Abstract
During acute Vesicular Stomatitis Virus (VSV) infection of the mouse central nervous system, neutrophils, natural killer (NK) cells, macrophages, and CD4+ and CD8+ T cells are recruited from the circulation in response to chemokines and cytokines. This study elucidated the production of these factors and infiltration of these peripheral cells. Chemokines that were observed included CCL1, CXCL10 (IP-10), CCL5 (RANTES), CCL3 (MIP-1α), CCL4 (MIP-1β), CXCL1 (MIP-2), CCL2 (MCP-1), and CCL11 (eotaxin). Cytokines produced in response to the infection include IL-1 and interferon-γ, but not type I interferons. Neutrophils are the first recruited cell type, appearing as early as 24 h after intranasal application of the virus. NK cells follow, but T cells are not detected until 6 days postinfection.
INTRODUCTION
Vesicular Stomatitis Virus (VSV) is an enveloped, single-stranded negative sense RNA virus of the family Rhabdoviridae. When infection of mice occurs by intranasal administration, VSV infects olfactory receptor neurons of the neuroepithelium and travels caudally through the cribriform plate to the olfactory bulbs. The virus then spreads caudally throughout the CNS, infecting many different cell types, including ependymal cells (23,29,30,47-49,51,53-56). In the absence of a vigorous early innate immune response, VSV infection can cause acute encephalitis resulting in hindlimb paralysis, with half the mice succumbing to the disease within 10 days post-infection. Mice that survive the disease recover and appear normal. Peripheral routes of infection of immunocompetent mice are rapidly cleared. In the CNS, the immune response is initiated by the innate arm of the immune response, with neutrophils infiltrating the CNS within 36 h postinfection (10,11). These neutrophils are quickly followed by natural killer (NK) cells (4,16), peaking 72 h postinfection; this innate response is quickly followed by the entry of T cells and macrophages, beginning day 6 post-infection, which is also required for clearance of the virus (29,51).
Cells are recruited from the peripheral circulation to the CNS in response to distinct chemoattractant signals. We had previously investigated, using genetic and pharmacologic approaches, the roles of leukotriene B4 (LTB4) and the small fragment of complement component C5a in contributing to the recruitment of neutrophils in response to VSV infection (10,11).
Chemokines are a family of small, soluble proteins that have been shown to play critical roles in the chemoattraction, maturation, and activation of immune cells to a site of infection, including into the CNS. Chemokines and their receptors are characterized by conserved N-terminal cysteine sequences, including the C, CC, CXC, and CX3C subfamilies. Unlike cytokines, the majority of which bind to only a single receptor, chemokines are much more promiscuous, with a single ligand binding multiple receptors. The function of a chemokine depends, in part, on the receptor expressed by the target cell. Therefore, a single chemokine may have multiple functions in a single target cell (3,57).
Many chemokines and chemokine receptors have been shown to be expressed by most resident cell types in the CNS, including microglia, astrocytes, and neurons (1,3,45,58,67). These chemokines are expressed both in the normal CNS and during times of immune stress. During immune responses to infection in the CNS, the cells that are attracted to the CNS also produce a large number of chemokines. Therefore, during an inflammatory response, the expression of both CNS intrinsic and extrinsic chemokines is present. Understanding the mechanisms governing the chemoattraction and subsequent infiltration of immune cells across the blood–brain barrier (BBB) and into the CNS during viral encephalitis or as a result of autoimmune disease is critical to our overall understanding of immune regulation in the CNS and the derivation of clinical methods to protect the CNS from inflammatory damage. In this study we have examined the expression of chemokines, inflammatory cytokines, and T cell-specific genes in brain tissue homogenates during the response of both BALB/c and B6 mice to VSV infection of the CNS.
MATERIALS AND METHODS
Virus
VSV, Indiana strain, San Juan serotype was propagated in Chinese hamster ovary (CHO) cells and purified over a sucrose gradient as previously described (4).
