Abstract
Although cellular caspase-8 (FLICE)-like inhibitory protein (c-FLIP) has been identified as an important player in the extrinsic (death receptor-induced) apoptosis pathway, its endogenous function in mature T lymphocytes remains undefined. c-FLIP may inhibit or promote T cell death as previous data demonstrate that the c-FLIPL isoform can promote or inhibit caspase 8 activation while the c-FLIPS isoform promotes or inhibit T cell death when overexpressed. Although the c-FLIPR isoform inhibits cell death in cell lines, its function in T cells remains unknown. To investigate the function of c-FLIP in mature T cells, we have generated several genetic mouse models with c-FLIP or its individual isoforms deleted in mature T cells. Surprisingly, we found that c-FLIP protects mature T cells not only from apoptosis induced by the death receptors Fas and TNFR but also from TCR-mediated and spontaneous apoptosis. Thus, c-FLIP plays an essential role in protecting mature T cells from a death signal induced through the TCR itself and is required for naïve T cell survival. Our results demonstrate that c-FLIP functions beyond the extrinsic death pathway.
Keywords: c-FLIP, apoptosis, mature T lymphocytes, Fas, TNFα
Introduction
c-FLIP (also named CLARP, FLME, I-FLICE, MRIT, usurpin, Casper or CASH) plays important roles in death receptor mediated signaling (1–8). Three isoforms of c-FLIP derived from mRNA alternative splicing have been identified in human cells: 55kD c-FLIPL, 24kD c-FLIPR, and 26kD c-FLIPS (9, 10) while only c-FLIPL and c-FLIPR but not c-FLIPS are expressed in mouse cells (11). c-FLIPL contains two death effector domains (DED) at the N-terminus and an inactive caspase-like domain at the C-terminus. c-FLIPS and c-FLIPR only contain two DEDs (9–11). All three isoforms inhibit death receptor induced apoptosis, or the extrinsic death pathway, by interfering with pro-caspase-8 recruitment to the adaptor protein FADD (Fas associated death domain) when overexpressed (9–12).
The roles of individual c-FLIP isoforms in mature T cells remain undefined as conditional deletion of c-FLIP in thymocytes prevents mature T cell development (13). Previous studies suggested that c-FLIPL may play a role in T helper cell differentiation in transgenic overexpression mouse models (14–16). c-FLIPL may promote or inhibit mature T cell death given that c-FLIPL can also promote pro-caspase-8 activation at low levels of expression (17–21). The roles of c-FLIPs (c-FLIPR in mouse) in mature T cells are controversial. For example, one report showed that overexpression of c-FLIPS/R decreases mature T cell survival in mouse (22) while another report using a similar system did not observe decreased T cell survival (23). Given these conflicting results, it is essential to assess the roles of endogenous c-FLIP in mature T cells using loss-of-function models. In this report, we have generated mouse models lacking c-FLIP or its individual isoforms in mature T cells. We show that the in vivo function of c-FLIP (c-FLIPL and c-FLIPR) is to protect mature T cells from death receptor induced apoptosis. Importantly, we found that c-FLIP protects mature T cells from TCR induced and spontaneous apoptosis. Thus, these results indicate that c-FLIP functions beyond the extrinsic death pathway.
Materials and Methods
Mice
c-FLIPf/f mice and c-FLIPR (previously termed c-FLIPS) bacterial artificial chromosome (BAC) transgenic mice were generated in our laboratory as described (13, 24). To generate c-FLIPL BAC transgenic mice, a mouse c-FLIP wild type bacterial artificial chromosome (BAC) DNA clone was modified using a system as described (25). Briefly, mouse c-FLIPL cDNA, including its own stop codon and poly A signal, was fused to the start codon in exon 1 of the c-FLIP gene. A 60kb BAC fragment that is 15kb downstream of the nearest 5′ gene (Als2cr12) and 10kb upstream of the nearest 3′ gene (caspase-8) was used for injection. 3 founders were obtained for each line and crossed to c-FLIPf/fER-Cre mice, and all mice displayed a similar phenotype. Animal usage was conducted according to protocols approved by the Duke University Institutional Animal Care and Use Committee.
PCR analysis
PCR to assess the presence of the c-FLIPR or c-FLIPL BAC transgene was performed with 35 cycles of 94°C for 30 s, 52°C for 30 s, and 72°C for 120s. The primers are: forward, 5′- GAGGTTGAGGGACTTGGCATG-3′; reverse, 5′-TCAGCAGGACCCTATAATCAG-3′.
In vitro deletion of c-FLIP
Pooled single cell suspensions from spleen and lymph nodes were lysed of RBCs by ACK buffer. Cells were cultured at 4×106/ml in complete RPMI-1640 medium in the presence of 0.2μM 4OH-tamoxifen (Sigma) for 3 days. Live cells were purified by Lympholyte centrifugation (Cedarlane) according to the manufactory instruction. Live cells were re-cultured at 2×106/ml in the presence of different stimuli or subjected to Western and RT-PCR analysis.
Detection of active caspase 8
One day or three days after in vitro deletion of c-FLIP, live cells were purified and recultured in the presence or absence of 10μg/ml anti-CD3 for 5 hours. Fluorescence-labeled cell permeable caspase 8 inhibitor (FAM-LETD-FMK from Immunochemistry Technologies) was added to the culture during the last hour of incubation according to the manufactory instruction. Cells were surface stained and analyzed by flow cytometry.
