conversion of S1P1 TG FoxP3− to FoxP3+ T cells, without affecting this conversion in T cells of WT mice [17]. The combination of anti-IL4 R antibody and anti-IL-4 antibody most significantly suppressed generation of S1P1 TG FoxP3+ T cells and also inhibited to a lesser extent the generation of WT FoxP3+ T cells.
Two related in vivo approaches established the involvement of the S1P-S1P1 axis and its augmentation of the IL-4-IL-4R system in antigen-driven generation of adaptive (FoxP3+) Treg cells in lymphoid tissues. First, when CD4+CD25−FoxP3− T cells from OTII and DTG mice were introduced separately into irradiated WT C57BL/6 host mice followed by intravenous OVA antigen intravenously, the level of FoxP3 in the DTG CD4 T cells was more than 14-fold higher than in OTII CD4 T cells seven days later [17]. Functional suppressive activity of the two purified sets of adaptive Tregs was examined by adding equal numbers to fresh WT CD4+25− T cells prior to stimulation and quantification of proliferation. The adaptive Treg cells from DTG mice were significantly more suppressive of proliferation than those from OTII mice. Thus adaptive Tregs generated in DTG mice in vivo have greater intrinsic suppressive activity than those from OTII mice, in contrast to the equivalent activity of adaptive Tregs of WT and S1P1 TG mice generated in vitro. Second, OTII and DTG mice were immunized with intravenous OVA antigen directly four days before recovery of CD4 T cells from blood, lymph nodes and spleen for analysis of FoxP3 expression. The levels of FoxP3 expression were higher in total CD4 T cells from all sources of DTG than OTII mice and these increments were statistically significant for blood and spleen, but not lymph nodes [17]. Further, only the levels of FoxP3 expression in blood and spleen CD4 T cells of DTG mice were higher than could be attributed to constitutive thymically-derived Treg cells alone. The role of IL-4 in S1P-S1P1 axis enhancement of antigen-induced generation of adaptive Tregs was proven in studies of two groups of OTII and DTG mice, that each received 30 ug of anti-IL-4 and 100 ug of anti-IL-4 R antibodies three days prior to harvesting splenic CD4 T cells for quantification of FoxP3 expression. The prevalence of splenic CD4 T cells expressing FoxP3 was significantly higher for DTG mice than for OTII mice three days after intravenous injection of isotype control rat IgG, whereas intravenous anti-IL-4 plus anti-IL-4 R antibodies reduced significantly the number of FoxP3 CD4 T cells in spleens of DTG mice to the same level as those in spleens of OTII mice, which did not decrease significantly [17].
3. The S1P-S1P1 axis in differentiation of Th17 effector cells
Numerous immune and other physiological factors separately, cooperatively, or oppositely influence differentiation of the Th17 effector T cell subset and adaptive Treg cell subset of CD4 T cells. Of the immune cytokines, IL-4 and IL-6 have the most prominent reciprocal influences on development of Th17 and adaptive Treg cells. Some other physiological factors act through effects on these cytokines and some act independently of the defining cytokines. Examples of factors that act reciprocally early in differentiation are pertussis toxin, that elicits generation of Th17 cells and inhibits development of Treg cells by inducing production of IL-6 [18], and retinoic acid, that suppresses IL-6-driven development of Th17 cells while enhancing differentiation of Treg cells [19]. In contrast to these factors that act reciprocally on early differentiation of Th17 and Treg cells, the later-acting S1P-S1P1 axis similarly promotes development of Th17 effector cells as well as Treg cells. The S1P-S1P1 axis acts in concert with a different array of cytokines to attain T cell subset specificity. In the same standardized in vitro system used for studies of mouse splenic CD4 T cell differentiation into adaptive CD4+CD25+FoxP3+ Treg cells, Il-6 was stimulatory and IL-4 was inhibitory of Th17 effector cell development, whereas their roles were the reverse in differentiation of adaptive Treg cells. The effects of the S1P-S1P1 axis were observed later in differentiation than the effects of IL-4 and IL-6, and promoted terminal development of both adaptive Treg cells and Th17 effector cells as an alternative stimulus to other types of immune cytokines that also act as terminal differentiation factors.
S1P is equal in activity to IL-23 as a terminal differentiation factor for WT Th17 effector T cells in an in vitro model where their development is initiated by TCR stimulation, TGF-β, IL-1, and IL-6 [20]. In some systems, the IL-21 secreted by Th17 cells provides positive-feedback stimulation of their development by serving as another alternative terminal differentiation factor. The introduction of IL-4 without and with IFN-γ in this culture model suppressed S1P-driven differentiation of Th17 cells as significantly as did addition of IL-27, another natural inhibitor of Th17 cell development [20].
