Abstract
Human cholangiocarcinomas evade apoptosis by overexpression of Mcl-1. The drug obatoclax (GX15–070) inhibits anti-apoptotic members of the Bcl-2 family including Mcl-1.
Purpose
To determine if obatoclax sensitizes human cholangiocarcinoma cells to apoptosis.
Experimental Design
The human cholangiocarcinoma cell lines, KMCH, KMBC, and TFK, were employed for these studies. Protein expression was assessed by immunoblot, and protein-protein interactions detected by co-precipitation of the polypeptide of interest with S-tagged Mcl-1. Activation of Bak and Bax was observed by immunocytochemistry with conformation specific antisera.
Results
Obatoclax induced minimal apoptosis alone; however, it increased apoptosis 3- to 13-fold in all three cancer cell lines when combined with Apo2L/TRAIL. Obatoclax did not alter cellular expression of Bid, Bim, Puma, Noxa, Bak, Bax, Mcl-1 or cFLIP. Mcl-1 binding to Bak was readily identified in untreated cells, and this association was disrupted by treating the cells with obatoclax. Additionally, Bim binding to Mcl-1 was markedly decreased by obatoclax treatment. We also identified alterations in Bak and Bax conformation following treatment with obatoclax plus Apo2L/TRAIL, but not with either Apo2L/TRAIL or obatoclax alone.
Conclusions
In conclusion, obatoclax releases Bak and Bim from Mcl-1 and sensitizes human cholangiocarcinoma cells to Apo2L/TRAIL-induced apoptosis. Obatoclax is a potentially promising adjunctive agent for the treatment of this cancer.
Keywords: BH3 mimetic, extrinsic pathway, Mcl-1, therapy, Apo2L/TRAIL
INTRODUCTION
Cholangiocarcinoma, a neoplasm arising from the biliary tract, is the second most common primary hepatobiliary cancer and its incidence is increasing in Western societies (1). Other than surgical extirpation, therapy has limited efficacy (2). Previous studies have demonstrated that this cancer is resistant to therapy because of its ability to evade apoptosis, as illustrated by its resistance to the death ligand Apo2L/TRAIL (3); Apo2L/TRAIL is also known as TNFSF10 and is distinct from the Apo2L/TRAIL receptors DR4/Apo2/TRAIL-R1/TNFRSF10A and DR5/TRAIL-R2/TNFRSF10B, here refered to as DR4 and DR5. Overexpression of Mcl-1, a potent antiapoptotic member of the Bcl-2 family plays a prominent role in apoptosis resistance upon Apo2L/TRAIL treatment in cholangiocarcinoma cells (4), as indicated by the observation that targeted knockdown of Mcl-1 by siRNA (4), cyclin dependent kinase inhibition (4, 5), or anti-Mcl-1 microRNA silencing (6) restores Apo2L/TRAIL sensitivity. Of note, neither Bcl-2 nor Bcl-xL siRNA saused sensitization to Apo2L/TRAIL. Further, Mcl-1 is over expressed in 70% of primary tumor samples as measured by immunohistochemistry (7). Accordingly, approaches to inhibit Mcl-1 function would be predicted to help sensitize this cancer to conventional therapeutics or even promote tumor-autonomous death signaling in vivo given the propensity of this cancer to express Apo2L/TRAIL (3).
Mcl-1 blocks apoptosis by preventing the mitochondrial dysfunction that results from activation of the multidomain, proapoptotic members of the Bcl-2 family, Bax and Bak (8). According to current models, activated Bax and/or Bak form homooligomers within the outer mitochondrial membrane that cause membrane permeabilization and release of apoptogenic, intermembrane space proteins such as cytochrome c and Smac/DIABLO (9). Once in the cytosol, these polypeptides act through caspase 9 to promote the activation of the effector caspases-3 and -7, which induce cell death.
The precise mechanisms responsible for Bax and Bak activation remain incompletely understood (10). There are two commonly discussed models of activation by BH3-only proteins, termed indirect and direct activation. In the indirect model, activated BH3-only proteins bind to Mcl-1 (or other anti-apoptotic Bcl-2 family proteins) causing displacement of Bak and allowing Bak activation by release of Mcl-1 inhibition (11). In the direct model, activated BH3-only proteins directly bind and activate Bak and Bax, while Mcl-1 acts to bind and neutralize the BH3-only proteins. The activation of both Bak and Bax involves a conformational change exposing the N-terminal regions of both polypeptides, observed by immunoreactivity to conformation-specific antisera (12, 13). Additionally, both proteins are thought to form dimers and higher order multimers which act to permeabilize the mitochondrial membrane.
Given the critical role of BH3-only proteins in mediating cell death, BH3 mimetics would seem to be promising agents to restore the cell death program in cancers (14, 15). Recent studies have shown that ABT-737, a BH3 mimetic that binds Bcl-2 and Bcl-xL but not Mcl-1 (16), restores sensitivity of Bcl-2 overexpressing cells but not Mcl-1 overexpressing cells to apoptotic stimuli (17, 18). Another BH3 mimetic, obatoclax, is less well characterized, but has been shown to inhibit Mcl-1 in addition to Bcl-2 and Bcl-xL, and is predicted to bind in the BH3-binding pocket of Bcl-2 (19, 20). Obatoclax is an indole bipyrrole compound that is being investigated as an anti-cancer agent in phase I and II clinical trials on hematologic and lymphoid malignancies as well as small cell and non-small cell lung cancers. Direct binding studies have been hampered by the hydrophobic nature of obatoclax, but an analog compound Gx-15 has been studied in fluorescence polarization assays and competes for a fluorescently-labeled Bid BH3-peptide binding to Mcl-1 as well as Bcl-2 and Bcl-xL (21). After treatment with obatoclax, other groups have observed changes in expression of the BH3-only proteins Bim (19, 21, 22) and Noxa (23), both of which are known to interact with Mcl-1 and may contribute to apoptosis induction. Because obatoclax disables Mcl-1, we sought to determine whether obatoclax sensitizes human cholangiocarcinoma cell lines to Apo2L/TRAIL-mediated cytotoxicity. Because the cell lines (in contrast to primary cholangiocarcinoma tumor samples) do not express Apo2L/TRAIL (3), exogenous Apo2L/TRAIL was added to mimic the in vivo expression of Apo2L/TRAIL. Our results indicate that obatoclax sensitizes human cholangiocarcinoma cells to Apo2L/TRAIL by inhibiting Mcl-1 binding to Bak and Bim.
MATERIALS AND METHODS
Cell lines and culture
Cholangiocarcinoma cell lines derived from mixed intrahepatic cholangiocellular-hepatocellular carcinoma KMCH (24), or extrahepatic cholangiocarcinoma KMBC (25) and TFK-1 (26) were used. Cholangiocarcinoma cell lines and mouse embryonic fibroblast (MEF) cell lines were cultured in DMEM supplemented with 10% fetal bovine serum, 100 units/mL penicillin, 100 μg/mL streptomycin and 100 μg/mL gentamicin. KMCH cells stably transfected with S-peptide tagged Mcl-1 (5) were cultured in DMEM supplemented with 10% fetal bovine serum, 100 units/mL penicillin, 100 μg/mL streptomycin, 100 μg/mL gentamicin, and 1200 μg/mL G418.