Flow cytometric analysis
Cells were incubated with an FcR blocker (2.4G2; eBioscience), stained on ice for 30 min with FITC-, PE/Cy5-, or APC-labeled mAb, and washed with PBS containing 2% FCS. 1–5 × 104 events were collected on a FACScan flow cytometer (BD Biosciences) and analyzed using CellQuest (Becton Dickinson) or FlowJo software. All fluorescence-labeled antibodies, including anti- -CD4 and -CD8 were obtained from eBioscience, Biolegend, or BD Biosciences. Apoptotic cells were defined by Annexin V and 7-AAD staining using an Annexin V-PE kit (BD Biosciences).
Antibodies
Antibodies used for Western blots were anti–c-FLIP (clone Dave-2, Alexis Biochemical Corp.) and anti-γ-tubulin (Santa Cruz Biotechnology, Inc.).
Semi-quantitative RT-PCR analysis
After in vitro deletion of c-FLIP, total RNA was extracted from live cells and subjected to RT-PCR analysis. The primers are: forward primer for both c-FLIPL and c-FLIPR: 5′GCTGCTGTGGTTCTGAACATG 3′; reverse primer for c-FLIPL: 5′CTTTGACTGTCACGGTATTCCAC 3′ and reverse primer for c-FLIPR: 5′GGTACTCCATACACTGGCTCC 3. To detect c-FLIPL, the PCR was performed with 35 cycles of 94°C for 30 s, 64°C for 30 s, and 72°C for 60s. To performed with 35 cycles of 94°C for 30 s, 68°C for 30 detect c-FLIPR, the reaction was s, and 72°C for 60s.
Results
Impaired survival of c-FLIP-deficient mature T lymphocytes after death receptor engagement
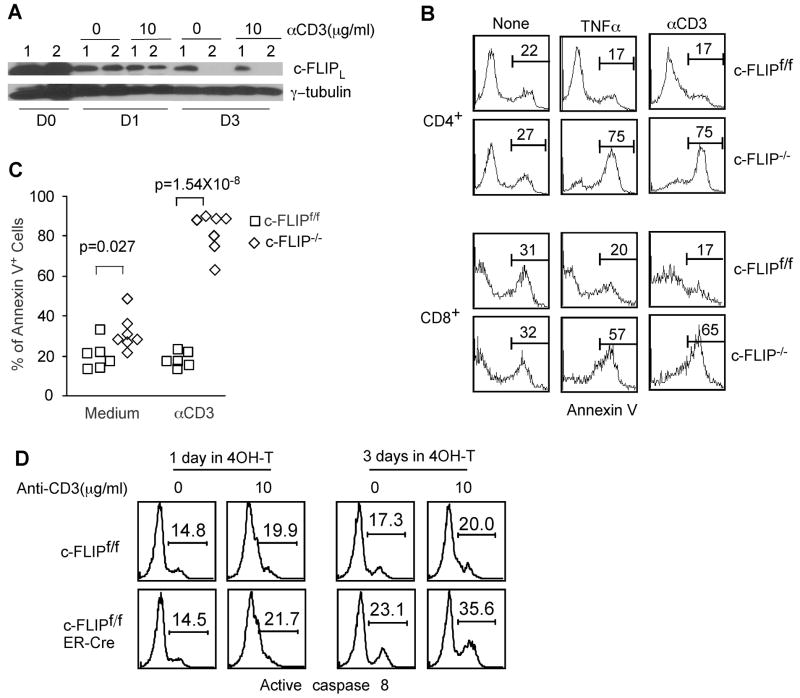
Our previous data have demonstrated that conditional deletion of c-FLIP (both c-FLIPL and c-FLIPR) in thymocytes results in severe impairment in the development of mature T lymphocytes in the periphery, excluding further analysis of c-FLIP function in these cells (13). To directly assess c-FLIP function in mature T cells, we crossed c-FLIPf/f mice with ER-Cre mice (26) to generate c-FLIPf/fER-Cre mice for the inducible deletion of c-FLIP. c-FLIPf/fER-Cre mice develop normally and have lymphoid compartments comparable to littermate controls (data not shown). As ER-Cre is expressed in all tissues, tamoxifen treatment of c-FLIPf/fER-Cre mice in vivo leads to lethality within three days, while all c-FLIPf/+ER-Cre littermates remain healthy (n=5), suggesting that c-FLIP plays an essential role in host survival in other tissues. We used in vitro deletion of c-FLIP to determine the role of c-FLIP in mature T cell survival. Total spleen and lymph node cells were pooled and cultured in the presence of 0.2 μM 4OH-tamoxifen for 3 days. Live cells were purified and recultured overnight with different stimuli. Under this culture condition, 4OH-tamoxifen exhibited no obvious toxicity to control T cells (data not shown) and induced efficient c-FLIP deletion in c-FLIPf/fER-Cre T cells (Fig. 1A). Consistent with previous results that c-FLIP functions as inhibitor of death receptor induced apoptosis (10, 27), c-FLIP−/− T cells exhibit markedly increased apoptosis upon TNFα and anti-Fas treatment compared with control T cells (Fig. 1B and 3A), indicating that c-FLIP protects T cells from death receptor induced death. After three days in vitro culture, 30–40% naïve T lymphocytes remained alive. To exclude the possibility that an abnormal T cell population was enriched after in vitro culture, we also performed the in vitro culture in the presence of IL-7. As expected, minimal T cell death was observed in the presence of IL-7. c-FLIP-deficient T cells from culture with or without IL-7 exhibited similar survival defect (data not shown).