Animals and infection
Male C57BL/6J and BALB/cJ mice, 6 weeks of age, were purchased from Jackson Laboratory (Bar Harbor, ME) and maintained in the New York University (New York, NY) animal facility under specific pathogen-free (SPF) conditions and in accordance with the Institutional Animal Care and Use Committee of New York University. Animals were given food and water ad libitum.
For infection, mice were anesthetized with 3% isoflurane (Fisher Scientific, Pittsburgh, PA) mixed with O2 (3 L/min). Mice were infected intranasally with 1.0 × 102 plaque-forming units (PFU) of VSV, Indiana strain, by placing 5 μL of VSV (1.0 × 104 PFU/mL) on each nostril for inhalation.
Tissue RNA extraction
VSV-infected mice were anesthetized by intraperitoneal injection of a lethal dose of Avertin. Each mouse was then perfused with 10 mL of sterile, RNase-free Hanks’ balanced salt solution (HBSS) and the brain was removed and placed in RNAlater RNA stabilizer (Ambion, Austin, TX). The tissue was homogenized in 1× RNA lysis buffer and total RNA was isolated with an RNAqueous-Midi kit, as per the manufacturer’s instructions (Ambion). The isolated RNA was stored in RNase-free double-distilled H2O in aliquots at −80°C to minimize freeze–thaw cycles.
Ribonuclease protection assay
Commercial multitemplate probe sets (mCD-1, mCK-2b, and mCK-5c; BD Biosciences Pharmingen, San Diego, CA) were transcribed with a MAXIscript T7 kit as per the manufacturer’s protocol (Ambion, Austin, TX), using [α-32P]dUTP (Perkin-Elmer, Norwalk, CT) to radiolabel single-stranded RNA probes for the ribonuclease protection assay (RPA). The probes were diluted to 3.2 × 105 and 2.5 × 105 cpm/μL for the mCD-1, mCK-2b, and mCK-5c probe sets, respectively, as per the manufacturer’s instructions (BD Biosciences).
The RPA was performed with an RPA III kit (Ambion), in accordance with the manufacturer’s recommended protocol for the BD Biosciences probe sets. Briefly, 40 μg of total RNA isolated from the brains of C57BL/6J and BALB/c mice (n = 4 per time point) killed on days 1, 2, 3, 6, 7, and 8 postinfection was concentrated by ammonium acetate–ethyl alcohol precipitation and resuspended in 8 μL of hybridization buffer. Sample RNA was mixed with 2 μL of diluted radiolabeled RNA probes. This mixture was incubated overnight at 50°C to allow the probes to hybridize with the sample mRNA. After hybridization, the samples were treated with RNase A/T1 for 30 min at 30°C to digest any single-stranded RNA remaining in the tube. The protected double-stranded RNA (dsRNA) hybrids were concentrated by precipitation and resuspended in 10 μL of gelloading buffer and denatured by heating at 95°C for 5 min.
The resulting RNA fragments were resolved by gel electrophoresis, using an 8 M urea–5% polyacrylamide gel (16 cm, 1-mm spacer) run at 180 V for approximately 3 h. The gels were dried onto Whatman filter paper (Whatman, Florham Park, NJ) and exposed to Kodak MS film in cassettes equipped with Kodak MS intensifying screens (Eastman Kodak, Rochester, NY). The radiographs were digitized with an HP Scanjet 6200Cxi flatbed scanner (Hewlett-Packard, Palo Alto, CA). The intensity of the bands was measured with Un-Scan-It gel densitometry software (Silk Scientific, Orem, UT) and normalized to the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) internal loading control. The mean values of four mice were calculated and plotted, using MS Excel software (Microsoft, Redmond, WA).
Staining of neutrophils
Tissue was prepared for hematoxylin and eosin staining as previously described (23). At least three sections from three individuals were examined in three replicate experiments.