S-peptide pulldown
KMCH cells stably transfected with S peptide-tagged Mcl-1 were incubated for 16 hrs with or without 0.5 μM obatoclax then Apo2L/TRAIL (1ng/mL) was added where indicated for 8 hours. After treatment, cells were washed with PBS and lysed at 4° C for 1 hr in lysis buffer consisting of 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1 mM dithiothreitol, 1 mM PMSF, 1 tablet Complete protease inhibitors/50 mL (Roche Diagnostics, Basel, Switzerland) 1 mM Na3VO4, 100 mM NaF, 20 nM microcystin, and 2% (w/v) CHAPS. After the protein concentration was determined by the Coomassie blue binding method (Pierce, Rockford, IL), aliquots containing 1 mg protein in 1 mL of lysis buffer were incubated with 50 L of S-protein agarose beads (Novagen, La Jolla, CA) at 4° C overnight. After at least 6 washes with lysis buffer, samples were released from the beads by boiling for 5–10 min in 50 L of Laemmli sample buffer (Bio-Rad Laboratories, Hercules, CA, USA) containing 5% 2-mercaptoethanol. Samples were resolved by SDS-PAGE, and transferred to nitrocellulose membranes.
To visualize Noxa binding to Mcl-1, a stable cell line derived from KMCH which expresses shRNA against endogenous Mcl-1 and also an shRNA-resistant phosphomimetic serine to glutamic acid (S64E) substituted S-tagged Mcl-1 protein was used, based on difficulty demonstrating Noxa immunoreactivity in pulldowns using wild-type human S-tagged Mcl-1. Our previous investigations suggested that Noxa binds much more strongly to S64E Mcl-1 than the unphosphorylatable S64A protein (5). The use of the S64E protein is biologically relevent, as mouse and rat Mcl-1 wild-type proteins contain a glutamic acid at this posistion. Otherwise, S-peptide pulldown experiments for Noxa binding were as above.
Immunoblot analysis
Whole cell lysates were obtained by incubating cells on ice with lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM PMSF, 1 tablet of Complete protease inhibitors/50 mL (Roche Diagnostics, Basel, Switzerland), 1 mM Na3VO4, 1 mM NaF, 1% Nonidet P-40, and 0.25% sodium deoxycholate. Insoluble particulates were removed by centrifugation at 14,000 x g for 15 min at 4° C. Samples were resolved on 4-20% gradient SDS-PAGE, transferred to nitrocellulose membrane and blotted with the indicated primary antibodies at a dilution of 1:1000. Peroxidase conjugated secondary antibodies (Biosource International, Camarillo, CA) were incubated at a dilution of 1:3000. Bound antibodies were visualized using Enhanced Chemiluminescence Reagents (ECL, Amersham, Arlington Heights, IL) and Kodak X-OMAT film. Primary antibodies used were those raised to Bak (Upstate, Charlottesville, VA), Bax (N-20, Santa Cruz Biotechnology, Inc, Santa Cruz, CA), c-FLIP (NT, Pro-Sci, Inc, Poway, CA), Caspase 8 (BD Biosciences, San Jose, CA), DR4 (Pro-Sci), DR5 (Pro-Sci), Bid (R&D Systems, Minneapolis, MN), Bim (BD Biosciences), Puma and Noxa (Pro-Sci) Mcl-1 (S-19, Santa Cruz Biotechnology) and Actin (C-11, Santa Cruz Biotechnology).
Cell death assays
Apoptosis was quantified by assessing the characteristic nuclear changes of apoptosis (i.e., chromatin condensation and nuclear fragmentation) using fluorescence microscopy after staining with DAPI (Sigma Chemical Co., St. Louis, MO). Caspase 3/7 activity in cell cultures was assessed using the Apo-ONE™ homogenous caspase-3/7 kit (Promega, Madison, WI) per the supplier’s protocol.
Clonogenic assays using wild-type MEFs and MEFs deficient for both Bak and Bax (DKO) were performed by plating cells at 100 cells/well followed by treatment with obatoclax (500 nM) overnight followed by media or Apo2L/TRAIL (10 ng/mL) for 4 hours, as indicated. Cells were then washed and allowed to form colonies (in media without obatoclax or Apo2L/TRAIL) for 10 days. Resultant colonies were stained with Coomassie blue and counted.
Immunofluorescence
KMCH cells were grown on glass coverslips and pretreated overnight with obatoclax (500 nM) or diluent followed by 8 hours of Apo2L/TRAIL or vehicle. Cells were washed once with PBS and fixed with 4% paraformaldehyde in buffer consisting of 100 mM PIPES (pH 6.95), 1 mM EGTA, and 3 mM magnesium sulfate. After permeabilization with 0.0125% (w/v) CHAPS in PBS and blocking of nonspecific protein binding with 5% normal goat serum (Sigma, St Louis, MO, USA) in PBS containing 5% glycerol and 0.04% sodium azide, cells were incubated overnight at 4°C with antibodies specific for activated Bax (6A7, Exalpha, Maynard, MA) or activated Bak (Bak-NT, Upstate) diluted 1:250 in blocking buffer. Cells were washed three times with PBS and Alexa 633-labeled anti-mouse (Bax) or anti-rabbit (Bak) secondary antibody (1:2000) was added for 1 hour at 37°C. Cells were then washed three times in PBS followed by a rinse with H2O, mounted onto slides using Prolong Gold Antifade Kit containing DAPI (Invitrogen, Carlsbad, CA) and examined on Zeiss LSM 510 confocal microscope (Carl Zeiss Inc., Thornwood, NJ, USA) using an excitation wavelength of 633 nm. Zeiss LSM Image Examiner was used to quantify fluorescence from manually outlined individual cells.
Materials
Reagents were obtained from the following suppliers: Recombinant human TRAIL was from R&D Systems (#375-TEC; Minneapolis, MN). Obatoclax was a generous gift from GeminX (Malvern, PA). Bax/Bak double knockout MEFs previously generated by Dr. Stanley J. Korsmeyer’s group were kindly provided by Dr. Douglas R. Green, St. Jude’sChildren’s Research Hospital, Memphis, TN.
Statistical Analysis
All data represent at least three independent experiments using cells from a minimum of three separate isolations and are expressed as mean ± standard error of the mean. Differences between groups were compared using ANOVA followed by pair-wise comparisons using the least significant difference by Tukey’s test.
