Abstract
Differences in the locomotor stimulating and rewarding properties of drugs of abuse have been described in several inbred strains of mice, and comparisons of inbred strains with differing responses to drugs of abuse may provide crucial insight into the question of individual vulnerability to the effects of drugs of abuse. The present study was designed to examine the rewarding and locomotor-stimulating effects of heroin in C57BL/6J and 129P3/J mice. Heroin produced a robust dose dependent locomotor stimulation in both strains. Both strains also developed conditioned place preference to heroin, again in a dose dependent manner. However C57BL/6J mice developed conditioned place preference to only the two lowest doses of heroin tested, while the 129P3/J counterparts showed conditioned place preference to only the three highest doses tested. These studies indicate that 129P3/J mice are less sensitive to the rewarding effects of heroin than are age-matched C57BL/6J mice.
Keywords: Mouse, Heroin, Behavior
Introduction
Many strains of mice differ in their baseline behaviors (e.g.[2, 5, 16, 24, 34]). Furthermore, differences in behavioral response to drugs of abuse have been reported in many strains of mice (e.g. [5, 6, 14, 15, 18, 28, 29, 31, 36]).
Individual vulnerability to the effects of drugs of abuse has been investigated in rodents by examining high or low responders of outbred strains (e.g. [11, 26]) or by comparing different inbred strains which had been shown to differ in their predisposition to self-administer drugs of abuse (e.g. [7, 13]). Behavioral response to drugs of abuse was studied in the C57BL/6J and different 129 inbred strains of mice, due to their importance in the generation of transgenic and knockout animals. In response to cocaine, mice of 129 substrains have consistently been reported to show limited or no locomotor response to cocaine, while C57BL/6J mice respond with robust locomotor activation [15, 18, 28, 29]. These results suggest that 129 mice are hyporesponsive to the locomotor stimulating effects of cocaine, relative to C57BL/6J mice. 129/OlaHsd mice failed to self-administer cocaine under conditions which supported this behavior in C57BL/6J and DBA mice [14], and 129P3/J mice did not develop conditioned place preference to cocaine administered in a “binge” pattern, while C57BL/6J mice did (although both strains developed conditioned place preference to a single daily injection of cocaine, [36]), again suggesting that the 129 strain is less responsive to the effects of drugs of abuse than C57BL/6J mice.
Opiate drugs produce locomotor activation in rodents (e.g. [1, 21, 25]). Some studies have shown strain differences in the locomotor stimulating and analgesic effects of opiates in mice (e.g. [22, 23]). C57BL/6J mice showed behavioral activation in response to opiates [5, 19, 31]. 129X1/sVJ mice showed a locomotor stimulatory effect of heroin, but they did not develop conditioned place preference to the dose administered, rather they expressed place aversion [31]. Interestingly, 129P3/J mice failed to develop physical dependence to morphine as assessed by naloxone precipitated jumping [12]. Thus, 129P3/J mice may be relatively insensitive to the effects of drugs of abuse in general. The present study was designed to extend our findings that 129P3/J mice are behaviorally hyporesponsive to cocaine, relative to C57BL/6J mice, by comparing the behavioral response to heroin in both strains. We examined both locomotor stimulating and rewarding effects of heroin in these strains in response to a wide range of heroin doses (0 -20 mg/kg) within a conditioned place preference (CPP) paradigm.
Materials and Methods
Animals
All studies were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Experimental Animals (NIH Publication No. 90-23) and experimental protocols were approved by the Rockefeller University Institutional Animal Care and Use Committee. A total of 128 age-matched male mice (6 weeks old on arrival), 56 C57BL/6J and 72 129P3/J mice were studied, in seven independent cohorts (Table 1). Saline controls were included in all cohorts. All animals were individually housed in an environmentally controlled room dedicated to this study. Food and water were available ad lib and animals were allowed two weeks to acclimate prior to the start of the experiments. Mice of each strain were randomly assigned to one of six groups, based on dose of heroin administered (0, 1.25, 2.5, 5, 10 or 20 mg/kg). Three animals died during the course of these studies (two 129P3/J and one C57BL/6J) and were not included in analyses. Additionally, one 129P3/J statistical outlier was deleted from the analysis of conditioned place preference. Heroin (3,6-Diacetyl-morphine HCl) was obtained from NIH - NIDA.
