Abstract
Most modern cochlear-implant speech processors convey speech-envelope information using amplitude-modulated pulse trains. The use of higher-rate carrier pulse trains allows more envelope detail in the signal. However, neural response properties could limit the efficacy of high-rate carriers. This study examined effects of carrier rate and stimulation site, on psychophysical modulation detection thresholds (MDTs). Both of these variables could affect the neural representation of the carrier and thus affect perception of the modulation. Twelve human subjects with cochlear implants were tested. Phase duration of symmetric biphasic pulses was modulated sinusoidally at 40 Hz. MDTs were determined for monopolar stimulation at two carrier rates [250 and 4000 pulses/s (pps)], three stimulation sites (basal, middle, and apical), and five stimulus levels (10%, 30%, 50%, 70%, and 90% of the dynamic range). MDTs were lower for 250 pps carriers than for 4000 pps carriers in 71% of the 180 cases studied. Effects of carrier rate were greatest at the apical stimulation site and effects of stimulation site on MDTs depended on carrier rate. The data suggest a distinct disadvantage to using carrier pulse rates as high as 4000 pps. Stimulation site should be considered in evaluating modulation detection ability.
I. INTRODUCTION
Most modern cochlear-implant speech processors function by filtering the auditory stimulus into discrete bands of frequencies, extracting the envelopes of the filtered signals, and using these envelopes to amplitude modulate trains of interleaved pulses. The use of amplitude-modulated pulse trains for cochlear prostheses was developed to enable stimulation with interleaved pulse trains (Wilson et al., 1991), which are needed to avoid problems with current interactions that occur with multichannel analog stimulation. The amplitude-modulation strategy is supported by the fact that subjects can achieve high levels of speech recognition using temporal-envelope cues, even with very limited spectral processing (Van Tasell et al., 1987; Shannon et al., 1995). Recent studies have demonstrated that modulation detection thresholds (MDTs), i.e., the just-detectable magnitudes of charge modulation, are strongly correlated with a subject’s speech recognition with cochlear and auditory-brainstem implants (Fu, 2002; Colletti and Shannon, 2005).
In charge-modulated pulse trains, the carrier pulse rate limits the temporal detail with which the modulation waveform is sampled. Higher carrier pulse rates allow more accurate representation of analog modulation waveform. For this reason, it has been assumed that higher carrier pulse rates will result in better information transmission. However, in the case of a cochlear implant, where temporal information must be transmitted by the temporal response patterns of neurons, neural response features such as adaptation and refractory properties must be considered. In studies of the representation of cochlear-implant stimulation in guinea pig auditory cortex, Middlebrooks (2005) found that the representation of the modulation waveform (20, 40, or 60 Hz sinusoids) in the activity of cortical neurons was better when low-rate (254 pps) carriers were used than when high-rate (4069 pps) carriers were used. This result suggests the hypothesis that lower-rate carriers will result in better modulation sensitivity in human subjects with cochlear implants. We tested that hypothesis in the current study by measuring MDTs in human subjects with Nucleus® prostheses using 250 and 4000 pulses/s (pps) carriers.
The 250 and 4000 pps carrier rates were chosen to produce very different patterns of neural activity in the auditory nerve and central auditory pathways. At 250 pps, the auditory nerve fibers are capable of entrainment to the pulse train, giving a neural discharge to every pulse, with the same temporal pattern in most or all of the activated neurons (Wilson et al., 1997) At pulse rates greater than 1000 pps, auditory nerve fibers cannot discharge to every pulse due to refractory properties. Because of across-fiber variation in time-to-recovery from refractory states, the across-fiber pattern of response to a high-rate pulse train will be more stochastic, which is a more natural across-fiber pattern. It has been suggested that this more stochastic pattern might result in better encoding of temporal information by cochlear implants (Rubinstein and Hong 2003). However, a recent comparison of low and high carrier rates did not support this hypothesis (Galvin and Fu, 2005).
For the current study, we chose a 40 Hz modulation frequency. Most current cochlear prosthesis speech processors provide temporal envelope cues low-pass filtered at about 200–400 Hz (Wilson, 2004).For English phoneme recognition, the most important envelope information is below 16–20 Hz (Drullman et al., 1994a, b; Fu and Shannon, 2000; Xu et al., 2005). Higher-frequency periodicity cues (50–500 Hz) have been found to benefit lexical-tone recognition (Fu et al., 1998; Xu et al., 2002) and voice gender recognition (Fu et al., 2004).
We hypothesized that effects of carrier rate on modulation detection might depend on stimulation site along the basal to apical extent of the electrode array and that stimulation site might interact with carrier rate. There are several reasons for this hypothesis. First, recent evidence suggests that the timing properties of spiral-ganglion neurons vary systematically along apical-basal dimension of the cochlea, with more apical neurons showing longer-latency, more slowly adapting responses than basal neurons (Adamson et al., 2002;Liu and Davis, 2006). Thus, we might expect that the apical end of the cochlear-implant electrode array would stimulate fibers that are better equipped to process signals with lower carrier rates. In addition, pathology of the deaf, implanted cochlea could affect the temporal response properties of the neurons. This pathology could be somewhat systematic because the base of the cochlea is often more vulnerable to pathology, but there can also be unsystematic, subject specific, variation in pathology along the cochlear length (Hinojosa and Lindsay, 1980; Nadol, 1997). If variation in modulation-detection ability depends in part on peripheral physiology, not just cognitive processes, we would expect to find variation across stimulation sites in modulation detection thresholds and possibly interactions between stimulation site and temporal properties of the stimulus. We tested the hypothesis that effects of carrier rate vary across stimulation sites by assessing the effects of carrier rate at three locations in each subject: Basal, middle, and apical regions of the electrode array.
Modulation detection and modulation-frequency discrimination improve as a function of level throughout the dynamic range of electrical hearing (Pfingst et al., 1994; Fu, 2002). Therefore, the level of the stimulus must be taken into account in comparing modulation detection across various stimulus conditions. Fu (2002) demonstrated that the mean MDTs averaged across five stimulus levels spaced throughout the dynamic range of electrical hearing correlated more highly with speech recognition than MDTs at only one level. Therefore, in the current study we assessed MDTs at five levels within the dynamic range of hearing for each condition.
II. METHOD
A. Subjects
Twelve postlingually deafened adults ranging in age from 37 to 71 years participated in this study. All had at least one year of experience with their cochlear implants. Five of the subjects had Nucleus 24M (straight array) implants and seven had Nucleus 24R(CS) (Contour) implants. Five of the subjects had participated in previous pilot studies involving modulation detection tasks. Details of the characteristics of each subject are summarized in Table I.
TABLE I.