RESULTS
Obatoclax sensitizes cholangiocarcinoma cells to Apo2L/TRAIL cytotoxicity
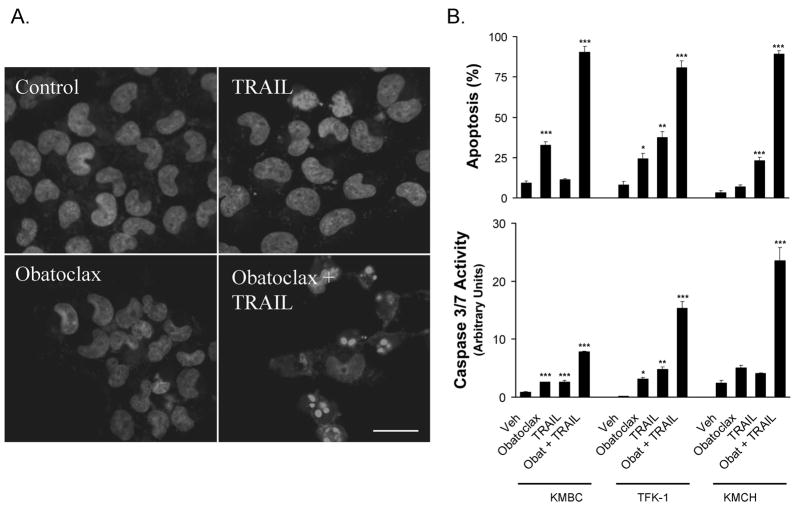
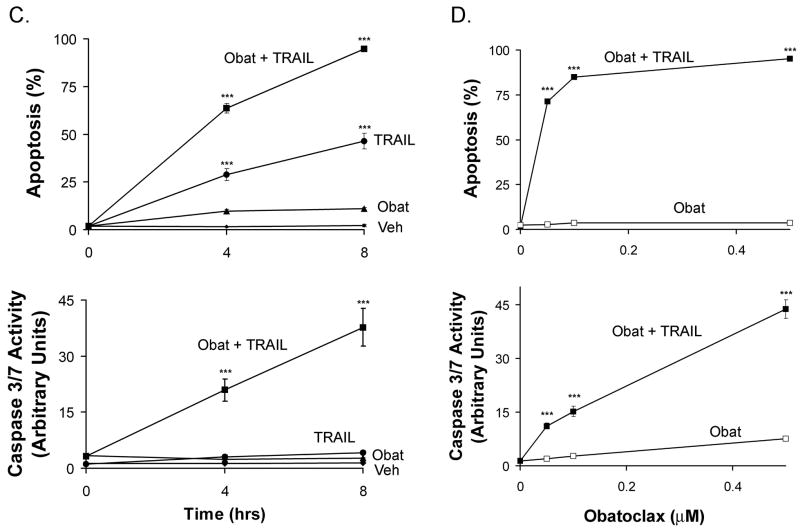
Previous studies from our laboratory have demonstrated that human cholangiocarcinoma cells are resistant to Apo2L/TRAIL (4, 5) and sensitivity to this death ligand can be restored by downregulation of Mcl-1 (4, 6) but not Bcl-2 or Bcl-xL (4). To build on these results, we examined the effect of obatoclax, a BH3 mimetic that targets Mcl-1 as well as Bcl-2 and Bcl-xL, on Apo2L/TRAIL sensitivity in the cholangiocarcinoma cell lines KMBC, KMCH, and TFK-1. All three lines were relatively resistant to apoptosis induced by exposure to the BH3 mimetic obatoclax or to Apo2L/TRAIL alone (Figure 1). However, pretreatment with obatoclax sensitized all three cell lines to Apo2L/TRAIL-mediated apoptosis (Figure 1A & B). Time course studies demonstrated that apoptotic changes were evident by 4 hours and increased at 8 hours (Figure 1C). Because KMCH cells underwent Apo2L/TRAIL-induced apoptosis at obatoclax concentrations as low as 50 nM (Figure 1D), this cell line was employed further for mechanistic studies.
Figure 1. Obatoclax sensitizes cholangiocarcinoma cell lines to Apo2L/TRAIL killing.
Panel A: KMCH cancer cells were pretreated with 0.5 μM obatoclax or vehicle overnight, followed by obatoclax plus Apo2L/TRAIL (1 ng/mL) for the final 8 hours where indicated. Cells were fixed and stained with the nuclear dye DAPI followed by imaging by confocal microscopy. Bar = 25 micrometers. Panel B: The cholangiocarcinoma cancer cell lines KMBC, KMCH and TFK-1 were treated as in panel A, and then stained with DAPI followed by fluorescence microscopy. Apoptotic and normal nuclei were quantitated and apoptotic cells were expressed as a percent of total. Cells treated in parallel were assayed for caspase 3/7-like activity (DEVDase activity), which is expressed in arbitrary units. For statistical analysis, treatments were compared to vehicle. Panel C. Following pretreatment with diluent or obatoclax, KMCH cells were treated with or without Apo2L/TRAIL (1 ng/mL) for the indicated times followed by quantitation of apoptosis and caspase 3/7 activity. For statistical analysis, treatments were compared to vehicle at the corresponding time point.Panel D. KMCH cells were pretreated with the indicated concentration of obatoclax followed by diluent (open symbol) or Apo2L/TRAIL (1 ng/mL; closed symbol) for 8 hours. Apoptosis and caspase 3/7 activity were measured as above. All data points represent the mean ± SE of triplicate experiments. For statistical analysis, Apo2L/TRAIL plus obatoclax was compared to obatoclax alone at the corresponding concentration. For all panels, statistical significance is indicated by * = p < 0.05, ** = p < 0.01, and *** = p < 0.001.
Obatoclax does not alter total cellular expression of anti- or pro-apoptotic proteins
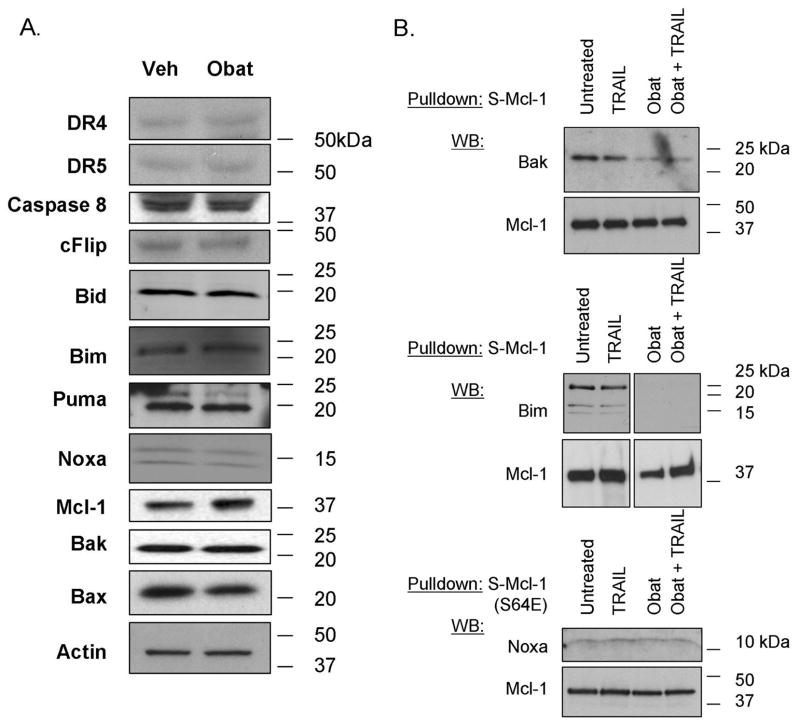
Sensitization of cell lines to Apo2L/TRAIL can be mediated by increased expression of pro-apoptotic, or decreased expression of anti-apoptotic mediators. We, therefore, next examined whole cell lysates from KMCH cholangiocarcinoma cells treated for 16 hours with obatoclax for expression of the Apo2L/TRAIL receptors DR4 and DR5, caspase 8, and cFLIP, as well as Bcl-2 family members Bid, Bim, Puma, Noxa, Mcl-1, Bak, and Bax. As examined by immunoblot analysis, no significant change in steady-state cellular levels of these proteins was observed (Figure 2A).