Table 1.
The total number of animals used, in each strain and in each group, is shown in Table 1. The cohort in which each group was studied is shown in parenthesis.
| Dose (mg/kg) | C57BL/6J | 129P3/J |
|---|---|---|
| 0 | n =14 (cohorts 1,4,7) | n =17 (cohorts 2, 3, 5, 6) |
| 1.25 | n = 6 (cohort 7) | n = 12 (cohorts 5, 6) |
| 2.5 | n = 6 (cohort 7) | n = 12 (cohorts 5, 6) |
| 5 | n = 9 (cohorts 1,4) | n = 9 (cohorts 2, 3) |
| 10 | n = 10 (cohorts 1,4) | n = 10 (cohorts 2, 3) |
| 20 | n = 10 (cohorts 1,4) | n = 10 (cohorts 2, 3) |
Conditioned Place Preference
Chambers
Commercially available conditioned place preference chambers (Med Associates, VT model ENV-3013) were used. The chambers consist of three compartments, separated from each other by a sliding door. Two “conditioning” compartments, one black and one white (16.8 × 12.7 × 12.7 cm) were separated from each other by a smaller, gray chamber. The white compartment had a stainless steel mesh floor while the floor of the black compartment was rolled steel bars. Each chamber was enclosed in a dimly lit, sound attenuated chamber. Data collection was automated and used infrared photo beams to determine time spent in each compartment and crossovers (breaking of photo beams at opposite ends of the chamber).
Conditioning
One half of the animals received heroin on the first conditioning day and the other half received saline (animals in the 0 mg/kg group received isotonic saline [0.9% NaCl] on all days). Half of the animals had heroin paired to the white chamber, the other half to the black chamber. The day before the first conditioning session, each animal was placed in the central gray compartment, with free access to all three compartments, and the time spent in each compartment was recorded over 30 minutes. Conditioning sessions were carried out as previously described [36] for a total of eight conditioning sessions, once a day for 30 minutes. On each conditioning day, mice were injected, i.p., with heroin at doses of 1.25, 2.5, 5, 10 or 20 mg/kg, dissolved in saline, or saline, and were immediately placed in the “paired” compartment where they were confined for 30 minutes, while crossovers were electronically recorded. On the next day, the procedure was repeated, alternating saline with heroin. Following each conditioning session, mice were weighed and returned to their home cage. Conditioning was carried out over eight days, four heroin conditioning sessions and four saline sessions. The day after the last conditioning session, animals were returned to the conditioning chamber, given free access to all three compartments and the time spent in each compartment was recorded over 30 minutes. The difference in time spent in the drug paired compartment during the pre- and post-conditioning sessions was calculated and used to determine the development of conditioned place preference.
Statistical Analysis
Pre-conditioning side bias was examined by comparing the time spent in the white and black chambers during the “pre-test” session. Pre-conditioning side bias was examined in C57BL/6J mice with unpaired two-tailed t-test. Due to a non-Gaussian distribution, pre-conditioning side bias in 129P3/J mice was analyzed using a Mann-Whitney U test. To rule out a possible effect of “Cohort,” the locomotor activity across the four conditioning sessions in the saline groups was analyzed within each strain by two-way ANOVA, Cohort by Session with repeated measures on the last variable, and conditioned place preference was analyzed by one-way ANOVA.
To rule out possible pre-conditioning bias, conditioned place preference was initially analyzed by three-way ANCOVA (Strain X Dose X Drug Paired Side) with pre-conditioning bias (time spent in black chamber - time spent in white chamber) as a covariate. Then, conditioned place preference in each strain was examined by one-way ANOVA (Dose). Locomotor activity was analyzed with three-way ANOVA, Strain X Dose X Session, with repeated measures on the last variable followed by Newman-Keuls post hoc tests where appropriate.