Roles of the S1P-S1P1 axis in development of Th17 cells also were examined in vivo by delineating differential effects and cytokine requirements of OVA antigen stimulation in OTII TCR transgenic and T cell-selective S1P1 x OTII DTG mice [21]. OVA-activated CD4 T cells from DTG mice manifest far greater chemotactic responses to S1P and inhibition of generation of IFN-γ by S1P than for those of OTII mice, which confirms their expected functionally higher expression of S1P1 [21]. Relative to OVA-stimulated CD4 T cells of OTII mice, those of DTG mice had higher levels of mRNA encoding IL-17, intracellular IL-17 by ELISpot assays, and secretion of IL-17. Greater activity of the S1P-S1P1 axis in CD4 T cells of DTG mice than OT-II mice led to this higher expression of IL-17 through an antigen- and IL-6-dependent pathway [21]. OVA challenge of subcutaneous air-pockets elicited influx of more OTII TCR-positive T cells generating a higher level of IL-17 in DTG mice than OTII mice. This transcriptionally-determined greater production of IL-17 by DTG CD4 T cells was prevented in vitro either by an S1P1-selective antagonist or by exogenous IL-4. IL-4 in this setting inhibited S1P-S1P1 axis augmentation of the number and activities of Th17 cells, whereas it mediates enhancement of development and activities of adaptive Treg cells by the S1P-S1P1 axis.
4. Complementary actions of immune cytokines and the S1P-S1P1 axis in terminal differentiation of adaptive Treg cells and Th17 effector cells
An initial conceptual model may be proposed to integrate recently-identified individual roles of the S1P-S1P1 axis in differentiation of CD4 T cell subsets (Fig. 1). These findings together suggest that the process of differentiation is initiated by TCR activation in a TGF-β-rich environment. Then the ratio of IL-4 to IL-6 concentrations at the major early branch-point determines which CD4 T cell subset will progress to terminal differentiation. IL-4 promotes adaptive Treg advancement and suppresses that of Th17 effector cells, whereas IL-6 promotes Th17 advancement and suppresses that of Treg cells.
Fig. 1.
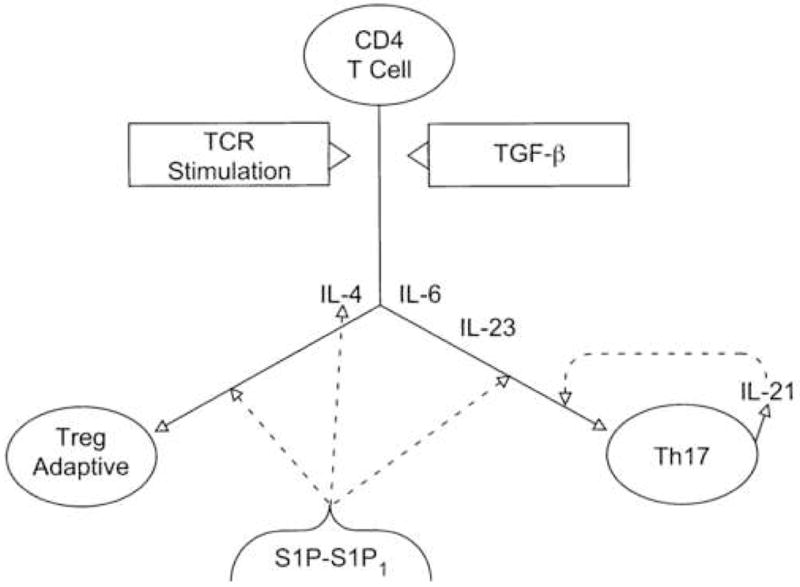
Complementary actions of cytokines and the S1P-S1P1 axis in terminal differentiation of Th17 effector cells and adaptive Treg cells. CD4 T cell= uncommitted T cell precursor of Th17 cell and adaptive Treg cell, and TCR= T cell antigen receptor. Solid lines depict the pathways of CD4 T cell differentiation or the generation of IL-21 and dashed lines show stimulation of production of IL-4 or of differentiation of Th17 and adaptive Treg cells.