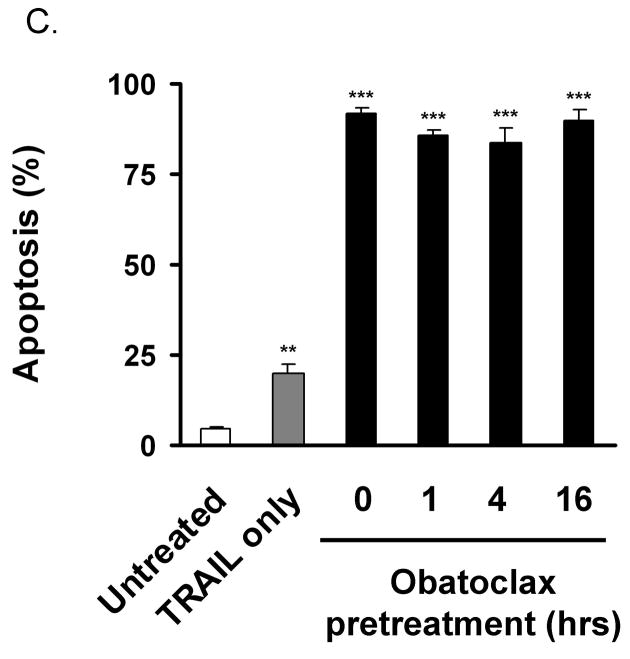
Figure 2. Obatoclax reduces Mcl-1 interaction with Bak and Bim.
Panel A: KMCH cells treated with 500 nM obatoclax for 16 hours were lysed and total protein separated by SDS-PAGE followed by immunoblot for the indicated proteins. Actin is shown as a loading control. Panel B. KMCH cells stably expressing S peptide-tagged Mcl-1 were treated overnight with medium or obatoclax followed by the addition of Apo2L/TRAIL (1 ng/mL, 8 hours) where indicated. S-peptide tagged Mcl-1 was enriched from whole cell lysates by S-protein agarose pulldown, and co-precipitating proteins examined by Western blot. The pulldown probed for Noxa (lower) used cells expressing S64E Mcl-1 (see text). Conditions are: untreated, Apo2L/TRAIL-treated, obatoclax-treated, and combined obatoclax plus Apo2L/TRAIL-treated. Images shown are cropped for clarity and the pulldown probed for Bim has been cropped to remove a redundant lane between the Apo2L/TRAIL-treated and obatoclax-treated lanes but were from the same experiment and blot. Full-length blots/gels are presented in Supplemental Figure 1. Panel C. KMCH cells were pretreated for the indicated times with medium or 0.5 M obatoclax and Apo2L/TRAIL added 8 hours before apoptosis was determined using fluorescence microscopy after DAPI staining. Statistical significance (compared to untreated) is indicated by ** = p < 0.01, and *** = p < 0.001. There was no statistical difference between cell death induced by Apo2L/TRAIL plus obatoclax pretreatment (1, 4, or 16 hours) versus Apo2L/TRAIL plus obatoclax cotreatment (0 hours pretreatment).
Obatoclax inhibits the association between Mcl-1 and Bak and Bim
Mcl-1 has been shown to inhibit apoptosis, in part, by binding and sequestering the proapoptotic multi-domain Bcl-2 protein Bak (11), an association which is antagonized by BH3-only proteins (27). Therefore, we next determined if the BH3-mimetic obatoclax also diminishes binding of Bak to Mcl-1. KMCH cells stably expressing S-peptide epitope-tagged Mcl-1 were utilized because the S-peptide/S-protein high affinity interaction provides a convenient and efficient method to selectively enrich for Mcl-1 and Mcl-1-bound polypeptides (5). Following S-peptide pulldown from CHAPS-lysed cells, Mcl-1 binding partners were assessed by immunoblot. In untreated KMCH cells, pulldown of S-peptide tagged Mcl-1 co-precipitated Bak (Figure 2B). In cells treated with obatoclax, reduced amounts of Bak were found associated with S-peptide tagged Mcl-1. This is consistent with a BH3-mimetic mode of action that displaces Bak from Mcl-1. Using this technique, we did not observe binding of Bax to Mcl-1 (data not shown).
Apo2L/TRAIL-mediated cell death in KMCH cells is dependent on the BH3-only protein Bim, as determined using siRNA silencing of Bim (28). Importantly, Bim co-precipitated with Mcl-1 and obatoclax treatment (in the presence or absence of Apo2L/TRAIL) prevented this interaction (Figure 2B). Thus, obatoclax interferes with binding of Bak and Bim, both pro-apoptotic, to Mcl-1.
The pro-apoptotic BH3-only protein Noxa specifically antagonizes Mcl-1 (11) and its invlovement in obatoclax sensitization was sought. No change in Noxa protein expression was observed after 16 hours of obatoclax treatment (Figure 2A). In addition, Noxa binding to Mcl-1 as tested by co-precipitation with S-tagged Mcl-1 was diminutive and difficult to visualize (not shown). We then tested a phosphomimetic Mcl-1 protein (serine 64 replaced by glutamic acid, S64E) previously shown to bind to Noxa (5). S-peptide pulldown of S64E Mcl-1 revealed Noxa binding was unchanged in the presence or absence of obatoclax (Figure 2B).
Obatoclax sensitization to apoptosis does not depend on pre-treatment
Treatment with obatoclax alone had little effect on cell death overnight, and thus in most of the experiments presented here cells were pretreated overnight with obatoclax followed by the addition of Apo2L/TRAIL after 16 hours. However, the suggested mechanism of action of obatoclax to occupy the BH3-binding pocket of anti-apoptotic proteins should not require pre-treatment. To test this, we added obatoclax (0.5 M final) to cells at 16, 4, and 1 hours prior to Apo2L/TRAIL treatment (1ng/mL) as well as adding both Apo2L/TRAIL and obatoclax simultaneously. Irrespective of the pre-incubation time, obatoclax sensitized to Apo2L/TRAIL treatment equally (Figure 2C). In addition, the proposed mechanism of action should not depend on de novo transcription/translation of an apoptogenic protein. To test this, we inhibited protein synthesis (cyclohexamide, 20 μg/mL) at the same time obatoclax was added (overnight treatment) and then added Apo2L/TRAIL for 8 hours. The inhibition of protein synthesis in contrast to a protective effect actually enhanced killing by Apo2L/TRAIL alone or in combination with obatoclax (not shown). Thus preventing translation did not decrease the injury, suggesting that new protein synthesis of pro-apoptotic proteins is not necessary for obatoclax activity.