Results
Since all doses of heroin were not represented in each cohort, we examined the effect of cohort on the locomotor response and the time in conditioning chambers in saline controls (which were represented in all cohorts). “Cohort” had no effect on locomotor activity in either C57BL/6J (p = 0.167) or 129P3/J (p = 0.943) mice nor on the time in conditioning chambers in C57BL/6J (p = 0.120 or 129P3/J (p = 0.605) mice.
On the pre-conditioning test day C57BL/6J mice did not show a preference for either compartment (p = 0.44), but 129P3/J mice did show a preference for the black chamber (U = 1698, p < 0.0005; data not shown).
Heroin increased locomotor activity in both strains, in a dose and time dependent manner across conditioning sessions (Figure 1). ANOVA showed significant main effects of Dose and Session (F(5,113) = 58.55, p < 0.0001 and F(3,339)=40.45, p < 0.0001 respectively) and a significant Strain X Dose X Session interaction (F(15,339)=2.54, p < 0.02). There was no significant difference in basal (saline) levels of locomotor activity between the two strains (p = 0.332, Newman-Keuls post hoc test). In C57BL/6J mice, the locomotor response to all doses of heroin was significantly greater than to saline (p < 0.005) and resembled an inverted “U” (Figure 1). The locomotor stimulating effects of heroin at doses of 2.5, 5 and 10 mg/kg were greater than the response to 1.25 mg/kg (p < 0.05). The locomotor response to 1.25 and 20 mg/kg were not significantly different (p = 0.22). 129P3/J mice also responded to all doses of heroin with increased locomotor activity (p < 0.05) and again the response resembled an inverted “U”. In 129P3/J mice, the locomotor responses to heroin doses of 5, 10 or 20 mg/kg were greater than those to 1.25 mg/kg (p < 0.002), and heroin doses of 5 and 10 mg/kg resulted in significantly greater levels of locomotion than did 2.5 mg/kg (p < 0.0005; Figure 1).
Figure 1.
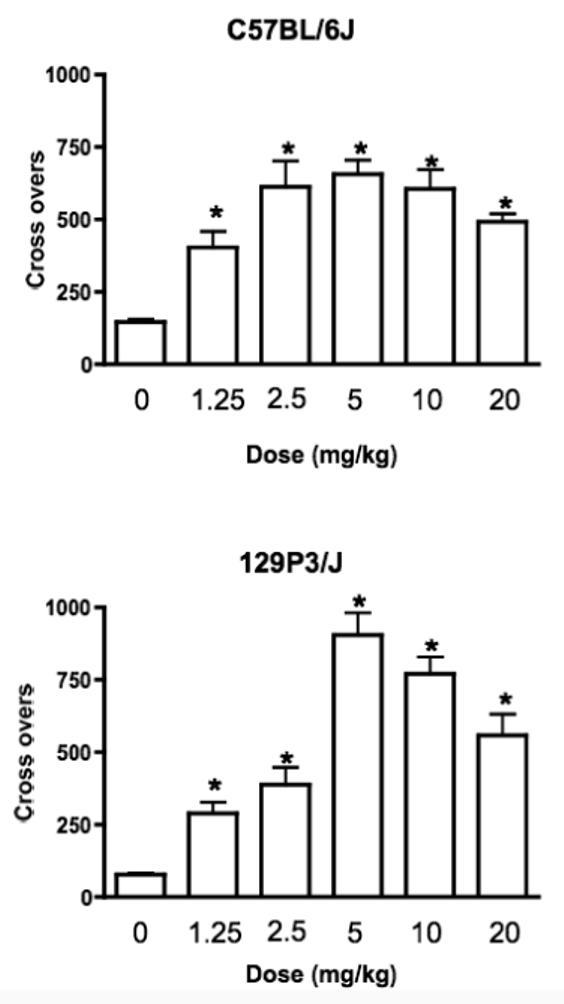
Heroin produced a dose dependent increase in locomotor activity in both C57BL/6J (top) and 129P3/J (bottom) mice. Data is mean + SEM of the number of crosses across the four 30 minute conditioning sessions. * = Significant increase compared to saline
Both strains showed a dose-dependent sensitization of heroin-induced locomotion; the locomotor response to heroin increased with subsequent administrations (Strain X Dose X Session interaction F(15,342) = 2.57 p < 0.005; Figure 2). In C57BL/6J mice, sensitization of locomotor response occurred at heroin doses of 1.25, 2.5, 5 and 10 mg/kg (Newman-Keuls post hoc tests). In 129P3/J mice, sensitization was observed only at the 5 and 10 mg/kg doses. Neither strain developed sensitization to the locomotor stimulating effects of 20 mg/kg of heroin.