Subject characteristics
| Subject | Age (years) | Sex | Implant type | Duration of profound deafness in implanted ear prior to implantation (years) | Duration of implant use (years) | Participated in pilot study |
|---|---|---|---|---|---|---|
| S1 | 49 | M | 24R(CS) | <1 | 4 | Yes |
| S2 | 58 | F | 24M | 1 | 7 | No |
| S3 | 70 | F | 24R(CS) | 35 | 3 | Yes |
| S4 | 56 | F | 24M | 25 | 8 | Yes |
| S5 | 66 | M | 24R(CS) | 2 | 2 | Yes |
| S6 | 37 | F | 24R(CS) | <1 | 1 | Yes |
| S7 | 53 | F | 24M | 12 | 16a | No |
| S8 | 66 | M | 24R(CS) | 29 | 2 | No |
| S9 | 45 | F | 24M | 11 | 7 | No |
| S10 | 64 | M | 24R(CS) | <1 | 5 | No |
| S11 | 71 | F | 24R(CS) | 30 | 5 | No |
| S12 | 67 | F | 24M | 3 | 4 | No |
S7 was explanted and reimplanted after 9.5 years with the first implant. Duration of use of the second implant at the time of this experiment was 6.5 years.
All subjects were paid an hourly rate for participation in the study plus travel expenses. The use of human subjects in this research was reviewed and approved by the University of Michigan Medical School Institutional Review Board.
B. Equipment and software
To assure uniformity in the external hardware, all listeners were tested with a laboratory-owned Sprint® processor (Cochlear Corporation) during the experiment. Communication with the processor was accomplished using an IF5 ISA card and a Processor Control Interface (PCI) from Cochlear Corporation. Sequences of frames were created and sent to the processor using the Nucleus Implant Communicator® (v. 3.7) software libraries. The software for the experiment was written locally and run on a personal computer.
C. Research design
The primary independent variables for this study were carrier rate and stimulation site. Two carrier rates were tested: 250 and 4000 pps. Three stimulation sites were tested in each subject. These sites were located in the apical (electrode 18), middle (electrode 11), or basal (electrode 4 or 6) 1 regions of the implant. Monopolar stimulation (MP 1+2) was used in all cases. The stimulation was between one scala tympani electrode and two external electrodes in parallel: (1) The plate electrode on the implanted receiver-stimulator and (2) the ball electrode implanted in the temporalis muscle. Psychophysical detection thresholds (T levels) and maximum comfortable loudness levels (C levels) were measured for each of the six conditions (2 pulse rates × 3 sites) to determine the dynamic range for testing modulation detection. Then MDTs were measured at five levels within the dynamic range for each of these six conditions. Details of the T level, C level, and MDT measurement procedures are given in the following.
Modern cochlear implants often use a combination of current-amplitude and phase-duration modulation to control the total charge per phase delivered. We used current amplitude to determine T and C levels and to set the stimulation level within the dynamic range. For determining MDTs, we used phase-duration modulation because the prosthesis design allows finer control of charge per phase using phase duration than is possible with current amplitude. Pulse phase duration was sinusoidally modulated at 40 Hz around a mean pulse duration of 50 µs per phase. Symmetric biphasic pulses were used with an 8 µs interphase gap. The gap was held constant while the durations of the positive and negative phases were modulated equally to maintain charge balance. The modulation index (m) was defined as
where PDmax and PDmin are the maximum and minimum phase durations, respectively. We report modulation values in percent modulation (m × 100) or in dB re 100% modulation (20 log m). All stimuli were 600 ms in duration.
D. T and C levels and dynamic ranges
For each of the six conditions (2 pulse rates × 3 sites), T levels and C levels were obtained at 0% modulation and at 50% modulation (i.e., −6.02 dB re 100% modulation). T levels and C levels were obtained using the method of adjustment in which the subjects adjusted the level of the biphasic pulses on individual electrodes using the keyboard arrow keys and our custom software program. For T levels, the listeners set the level as just barely audible. For C levels, the subjects adjusted the level to the maximum comfortable loudness level at which the subjects felt they could listen for a long period of time without discomfort. T and C levels were measured for the unmodulated signal and for the 50% modulated signals for the six conditions in random order and the set was then repeated twice using a different randomization each time for a total of three measurements for each condition. The means of the sets of three measurements were used as the estimates of the T and C levels.
The variation of the repeated T and C level measurements was computed using the following procedures. First, for each set of the three measurements, the values were normalized to the median of the three. All normalized estimates pooled across pulse rates, modulation depths, sites, and subjects showed a normal distribution. The standard deviation of the normalized estimates was then obtained as a measure of the variation of the repeated T and C level measurements.
Across the 12 subjects times three sites tested in this experiment, the T levels for the unmodulated pulse train were an average of 1.31 dB higher than those for the 50% phase-duration modulated pulse train. C levels for the unmodulated pulse train were an average of 1.06 dB higher than those for the 50% modulated pulse train. Note that as modulation depths became shallower, T levels and C levels for modulated and unmodulated signals became indistinguishable, as detailed in Sec. III. To assure that the subject could hear all stimuli and that none would be too loud during the tracking procedure for determining MDTs, dynamic range for each condition was conservatively defined as the lower of the two C levels (modulated or unmodulated) minus the higher of the two T levels. MDTs were obtained at 10%, 30%, 50%, 70%, and 90% of these dynamic ranges, as detailed in the following.
E. Modulation detection thresholds
MDTs were obtained using a two-interval forced-choice paradigm with flanking cues. On each trial, subjects were presented with four sequential observation intervals marked by buttons on the computer screen which were highlighted in sequence. An electrical stimulus to the implant (a pulse train) was presented during each interval. The first and fourth intervals contained identical unmodulated pulse trains which served as flanking cues. One of the other intervals (interval 2 or interval 3), chosen at random on each trial, also contained this unmodulated signal. The modulated pulse train occurred in the remaining interval and the subject was instructed to choose the interval that sounded different from the other three. Stimulus duration was 600 ms with a 600 ms interval between stimuli.
A two-down one-up adaptive tracking procedure (Levitt, 1971), was used, starting with a modulation depth of 50% and decreasing in steps of 6 dB to the first reversal, 2 dB for the next two reversals, and 1 dB for the next 10 reversals. The MDT was defined as the mean of the levels at the last 8 reversal points.
MDTs were measured in each subject for a total of 30 conditions (2 carrier rates × 3 stimulation sites × 5 levels). MDTs for these conditions were measured in random order and then the complete set of tests was repeated twice using a different randomization each time for a total of three estimates per condition. If trial-to-trial variability in any condition seemed high, additional MDTs for that condition were obtained. These data were later screened for outliers as described in the following.
After collecting the estimates of MDTs for each condition, the data were examined to determine if there were any outliers. First, for each set of the three estimates the values were normalized to the median of the three. All normalized estimates across carrier rates, levels, sites, and subjects showed a normal distribution. The standard deviation (s.d.) of the normalized estimates was obtained, which was equal to 2.37 dB. Then, the outliers were defined as estimates that were more than 7.1 dB (i.e., 3 × s.d.) of the median of each set of the three estimates. Once an outlier was identified, which occurred only rarely (approximately 1.9% of all measurements), we excluded the outlier, replaced it with a fourth collected MDT, and took the mean of the resulting three values.