Figure 1. c-FLIP protects mature T lymphocytes from death receptor induced, spontaneous and TCR-induced apoptosis.
Total splenocytes and LN cells from c-FLIPf/f and c-FLIPf/fER-Cre mice were cultured with 0.2μM 4OH-tamoxifen for 3 days in complete RPMI-1640 medium. (A) Deletion efficiency of c-FLIP in total splenocytes or LN cells as assessed by Western blot analysis. Live cells were purified at different days (D0–D3) after 4OH-tamoxifen culture and subjected to Western blot analysis. γ-tubulin serves as a loading control. c-FLIPf/f: lane 1 and c-FLIPf/fER-Cre: lane 2. (B) Enhanced apoptosis of c-FLIP-deficient T cells (c-FLIP−/−) with or without stimulation. After 3 days in 4OH-tamoxifen treated culture, live cells were purified and recultured for another day in the presence or absence of 5μg/ml anti-CD3 or 40ng/ml TNFα. Apoptotic rate was determined by Annexin V staining and FACS analysis for CD4+ and CD8+ cells. (C) Apoptosis results for CD4+ T cells as shown in (B) were combined from six independent experiments. Each dot represents the result from an individual mouse. (D) Caspase 8 activity in c-FLIP-deficient T cells. One or three days after 4OH-tamoxifen treatment, live cells were purified and recultured for 5 hours in the presence or absence of 10μg/ml anti-CD3. During the last hour of incubation, fluorescence labeled cell permeable caspase 8 inhibitor was added. Active caspase 8 was determined by FACS. FACS profiles were pre-gated on CD4+ cells.
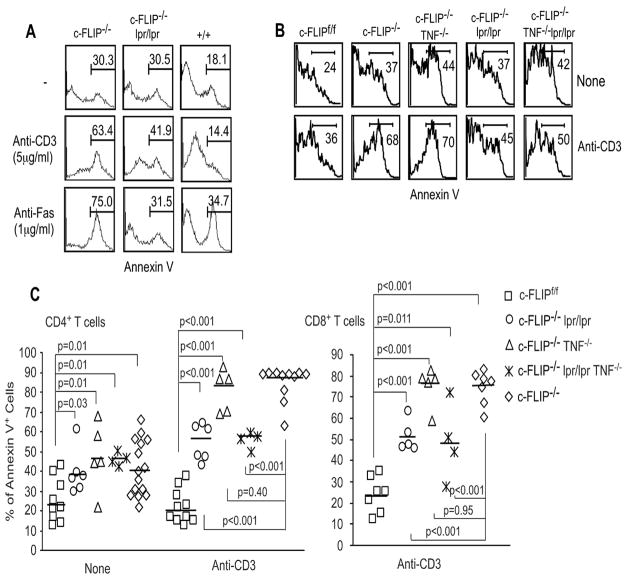
Figure 3. Role of Fas and TNFα signaling in the apoptosis of c-FLIP−/− T lymphocytes.
(A) Role of Fas in the apoptosis of c-FLIP−/− T cells. Splenocytes and LN cells from c-FLIPf/fER-Cre (c-FLIP−/−), c-FLIPf/fER-Cre.lpr/lpr, and wild type mice were cultured in 4OH-tamoxifen for 3 days. Live cells were purified and recultured under different conditions for another day. CD4+ T cell apoptotic rate was determined by Annexin V staining. (B) Role of TNFα in the apoptosis of c-FLIP−/− T cells. Splenocytes and LN cells from c-FLIPf/f, c-FLIPf/fER-Cre (c-FLIP−/−), c-FLIPf/fER-Cre.lpr/lpr, c-FLIPf/fER-Cre.TNF−/−, and c-FLIPf/fER-Cre.lpr/lpr.TNF−/− mice were cultured and tested as in (A). (C) Accumulative results of apoptosis of c-FLIP-deficient T cells in different genetic backgrounds. Data are a collection of four independent experiments as shown in (A) and (B) to show the apoptotic rate for CD4+ (left) and CD8+ (right) T cells. Each dot represents one mouse.