Figure 2.
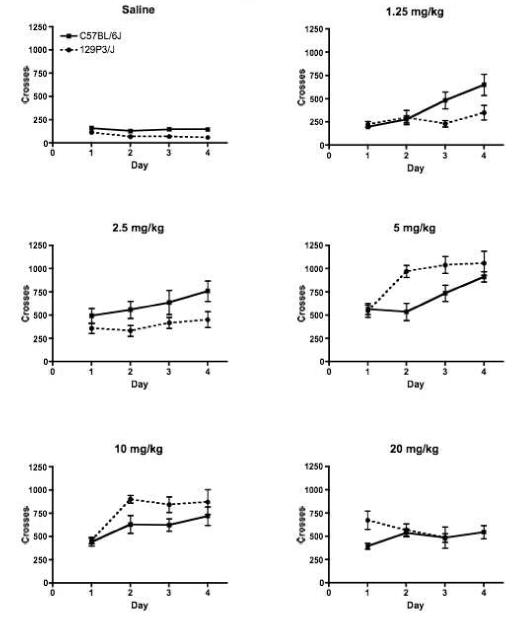
Heroin produced dose and time dependent sensitization of the locomotor stimulating effects in both C57BL/6J and 129P3/J mice (Strain X Dose X Session main effect F(15,342) = 2.57, p < 0.005). C57BL/6J mice are represented by the solid lines, while 129P3/J mice are represented by the dashed lines. Data is mean +/- SEM.
Since 129P3/J mice showed a distinct pre-conditioning bias for the black chamber of the conditioned place preference apparatus, we first examined conditioned place preference by three-way ANCOVA, and found main effects of Strain (F(1,100) = 9.94 p < 0.005) and Dose (F(5,100) = 3.23, p < 0.01) but no effect of Drug Paired Side (F(1,100) < 1.0 p = 0.94). We then analyzed conditioned place preference by one-way ANOVA within each strain. Both strains of mice developed conditioned place preference to heroin in a dose dependent manner. In C57BL/6J mice, there was a significant main effect of Dose (F(5,49) = 3.51, p < 0.01). C57BL/6J mice developed conditioned place preference to the two lowest doses of heroin tested, 1.25 and 2.5 mg/kg (p < 0.05), but not to the higher doses of 5, 10, or 20 mg/kg (Newman-Keuls post hoc tests; Figure 3). 129P3/J mice also developed conditioned place preference (main effect of Dose, F(5,63) = 4.93, p < 0.001), but 129P3/J mice developed conditioned place preference to the 5, 10 and 20 mg/kg doses of heroin (p < 0.02, 0.05 and 0.01 respectively; Newman-Keuls post hoc tests; Figure 3).
Figure 3.
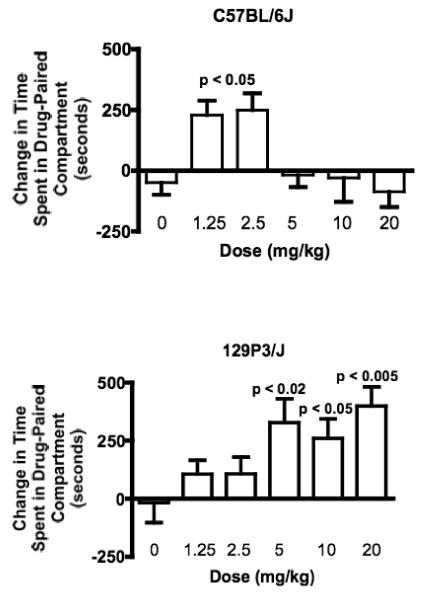
Heroin induced conditioned place preference in C57BL/6J (top) and 129P3/J (bottom) mice. C57BL/6J mice developed conditioned place preference to the two lowest doses of heroin tested (1.25 and 2.5 mg/kg), but not to higher doses. 129P3/J mice developed conditioned place preference to the 5, 10 and 20 mg/kg doses of heroin but not to the lower doses. Data is mean + SEM.