III RESULTS
A. Dynamic ranges
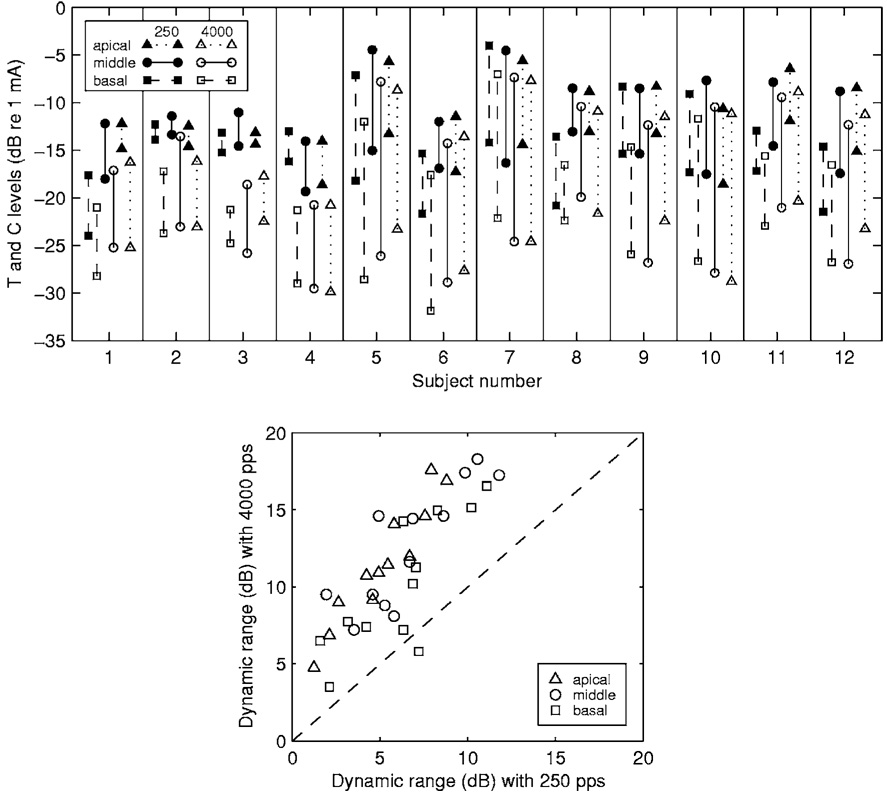
As noted in Sec. I, modulation detection improves as a function of stimulus level throughout much or all of the dynamic range of electrical hearing. An important consideration in the current experiment is that the dynamic range for stimulation at 4000 pps was typically larger than that at 250 pps. Figure 1 (upper panel) shows the T and C levels of all 12 subjects. Stimulation at 4000 pps (open symbols) produced lower C levels and lower T levels than did stimulation at 250 pps (closed symbols). However, the effects of pulse rate were usually greater for T levels than for C levels on the decibel (dB) scale. That is, when the pulse rate was increased from 250 to 4000 pps, T levels decreased more than C levels. Thus, the dynamic range in dB of current for stimulation at 4000 pps was usually larger than that at 250 pps. In these data, the dynamic range was larger for the 4000 pps stimulus in all but one (the basal site of S8) of the 36 cases (12 subjects × 3 stimulation sites) studied (Fig. 1, lower panel). To compensate for these differences in dynamic range for the two carriers as well as large across-subject differences in dynamic range, we compared MDT versus level functions using percent of dynamic range as the measure of level. This scale was based on dynamic ranges in dB of current. This scale was chosen because it corresponds roughly to the scale used in delivering current in the subjects’ normal everyday speech processors.
FIG. 1.
T and C levels and dynamic ranges. Upper panel: T and C levels obtained from the 12 subjects. In each pair of points connected by a vertical line, the upper point is the C level and the lower point is the T level. Means for three repeated measurements are shown. The standard deviations, calculated as described in Sec. II, were 1.39 and 0.86 dB for repeated T and C level measurements, respectively. Lower panel: Comparison of the dynamic ranges obtained with 250 pps carriers to those obtained with 4000 pps carriers. Each point indicates the dynamic ranges for the 4000 pps carrier (ordinate) and the 250 pps carrier (abscissa) for one stimulation site in one subject, calculated from the T and C levels shown in the upper panel. Points above the diagonal indicate that dynamic ranges were larger for the 4000 pps carrier than for the 250 pps carrier.
B. MDT-versus-level functions
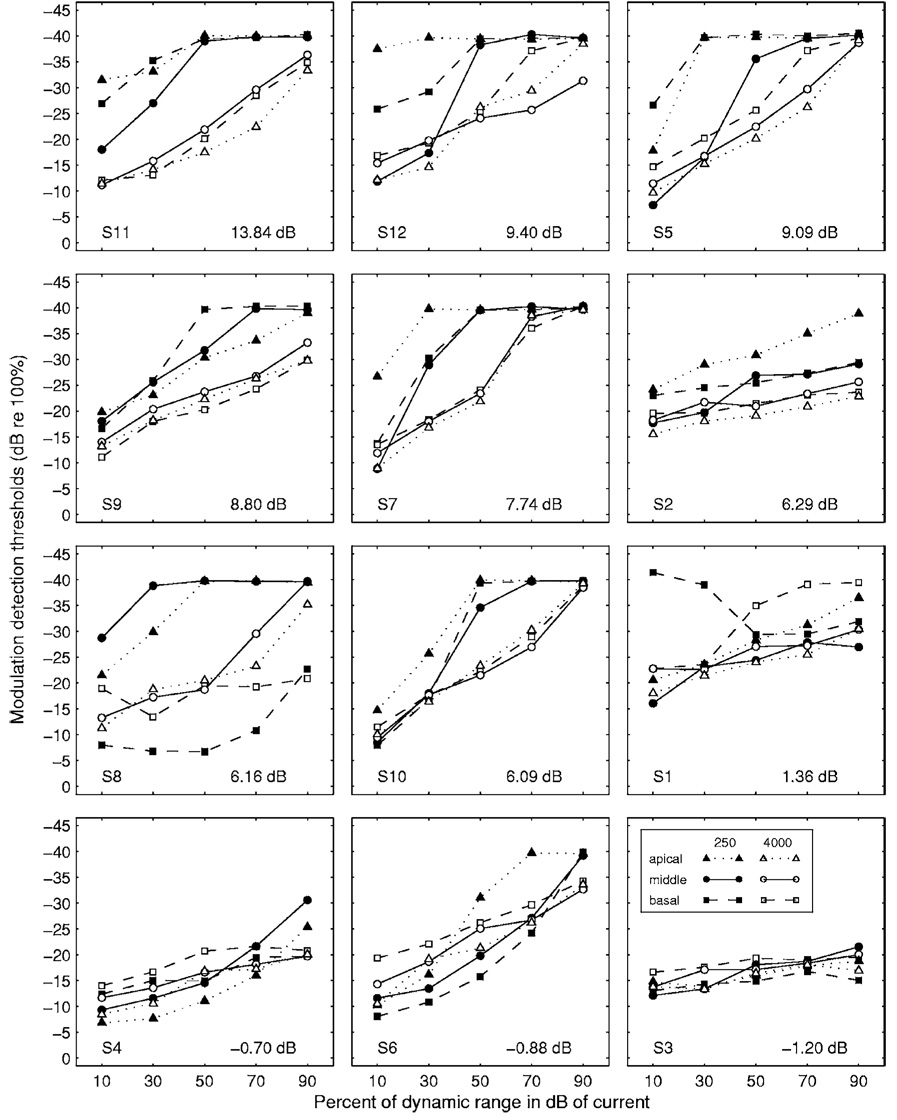
MDTs for the 12 subjects are shown in Fig. 2. For each subject, MDT-versus-level functions for the two carrier rates at each of three stimulation sites are shown. There is considerable variability across subjects and conditions in the details of the MDT-versus-level functions but there are some clear and consistent trends. MDTs improve as a function of level in almost all cases. In most cases, the MDTs for the 250 pps carrier are better than those for 4000 pps carrier when compared at equal levels in percent of dynamic range. Differences between MDTs obtained for the 4000 pps carrier and the 250 pps carrier averaged over all three stimulation sites and all five levels are shown for each subject in the lower right corner of each panel. These values are positive for 9 of the 12 subjects. The positive values indicate better average modulation detection when the 250 pps carrier was used. Note that the MDT data for the three subjects with negative values (4000 pps carrier better) were generally poor compared to those for the other subjects in the study. They were particularly poor, relative to those for other subjects, at higher levels for both carriers and for all levels for the 250 pps carrier. The stimulation site itself did not produce a consistent effect on the MDTs. However, if the effects of stimulation site were considered together with level, as detailed in Sec. III D, we found some interesting interactions of the two variables affecting the MDTs.