Obatoclax-facilitated killing is associated with Bak and Bax activation
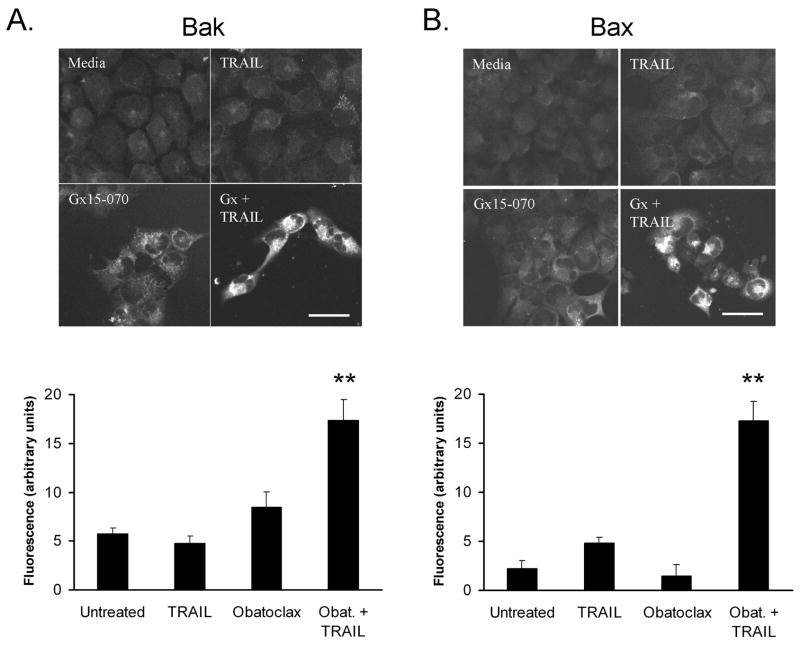
The pro-apoptotic proteins Bak and Bax undergo a conformational change upon activation that can be visualized using conformation-specific antibodies (12, 13). We tested the effect of obatoclax in the absence and presence of Apo2L/TRAIL on Bak and Bax N-terminal conformational alterations. Because initial studies demonstrated that obatoclax exhibits fluorescence at fluorescein and rhodamine wavelengths, but not at the far red end of the spectrum, we employed secondary antibodies labeled with the Alexa633 fluorophore (λexcitation and λemission of 633 and 650 nm, respectively). Bak conformational changes were observed in the majority of cells treated with Apo2L/TRAIL plus obatoclax, but not cells treated with diluent, Apo2L/TRAIL, or obatoclax (Figure 3A). The number of cells positive for staining with the conformation-specific Bak antiserum is consistent with the number of cells undergoing apoptosis (Figure 1B). Similarly, Bax conformational changes were observed in cells treated with obatoclax plus Apo2L/TRAIL, but not in the majority of cells treated with diluent or either stimulus alone (Figure 3B).
Figure 3. Bax and Bak undergo conformational change upon obatoclax and Apo2L/TRAIL treatment.
Panel A: KMCH cells were untreated or pretreated with 500 nM obatoclax with or without Apo2L/TRAIL (1 ng/ml) treatment for the final 8 hours and immunostained with antiserum specific for the N-terminus of Bak. Minimal staining was observed in untreated or Apo2L/TRAIL-treated cells while obatoclax-treated cells showed a slight increase in background fluorescence. Cells demonstrating N-terminus accessible Bak exhibited high levels of fluorescent staining, readily distinguished from background. Bar = 25 micrometers. Cellular fluorescence was quantitated (Zeiss LSM software) as the average signal within manually outlined cells and corrected by subtracting the average fluorescence of cells stained with secondary antibody only. To correct for residual fluorescence from obatoclax, signal for obatoclax and Apo2L/TRAIL + obatoclax was corrected by subtracting the average fluorescence of cells pretreated with obatoclax and stained with secondary antibody only. Random cell fields were photographed and fluorescence was quantitated for all cells (n = 31 to 75 cells per condition); **p <0.01 compared to untreated cells, ANOVA with Tukey’s least significant difference post-test. Panel B. Immunofluorescent staining with a monoclonal antibody (6A7) specific for the Bax N-terminus was performed similar to the experiments in panel A. Again, there was a slight increase in background with obatoclax alone, but Apo2L/TRAIL plus obatoclax induced strong immunoreactivity. Bar = 25 micrometers. Fluorescent signal was quantitated as in Panel A and is presented in a graph below the fluorescence images. **p <0.01 compared to untreated cells, ANOVA with Tukey’s least significant difference post-test.
Obatoclax killing is partially dependent upon Bax or Bak
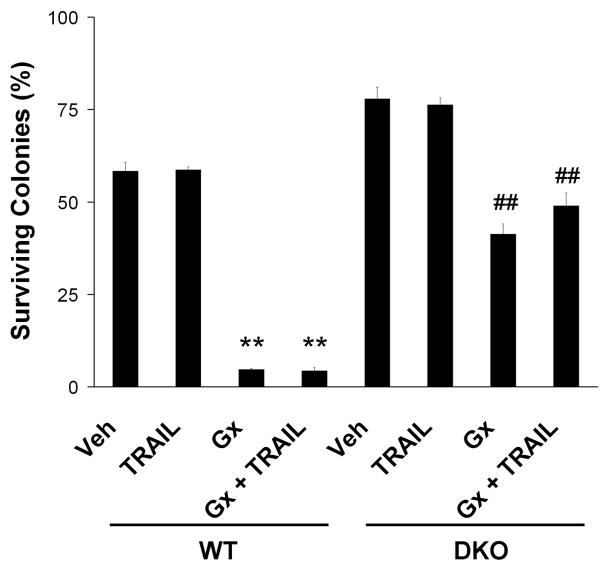
The activation of Bak and Bax in cells treated with obatoclax plus Apo2L/TRAIL is suggestive, but does not demonstrate that obatoclax cytotoxicity is dependent upon Bak or Bax. We tried several siRNA constructs unsuccessfully to knock Bax and/or Bak in KMCH cells. Therefore, to directly test the mechanism by which obatoclax sensitizes cells to apoptosis we employed MEFs doubly-deficient in Bak and Bax (DKO). Cell death was quantified in clonogenic assays, a rigorous method for assessing cell survival. This assay determines long-term survival after treatment, as cells must survive and proliferate to form colonies; colonies, not individual cells, are then counted after 10 days in culture. Wild-type MEFs were sensitive to obatoclax plus Apo2L/TRAIL treatment, and, in fact, were sensitive to obatoclax as a single agent (Figure 4). Single agent activity suggests that wild-type MEFs are under chronic BH3 stress that is mitigated by Bcl-2 family members and when treated with obatoclax, the wild-type MEFs can no longer resist apoptosis. DKO MEFs on the other hand, were partially resistant to apoptosis induction by either obatoclax alone or combined obatoclax plus Apo2L/TRAIL. Thus, cell killing depends, at least in part, on Bax/Bak protein expression.
Figure 4. Bax/Bak deficient cells are resistant to obatoclax mediated apoptosis.