RESULTS AND DISCUSSION
Chemokine expression in the CNS of mice during VSV infection
Preliminary experiments using the ribonuclease protection assay (RPA) performed every other day during the course of VSV encephalitis (data not shown) indicated periods of change in chemokine expression. Significant changes in chemokine mRNA expression were observed during two periods. The first period of chemokine expression occurred in the first 3 days after infection. The second period occurred at the peak of disease (days 6–8 postinfection), independent of mouse strain. In contrast, naive age- and sex-matched controls tested negative for expression of all chemokines tested.
At 24 h postinfection, significant expression of two chemokines was observed in the CNS: CCL1 (TCA-3) and CXCL10 (IP-10) (Fig. 1, top). CCL1 is a known chemoattractant of neutrophils (20,22) and macrophages. Expression of CCL1 by microglia has been observed in experimental autoimmune encephalomyelitis (EAE) (43,62), supporting the early expression of CCL1 by CNS-resident cells to attract innate immune factors into the CNS.
FIG. 1.
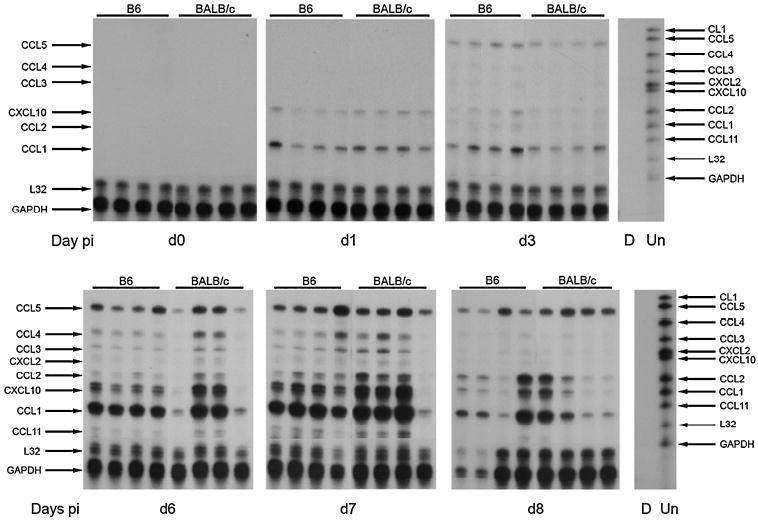
Chemokine expression. Representative radiographs demonstrate the expression of chemokine mRNAs in the CNS during VSV encephalitis during early and late VSV encephalitis. Each lane represents an individual mouse, killed on the indicated day postinfection (day 0, uninfected controls). Lane D, digested probe control set; lane Un, undigested probe set, with the chemokine represented by each undigested band listed on the right. Arrows on the left mark the indicated chemokine in each lane of the radiograph (digested size). These data are representative of three individual experiments.
The expression of CXCL10, which recruits macrophages and monocytes, has been observed early in the course of other viral encephalitis models, including Theiler’s murine encephalitis virus (TMEV), lymphocytic choriomeningitis virus (LCMV), human immunodeficiency virus (HIV), and JHM strain of mouse hepatitis virus (JHMV) infections (2,3,8,9,21,25,26,28,42,44,45,50,66,68); as well as in EAE, the mouse model for multiple sclerosis (MS). In these models, it has been shown to be expressed by the CNS-resident cell population, including microglia, astrocytes, and neurons.
At the peak of VSV encephalitis (days 6–8 postinfection), we observed the expression of many other chemokines in the CNS (Fig. 1, bottom) in addition to CCL1 and CXCL10, including the following: CCL5 (RANTES), CCL3 (MIP-1α), CCL4 (MIP-1β), CXCL1 (MIP-2), CCL2 (MCP-1), and CCL11 (eotaxin).
CCL1, CCL2, CCL5, and CXCL10 are expressed at the most significant levels in the CNS of VSV-infected mice at the peak of inflammation. CCL1, CCL2, and CXCL10 all appear to be expressed in a biphasic pattern, with CCL2 being expressed only at lower levels in the early phase (days 1–3 postinfection) and at much greater levels at the peak of inflammation. CCL5 expression increases throughout the course of infection. This profile of chemokine expression is consistent with the chemoattraction and activation of multiple cell types into the CNS, including CD4- and CD8-positive T cells, which are important for the clearance of VSV infection, but may also cause significant immune-mediated damage to CNS-resident cells.