FIG. 2.
Modulation detection threshold vs level functions for the 12 subjects. Each panel shows MDT-versus-level functions for a single subject for three stimulation sites (basal, middle, and apical) at two carrier rates (250 and 4000 pps). The legend is shown in the lower right panel. Subject numbers are indicated in the lower left corner of each panel. For each subject, the mean effect of carrier rate, calculated as the mean difference in MDTs (MDT at 4000 pps minus MDT at 250 pps) for all 15 conditions (3 sites × 5 levels), is shown in the lower right corner of the panel. The panels are arranged in order from highest mean difference value (upper left panel) to the lowest mean difference value (lower right panel). The abscissa gives the stimulus level in percent of dynamic range where dynamic range is in dB of current.
A three-way ANOVA was performed to compare the means of the MDTs produced by the three variables, i.e., level, carrier rate, and stimulation site. The results indicated that both level and carrier rate produced significantly different mean MDTs (level: F =63.4, d.f.=4, p < 0.000; carrier rate: F =48.5, d.f.= 1, p<0.000) whereas stimulation site did not yield significantly different mean MDTs (F =0.44, d.f.=2, p=0.644).
C. Effects of carrier rate
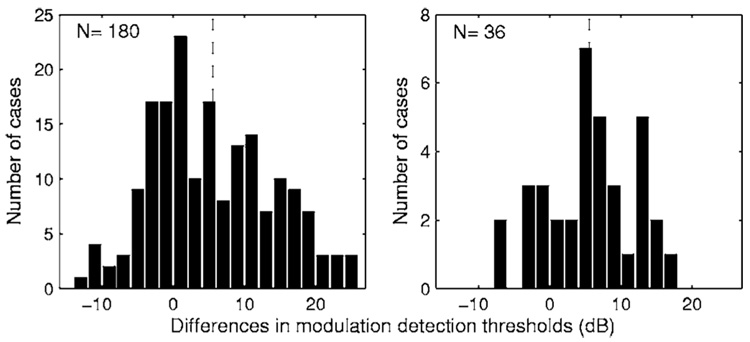
From the data in Fig. 2, effects of carrier rate on MDTs (MDT for the 4000 pps carrier minus MDT for the 250 pps carrier) were computed for the 180 cases (12 subjects × 3 sites per subject × 5 levels per site). Of these 180 cases, MDTs were better for the 250 pps carrier in 70.8% of the cases. The differences between MDTs for the two carriers ranged from +25.3 dB (MDT for the 250 pps carrier better) to −12.8 dB (MDT for the 4000 pps carrier better). The mean difference was 5.5 dB. The distribution of these differences is shown in the left panel of Fig. 3.
FIG. 3.
Distribution of the differences between MDTs obtained with the two carriers (MDT for the 4000 pps carrier minus MDT for the 250 pps carrier). In the left panel, the differences were calculated for 180 cases (12 subjects × 3 sites/subject × 5 levels/site). In the right panel, the differences were calculated using mean MDTs (averaged across the five tested levels) for 36 cases (12 subjects × 3 sites/subject). Positive values indicate that MDTs were lower (better) for the 250 pps carrier. The vertical dashed lines indicate the means of the distributions.
We also computed mean MDTs, averaged across the five stimulus levels, for the 36 cases (12 subjects × 3 sites). Mean MDTs averaged across multiple stimulation levels have been shown by Fu (2002) to be highly correlated across subjects with consonant recognition and well correlated with vowel recognition. Mean MDTs for the 250 pps carrier were better than those for the 4000 pps carrier in 28 of the 36 cases tested. The distribution of these differences is shown in the right panel of Fig. 3. The mean of this distribution was 5.5 dB.
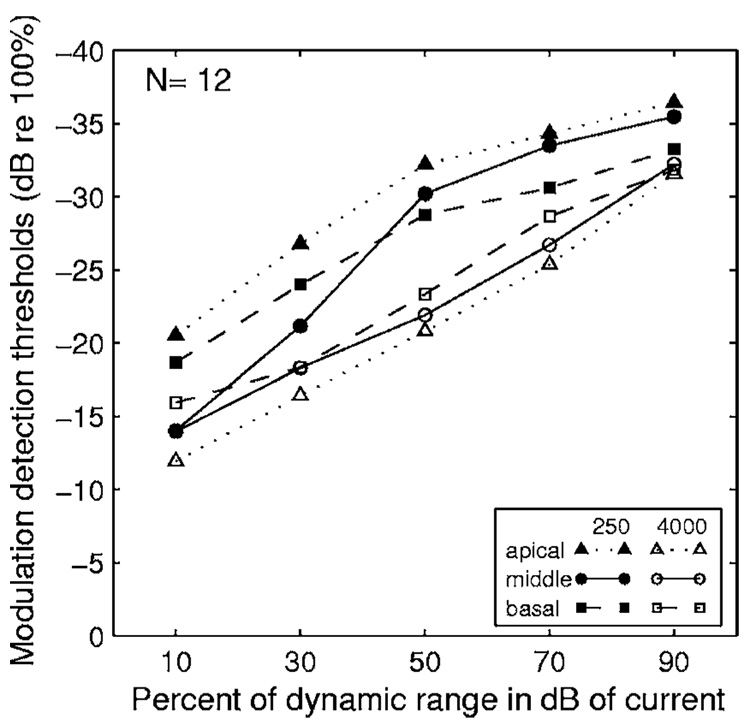
Group-mean MDT-versus-level functions averaged across all 12 subjects are shown in Fig. 4 for each of the two carrier rates at each of the three stimulation sites. In these group-mean data, MDTs for the 250 pps carrier (closed symbols) were lower (better) than those for the 4000 pps carrier (open symbols) in the corresponding conditions across three tested stimulation sites and across all five tested levels with the only exception being the middle site at 10% of the dynamic range.