The S1P-S1P1 axis is one major stimulus of terminal differentiation of CD4 T cell subsets, either as a direct alternative to cytokine mechanisms or indirectly by enhancing the local concentration of a stimulatory cytokine (Fig. 1). The introduction of S1P after initial addition of IL-6 with TGF-β to cultures of CD4 T cells activated by TCR stimulation enhances differentiation of Th17 cells, but not Treg cells [20]. In this setting, S1P augmentation of terminal differentiation is a direct alternative to the enhancing effect of IL-23 or of IL-21 released by Th17 cells in an auto-enhancing mechanism. There is no information yet about whether S1P also resembles IL-23 in its capacity to improve survival of Th17 cells. In contrast, the S1P-S1P1 axis enhances differentiation of Treg cells in vivo predominantly by increasing local production and thereby concentration of IL-4 [21]. Further augmentation of adaptive Treg generation by addition of S1P after initial introduction of optimal levels of exogenous IL-4 in vitro suggests a second direct Treg-stimulatory mechanism of the S1P-S1P1 axis. However, increased production of endogenous IL-4 in S1P1 TG and DTG mice, as a result of upregulation of CD4 T cell expression of S1P1 in vivo, has a maximal stimulatory effect on development of adaptive Treg cells that is nearly completely eliminated by concurrent administration of neutralizing anti-IL-4 Abs and anti-IL-4 R Abs [17]. The direct IL4-independent S1P-S1P1 axis effect on Treg differentiation therefore is probably a minor mechanism.
Further proof of the importance of these effects of the S1P-S1P1 axis on differentiation CD4 T cell subsets will require application of selective S1P1 antagonists in both physiological circumstances and immune inflammatory diseases. What also remains is to fully characterize the full range of specific functional capabilities and mechanisms of action of Treg cells and Th17 cells induced to develop under the influence of the S1P-S1P1 axis in comparison with these properties of Treg and Th17 cells derived entirely by cytokine-dependent mechanisms.
5. Levels of regulation of S1P-S1P1 axis effects on T cell traffic and functions
Many pharmacological agents that either alter concentrations of S1P in physiological compartments, mimic or alter the interaction of S1P with S1P1, or modify the level of cellular expression of S1P1 have been designed to influence specifically the effects of the S1P-S1P1 axis on immunity. These may be considered in three different categories (Fig. 2). Their relevance to control of S1P-S1P R axes in many organ systems has stimulated intense interest in development of Sph K inhibitors (Fig. 2- level 1). The ability of a sphingosine kinase (Sph K) inhibitor to decrease concentrations of S1P in lymph and blood predicts that the immunological effects would include a lower rate of thymocyte emigration, reduced lymphocyte traffic in lymphoid organs, decreased effective interactions of T cells and B cells in lymphoid follicles, and facilitation of T cell return from tissue sites to lymph. The net effect on immune responses should be inhibition of T cell-mediated immunity and to a lesser extent antibody generation, probable sparing of immune responses to most microbes, and possible increases in some types of T cell-mediated tissue inflammation. The broad involvement of the S1P-S1P R axes in most physiological systems suggests that this would be an approach with less than desirable specificity and many undesirable side-effects. Another possibly additive approach to lowering concentrations of S1P in physiological fluids would be augmentation of the levels of activity of the S1P-degrading sphingosine phosphate phosphatases (SPP1 and SPP2) and/or sphingosine phosphate lyase (SPL), but this strategy has received less attention in mammals (Fig. 2- level 2).
Fig. 2.
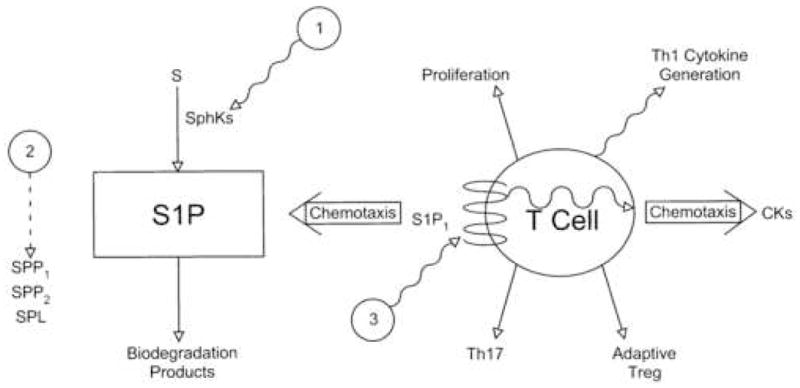
Levels of regulation of control of T cell traffic and functions by the S1P- S1P1 axis. S= sphingosine, SPP= sphingosine phosphate phosphatase, SPL= sphingosine phosphate lyase, and CKs = chemokines. Solid arrows depict a process of T cell differentiation, a T cell functional response, or a metabolic conversion, the dashed arrow shows stimulation, and wavy arrows or lines indicate inhibition or suppression of a component of the S1P-S1P1 axis or of a T cell response.