The chemokines expressed during VSV encephalitis have also been observed in other models of viral encephalitis, and in EAE and ischemia–reperfusion injury (2,3,8,9,21,25,26,28,42,44,45,50,66,68). This commonality underlines the importance of understanding the role chemokines play in the regulation of immune response in the CNS and potentially their use as therapeutic targets to control disease (67).
During acute TMEV, West Nile virus (WNV), mouse hepatitis virus (MHV), HIV, and LCMV infections (2,3,9,25,26,28,42,45,66,68), several chemokines are expressed by brain-resident cells. Common to many of these models are the following: CCL1, CCL2, CCL5, and CXCL10. In our intranasal VSV model, we confirm and expand on previous findings. In the brain of naive mice, we did not detect the expression of any chemokine tested. However, within 24 h postinfection, CXCL10 and CCL1 mRNAs were expressed.
CCL1 is a known chemotactic factor of neutrophils but not of lymphocytes in mice (20,22). The kinetics of CCL1 correlate well with the observed infiltration of neutrophils into periventricular regions of the VSV-infected brain. Expression of CCL1 and the presence of neutrophils may play important roles in BBB regulation, as research has indicated that neutrophils in the CNS act to disrupt BBB permeability (24,31). CXCL10 is a potent chemotactic factor of monocytes and NK cells to a site of infection. Expression of this chemokine has been shown to be in neurons, microglia, and astrocytes in other models of brain inflammation (25,42,45,58,67). The exact role of this chemokine is still a matter of intense study in several inflammatory models. The function of CXCL10 depends in part on the model in which it is studied (66). The rapidity with which the RNAs for CCL1 and CXCL10 are expressed implies that brain-resident cells are the cellular source, as cells from the peripheral immune response are not observed in significant numbers until at least 24 h postinfection, when neutrophils are present at periventricular locations. After day 3 post-infection, expression of many more chemokines is observed in the brain, including CCL5, CCL2, CCL3, and CCL4. This expression could also be the result of the activation of microglial cells, or expression by immune cells that are infiltrating into the CNS, including neutrophils and macrophages.
CCL5 is a potent chemotactic factor drawing T cells into sites of infection. It is expressed by endothelial cells as well as macrophages and microglia. CCL5 has also been shown to increase macrophage adherence to endothelial cells at inflammatory sites, causing blood-borne cells to stop and enter sites of inflammation (44). The absence of CCR5 (the receptor for CCL5) results in increased virus load and decreased lymphocyte infiltration during West Nile virus infection of the brain (26).
Mice deficient in the CX3C chemokine, which is expressed by neurons, and whose receptor is expressed by microglia and NK cells, were examined for their response to VSV infection. No difference between Fractalkine-deficient and wild-type mice was noted (C.S. Reiss and S. Lira, unpublished observation).
Neutrophil infiltration
On the basis of previous studies these time periods correspond to the observation of infiltration of innate cells (neutrophils, natural killer cells, and macrophages) and lymphocytes into the brain parenchyma (4,10,11,16,29). The first phase of expression corresponds to observed positive staining for neutrophils (peak, 36 h postinfection; Fig. 2) and infiltrating NK cells (peak, approximately 3–4 days postinfection). The second phase of expression corresponds to the infiltration of macrophages and CD4+ and CD8+ T cells (Figs. 3 and 4) (29,36).
FIG. 2.