FIG. 4.
Mean MDT-vs-level functions for two carrier rates and three stimulation sites. Means were averaged across the 12 subjects. The abscissa gives the stimulus level in percent of dynamic range where dynamic range is in dB of current.
D. Effects of stimulation site
On average, the best MDTs for the 250 pps carrier were at the apical stimulation site and the best MDTs for the 4000 pps carrier were at the basal site (Fig. 4). The poorest MDTs were for the 4000 pps carrier at the apical site. Thus, the largest difference between MDTs for the 250 pps carrier and the 4000 pps carrier was at the apical stimulation site. The data for the differences in MDTs between the two carriers were organized in a matrix with stimulus level being one factor and stimulation site being another factor. A two-way ANOVA of the data revealed a statistically significant effect of stimulation site on differences between MDTs for the two carriers (F=7.7, d.f.=2, p=0.0007) but no statistically significant effect of stimulus level on these differences (F=2.4, d.f.=4,p=0.056) No interaction between levels and sites was found to be statistically significant (F=0.7, d.f.=8, p=0.675).
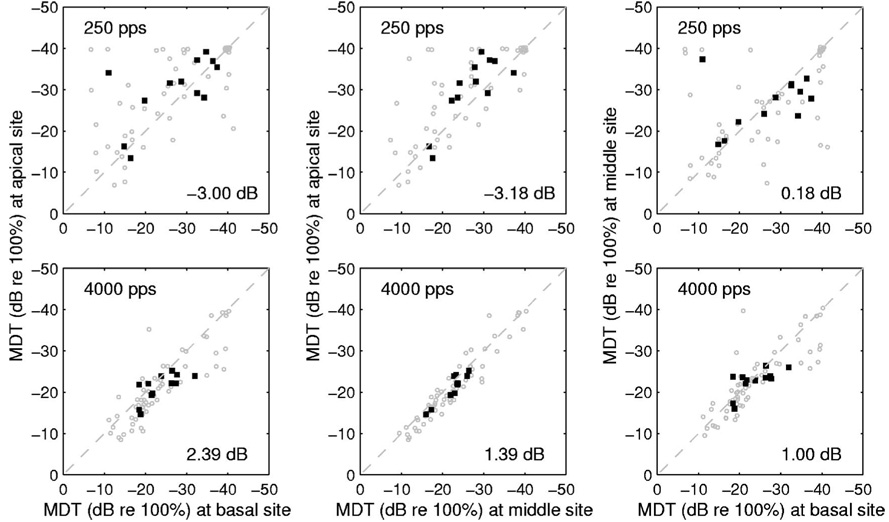
Interaction between stimulation site and carrier rate in determining MDTs is also illustrated in Fig. 5, which compares MDTs for the three possible pairings of the three stimulation sites (apical, middle, and basal) at the two carrier rates (250 and 4000 pps). The left column of Fig. 5 compares MDTs at the apical and basal sites for 60 cases (12 subjects × 5 stimulation levels) (gray circles) and the mean MDTs across the 5 levels for each of the 12 subjects (closed squares). At 250 pps (Fig. 5, upper left panel), MDTs were smaller (better) at the apical site in the majority of the cases: 63.3% of the 60 cases fell above the diagonal and the average apical-basal difference was −3.00 dB. At 4000 pps (Fig. 5, lower left panel), MDTs were better at the basal site in the majority of the cases: 76.7% of the 60 cases were below the diagonal and the average apical-basal difference was +2.39 dB. The differences between the stimulation sites in the means across the 5 levels (closed squares) in the upper panel were compared with those in the lower panel (leftmost column of Fig. 5). It was found that the apical-basal differences in the two panels were statistically significant (paired t test, t=3.075, p=0.0106). Thus, the effect of stimulation site (apical versus basal) on MDTs depended on the carrier rate.
FIG. 5.
Comparisons of MDTs vs stimulation site. MDTs for the 60 cases (12 subjects × 5 stimulation levels per subject) are plotted using gray circles and the means across the 5 levels for the 12 subject are plotted using closed squares. The top and bottom rows represent the 250 and the 4000 pps carriers, respectively. Comparisons are shown for the three possible pairings of the three stimulation sites: Apical vs basal in the left column, apical vs middle in the center column, and middle vs basal in the right column. Mean differences in MDTs between the pairs of sites are shown in the lower-right corner of each panel.
Comparison of the apical and middle sites (middle column in Fig. 5) showed results similar to the comparison of apical and basal sites. Statistically significant differences were found between the apical-middle differences in the two panels in the middle column of Fig. 5 (paired t test, t=3.555, p=0.0045). Comparison of the middle and basal sites (right column in Fig. 5) showed little consistency in effects of site on MDTs. The middle-basal differences were not statistically significant (paired t test, t=0.382, p =0.7095).
E. Relation between MDTs and dynamic ranges
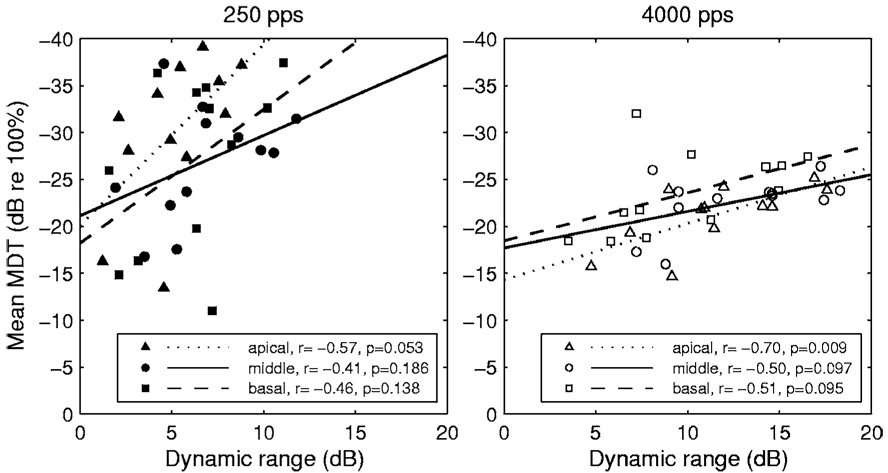
As noted earlier, the dynamic ranges for the 250 pps carrier were smaller than those for the 4000 pps carrier in all but one of the 36 cases studied (Fig. 1), and the MDTs for the 250 pps carrier were smaller (better) than those for the 4000 pps carrier in 28 of these 36 cases (Fig. 3, right panel). Thus, under these circumstances, it seems that small dynamic ranges were associated with better MDTs. To determine if small dynamic ranges were associated with better MDTs as a general rule, we compared mean MDTs to dynamic ranges across subjects, utilizing the commonly observed variation in dynamic ranges across implanted subjects. We make these comparisons in the 12 subjects for each of the three stimulation sites and two carrier rates (Fig. 6). These data do not support the hypothesis that smaller dynamic ranges are associated with better modulation detection under these circumstances. In fact, across subjects, there is a trend in the opposite direction. As shown in Fig. 6, regression lines for these data had a positive slope in all cases, i.e., larger dynamic ranges tended to be associated across subjects with better MDTs. However, the correlations were statistically significant at the p<0.05 level in only one case (the apical site for the 4000 pps carrier).