Impaired survival of c-FLIP-deficient T cells after TCR engagement
As TCR signaling induces caspase 8 activation (28, 29) and c-FLIP can either promote or inhibit caspase 8 activation (10, 17–19), and c-FLIP deficient T cells have been found to have increased apoptosis following TCR stimulation in a Rag chimeric mutant model(30), we tested what roles c-FLIP might play after TCR stimulation of mature T cells in an ER-cre inducible system. We stimulated mature T cells that were deleted of c-FLIP in vitro with anti-CD3. Remarkably, TCR stimulation induced the death of 60-90% of CD4+ and CD8+ c-FLIP-deficient T cells (Fig. 1B and 1C). Since it is possible that naïve T cells could be more sensitive to tamoxifen-induced toxicity than activated cells, resulting in a cell population enriched for activated cells that would undergo activation induced cell death (AICD) upon TCR stimulation, we examined wild type and c-FLIPf/fER-cre T cells cultured for three days with and without tamoxifen. We saw no differences in the activation profile (CD62L and CD44 expression) in cells treated with or without tamoxifen, indicating that the results we see are due to a defect in T cell survival following initial TCR stimulation in c-FLIP deficient cells (data not shown). Interestingly, c-FLIP−/− T cells also exhibit a slightly but significantly elevated apoptotic rate without any stimuli (Fig. 1B and 1C). It should be noted that in the absence of anti-CD3 stimulation, T cells were still in the resting stage after in vitro culture as determined by CD25 and CD69 staining (data not shown). These results suggest that c-FLIP regulates the survival of both resting and activated T cells. We then tested whether caspase 8 activity is enhanced in c-FLIP−/− T cells. One day after 4OH-tamoxifen induced deletion, c-FLIPf/fER-Cre T cells exhibited comparable caspase 8 activity as WT T cells due to the incomplete deletion of c-FLIP at this time point (Fig. 1A and 1D). However, when c-FLIP deletion was completed at day 3, c-FLIP−/− T cells exhibited a 33% increase (17.3% vs 23.1%) without TCR stimulation and a 78% increase (20% vs 35.6%) with TCR stimulation in the percentage of cells containing active caspase 8 during a 5-hour culture (Fig. 1D). Furthermore, addition of zVAD restored the survival defects in c-FLIP−/− T cells with or without TCR stimulation (Fig. 2A and 2B), suggesting that these cells die by apoptosis. These results suggest that c-FLIP plays a critical role in the survival of resting and activated T cells likely through the inhibition of caspase 8 activation.
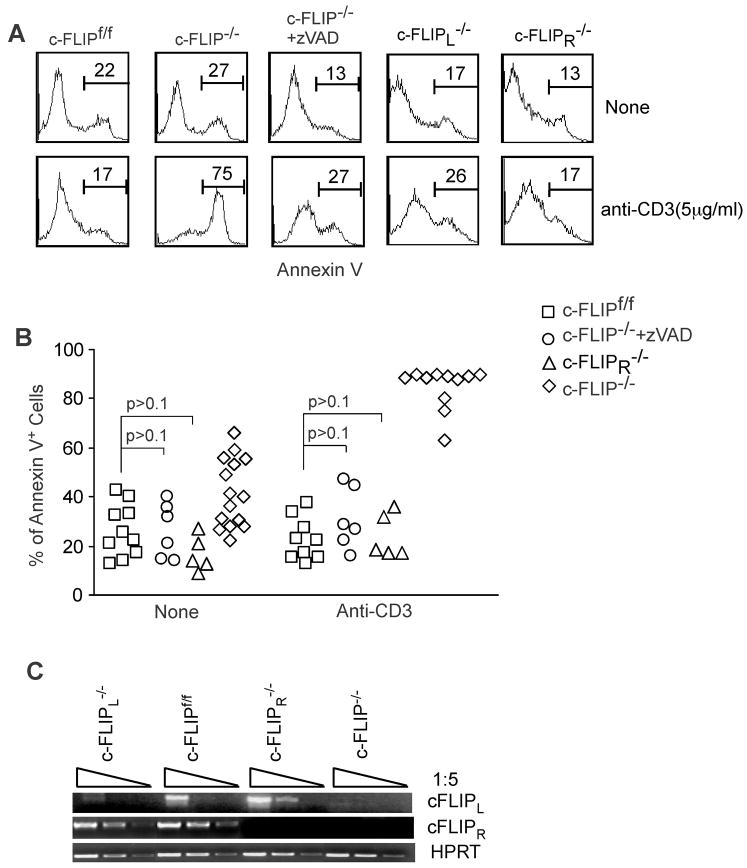
Figure 2. Both c-FLIPL and c-FLIPR are anti-apoptotic in TCR-induced death of c-FLIP−/− T cells.
(A) Expression of c-FLIPL or c-FLIPR in c-FLIP-deficient T cells or addition of the caspase inhibitor zVAD rescues the defective survival of c-FLIP-deficient T cells. Total splenocytes and LN cells from c-FLIPf/f, c-FLIPf/fER-Cre (c-FLIP−/−), c-FLIPf/fER-Cre.c-FLIPR BAC Tg (c-FLIPL−/−), and c-FLIPf/fER-Cre.c-FLIPL BAC Tg (c-FLIPR−/−) were cultured for three days with 4OH-tamoxifen. Live cells were purified and recultured under different conditions for another 24 hours. 10 μM zVAD was used in the reculture period. The apoptotic rate of CD4+ T cells was determined by Annexin V staining. (B) Cumulative data on CD4+ T cell apoptosis from different treatments. Data are from five independent experiments as shown in (A). Each dot represents one mouse. (C) Expression of c-FLIPL or c-FLIPR in T cells lacking c-FLIP, c-FLIPL or c-FLIPR Three days after 4OH-tamoxifen culture as described in (A), live cells were purified and subjected to RT-PCR analysis. HPRT serves as a loading control.