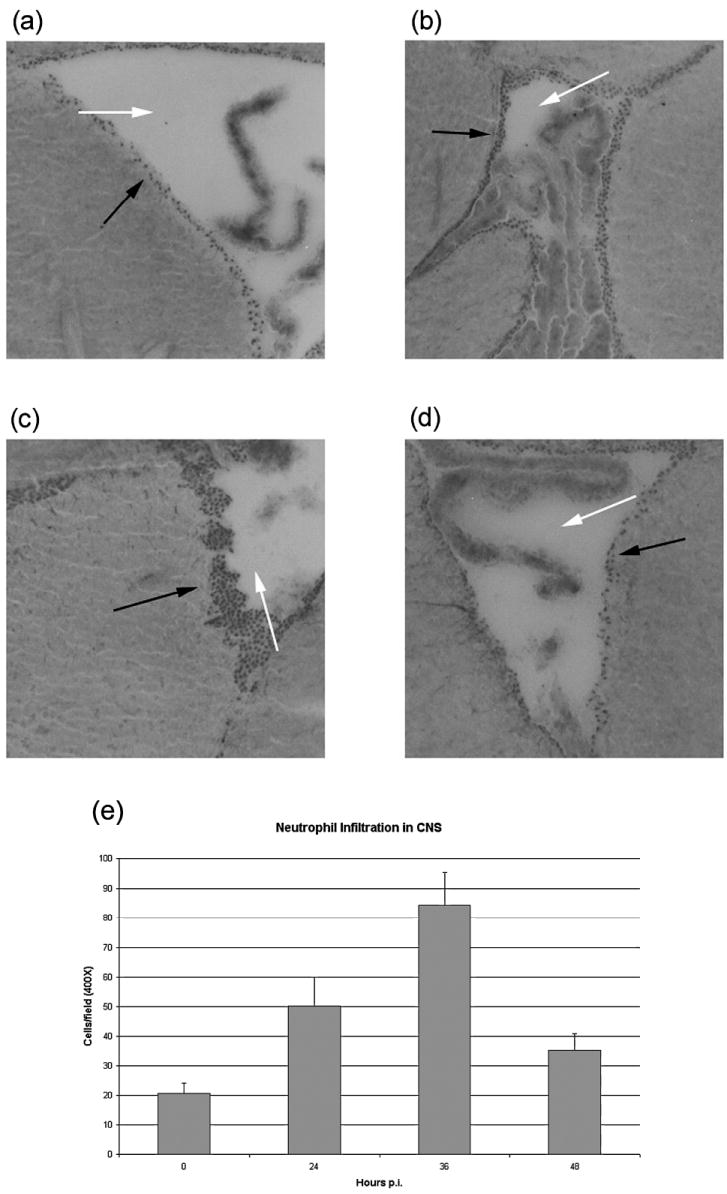
Infiltration of neutrophils into the CNS. (a) Uninfected; (b) 24 h postinfection; (c) 36 h postinfection; (d) 48 h postinfection. Neutrophils (solid arrows) were counted in the periventricular region (open arrows mark the lumen of the lateral ventricle). (e) Quantification of neutrophils. Three individual mice per time point were used for immunohistochemical staining to determine the influx of neutrophils into the CNS. Cells were counted in four high-power fields (×400), three sections per donor, and average counts ±S.E. are shown.
FIG. 3.
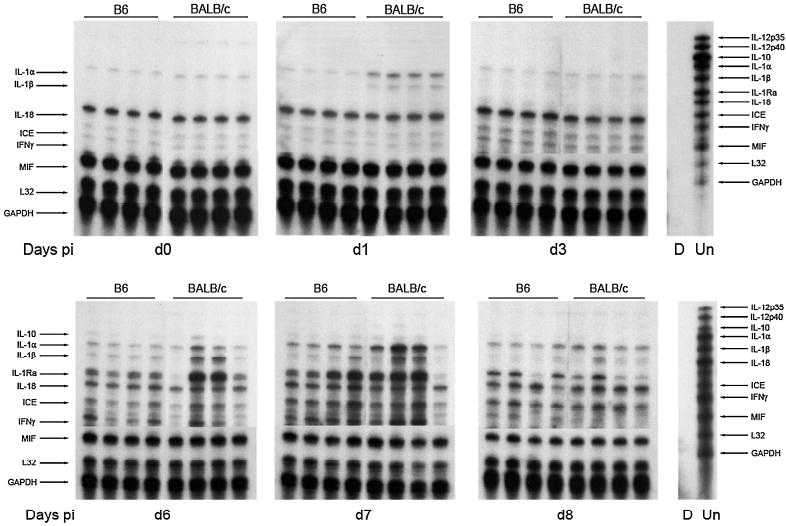
Inflammatory cytokine expression. Representative radiographs demonstrate the expression of inflammatory mRNAs in the CNS during VSV encephalitis. Each lane represents an individual mouse, killed on the indicated day post-infection. Lane D, digested probe control; lane Un, undigested probe set, with the chemokine represented by each undigested band listed on the right. Arrows on the left mark the indicated cytokine in each lane of the radiograph (digested size). These data are representative of three individual experiments.