FIG. 6.
Relationship between mean MDTs and dynamic ranges for the three stimulation sites (apical, middle, and basal). The left and right panels represent data for the 250 and 4000 pps carriers, respectively. The dotted, solid, and dashed lines represent the least-square fits of the data for the apical, middle, and basal stimulation sites, respectively. The legend shows the correlation coefficients and the statistical significance of the test results.
F. Effects of level
From the data in Fig. 2 and Fig. 4, it is evident that the shapes of the MDT-versus-level functions are often different for the two carriers. On average, MDTs (in dB) obtained with the 250 pps carrier improved more rapidly as a function of level (in percent of dB dynamic range) than those for the 4000 pps carrier. Given this consideration, it is not obvious precisely what relative levels are appropriate for comparison of MDTs across conditions. A practical approach to this issue is to determine how much one would need to change the presentation level in one condition to obtain the same MDT as that obtained at a given level in another condition. For example, for subject S11 at the middle stimulation site (the solid lines in the upper left panel of Fig. 2), the MDT at 30% of the dynamic range with a 250 pps arrier was −27 dB re. 100% modulation. To achieve this MDT with a 4000 pps carrier at the same stimulation site, the stimulus level would need to be raised to about 60% of the dynamic range, i.e., an increase in level of about 30% of the dynamic range. In the group-average data in Fig. 4, the level for stimulation with the 4000 pps carrier needed to achieve similar MDTs to those obtained with the 250 pps carrier at 30% of the dynamic range would be about 22%, 16%, and 45% of the dynamic range higher for the basal, middle, and apical sites, respectively. Thus, minor errors in estimating the appropriate levels for comparison would not be sufficient to account for the differences in MDTs observed between data for the two carriers.
G. Loudness cues
Amplitude modulation of pulse trains results in pitch-like perceptions and other perceptual cues that may be useful to subjects in recognizing speech signals. As noted in Sec. II, phase-duration modulation of pulse trains can produce a slight lowering of T levels and C levels, suggesting the possibility that loudness cues can contribute to modulation detection. In order to estimate the possible contribution of the loudness cues to modulation detection, we measured the difference in T levels and C levels between unmodulated signals and modulated signals at various modulation depths (0%–50%) using six of the subjects from this study (S1, S3, S5, S6, S10, and S12). Results of these measurements are shown in Fig. 7. The differences in T and C levels between the unmodulated signal and the 50% modulated signal ranged between 1.25 and 1.12 dB, similar to the mean differences for all 12 subjects reported in Sec. II. However, for modulation depths of 25% or less, these mean differences between the unmodulated and modulated signals were less than 0.3 dB. These differences are well below the range of variation in repeated T and C level measurements for the unmodulated signals and the modulated signals, so if any loudness differences exist at these modulation depths they are below the resolution of our measurement procedures.
FIG. 7.
Differences of T and C levels between modulated and unmodulated carriers. The data were pooled across sites and carrier rates since no systematic differences were found in the data among different stimulation sites or between the low and high carrier rates. The boxes have lines at the lower quartile, median, and upper quartile values. The whiskers are lines extending from each end of the boxes to show the extent of the rest of the data.
IV DISCUSSION
A. Summary of results
The major findings in this study were (1) that MDTs were usually better when a low rate (250 pps) carrier was used than when a high rate (4000 pps) carrier was used; (2) that the effects of carrier rate on MDTs are affected by the apical to basal location of the electrical-stimulation site, and (3) the effects of stimulation site on MDTs depend on carrier rate. We also confirmed, as expected, that MDTs improved as a function of stimulus level and found that the shapes and slopes of the MDT-versus-level functions varied as a function of carrier rate in most subjects. Finally, we found that the relationship between dynamic range and MDTs depended on the variable (carrier rate or subject) associated with differences in the dynamic range.
B. Effects of carrier rate
The increase in MDTs (reduced sensitivity) resulting from the higher carrier rate averaged 5.5 dB (almost a factor of 2 in percent of modulation) and ranged up to 25.3 dB (a factor of about 18 in percent of modulation) (Fig. 3). The effects of carrier rate were not as large as the effects of level, which averaged 18.0 dB over levels from 10% to 90% of the dynamic range and ranged up to 32.8 dB. Nevertheless, as noted in Sec. III F, very large increases in level of the 4000 pps carrier signal would be needed in many cases to achieve MDTs that were equivalent to those achieved with the 250 pps carrier.
There was considerable variability in effects of pulse rate from subject to subject. A few subjects showed little or no effect of carrier rate or showed small effects in the opposite direction to that observed for the majority. In general, the subjects with small effects of carrier rate had poor MDTs for both carriers. Similar across-subject variability in effects of pulse rate on psychophysical performance has been observed in a study of intensity discrimination in the context of interleaved multichannel stimulation (Drennan and Pfingst 2006). This across-subject variability in effects of pulse rate on basic psychophysical functions suggests a basis for across-subject variability in effects of pulse rate on speech recognition, which has been observed in several studies (Vandali et al., 2000; Holden et al., 2002).
Some of our subjects showed the largest effects of carrier rate at the intermediate levels within the dynamic range. In these cases, the smaller effects at the highest and lowest levels were probably due to ceiling effects and floor effects, respectively.
Effects of carrier rate similar to those seen in our study were demonstrated in a recently published study by Galvin and Fu (2005). That study used six subjects with Nucleus-22 or Nucleus-24 implants. There were a number of minor differences between their study and ours: They compared 250 and 2000 pps carriers and used a 20 Hz modulation frequency. They used primarily bipolar configurations (BP+3 and BP+13) and one stimulation site (i.e., one reference-electrode site) per subject. One interesting, but probably minor procedural difference was that their study compared MDTs for the two carrier rates at levels that were matched in loudness to various levels in the dynamic range of a 1000 pps pulse train on a common BP+3 electrode pair, whereas in our study the levels were matched in terms of the percent of the dynamic range for each carrier and each stimulation site. While it is clear that perceived loudness increases as a function of stimulus level and modulation detection thresholds decrease as a function of level, the precise relationship between perceived loudness and modulation detection is not known. The shapes of the MDT versus percent of dynamic range functions reported by Galvin and Fu (2005) were not noticeably different from the functions reported in our study. In both cases the intersubject variability was much larger than any subtle differences that might be based on the two level scales.