Both c-FLIPL and c-FLIPR isoforms protect T cells from death
Although two isoforms of c-FLIP, c-FLIPL and c-FLIPR, have been identified in mouse T cells, their function remains undetermined. It was shown that c-FLIPL inhibits caspase 8 activation at high expression levels, while it promotes caspase 8 activation at low expression levels (17–19). An enhanced caspase 8 activity was observed in c-FLIPL transgenic T lymphocytes (20, 21). Although c-FLIPR is considered a caspase 8 inhibitor (9–11), its role has not been examined in mouse T lymphocytes. Furthermore, a recent report showed that overexpression of human c-FLIPS, which is very similar human c-FLIPR at both sequence and function level, promotes T cell death (22) and this result is contradictory to a previous publication (23). Thus, it is essential to examine the role of endogenous c-FLIPL and c-FLIPR in mature T cell survival. To achieve this, we have generated conditional knockout mice lacking either isoform by crossing c-FLIPf/fER-cre to BAC transgenic mice expressing either mouse c-FLIPL or mouse c-FLIPR cDNA. As these cDNAs were inserted into the c-FLIP locus in BAC DNA, the expression of c-FLIPL or c-FLIPS in these mice is controlled by the endogenous c-FLIP promoter and regulatory elements. T lymphocytes from c-FLIPf/fER-cre.c-FLIPL BAC Tg mice that are treated with 4OH-tamoxifen express c-FLIPL but not c-FLIPR and therefore referred to as c-FLIPR−/− T cells (Fig. 2C). Similarly, T lymphocytes from c-FLIPf/fER-cre.c-FLIPR BAC Tg mice that are treated with 4OH-tamoxifen express c-FLIPR but not c-FLIPL and therefore referred to as c-FLIPL−/− T cells (Fig. 2C). Expression of either c-FLIPR or c-FLIPL in T lymphocytes was sufficient to rescue the survival defects in c-FLIP−/− T cells in the presence or absence of TCR stimulation (Fig. 2A and 2B). These data suggest that both c-FLIPR and c-FLIPL isoforms are anti-apoptotic caspase 8 inhibitors in mature T cells at endogenous expression levels.
The role of death receptors in TCR-induced apoptosis of c-FLIP−/− T cells
Previous data suggests that TCR-mediated apoptosis is mediated through Fas/FasL or TNFα/TNFRs in CD4+ and CD8+ T cells (31–39). The massive death of c-FLIP-deficient T cells upon TCR stimulation may be due to Fas and/or TNFR induced death signal. To determine the contribution of Fas and TNFα during the apoptosis of c-FLIP−/− T cells upon TCR stimulation, c-FLIPf/fER-Cre mice were crossed onto lpr (Fas) mutant, TNFα-deficient and lpr/lpr/TNFα double deficient backgrounds. Fas mutation partially rescued the TCR-induced apoptosis while it completely rescued anti-Fas induced apoptosis in c-FLIP−/− T cells (Fig. 3A and 3C). In contrast, deletion of TNFα had no obvious effect on the high apoptosis of c-FLIP−/− T cells (Fig. 3B and 3C). Furthermore, c-FLIP-deficient T cells from an lpr/lpr/TNFα double deficient background had apoptotic rates similar to those from an lpr single mutation background (Fig. 3B and 3C). Thus, these results suggest that Fas-mediated death signaling only contribute partially to TCR-induced apoptosis in c-FLIP-deficient T cells, while TNF-mediated death signaling has no obvious role in this process.
c-FLIP is critical for maintaining T cell homeostasis in vivo
To directly test whether c-FLIP is required for mature T cell survival in vivo, T cells from c-FLIPf/fER-Cre and c-FLIPf/+ER-cre mice were labeled with high or low amounts of CFSE, mixed at a 1:1 ratio and transferred into unmanipulated WT hosts. Similar results were seen when host lymph node and spleen were analyzed, and the panel shown is from host lymph node. Five days after in vivo tamoxifen administration, the number of c-FLIP−/− T cells was decreased to 10–40% of that of transferred control T cells (Fig. 4). Thus, c-FLIP is required for T cell homeostasis in vivo.
Figure 4. c-FLIP is required for mature T cell homeostasis in vivo.
Equal numbers of splenocytes from c-FLIPf/+ER-Cre and c-FLIPf/fER-Cre mice were labeled with 0.5 and 5 μM of CFSE, mixed and i.v. injected into c-FLIPf/f mice. 2 mg tamoxifen were administered to the host mice after cell transfer. 5 days later, the host mice were sacrificed and the CFSE+ cell percentage was examined in spleen and lymph node by FACS analysis. (A) FACS profiles show the percent of CD4+ T cell population before and after cell transfer. Results shown are from lymph node, but similar results were seen in the spleen. (B) Ratio of c-FLIP−/− versus c-FLIP+/− T cells after transfer at day 0 and day 5. Each line represents one mouse.
Discussion
Although c-FLIP has been identified as a critical player in death receptor induced signaling for more than a decade, the function of endogenous c-FLIP in mature T cells remains unclear. Conflicting results suggest that c-FLIPL might inhibit or promote mature T cell death (17–19, 22, 23). We have generated several genetic models to investigate the function of c-FLIP in mature T cells. As expected, we found that c-FLIP protects T cells from death receptor induced apoptosis via Fas and TNFR. However, we also found that c-FLIP protects mature T cells from TCR-induced as well as spontaneous cell death. Furthermore, both c-FLIPL and c-FLIPR isoforms function as anti-apoptotic proteins in mature T cells.
The likely target for c-FLIP in the survival of resting and TCR-stimulated mature T cells is caspase 8. Caspase 8 is actively involved in T cell homeostasis. TCR signaling rapidly upregulates caspase 8 activity (28, 29). Considering the fact that weak TCR/MHC interaction is required for naïve T cell survival, basal caspase 8 activity may also be present in resting T lymphocytes. Indeed, c-FLIP−/− T cells exhibit a marked increase of caspase 8 activity with or without TCR stimulation. Furthermore, because c-FLIP−/− T cells exhibit enhanced spontaneous and TCR-induced apoptosis, and zVAD completely rescues both defects, c-FLIP is the essential caspase 8 inhibitor in T lymphocytes. In the absence of c-FLIP, caspase 8 activity may elevate to a harmful level even without TCR stimulation. Thus, the major function of c-FLIP in resting naïve T cells is likely to prevent the constitutively low levels of active caspase 8 from killing the cells.