FIG. 4.
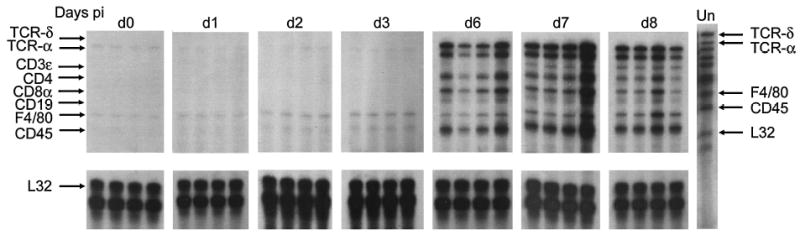
T cell marker expression. Representative radiographs demonstrate the expression of T cell mRNAs in the CNS during VSV encephalitis. Each lane represents an individual mouse, killed on the indicated day postinfection. Lane Un, undigested probe set, with the chemokine represented by each undigested band listed on the right. The arrows on the left mark the indicated cell marker in each lane of the radiograph (digested size). These data are representative of three individual experiments.
This rapid expression of CCL1 in the CNS (Fig. 1) correlates well with the infiltration of neutrophils, which peaks at periventricular locations within the infected CNS at 36 h postinfection, as indicated by both hematoxylin and eosin staining (Fig. 2) as well as immunohistochemistry for myeloperoxidase (data not shown). In other experiments, we have depleted neutrophils with the monoclonal antibody (mAb) RB6-8C5 (the generous gift of R. Coffman, DNAX/Schering-Plough Biopharma, Palo Alto, CA). These unpublished data are consistent with a critical early contribution of neutrophils in the immune response to VSV encephalitis. Similarly, the increased morbidity and mortality observed in infected mice that received zileuton treatment highlights the contribution of these cells (10).
Expression of inflammatory cytokines during VSV infection
We also examined the expression of inflammatory cytokines in RNA samples obtained from VSV-infected brains (Fig. 3), as well. As we have previously observed (27), interleukin (IL)-18, and caspase-1 (also known as ICE, the IL-1-converting enzyme), which activates the IL-18 zymogen, are constitutively expressed by mice and their levels of expression are not regulated by infection. Macrophage migration inhibitory factor (MIF) is also constitutively expressed.
IL-1α and IL-1β mRNAs are rapidly induced by infection and are expressed in waves consistent with production 24 h post-infection by a parenchymal cell, probably microglia. When macrophages are recruited from the circulation, starting on day 6, IL-1 mRNA levels rebound and peak on day 7 postinfection.
Although others have observed IL-12 and IL-23 mRNA production by microglia in response to infections and EAE (17,18,46,59-61), the mRNA levels for IL-12 p35 and IL-12 p40 were below the level of detection during VSV infection. We have observed beneficial effects of exogenous IL-12 administration (5,7,14,32,34,35,37,39,52), but failed to find a requirement for IL-12Rβ1 or IL-12Rβ2 during infection (32,33,34).