Discussion
C57BL/6J and 129P3/J mice show differences in the rewarding effects of heroin, as measured by conditioned place preference. This finding is consistent with the report of dvelopment of conditioned place preference to low doses of heroin (50 or 100 μg/kg) in C57BL/6J mice, but not in 129X1/sVJ mice [31]. Our study extends this finding by expanding the dose range and demonstrating the development of conditioned place preference in 129P3/J mice to high doses of heroin, which were not rewarding in C57BL/6J counterparts.
In contrast to earlier studies which examined the locomotor stimulating effects of cocaine in C57BL/6J and 129 mice (e.g. [15, 18, 28, 29]), this study found a robust locomotor response to heroin and the development of locomotor sensitization in both strains. All doses of heroin stimulated locomotor activity in both strains of mice, on all days of the study. Significant strain differences were observed at the 2.5 mg/kg and the 5 mg/kg doses (Figure 2).
In the present study, strain and dose dependent sensitization to the locomotor stimulating effects of heroin was observed. Locomotor sensitization in 129P3/J mice, occurred more rapidly than in C57BL/6J mice and appeared more robust. Our finding of behavioral sensitization in C57BL/6J mice is in agreement with earlier findings [31]. Our finding of sensitization to the locomotor stimulating effects of heroin in 129P3/J mice, however is in contrast to that study which did not find sensitization in 129X1/sVJ mice [31]. This difference may be related to substrain differences or, more likely, to differences in the doses of heroin administered. Our report examined behavioral effects of a wide range of heroin doses (0-20 mg/kg) and, in 129P3/J mice, we found locomotor sensitization only to the 5 and 10 mg/kg doses. The earlier study used a single dose of 100 μg/kg (below our lowest dose of 1.25 mg/kg; [31]).
The finding of robust locomotor activation in 129P3/J mice in response to heroin is in sharp contrast to reports of the locomotor stimulating effects of cocaine. We have reported that, while C57BL/6J mice showed a dose-dependent increase in home cage locomotor activity in response to cocaine, 129/J (now 129P3/J) mice did not [28, 29]. Similarly, 129/OlaHsd mice failed to express a locomotor response to cocaine administration [15]. Miner reported that mice of the 129/SvJ strain were hypoactive relative to C57BL/6J mice [18].
We report herein that both C57BL/6J and 129P3/J mice develop conditioned place preference to heroin in a dose dependent manner. This is in partial agreement with studies reporting that both C57BL/6J mice and substrains of 129 mice develop conditioned place preference and locomotor activation in response to morphine, although with significant strain differences in the response (e.g. [6, 20]), and a study showing that C57BL/6J mice developed conditioned place preference to a very low dose of heroin (100 μg/kg; [31]). Interestingly, the same dose of heroin was reported to produce conditioned place aversion in 129X1/sVJ mice [31]. We did not find conditioned place aversion to any dose of heroin tested, although in 129P3/J mice, place preference was formed only to high doses of heroin. C57BL/6J mice formed place preference to lower doses of heroin but, while not showing preference, did not form aversion to the higher doses. The reasons for these strain differences are not clear, although heroin-induced conditioned place preference was not observed in rats that received naloxone pre-treatment [9] nor was morphine induced locomotor activation (e.g.[25, 32]) and mice with life-long deletion of the MOP-r show no heroin induced locomotion or reward (e.g.[3]). Therefore, differences in the MOP-r might explain the observed strain difference in the rewarding effects of heroin described in the present report. Other studies suggest that dopaminergic (e.g. [8, 20, 33, 35]) and glutamatergic (e.g. [17, 27, 30]) systems may also mediate opiate-induced reward or locomotor activity. Strain differences in basal anxiety level may also be important determinants in the expression of conditioned place preference. 129/SvJ mice showed greater levels of anxiety-like behaviors than did C57BL/6J mice [10] and anxiety has been shown to attenuate the expression of conditioned place preference [6]. We found that during the preconditioning baseline day 129P3/J, mice spent significantly more time in the black side of the conditioning chamber than on the white side,which may indicate higher levels of basal anxiety. Morphine attenuates anxiety in mice [4] and it is possible that the expression of conditioned place preference to high doses of heroin in 129P3/J mice might be due to an anxiolytic effect. It is possible that differences in heroin-induced behaviors may also be due to strain dependent differences in the metabolism of heroin (e.g deacetylation to morphine) or differences in the ability of heroin to cross the blood brain barrier. While the neural substrates underlying the strain specific differences in heroin-induced locomotion and reward reported here remain to be elucidated, our results indicate that 129P3/J mice may be less susceptible to the rewarding effects of heroin than are C57BL/6J mice. Thus, these strains may be useful tools to investigate determinants of an individual’s vulnerability to develop addiction.