Wild-type MEFs and MEFs deficient for both Bak and Bax (DKO) were treated with obatoclax (500 nM) overnight followed by media or Apo2L/TRAIL (10 ng/mL) for 4 hours, as indicated, and assayed for cell death by clonogenic assay (see methods). Resultant colonies were counted. Wild-type MEFs were sensitive to cell death induced by either obatoclax or obatoclax plus Apo2L/TRAIL. DKO MEFs on the other hand, were significantly more resistant than wild-type to apoptosis induction by either obatoclax alone or combined obatoclax plus Apo2L/TRAIL. Data represent the mean ± SE of three independent experiments. **p<0.01 compared to vehicle-treated wild-type MEFs; ##p<0.01 compared to vehicle-treated DKO MEFs and obatoclax-treated wild-type MEFs; ANOVA with Tukey’s least significant difference post-test.
DISCUSSION
BH3 mimetics represent a new therapeutic tool in the treatment of human cancers (14). These agents have been shown by competition studies and X-ray crystallography to bind anti-apoptotic proteins in the Bcl-2 family. For instance, the BH3 mimetic ABT-737 competes with a BH3 peptide (derived from Bad) for binding to Bcl-2, Bcl-xL, and Bcl-w but not Mcl-1 nor A1 (16). Indeed, Mcl-1 expression is now recognized as a common mechanism for ABT-737 resistance (17, 18). Obatoclax is a structurally dissimilar molecule that antagonizes Mcl-1 function in addition to Bcl-2 and Bcl-XL (19, 20). Further, the related compound Gx-15 competes for BH3-peptide binding to Mcl-1, Bcl-2, and Bcl-xL, using a fluorescence polarization assay (21). In recent studies, obatoclax has been reported to synergize with the proteasome inhibitor bortezomib in lymphoma cell lines (29), with tyrosine kinase inhibitors in breast cancer cells (30), and with cisplatin in non-small cell lung cancer cells (31). Our studies extend these observations by demonstrating that obatoclax increases the cytotoxicity of Apo2L/TRAIL in cholangiocarcinoma cells. Because obatoclax does not appreciably kill these cells by itself, the enhanced effects of the combination meet the definition of synergy, as put forth by Berenbaum (32). This synergy is consistent with several observations by us and others that Mcl-1 is a key mediator of Apo2L/TRAIL resistance (4, 6, 33). This is also the first report, to our knowledge, demonstrating that obatoclax can potentiate death ligand-induced apoptosis.
The precise mechanism of action of BH3-only proteins remains unsettled. One model posits that selected BH3-only proteins directly activate Bax and Bak (34), whereas another suggests that BH3-only proteins bind and inactivate anti-apoptotic Bcl-2 family members, thereby allowing release of inhibition of Bak and Bax (35). Treatment of cells with obatoclax alone did not induce activation of Bax and Bak (measured as an increase in immunoreactivity to conformation-specific antisera, Figure 3) making it unlikely obatoclax is a direct agonist of these pro-apoptotic proteins. Release of Bak and Bim binding to Mcl-1, however, was observed following obatoclax treatment (Figure 2), consistent with obatoclax binding to Mcl-1 and disabling its anti-apoptotic function. The lack of Bak activation despite its release from Mcl-1 by obatoclax (cf. Figure 2B and 3A) suggests that release from Mcl-1 is insufficient to induce full activation of Bak. These observations are consistent with the report that Bak mutants which do not bind Mcl-1 are not spontaneously lethal, but still require further activation to induce cell death (36). We also observed Bax activation by Apo2L/TRAIL in obatoclax treated cells, and Bax-dependent killing by Apo2L/TRAIL has also been reported (37). Because we were unable to get a >50% knockdown of Bax and Bak in these cells (S.F.B. and G.J.G., unpublished observations), we were unable to determine whether Bak and/or Bax is the principal mediator of cell death in cells treated with obatoclax and Apo2L/TRAIL.
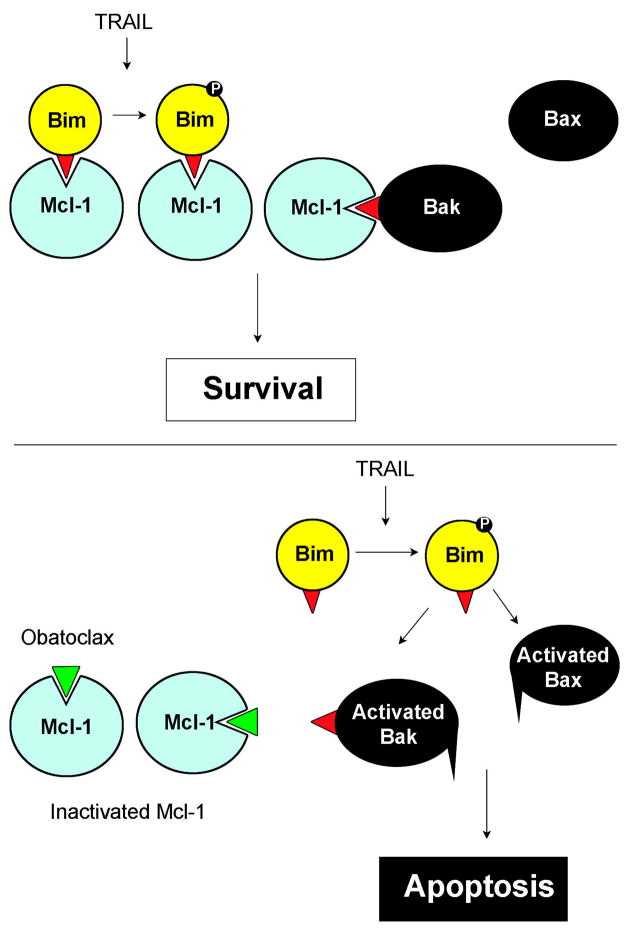
The effector causing activation of Bak after release from Mcl-1 is unknown, but recent studies in our lab demonstrated that Apo2L/TRAIL-dependent killing of KMCH cholangiocarcinoma cells is dependent on the BH3-only protein Bim (28). Interestingly, in addition to siRNA against Bim, the JNK pathway inhibitor SP600125 also prevented Apo2L/TRAIL killing. Thus, it is reasonable to postulate that Apo2L/TRAIL-induces activation of Bim through JNK-dependent phosphorylation (38). This activated Bim can still be held in check by Mcl-1 which may also act by neutralizing Bak. However, Apo2L/TRAIL treatment in the presence of obatoclax, which causes release of Bim and Bak from Mcl-1, facilitates apoptotic signaling (Figure 5). Thus Apo2L/TRAIL alone does not cause apoptosis as Bim (potentially phospho-Bim) and Bak are bound to Mcl-1. Similarly, obatoclax alone does not result in apoptosis, because the release of unactivated Bim and Bak does not cause death. The combination, though, results in release of Bak and Bim, and activation of Bim, resulting in Bak conformational change and mitochondrial permeabilization. We recognize that this mechanism of action (i.e. direct activation of Bak) is contentious. Indeed, alternative mechanisms of Bak activation remain possible. However, our data are inconsistent with spontaneous Bak activation upon release from Mcl-1, as obatoclax treatment alone induced Bak release (Figure 2B), but not Bak conformational change (Figure 3) or apotosis (Figure 1).