C. Effects of electrode location
Our study showed some interesting interactions between carrier rate and stimulation site. These relationships have not been studied previously to our knowledge. On average, the best MDTs at 250 pps were found at the apical stimulation site and the greatest effects of carrier rate on MDTs were also found at this apical site. However, not all subjects showed the same pattern. Thus, we must consider both systematic and more seemingly random variation in MDTs across stimulation sites.
The finding of interactions between carrier rate and the apical versus basal location of the stimulation site is consistent with the finding of apical-basal differences in temporal properties of auditory nerve fibers (Adamson et al., 2002; Liu and Davis, 2006). Alternatively, across-site variation in the effects of carrier rate on MDTs could be due to variation in pathology along the length of the cochlea. The diversity of effects across subjects and stimulation sites suggests that there are interactions between carrier pulse rate and other variables that are specific to individual subjects and individual stimulation sites within subjects. Possible candidates for the variables underlying this observation include the nerve survival pattern and the condition of the implanted scala tympani. Nerve survival pattern in a hearing-impaired patient with a cochlear implant is never complete and the pattern of nerve loss as well as the condition of the surviving neurons varies considerably from patient to patient (Hinojosa and Lindsay, 1980; Nadol, 1997). In addition, fibrous tissue and new bone frequently grow near the implant in the scala tympani, potentially resulting in alterations in the pathways from individual electrodes to the excitable neural elements (Kawano et al., 1998). Finally, the radial position of the electrode array with respect to the modiolus varies along the length of the cochlear implant in ways that are not always predictable (Saunders et al., 2002). These three variables (nerve survival pattern, pattern of tissue growth, and pattern of electrode location with respect to the modiolus) can combine to create seemingly random variation in the number and position of neural elements excited by individual stimulation sites along the length of the electrode array. The pathology that influences these factors could vary systematically from base to apex contributing to the apical-basal differences that we observed and they could also be responsible for the less systematic across-site variation that we also observed.
The magnitudes of differences in MDTs between the apical and basal site for the 250 pps carrier averaged 3.0 dB and were as large as 33.0 dB (Fig. 5). Thus, when comparing modulation detection ability across subjects, it is important to sample MDTs at several sites in order to get an accurate estimate of each subject’s relative ability.
D. Relation to dynamic range
Dynamic ranges for electrical stimulation of cochlear implants are typically small and are highly variable across subjects. The smallest dynamic ranges have been found to be associated with poor electrode discrimination, poor place-pitch perception, and poor speech recognition (Blamey et al., 1992; Pfingst et al., 1999; Donaldson and Nelson, 2000). In this study, the subjects with dynamic ranges greater than 7.2 dB all had relatively good MDTs when the 250 pps carrier was used, while those with dynamic ranges less than 7.2 dB showed a range of performance from good to poor. Dynamic ranges were usually much larger for the 4000 pps carrier rate, but MDT performance was typically much poorer for this carrier compared to that for the 250 pps carrier. Thus, large dynamic range per se does not assure better MDT performance.
Galvin and Fu (2005) found no significant correlation between dynamic ranges and mean MDTs. However, three factors that affect dynamic range (subjects, electrode configuration, and pulse rate) were confounded in that analysis. As noted in our study, the relationship between MDTs and dynamic range depends on the variable (carrier rate or subject) that is associated with differences in the dynamic range. The relation between MDTs and dynamic ranges across carrier rates was opposite to the relation between MDTs and dynamic range across subjects with carrier rate held constant, suggesting that at least two different mechanisms underly these relationships.
V. CONCLUSIONS
High carrier pulse rates pose a distinct disadvantage for detection of amplitude-modulation.
Modulation detection ability depends to some extent on the location of the stimulation site along the tonotopic axis of the cochlea.
Presentation of stimuli in the upper regions of the dynamic range results in better modulation detection.
Subjects with larger dynamic ranges tend to have better modulation detection thresholds. However, increasing dynamic range by using higher carrier rates is detrimental to modulation detection.
ACKNOWLEDGMENTS
We express appreciation to our research subjects for their cheerful participation in these studies, to John Middle-brooks for his helpful suggestions and insightful comments, and to Chenfei Ma and Rose Burkholder for assistance with data analysis and presentation. We also express appreciation to Charles Kowalski at the University of Michigan Center for Statistical Consultation and Research for statistical consultation and to Thyag Sadasiwan for programming. This work was supported by NIH NIDCD Grant Nos. R01 DC03808, R01 DC04312, and P30 DC05188.
Footnotes
Initial reports of these data were presented previously [Pfingst et al., 2005 Conference on Implantable Auditory Prostheses, Asilomer Conference Grounds, Pacific rove, CA; Xu and Pfingst, The 25th Politzer Society Meeting, Seoul, Korea (2005)].
For the basal site, the default was electrode 4, but that electrode was unusable in two subjects (S2 and S3), so electrode 6 was used in those cases.
Contributor Information
Bryan E. Pfingst, Kresge Hearing Research Institute, Department of Otolaryngology, University of Michigan, Ann Arbor, Michigan 48109-0506.
Li Xu, School of Hearing, Speech and Language Sciences, Ohio University, Athens, Ohio 45701 and Kresge Hearing Research Institute, Department of Otolaryngology, University of Michigan, Ann Arbor, Michigan 48109-0506.