Acknowledgements
This work was supported by NIH–NIDA P60-DA05130 to MJK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Babbini M, Davis WM. Time-dose relationships for locomotor activity effects of morphine after acute or repeated treatment. Br J Pharmacol. 1972;46:213–24. doi: 10.1111/j.1476-5381.1972.tb06866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Balogh SA, McDowell CS, Stavnezer AJ, Denenberg VH. A behavioral and neuroanatomical assessment of an inbred substrain of 129 mice with behavioral comparisons to C57BL/6J mice. Brain Res. 1999;836:38–48. doi: 10.1016/s0006-8993(99)01586-3. [DOI] [PubMed] [Google Scholar]
- [3].Contarino A, Picetti R, Matthes HW, Koob GF, Kieffer BL, Gold LH. Lack of reward and locomotor stimulation induced by heroin in mu-opioid receptor-deficient mice. Eur J Pharmacol. 2002;446:103–9. doi: 10.1016/s0014-2999(02)01812-5. [DOI] [PubMed] [Google Scholar]
- [4].Costall B, Jones BJ, Kelly ME, Naylor RJ, Tomkins DM. Exploration of mice in a black and white test box: validation as a model of anxiety. Pharmacol Biochem Behav. 1989;32:777–85. doi: 10.1016/0091-3057(89)90033-6. [DOI] [PubMed] [Google Scholar]
- [5].Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl) 1997;132:107–24. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- [6].Dockstader CL, van der Kooy D. Mouse strain differences in opiate reward learning are explained by differences in anxiety, not reward or learning. J Neurosci. 2001;21:9077–81. doi: 10.1523/JNEUROSCI.21-22-09077.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Flores G, Wood GK, Barbeau D, Quirion R, Srivastava LK. Lewis and Fischer rats: a comparison of dopamine transporter and receptors levels. Brain Res. 1998;814:34–40. doi: 10.1016/s0006-8993(98)01011-7. [DOI] [PubMed] [Google Scholar]
- [8].Flores JA, Galan-Rodriguez B, Ramiro-Fuentes S, Fernandez-Espejo E. Role for dopamine neurons of the rostral linear nucleus and periaqueductal gray in the rewarding and sensitizing properties of heroin. Neuropsychopharmacology. 2006;31:1475–88. doi: 10.1038/sj.npp.1300946. [DOI] [PubMed] [Google Scholar]
- [9].Hand TH, Stinus L, Le Moal M. Differential mechanisms in the acquisition and expression of heroin-induced place preference. Psychopharmacology (Berl) 1989;98:61–7. doi: 10.1007/BF00442007. [DOI] [PubMed] [Google Scholar]
- [10].Homanics GE, Quinlan JJ, Firestone LL. Pharmacologic and behavioral responses of inbred C57BL/6J and strain 129/SvJ mouse lines. Pharmacol Biochem Behav. 1999;63:21–6. doi: 10.1016/s0091-3057(98)00232-9. [DOI] [PubMed] [Google Scholar]
- [11].Hooks MS, Juncos JL, Justice JB, Jr., Meiergerd SM, Povlock SL, Schenk JO, Kalivas PW. Individual locomotor response to novelty predicts selective alterations in D1 and D2 receptors and mRNAs. J Neurosci. 1994;14:6144–52. doi: 10.1523/JNEUROSCI.14-10-06144.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kest B, Palmese CA, Hopkins E, Adler M, Juni A, Mogil JS. Naloxone-precipitated withdrawal jumping in 11 inbred mouse strains: evidence for common genetic mechanisms in acute and chronic morphine physical dependence. Neuroscience. 2002;115:463–9. doi: 10.1016/s0306-4522(02)00458-x. [DOI] [PubMed] [Google Scholar]