Figure 5. Schematic model illustrating the potential mechanism by which Apo2L/TRAIL plus obatoclax causes apoptosis.
Previous studies have demonstrated Bim phosphorylation/activation by Apo2L/TRAIL (38). In the presence of Mcl-1, Apo2L/TRAIL does not kill these cells, possibly due to the sequestration of Bim and Bak, and neither Bak nor Bax is activated. In contrast, in the presence of obatoclax Bak and Bim are not sequestered and Apo2L/TRAIL treatment leads to Bax and Bak conformational changes and apoptosis.
Other groups have described alterations in BH3-only protein levels after obatoclax treatment (19, 22, 23). In cholangiocarcinoma cells we do not see these changes after 16 hours of treatment. Thus, there may be cell-type specific differences in the transcriptional/translational response to obatoclax. Additional evidence that increased protein synthesis is not required for Apo2L/TRAIL-sensitization in cholangiocarcinoma cells includes the observation that pretreatment is not necessary for the full effect (Figure 2C). Preventing protein synthesis with cyclohexamide led to increased apoptosis further suggesting that de novo protein synthesis is not required for death signaling with this combination, and indeed newly synthesized proteins (possibly the short lived antiapoptotic protein c-FLIP) may instead play a protective role.
Although many molecules have been reported to function as BH3 mimetics, a number of these agents kill cells deficient in both Bax and Bak at the same efficiency as wiltype cells (18). In contract, it has been reported that obatoclax activity is dependent upon Bax and/or Bak, with baby kidney epithelial cells lacking Bax and Bak resistant to oligosomal DNA fragmentation and caspase-3 cleavage after obatoclax treatment compared to wild-type cells (19). We found partial protection of Bax/Bak double knockout fibroblasts from obatoclax using a clonogenic assay. Of note, there was an unexpected decrease in colony-forming ability of the DKO MEFs upon treatment with obatoclax. This may represent non-specific (ie. Bax/Bak independent) killing by obatoclax. This decrease was significantly less than in wild-type MEFs (53% survival for DKO versus 8% survival for wild-type MEFs). What sets obatoclax apart from ABT-737, another agent that is a bona fide BH3 mimetic, is the ability of the former to antagonize the anti-apoptotic function of Mcl-1 as well as Bcl-2 and Bcl-xL.
Of interest, Apo2L/TRAIL treatment of cholangiocarcinoma cells is not sufficient to cause apoptosis, but instead promotes cell migration and invasion, acting potentially to promote a malignant phenotype (3). This is consistent with alternate signaling pathways activated by Apo2L/TRAIL, and indeed we demonstrated that the increased migration and invasion of cholangiocarcinoma cells was caused by Apo2L/TRAIL-induced NF-κB activation (3). NF-κB can induce apoptosis resistance by transcriptional increases in anti-apoptotic Bcl-2 family members. This raises the possibility that obatoclax acts to sensitize cholangiocarcinoma cells to Apo2L/TRAIL by inhibiting an NF-κB-mediated survival signal, in effect converting the Apo2L/TRAIL effect from pro-survival to apoptogenic, as indeed NF-κB inhibition sensitizes hepatoma cells to Apo2L/TRAIL killing (39). However, in cholangiocarcinoma cells this is unlikely to be the operative mechanism of action, as NF-κB inhibition does not sensitize these cells to Apo2L/TRAIL killing (3).
In summary, our studies suggest that obatoclax reverses Apo2L/TRAIL resistance in cholangiocarcinoma cells. Because human cholangiocarcinomas (as compared to cell lines) express Apo2L/TRAIL (3), it is possible that obatoclax might have single agent antitumor activity in vivo. Accordingly, obatoclax or analogous compounds merit further preclinical investigation and possible evaluation for the treatment of cholangiocarcinoma.
Supplementary Material
Acknowledgments
The authors thank Erin Nystuen-Bungum for assistance preparing the manuscript. This work was supported by NIH grants (DK 79875 to J.L.M., K23 CA 96780 to R.A.M., CA 69008 to S.H.K., and DK 63947 to G.J.G.), the Palumbo Foundation and the Mayo Foundation.
Abbreviations
- BH3
Bcl-2 homology domain 3
- DAPI
4′ 6-diamidino-2-pheylindole dihydrochloride
- CHAPS
3-((3-cholamidopropyl)dimethylammonio)-1-propanesulfonic acid
- DMEM
Dulbecco’s modified Eagle’s medium
- MEF
mouse embryonic fibroblasts
- Apo2L/TRAIL
TNF-alpha-related apoptosis inducing ligand
Footnotes
This work was supported by NIH grants (DK 79875 to J.L.M., K23 CA 96780 to R.A.M., CA 69008 to S.H.K., and DK 63947 to G.J.G.), the Palumbo Foundation and the Mayo Foundation.
The authors declare no conflicts of interest.
References
- 1.Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353–1357. doi: 10.1053/jhep.2001.25087. [DOI] [PubMed] [Google Scholar]
- 2.de Groen PC, Gores GJ, LaRusso NF, Gunderson LL, Nagorney DM. Biliary tract cancers. N Engl J Med. 1999;341:1368–1378. doi: 10.1056/NEJM199910283411807. [DOI] [PubMed] [Google Scholar]
- 3.Ishimura N, Isomoto H, Bronk SF, Gores GJ. Trail induces cell migration and invasion in apoptosis-resistant cholangiocarcinoma cells. Am J Physiol Gastrointest Liver Physiol. 2006;290:G129–136. doi: 10.1152/ajpgi.00242.2005. [DOI] [PubMed] [Google Scholar]
- 4.Taniai M, Grambihler A, Higuchi H, et al. Mcl-1 mediates tumor necrosis factor-related apoptosis-inducing ligand resistance in human cholangiocarcinoma cells. Cancer Res. 2004;64:3517–3524. doi: 10.1158/0008-5472.CAN-03-2770. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi S, Lee SH, Meng XW, et al. Serine 64 phosphorylation enhances the antiapoptotic function of mcl-1. J Biol Chem. 2007;282:18407–18417. doi: 10.1074/jbc.M610010200. [DOI] [PubMed] [Google Scholar]
