Figure 3. Enantioselective C(sp3)–C(sp3) cross-coupling toward fluvirucinine A1 (17).
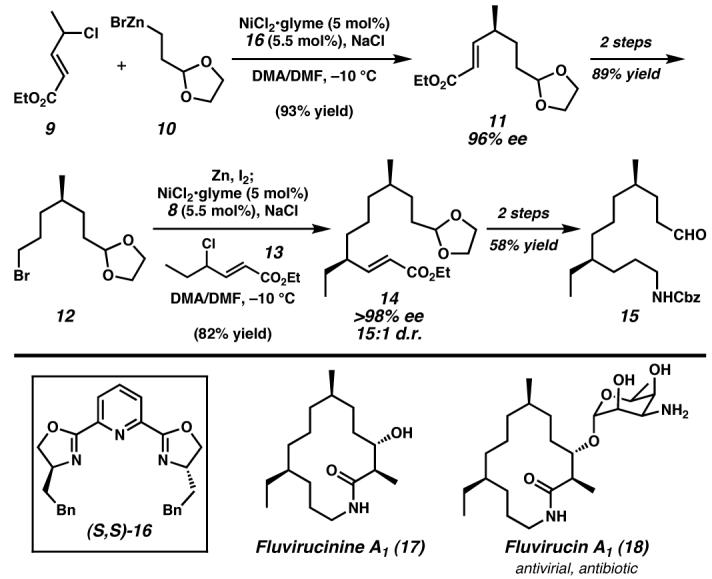
Sequential asymmetric C(sp3)–C(sp3) Negishi cross-couplings of racemic allylic chlorides and alkylzinc reagents catalyzed by nickel/(S,S)-16 enabled the rapid formal synthesis of Fluvirucinine A1 with excellent enantio- and diastereoselectivity, highlighting a creative solution to remote stereochemical control in unfunctionalized systems.51 Glyme, 1,2-dimethoxyethane; DMF, N,N-dimethylformamide. (Reduced to 45%)