Our data demonstrate that c-FLIPL and c-FLIPR, when expressed at endogenous levels, inhibit apoptosis. The role of c-FLIPL in regulating caspase 8 activity and cell death has been controversial. c-FLIPL was shown to promote caspase 8 activation at low expression levels and inhibit caspase 8 activation at high expression levels (17–21). Although c-FLIPR is generally considered a caspase 8 inhibitor (9–11), recent reports showed that overexpression of c-FLIPS, which is very similar to c-FLIPR, in T cells generated conflicting results regarding T cell death (22, 23). These controversial results are likely derived from the various over expression systems used. By expressing the c-FLIPL or c-FLIPR isoform under its own regulatory elements, our data clearly show that both c-FLIPL and c-FLIPR are anti-apoptotic in resting and TCR-stimulated mature T lymphocytes.
Our data demonstrate that the Fas/FasL interaction, but not TNFα contributes only partially to the apoptosis of c-FLIP-deficient CD4+ and CD8+ T cells upon TCR engagement. Previous studies have demonstrated that pre-activated T cells and T cell hybridoma cell lines undergo rapid apoptosis upon TCR restimulation through a Fas/FasL or TNFα/TNFR dependent mechanism (31–39). However, our genetic data has clearly established that Fas does not cause all the cell death in TCR-stimulated c-FLIP-deficient T cells. Our results suggest that TCR engagement may activate caspase 8 independent of death receptors and Fas/FasL may function as an amplifier, but not an initiator, of caspase 8 activation in T lymphocytes. Although lpr mutation does not completely eliminate Fas signaling (40), it is unlikely that the mutant Fas still delivers a critical death signal during TCR-induced cell death in our system based on three lines of evidences. First, lpr mutation completely restores the survival defects upon the anti-Fas stimulation in c-FLIP−/− T cells (Fig 3A). Second, extensive previous investigations have shown that lpr mutation rescues the pre-activated T cells from TCR-induced apoptosis (33, 35, 36, 38, 39). Third, in the report showing that lpr mutant is partially functional, the authors clearly demonstrated that lpr mutant completely loses the capacity to induce cell death while it can activate NF-κB signaling only in heterozygous (lpr/+) mice(40). In our experiments, only lpr/lpr mice were employed. Therefore, lpr mutant can be considered as a complete loss-of-function of the death inducing capacity of Fas. Since death receptor mutations partially restore and zVAD completely restores the survival defects in c-FLIP−/− T cells upon TCR stimulation, this suggests that TCR signaling induces caspase activation through a Fas- (and TNFR) independent mechanism. Thus, c-FLIP is critically needed to protect T cells from capase activity during resting as well as activation phases.
Acknowledgments
We thank T. Matt Holl and Cheryl Bock for help in generating the c-FLIPL BAC tansgenic mice.
This work was supported by NIH grants CA92123 and AI54683.
References
- 1.Goltsev YV, Kovalenko AV, Arnold E, Varfolomeev EE, Brodianskii VM, Wallach D. CASH, a novel caspase homologue with death effector domains. J Biol Chem. 1997;272:19641–19644. doi: 10.1074/jbc.272.32.19641. [DOI] [PubMed] [Google Scholar]
- 2.Han DK, Chaudhary PM, Wright ME, Friedman C, Trask BJ, Riedel RT, Baskin DG, Schwartz SM, Hood L. MRIT, a novel death-effector domain-containing protein, interacts with caspases and BclXL and initiates cell death. Proc Natl Acad Sci U S A. 1997;94:11333–11338. doi: 10.1073/pnas.94.21.11333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu S, Vincenz C, Ni J, Gentz R, Dixit VM. I-FLICE, a novel inhibitor of tumor necrosis factor receptor-1- and CD-95-induced apoptosis. J Biol Chem. 1997;272:17255–17257. doi: 10.1074/jbc.272.28.17255. [DOI] [PubMed] [Google Scholar]
- 4.Inohara N, Koseki T, Hu Y, Chen S, Nunez G. CLARP, a death effector domain-containing protein interacts with caspase-8 and regulates apoptosis. Proc Natl Acad Sci U S A. 1997;94:10717–10722. doi: 10.1073/pnas.94.20.10717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer JL, Schroter M, Burns K, Mattmann C, Rimoldi D, French LE, Tschopp J. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 6.Rasper DM, Vaillancourt JP, Hadano S, Houtzager VM, Seiden I, Keen SL, Tawa P, Xanthoudakis S, Nasir J, Martindale D, Koop BF, Peterson EP, Thornberry NA, Huang J, MacPherson DP, Black SC, Hornung F, Lenardo MJ, Hayden MR, Roy S, Nicholson DW. Cell death attenuation by ‘Usurpin’, a mammalian DED-caspase homologue that precludes caspase-8 recruitment and activation by the CD-95 (Fas, APO-1) receptor complex. Cell Death Differ. 1998;5:271–288. doi: 10.1038/sj.cdd.4400370. [DOI] [PubMed] [Google Scholar]