- 6.Mott JL, Kobayashi S, Bronk SF, Gores GJ. mir-29 regulates Mcl-1 protein expression and apoptosis. Oncogene. 2007 doi: 10.1038/sj.onc.1210436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobayashi S, Werneburg NW, Bronk SF, Kaufmann SH, Gores GJ. Interleukin-6 contributes to Mcl-1 up-regulation and TRAIL resistance via an Akt-signaling pathway in cholangiocarcinoma cells. Gastroenterology. 2005;128:2054–2065. doi: 10.1053/j.gastro.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Michels J, Johnson PW, Packham G. Mcl-1. Int J Biochem Cell Biol. 2005;37:267–271. doi: 10.1016/j.biocel.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Deng Y, Lin Y, Wu X. TRAIL-induced apoptosis requires Bax-dependent mitochondrial release of Smac/DIABLO. Genes Dev. 2002;16:33–45. doi: 10.1101/gad.949602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willis SN, Adams JM. Life in the balance: how BH3-only proteins induce apoptosis. Curr Opin Cell Biol. 2005;17:617–625. doi: 10.1016/j.ceb.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willis SN, Chen L, Dewson G, et al. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–1305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nechushtan A, Smith CL, Hsu YT, Youle RJ. Conformation of the Bax C-terminus regulates subcellular location and cell death. Embo J. 1999;18:2330–2341. doi: 10.1093/emboj/18.9.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffiths GJ, Dubrez L, Morgan CP, et al. Cell damage-induced conformational changes of the pro-apoptotic protein Bak in vivo precede the onset of apoptosis. J Cell Biol. 1999;144:903–914. doi: 10.1083/jcb.144.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Letai A. Restoring cancer’s death sentence. Cancer Cell. 2006;10:343–345. doi: 10.1016/j.ccr.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 15.Meng XW, Lee SH, Kaufmann SH. Apoptosis in the treatment of cancer: a promise kept? Curr Opin Cell Biol. 2006;18:668–676. doi: 10.1016/j.ceb.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Oltersdorf T, Elmore SW, Shoemaker AR, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 17.Konopleva M, Contractor R, Tsao T, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–388. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 18.van Delft MF, Wei AH, Mason KD, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen M, Marcellus RC, Roulston A, et al. Small molecule obatoclax (GX15–070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci U S A. 2007;104:19512–19517. doi: 10.1073/pnas.0709443104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shore GC, Viallet J. Modulating the bcl-2 family of apoptosis suppressors for potential therapeutic benefit in cancer. Hematology Am Soc Hematol Educ Program. 2005:226–230. doi: 10.1182/asheducation-2005.1.226. [DOI] [PubMed] [Google Scholar]
- 21.Zhai D, Jin C, Satterthwait AC, Reed JC. Comparison of chemical inhibitors of antiapoptotic Bcl-2-family proteins. Cell Death Differ. 2006;13:1419–1421. doi: 10.1038/sj.cdd.4401937. [DOI] [PubMed] [Google Scholar]
- 22.Trudel S, Li ZH, Rauw J, et al. Preclinical studies of the pan-Bcl inhibitor obatoclax (GX015–070) in multiple myeloma. Blood. 2007;109:5430–5438. doi: 10.1182/blood-2006-10-047951. [DOI] [PubMed] [Google Scholar]
- 23.Gomez-Bougie P, Maiga S, Pellat-Deceunynck C, et al. The pan-Bcl-2 inhibitor GX15–070 induces apoptosis in human myeloma cells by Noxa induction and strongly enhances melphalan, Bortezomib or TRAIL-R1 antibody apoptotic effect. Blood. 2006;108:991a–991a. [Google Scholar]
- 24.Murakami T, Yano H, Maruiwa M, Sugihara S, Kojiro M. Establishment and characterization of a human combined hepato cholangiocarcinoma cell line and its heterologous transplantation in nude mice. Hepatology. 1987;7:551–556. doi: 10.1002/hep.1840070322. [DOI] [PubMed] [Google Scholar]
- 25.Yano H, Maruiwa M, Iemura A, Mizoguchi A, Kojiro M. Establishment and characterization of a new human extrahepatic bile duct carcinoma cell line (KMBC) Cancer. 1992;69:1664–1673. doi: 10.1002/1097-0142(19920401)69:7<1664::aid-cncr2820690705>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 26.Saijyo S, Kudo T, Suzuki M, et al. Establishment of a new extrahepatic bile duct carcinoma cell line, TFK-1. Tohoku J Exp Med. 1995;177:61–71. doi: 10.1620/tjem.177.61. [DOI] [PubMed] [Google Scholar]
- 27.Han J, Goldstein LA, Hou W, Rabinowich H. Functional Linkage between NOXA and Bim in Mitochondrial Apoptotic Events. J Biol Chem. 2007;282:16223–16231. doi: 10.1074/jbc.M611186200. [DOI] [PubMed] [Google Scholar]
- 28.Werneburg NW, Guicciardi ME, Bronk SF, Kaufmann SH, Gores GJ. Tumor necrosis factor-related apoptosis-inducing ligand activates a lysosomal pathway of apoptosis that is regulated by Bcl-2 proteins. J Biol Chem. 2007;282:28960–28970. doi: 10.1074/jbc.M705671200. [DOI] [PubMed] [Google Scholar]
- 29.Perez-Galan P, Roue G, Villamor N, Campo E, Colomer D. The BH3-mimetic GX15–070 synergizes with Bortezomib in Mantle Cell Lymphoma by enhancing Noxa-mediated activation of Bak. Blood. 2007 doi: 10.1182/blood-2006-07-034173. [DOI] [PubMed] [Google Scholar]
- 30.Witters LM, Witkoski A, Planas-Silva MD, et al. Synergistic inhibition of breast cancer cell lines with a dual inhibitor of EGFR-HER-2/neu and a Bcl-2 inhibitor. Oncol Rep. 2007;17:465–469. [PubMed] [Google Scholar]
- 31.Li J, Viallet J, Haura EB. A small molecule pan-Bcl-2 family inhibitor, GX15–070, induces apoptosis and enhances cisplatin-induced apoptosis in non-small cell lung cancer cells. Cancer Chemother Pharmacol. 2007 doi: 10.1007/s00280-007-0499-3. [DOI] [PubMed] [Google Scholar]
- 32.Berenbaum MC. What is synergy? Pharmacol Rev. 1989;41:93–141. [PubMed] [Google Scholar]
- 33.Han J, Goldstein LA, Gastman BR, Rabinowich H. Interrelated roles for Mcl-1 and BIM in regulation of TRAIL-mediated mitochondrial apoptosis. J Biol Chem. 2006;281:10153–10163. doi: 10.1074/jbc.M510349200. [DOI] [PubMed] [Google Scholar]
- 34.Walensky LD, Pitter K, Morash J, et al. A stapled BID BH3 helix directly binds and activates BAX. Mol Cell. 2006;24:199–210. doi: 10.1016/j.molcel.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 35.Willis SN, Fletcher JI, Kaufmann T, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- 36.Kim H, Rafiuddin-Shah M, Tu HC, et al. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat Cell Biol. 2006;8:1348–1358. doi: 10.1038/ncb1499. [DOI] [PubMed] [Google Scholar]
- 37.LeBlanc H, Lawrence D, Varfolomeev E, et al. Tumor-cell resistance to death receptor--induced apoptosis through mutational inactivation of the proapoptotic Bcl-2 homolog Bax. Nat Med. 2002;8:274–281. doi: 10.1038/nm0302-274. [DOI] [PubMed] [Google Scholar]
- 38.Corazza N, Jakob S, Schaer C, et al. TRAIL receptor-mediated JNK activation and Bim phosphorylation critically regulate Fas-mediated liver damage and lethality. J Clin Invest. 2006;116:2493–2499. doi: 10.1172/JCI27726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim YS, Schwabe RF, Qian T, Lemasters JJ, Brenner DA. TRAIL-mediated apoptosis requires NF-kappaB inhibition and the mitochondrial permeability transition in human hepatoma cells. Hepatology. 2002;36:1498–1508. doi: 10.1053/jhep.2002.36942. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.