- [13].Kosten TA, Miserendino MJ, Haile CN, DeCaprio JL, Jatlow PI, Nestler EJ. Acquisition and maintenance of intravenous cocaine self-administration in Lewis and Fischer inbred rat strains. Brain Res. 1997;778:418–29. doi: 10.1016/s0006-8993(97)01205-5. [DOI] [PubMed] [Google Scholar]
- [14].Kuzmin A, Johansson B. Reinforcing and neurochemical effects of cocaine: differences among C57, DBA, and 129 mice. Pharmacol Biochem Behav. 2000;65:399–406. doi: 10.1016/s0091-3057(99)00211-7. [DOI] [PubMed] [Google Scholar]
- [15].Kuzmin A, Johansson B, Fredholm BB, Ogren SO. Genetic evidence that cocaine and caffeine stimulate locomotion in mice via different mechanisms. Life Sci. 2000;66:PL113–8. doi: 10.1016/s0024-3205(99)00647-5. [DOI] [PubMed] [Google Scholar]
- [16].Logue SF, Owen EH, Rasmussen DL, Wehner JM. Assessment of locomotor activity, acoustic and tactile startle, and prepulse inhibition of startle in inbred mouse strains and F1 hybrids: implications of genetic background for single gene and quantitative trait loci analyses. Neuroscience. 1997;80:1075–86. doi: 10.1016/s0306-4522(97)00164-4. [DOI] [PubMed] [Google Scholar]
- [17].McGeehan AJ, Olive MF. The mGluR5 antagonist MPEP reduces the conditioned rewarding effects of cocaine but not other drugs of abuse. Synapse. 2003;47:240–2. doi: 10.1002/syn.10166. [DOI] [PubMed] [Google Scholar]
- [18].Miner LL. Cocaine reward and locomotor activity in C57BL/6J and 129/SvJ inbred mice and their F1 cross. Pharmacol Biochem Behav. 1997;58:25–30. doi: 10.1016/s0091-3057(96)00465-0. [DOI] [PubMed] [Google Scholar]
- [19].Mogil JS, Sternberg WF, Marek P, Sadowski B, Belknap JK, Liebeskind JC. The genetics of pain and pain inhibition. Proc Natl Acad Sci U S A. 1996;93:3048–55. doi: 10.1073/pnas.93.7.3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Murphy NP, Lam HA, Maidment NT. A comparison of morphine-induced locomotor activity and mesolimbic dopamine release in C57BL6, 129Sv and DBA2 mice. J Neurochem. 2001;79:626–35. doi: 10.1046/j.1471-4159.2001.00599.x. [DOI] [PubMed] [Google Scholar]
- [21].Oka T, Hosoya E. Effects of humoral modulators and naloxone on morphine-induced changes in the spontaneous locomotor activity of the rat. Psychopharmacology (Berl) 1976;47:243–8. doi: 10.1007/BF00427608. [DOI] [PubMed] [Google Scholar]
- [22].Oliverio A, Castellano C. Behavioral effects of opiates: a pharmacogenetic analysis. Curr Dev Psychopharmacol. 1981;6:45–64. doi: 10.1007/978-94-011-8123-5_2. [DOI] [PubMed] [Google Scholar]
- [23].Oliverio A, Castellano C. Genotype-dependent sensitivity and tolerance to morphine and heroin: dissociation between opiate-induced running and analgesia in the mouse. Psychopharmacologia. 1974;39:13–22. doi: 10.1007/BF00421454. [DOI] [PubMed] [Google Scholar]