The most potentially specific approach is directed at the S1P1 R level because of the many possibilities for altering S1P1 R expression, S1P binding, signaling mechanisms, and selective coupling to individual functions. FTY720, a range of analogs, and their phosphorylated derivatives are partial agonists and down-regulators for most of the known S1P GPCRs, that have been extensively characterized experimentally and clinically [22–24]. This comprehensive experience has proven that pharmacological downregulation of lymphocyte and endothelial cell S1P1 Rs, without or with prior stimulation of cellular signaling, alters lymphocyte traffic and functions sufficiently to suppress immunity [25]. FTY720-induced immunosuppression is significant as evidenced by depressed in vivo T cell-mediated immunity, suppressed organ graft rejection, and alleviation of many autoimmune diseases in animal models as well as multiple sclerosis in humans. Maintenance of immunosuppression by FTY720, however, is associated with such a broad spectrum of serious ocular, pulmonary and cardiac complications that alternative agents and approaches are being sought which have S1P1 R selectivity, a greater ratio of antagonist and downregulator functions to agonist activity, and preferential organ system effects. One very hopeful therapeutic possibility is a recently discovered cluster of monoclonal anti-S1P1 Abs that inhibit lymphocyte functional responses to S1P. Two clear advantages of these monoclonal antibodies are absolute specificity and the greater ability to focus effective inhibitory concentrations of a protein than a small organic compound at a tissue site of involvement of the S1P-S1P1 axis.
Acknowledgments
The research described was supported by grant RO-1 HL31809 from the National Institutes of Health and the Kenneth Rainin Fellowship Endowment Fund.
Abbreviations
- S
sphingosine
- S1P
sphingosine 1-phosphate
- GPCR
G protein-coupled receptor
- S1P1
type 1 GPCR for S1P
- TG
transgenic
- WT
wild-type
- TCR
T cell antigen receptor
- OVA
a distinct substituent peptide epitope of ovalbumin
- OTII
a line of C57BL/6 mice in which all TCRs are specific for OVA
- DTG
double TG mice generated by crossing OTII mice with S1P1 TG mice, in which T cells selectively express higher than normal levels of S1P1
- Treg cells
regulatory T cells
- PHA
phytohemagglutinin mitogen
- Ab
antibody
- R
receptor
- Sph K
sphingosine kinase
- SPP
sphingosine phosphate phosphatase
- SPL
sphingosine phosphate lyase
- CK
chemokine
- FTY720
a pharmacological partial agonist of multiple S1P GPCRs
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Olivera A, Rivera J. Sphingolipids and the balancing of immune cell function: lessons from the mast cell. J Immunol. 2005;174:1153–1158. doi: 10.4049/jimmunol.174.3.1153. [DOI] [PubMed] [Google Scholar]
- 2.Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, Camerer E, Zheng YW, Huang Y, Cyster JG, Coughlin SR. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 3.Rosen H, Goetzl EJ. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat Rev Immunol. 2005;5:560–570. doi: 10.1038/nri1650. [DOI] [PubMed] [Google Scholar]
- 4.Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–1739. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- 5.Wang W, Huang MC, Goetzl EJ. Type 1 sphingosine 1-phosphate G protein-coupled receptor (S1P1) mediation of enhanced IL-4 generation by CD4 T cells from S1P1 transgenic mice. J Immunol. 2007;178:4885–4890. doi: 10.4049/jimmunol.178.8.4885. [DOI] [PubMed] [Google Scholar]
- 6.Ledgerwood LG, Lal G, Zhang N, Garin A, Esses SJ, Ginhoux F, Merad M, Peche H, Lira SA, Ding Y, Yang Y, He X, Schuchman EH, Allende ML, Ochando JC, Bromberg JS. The sphingosine 1-phosphate receptor 1 causes tissue retention by inhibiting the entry of peripheral tissue T lymphocytes into afferent lymphatics. Nat Immunol. 2008;9:42–53. doi: 10.1038/ni1534. [DOI] [PubMed] [Google Scholar]