- 7.Shu HB, Halpin DR, Goeddel DV. Casper is a FADD- and caspase-related inducer of apoptosis. Immunity. 1997;6:751–763. doi: 10.1016/s1074-7613(00)80450-1. [DOI] [PubMed] [Google Scholar]
- 8.Srinivasula SM, Ahmad M, Ottilie S, Bullrich F, Banks S, Wang Y, Fernandes-Alnemri T, Croce CM, Litwack G, Tomaselli KJ, Armstrong RC, Alnemri ES. FLAME-1, a novel FADD-like anti-apoptotic molecule that regulates Fas/TNFR1-induced apoptosis. J Biol Chem. 1997;272:18542–18545. doi: 10.1074/jbc.272.30.18542. [DOI] [PubMed] [Google Scholar]
- 9.Golks A, Brenner D, Fritsch C, Krammer PH, Lavrik IN. c-FLIPR, a new regulator of death receptor-induced apoptosis. J Biol Chem. 2005;280:14507–14513. doi: 10.1074/jbc.M414425200. [DOI] [PubMed] [Google Scholar]
- 10.Thome M, Tschopp J. Regulation of lymphocyte proliferation and death by FLIP. Nat Rev Immunol. 2001;1:50–58. doi: 10.1038/35095508. [DOI] [PubMed] [Google Scholar]
- 11.Ueffing N, Keil E, Freund C, Kuhne R, Schulze-Osthoff K, Schmitz I. Mutational analyses of c-FLIP(R), the only murine short FLIP isoform, reveal requirements for DISC recruitment. Cell Death Differ. 2008;15:773–782. doi: 10.1038/sj.cdd.4402314. [DOI] [PubMed] [Google Scholar]
- 12.Budd RC, Yeh WC, Tschopp J. cFLIP regulation of lymphocyte activation and development. Nat Rev Immunol. 2006;6:196–204. doi: 10.1038/nri1787. [DOI] [PubMed] [Google Scholar]
- 13.Zhang N, He YW. An essential role for c-FLIP in the efficient development of mature T lymphocytes. The Journal of experimental medicine. 2005;202:395–404. doi: 10.1084/jem.20050117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tseveleki V, Bauer J, Taoufik E, Ruan C, Leondiadis L, Haralambous S, Lassmann H, Probert L. Cellular FLIP (long isoform) overexpression in T cells drives Th2 effector responses and promotes immunoregulation in experimental autoimmune encephalomyelitis. J Immunol. 2004;173:6619–6626. doi: 10.4049/jimmunol.173.11.6619. [DOI] [PubMed] [Google Scholar]
- 15.Tseveleki V, Tsagozis P, Koutsoni O, Dotsika E, Probert L. Cellular FLIP long isoform transgenic mice overcome inherent Th2-biased immune responses to efficiently resolve Leishmania major infection. Int Immunol. 2007;19:1183–1189. doi: 10.1093/intimm/dxm089. [DOI] [PubMed] [Google Scholar]
- 16.Wu W, Rinaldi L, Fortner KA, Russell JQ, Tschopp J, Irvin C, Budd RC. Cellular FLIP long form-transgenic mice manifest a Th2 cytokine bias and enhanced allergic airway inflammation. J Immunol. 2004;172:4724–4732. doi: 10.4049/jimmunol.172.8.4724. [DOI] [PubMed] [Google Scholar]
- 17.Boatright KM, Deis C, Denault JB, Sutherlin DP, Salvesen GS. Activation of caspases-8 and -10 by FLIP(L) Biochem J. 2004;382:651–657. doi: 10.1042/BJ20040809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang DW, Xing Z, Pan Y, Algeciras-Schimnich A, Barnhart BC, Yaish-Ohad S, Peter ME, Yang X. c-FLIP(L) is a dual function regulator for caspase-8 activation and CD95-mediated apoptosis. Embo J. 2002;21:3704–3714. doi: 10.1093/emboj/cdf356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Micheau O, Thome M, Schneider P, Holler N, Tschopp J, Nicholson DW, Briand C, Grutter MG. The long form of FLIP is an activator of caspase-8 at the Fas death-inducing signaling complex. J Biol Chem. 2002;277:45162–45171. doi: 10.1074/jbc.M206882200. [DOI] [PubMed] [Google Scholar]
- 20.Dohrman A, Kataoka T, Cuenin S, Russell JQ, Tschopp J, Budd RC. Cellular FLIP (long form) regulates CD8+ T cell activation through caspase-8-dependent NF-kappa B activation. J Immunol. 2005;174:5270–5278. doi: 10.4049/jimmunol.174.9.5270. [DOI] [PubMed] [Google Scholar]
- 21.Dohrman A, Russell JQ, Cuenin S, Fortner K, Tschopp J, Budd RC. Cellular FLIP long form augments caspase activity and death of T cells through heterodimerization with and activation of caspase-8. J Immunol. 2005;175:311–318. doi: 10.4049/jimmunol.175.1.311. [DOI] [PubMed] [Google Scholar]
- 22.Hinshaw-Makepeace J, Huston G, Fortner KA, Russell JQ, Holoch D, Swain S, Budd RC. c-FLIP(S) reduces activation of caspase and NF-kappaB pathways and decreases T cell survival. Eur J Immunol. 2008;38:54–63. doi: 10.1002/eji.200636956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oehme I, Neumann F, Bosser S, Zornig M. Transgenic overexpression of the Caspase-8 inhibitor FLIP(short) leads to impaired T cell proliferation and an increased memory T cell pool after staphylococcal enterotoxin B injection. Eur J Immunol. 2005;35:1240–1249. doi: 10.1002/eji.200425564. [DOI] [PubMed] [Google Scholar]