- [24].Owen EH, Logue SF, Rasmussen DL, Wehner JM. Assessment of learning by the Morris water task and fear conditioning in inbred mouse strains and F1 hybrids: implications of genetic background for single gene mutations and quantitative trait loci analyses. Neuroscience. 1997;80:1087–99. doi: 10.1016/s0306-4522(97)00165-6. [DOI] [PubMed] [Google Scholar]
- [25].Pert A, Sivit C. Neuroanatomical focus for morphine and enkephalin-induced hypermotility. Nature. 1977;265:645–7. doi: 10.1038/265645a0. [DOI] [PubMed] [Google Scholar]
- [26].Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–3. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- [27].Popik P, Wrobel M. Morphine conditioned reward is inhibited by MPEP, the mGluR5 antagonist. Neuropharmacology. 2002;43:1210–7. doi: 10.1016/s0028-3908(02)00309-x. [DOI] [PubMed] [Google Scholar]
- [28].Schlussman SD, Ho A, Zhou Y, Curtis AE, Kreek MJ. Effects of “binge” pattern cocaine on stereotypy and locomotor activity in C57BL/6J and 129/J mice. Pharmacol Biochem Behav. 1998;60:593–9. doi: 10.1016/s0091-3057(98)00047-1. [DOI] [PubMed] [Google Scholar]
- [29].Schlussman SD, Zhang Y, Kane S, Stewart CL, Ho A, Kreek MJ. Locomotion, stereotypy, and dopamine D1 receptors after chronic “binge” cocaine in C57BL/6J and 129/J mice. Pharmacol Biochem Behav. 2003;75:123–31. doi: 10.1016/s0091-3057(03)00067-4. [DOI] [PubMed] [Google Scholar]
- [30].Sharp FR, Liu J, Nickolenko J, Bontempi B. NMDA and D1 receptors mediate induction of cfos and junB genes in striatum following morphine administration: implications for studies of memory. Behav Brain Res. 1995;66:225–30. doi: 10.1016/0166-4328(94)00146-7. [DOI] [PubMed] [Google Scholar]
- [31].Szumlinski KK, Lominac KD, Frys KA, Middaugh LD. Genetic variation in heroin-induced changes in behaviour: effects of B6 strain dose on conditioned reward and locomotor sensitization in 129-B6 hybrid mice. Genes Brain Behav. 2005;4:324–36. doi: 10.1111/j.1601-183X.2004.00111.x. [DOI] [PubMed] [Google Scholar]
- [32].Vaccarino FJ, Corrigall WA. Effects of opiate antagonist treatment into either the periaqueductal grey or nucleus accumbens on heroin-induced locomotor activation. Brain Res Bull. 1987;19:545–9. doi: 10.1016/0361-9230(87)90071-2. [DOI] [PubMed] [Google Scholar]
- [33].Vanderschuren LJ, Schoffelmeer AN, Mulder AH, De Vries TJ. Dopaminergic mechanisms mediating the long-term expression of locomotor sensitization following pre-exposure to morphine or amphetamine. Psychopharmacology (Berl) 1999;143:244–53. doi: 10.1007/s002130050943. [DOI] [PubMed] [Google Scholar]
- [34].Voikar V, Koks S, Vasar E, Rauvala H. Strain and gender differences in the behavior of mouse lines commonly used in transgenic studies. Physiol Behav. 2001;72:271–81. doi: 10.1016/s0031-9384(00)00405-4. [DOI] [PubMed] [Google Scholar]
- [35].Wise RA. Opiate reward: sites and substrates. Neurosci Biobehav Rev. 1989;13:129–33. doi: 10.1016/s0149-7634(89)80021-1. [DOI] [PubMed] [Google Scholar]
- [36].Zhang Y, Mantsch JR, Schlussman SD, Ho A, Kreek MJ. Conditioned place preference after single doses or “binge” cocaine in C57BL/6J and 129/J mice. Pharmacol Biochem Behav. 2002;73:655–62. doi: 10.1016/s0091-3057(02)00859-6. [DOI] [PubMed] [Google Scholar]