- 7.Graeler M, Shankar G, Goetzl EJ. Cutting edge: suppression of T cell chemotaxis by sphingosine 1-phosphate. J Immunol. 2002;169:4084–4087. doi: 10.4049/jimmunol.169.8.4084. [DOI] [PubMed] [Google Scholar]
- 8.Dorsam G, Graeler MH, Seroogy C, Kong Y, Voice JK, Goetzl EJ. Transduction of multiple effects of sphingosine 1-phosphate. S1P on T cell functions by the S1P1 G protein-coupled receptor. J Immunol. 2003;171:3500–3507. doi: 10.4049/jimmunol.171.7.3500. [DOI] [PubMed] [Google Scholar]
- 9.Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D, Keohane C, Rosenbach M, Hale J, Lynch CL, Rupprecht K, Parsons W, Rosen H. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 10.Cinamon G, Matloubian M, Lesneski MJ, Xu Y, Low C, Lu T, Proia RL, Cyster JG. Sphingosine 1-phosphate receptor 1 promotes B cell localization in the splenic marginal zone. Nat Immunol. 2004;5:713–720. doi: 10.1038/ni1083. [DOI] [PubMed] [Google Scholar]
- 11.Graler MH, Huang MC, Watson S, Goetzl EJ. Immunological effects of transgenic constitutive expression of the type 1 sphingosine 1-phosphate receptor by mouse lymphocytes. J Immunol. 2005;174:1997–2003. doi: 10.4049/jimmunol.174.4.1997. [DOI] [PubMed] [Google Scholar]
- 12.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 14.Shevach EM. From vanilla to 28 flavors: multiple varieties of T regulatory cells. Immunity. 2006;25:195–201. doi: 10.1016/j.immuni.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nat Immunol. 2007;8:457–462. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez-Guajardo V, Tanchot C, O’Malley JT, Kaplan MH, Garcia S, Freitas AA. Agonist-driven development of CD4+CD25+Foxp3+ regulatory T cells requires a second signal mediated by Stat6. J Immunol. 2007;178:7550–7556. doi: 10.4049/jimmunol.178.12.7550. [DOI] [PubMed] [Google Scholar]
- 17.Wang W, Huang MC, Marquez CP, Liao JJ, Goetzl EJ. IL-4 Requirement for Sphingosine 1-Phosphate Enhancement of Treg Cell Differentiation. J Immunol. 2008 in press. [Google Scholar]
- 18.Chen X, Howard OM, Oppenheim JJ. Pertussis toxin by inducing IL-6 promotes the generation of IL-17-producing CD4 cells. J Immunol. 2007;178:6123–6129. doi: 10.4049/jimmunol.178.10.6123. [DOI] [PubMed] [Google Scholar]
- 19.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 20.Liao JJ, Huang MC, Goetzl EJ. Cutting edge: Alternative signaling of Th17 cell development by sphingosine 1-phosphate. J Immunol. 2007;178:5425–5428. doi: 10.4049/jimmunol.178.9.5425. [DOI] [PubMed] [Google Scholar]
- 21.Huang MC, Watson SR, Liao JJ, Goetzl EJ. Th17 augmentation in OTII TCR plus T cell-selective type 1 sphingosine 1-phosphate receptor double transgenic mice. J Immunol. 2007;178:6806–6813. doi: 10.4049/jimmunol.178.11.6806. [DOI] [PubMed] [Google Scholar]
- 22.Brinkmann V, Pinschewer DD, Feng L, Chen S. FTY720: altered lymphocyte traffic results in allograft protection. Transplantation. 2001;72:764–769. doi: 10.1097/00007890-200109150-00002. [DOI] [PubMed] [Google Scholar]
- 23.Hale JJ, Neway W, Mills SG, Hajdu R, Ann Keohane C, Rosenbach M, Milligan J, Shei GJ, Chrebet G, Bergstrom J, Card D, Koo GC, Koprak SL, Jackson JJ, Rosen H, Mandala S. Potent S1P receptor agonists replicate the pharmacologic actions of the novel immune modulator FTY720. Bioorg Med Chem Lett. 2004;14:3351–3355. doi: 10.1016/j.bmcl.2004.02.106. [DOI] [PubMed] [Google Scholar]
- 24.Graler MH, Goetzl EJ. The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G-protein-coupled receptors. FASEB J. 2004;18:551–553. doi: 10.1096/fj.03-0910fje. [DOI] [PubMed] [Google Scholar]
- 25.Goetzl EJ, Rosen H. Regulation of immunity by lysosphingolipids and their G protein-coupled receptors. J Clin Invest. 2004;114:1531–1537. doi: 10.1172/JCI23704. [DOI] [PMC free article] [PubMed] [Google Scholar]