- 24.Zhang N, Hopkins K, He YW. The long isoform of cellular FLIP is essential for T lymphocyte proliferation through an NF-kappaB-independent pathway. J Immunol. 2008;180:5506–5511. doi: 10.4049/jimmunol.180.8.5506. [DOI] [PubMed] [Google Scholar]
- 25.Sparwasser T, Gong S, Li JY, Eberl G. General method for the modification of different BAC types and the rapid generation of BAC transgenic mice. Genesis. 2004;38:39–50. doi: 10.1002/gene.10249. [DOI] [PubMed] [Google Scholar]
- 26.Shapiro-Shelef M, Lin KI, Savitsky D, Liao J, Calame K. Blimp-1 is required for maintenance of long-lived plasma cells in the bone marrow. The Journal of experimental medicine. 2005;202:1471–1476. doi: 10.1084/jem.20051611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeh WC, Itie A, Elia AJ, Ng M, Shu HB, Wakeham A, Mirtsos C, Suzuki N, Bonnard M, Goeddel DV, Mak TW. Requirement for Casper (c-FLIP) in regulation of death receptor-induced apoptosis and embryonic development. Immunity. 2000;12:633–642. doi: 10.1016/s1074-7613(00)80214-9. [DOI] [PubMed] [Google Scholar]
- 28.Alam A, Cohen LY, Aouad S, Sekaly RP. Early activation of caspases during T lymphocyte stimulation results in selective substrate cleavage in nonapoptotic cells. The Journal of experimental medicine. 1999;190:1879–1890. doi: 10.1084/jem.190.12.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kennedy NJ, Kataoka T, Tschopp J, Budd RC. Caspase activation is required for T cell proliferation. The Journal of experimental medicine. 1999;190:1891–1896. doi: 10.1084/jem.190.12.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chau H, Wong V, Chen NJ, Huang HL, Lin WJ, Mirtsos C, Elford AR, Bonnard M, Wakeham A, You-Ten AI, Lemmers B, Salmena L, Pellegrini M, Hakem R, Mak TW, Ohashi P, Yeh WC. Cellular FLICE-inhibitory protein is required for T cell survival and cycling. The Journal of experimental medicine. 2005;202:405–413. doi: 10.1084/jem.20050118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alderson MR, Tough TW, Davis-Smith T, Braddy S, Falk B, Schooley KA, Goodwin RG, Smith CA, Ramsdell F, Lynch DH. Fas ligand mediates activation-induced cell death in human T lymphocytes. The Journal of experimental medicine. 1995;181:71–77. doi: 10.1084/jem.181.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brunner T, Mogil RJ, LaFace D, Yoo NJ, Mahboubi A, Echeverri F, Martin SJ, Force WR, Lynch DH, Ware CF, et al. Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature. 1995;373:441–444. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- 33.Dhein J, Walczak H, Baumler C, Debatin KM, Krammer PH. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95) Nature. 1995;373:438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 34.Ju ST, Panka DJ, Cui H, Ettinger R, el-Khatib M, Sherr DH, Stanger BZ, Marshak-Rothstein A. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373:444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 35.Mixter PF, Russell JQ, Budd RC. Delayed kinetics of T lymphocyte anergy and deletion in lpr mice. J Autoimmun. 1994;7:697–710. doi: 10.1006/jaut.1994.1055. [DOI] [PubMed] [Google Scholar]
- 36.Russell JH, Wang R. Autoimmune gld mutation uncouples suicide and cytokine/proliferation pathways in activated, mature T cells. Eur J Immunol. 1993;23:2379–2382. doi: 10.1002/eji.1830230951. [DOI] [PubMed] [Google Scholar]
- 37.Speiser DE, Sebzda E, Ohteki T, Bachmann MF, Pfeffer K, Mak TW, Ohashi PS. Tumor necrosis factor receptor p55 mediates deletion of peripheral cytotoxic T lymphocytes in vivo. Eur J Immunol. 1996;26:3055–3060. doi: 10.1002/eji.1830261235. [DOI] [PubMed] [Google Scholar]
- 38.Sytwu HK, Liblau RS, McDevitt HO. The roles of Fas/APO-1 (CD95) and TNF in antigen-induced programmed cell death in T cell receptor transgenic mice. Immunity. 1996;5:17–30. doi: 10.1016/s1074-7613(00)80306-4. [DOI] [PubMed] [Google Scholar]
- 39.Zheng L, Fisher G, Miller RE, Peschon J, Lynch DH, Lenardo MJ. Induction of apoptosis in mature T cells by tumour necrosis factor. Nature. 1995;377:348–351. doi: 10.1038/377348a0. [DOI] [PubMed] [Google Scholar]
- 40.Legembre P, Barnhart BC, Zheng L, Vijayan S, Straus SE, Puck J, Dale JK, Lenardo M, Peter ME. Induction of apoptosis and activation of NF-kappaB by CD95 require different signalling thresholds. EMBO Rep. 2004;5:1084–1089. doi: 10.1038/sj.embor.7400280. [DOI] [PMC free article] [PubMed] [Google Scholar]