Catherine S. Thompson, Kresge Hearing Research Institute, Department of Otolaryngology, University of Michigan, Ann Arbor, Michigan 48109-0506
References
- Adamson CL, Reid MA, Mo ZL, Bowne-English J, Davis RL. Firing features and potassium channel content of murine spiral ganglion neurons vary with cochlear location. J. Comp. Neurol. 2002;447:331–350. doi: 10.1002/cne.10244. [DOI] [PubMed] [Google Scholar]
- Blamey PJ, Pyman BC, Gordon M, Clark GM, Brown AM, Dowell RC, Hollow RD. Factors predicting postoperative sentence scores in postlinguistically deaf adult cochlear implant patients. Ann. Otol. Rhinol. Laryngol. 1992;101:342–348. doi: 10.1177/000348949210100410. [DOI] [PubMed] [Google Scholar]
- Colletti V, Shannon RV. Open set speech perception with auditory brainstem implant? Laryngoscope. 2005;115:1974–1978. doi: 10.1097/01.mlg.0000178327.42926.ec. [DOI] [PubMed] [Google Scholar]
- Donaldson GS, Nelson DA. Place-pitch sensitivity and its relation to consonant recognition by cochlear implant listeners using the MPEAK and SPEAK speech processing strategies. J. Acoust. Soc. Am. 2000;107:1645–1658. doi: 10.1121/1.428449. [DOI] [PubMed] [Google Scholar]
- Drennan WR, Pfingst BE. Current-level discrimination in the context of interleaved multichannel stimulation in cochlear implants: Effects of number of stimulated electrodes, pulse rate and electrode separation. J. Assoc. Res. Otolaryngol. 2006;7:308–316. doi: 10.1007/s10162-006-0045-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drullman R, Festen JM, Plomp R. Effect of temporal envelope smearing on speech perception. J. Acoust. Soc. Am. 1994a;95:1053–1064. doi: 10.1121/1.408467. [DOI] [PubMed] [Google Scholar]
- Drullman R, Festen JM, Plomp R. Effect of reducing slow temporal modulations on speech reception. J. Acoust. Soc. Am. 1994b;95:2670–2680. doi: 10.1121/1.409836. [DOI] [PubMed] [Google Scholar]
- Fu Q-J. Temporal processing and speech recognition in cochlear implant users. NeuroReport. 2002;13:1635–1639. doi: 10.1097/00001756-200209160-00013. [DOI] [PubMed] [Google Scholar]
- Fu Q-J, Chinchilla S, Galvin JJ. The role of spectral and temporal cues in voice gender discrimination by normal-hearing listeners and cochlear implant users. J. Assoc. Res. Otolaryngol. 2004;5:253–260. doi: 10.1007/s10162-004-4046-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q-J, Shannon RV. Effect of stimulation rate on phoneme recognition by Nucleus-22 cochlear implant listeners. J. Acoust. Soc. Am. 2000;107:589–597. doi: 10.1121/1.428325. [DOI] [PubMed] [Google Scholar]
- Fu Q-J, Zeng FG, Shannon RV, Soli SD. Importance of tonal envelope cues in Chinese speech recognition. J. Acoust. Soc. Am. 1998;104:505–510. doi: 10.1121/1.423251. [DOI] [PubMed] [Google Scholar]
- Galvin JJ, III, Fu Q-J. Effects of stimulation rate, mode and level on modulation detection by cochlear implant users. J. Assoc. Res. Otolaryngol. 2005;6:269–279. doi: 10.1007/s10162-005-0007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinojosa R, Lindsay JR. Profound deafness: Associated sensory and neural degeneration. Arch. Otolaryngol. Head Neck Surg. 1980;106:193–209. doi: 10.1001/archotol.1980.00790280001001. [DOI] [PubMed] [Google Scholar]
- Holden LK, Skinner MW, Holden TA, Demorest ME. Effects of stimulation rate with the Nucleus 24 ACE speech coding strategy. Ear Hear. 2002;23:463–476. doi: 10.1097/00003446-200210000-00008. [DOI] [PubMed] [Google Scholar]
- Kawano A, Seldon HL, Clark GM, Ramsden RT, Raine CH. Intracochlear factors contributing to psychophysical percepts following cochlear implantation. Acta Oto-Laryngol. 1998;118:313–326. doi: 10.1080/00016489850183386. [DOI] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. J. Acoust. Soc. Am. 1971;49:467–477. [PubMed] [Google Scholar]
- Liu Q, Davis RL. From apex to base: How endogenous neuronal membrane properties are distributed in the spiral ganglion. J. Assoc. Res. Otolaryngol. Abst. 2006;29:305. [Google Scholar]
- Middlebrooks JC. Transmission of temporal information from a cochlear implant to the auditory cortex. J. Assoc. Res. Otolaryngol. Abst. 2005;28:91. [Google Scholar]
- Nadol J. Patterns of neural degeneration in the human cochlea and auditory nerve: Implications for cochlear implantation. Otolaryngol.- Head Neck Surg. 1997;117:220–228. doi: 10.1016/s0194-5998(97)70178-5. [DOI] [PubMed] [Google Scholar]
- Pfingst BE, Holloway LA, Poopat N, Subramanya AR, Warren MF, Zwolan TA. Effects of stimulus level on nonspectral frequency discrimination by human subjects. Hear. Res. 1994;78:197–209. doi: 10.1016/0378-5955(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Pfingst BE, Holloway LA, Zwolan TA, Collins LM. Effects of stimulus level on electrode-place discrimination in human subjects with cochlear implants. Hear. Res. 1999;134:105–115. doi: 10.1016/s0378-5955(99)00079-9. [DOI] [PubMed] [Google Scholar]
- Pfingst BE, Xu L, Thompson CS, Ma C. Effects of carrier pulse rate on modulation detection in subjects with cochlear implants. 2005 Abst. Conf. Implant. Aud. Prost. 2005:139. doi: 10.1121/1.2537501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein JT, Hong R. Signal coding in cochlear implants: Exploiting stochastic effects of electrical stimulation. Ann. Otol. Rhinol. Laryngol. 2003;112:14–19. doi: 10.1177/00034894031120s904. [DOI] [PubMed] [Google Scholar]
- Saunders E, et al. Threshold, comfortable level and impedance changes as a function of electrode-modiolar distance. Ear Hear. 2002;23:28S–40S. doi: 10.1097/00003446-200202001-00004. [DOI] [PubMed] [Google Scholar]
- Shannon RV, Zeng FG, Kamath V, Wygonski J, Ekelid M. Speech recognition with primarily temporal cues. Science. 1995;270:303–304. doi: 10.1126/science.270.5234.303. [DOI] [PubMed] [Google Scholar]
- Vandali AE, Whitford LA, Plant KL, Clark GM. Speech perception as a function of electrical stimulation rate: Using the Nucleus 24 cochlear implant system. Ear Hear. 2000;21:608–624. doi: 10.1097/00003446-200012000-00008. [DOI] [PubMed] [Google Scholar]
- Van Tasell DJ, Soli SD, Kirby VM, Widin GP. Speech waveform envelope cues for consonant recognition. J. Acoust. Soc. Am. 1987;82:1152–1161. doi: 10.1121/1.395251. [DOI] [PubMed] [Google Scholar]
- Wilson BS. Engineering design of cochlear implants. In: Zeng F-G, Popper AN, Fay RR, editors. Cochlear Implants: Auditory Prostheses and Electrical Hearing. New York: Springer; 2004. pp. 14–52. [Google Scholar]
- Wilson BS, Finley CC, Lawson DT, Wolford RD, Eddington DK, Rabinowitz WM. Better speech recognition with cochlear implants. Nature (London) 1991;352:236–238. doi: 10.1038/352236a0. [DOI] [PubMed] [Google Scholar]
- Wilson BS, Finley CC, Lawson DT, Zerbi M. Temporal representations with cochlear implants. Am. J. Otol. 1997;18:S30–S34. [PubMed] [Google Scholar]
- Xu L, Pfingst BE. Effects of carrier pulse rate on modulation detection in subjects with cochlear implants; The 25th Politzer Society Meeting; Seoul, Korea. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Thompson C, Pfingst BE. Relative contributions of spectral and temporal cues for phoneme recognition. J. Acoust. Soc. Am. 2005;117:3255–3267. doi: 10.1121/1.1886405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Tsai Y, Pfingst BE. Features of stimulation affecting tonal-speech perception: Implications for cochlear prostheses. J. Acoust. Soc. Am. 2002;112:247–258. doi: 10.1121/1.1487843. [DOI] [PMC free article] [PubMed] [Google Scholar]