IFN-γ is not detected early, but is rarely seen in B6 mice on day 3 post-infection, possibly the product of inflammatory NK cells. IFN-γ expression is closely tied to infiltration of CD4+ and CD8+ T cells (Fig. 3), late in infection. IFN-γ has been shown to promote the clearance of virus from infected neurons via induction of nitric oxide synthase type 1 (NOS-1) (6,7,12-15,38,40, 41,54). Mice deficient in IFN-γR fared well (J.L. Hodges, N. Chen, and C.L. Reiss, unpublished observation), indicating that IFN-γ is not essential for host responses to infection. There was no difference between B6 and BALB/c mice in the helper T cell type 1 (Th1) response to infection (data not shown).
The anti-inflammatory Th2-derived cytokine IL-10 was observed first in a few BALB/c mice on day 6 postinfection, but both B6 and BALB/c mice downregulate the mRNA production by day 8 postinfection. The disease course does not differ between BALB/c and B6 mice in terms of clearance of infection, morbidity and mortality, infiltration of inflammatory cells, and the other mRNAs examined (Figs. 1 and 3); thus, it is unlikely that this IL-10 mRNA observation indicates a regulatory or essential contribution of Th2 cells to the pathology or resolution of the infection.
Type 1 IFNs are not detected in the brains of VSV-infected mice (65). VSV evades this powerful antiviral cytokine by shutting off host cell synthesis and nuclear export. VSV is exquisitely sensitive to the protective effects of IFN-β on neurons (64).
Expression of T cell markers in the CNS after VSV infection
To determine the kinetics of expression of T cell, B cell, and macrophage mRNAs during VSV infection, we examined the mRNAs isolated from infected brains. The earliest detected marker that was upregulated is F4/80, which is expressed primarily by macrophages and activated microglia (Fig. 4). B cells had not been detected in our immunohistochemical studies (4,16), and CD19 was also below the level of sensitivity of the RNase protection assay.
CD4+ and CD8+ T cells enter the CNS at the same time, first detected 6 days postinfection; both CD4+ and CD8+ T cells are essential for recovery (29,51). Furthermore, γδ T cells are not observed in the CNS at the same time points.
We have examined the induction and expression of essential cytokines and chemokines and associated them with recruitment of inflammatory cells during the course of acute VSV encephalitis in BALB/c and B6 mice. Although chemokines are unambiguously important for infiltration, we also recognize the essential roles of other chemoattracting molecules including the lipid mediator LTB4 and anaphylotoxins, and complement fragments such as C5a (10,11). Cytokines such as IL-12 can also have recruiting as well as activating roles for NK cells (19,63).
Acknowledgments
The authors are grateful to Jane A. McCutcheon, D.D.S., Ph.D. (NYU School of Dental Medicine) for thoughtful discussions and suggestions while this work was underway; to Sergio Lira, M.D., Ph.D. (then at Schering-Plough) for Fractalkine-deficient mice; to Robert Coffman (DNAX/Schering-Plough Biopharma) for mAb RB6-2C5; to Steve Zimmer for technical assistance; to Muneeb Samma and Eugene Friedman for performing the immunohistochemical experiments to identify neutrophils in the brains of VSV infected mice; and to Paul M. D’Agostino for assistance with manuscript preparation. This work was supported by grants from the NIH to C.S.R. (DC003536 and NS039746), and by a grant from NYU (N5385).
References
- 1.Adler MW, Rogers TJ. Are chemokines the third major system in the brain? J Leukoc Biol. 2005;78:1204–1209. doi: 10.1189/jlb.0405222. [DOI] [PubMed] [Google Scholar]
- 2.Asensio VC, Campbell IL. Chemokine gene expression in the brains of mice with lymphocytic choriomeningitis. J Virol. 1997;71:7832–7840. doi: 10.1128/jvi.71.10.7832-7840.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asensio VC, Campbell IL. Chemokines in the CNS: plurifunctinal mediators in diverse states. Trends Neurosci. 1999;22:504–512. doi: 10.1016/s0166-2236(99)01453-8. [DOI] [PubMed] [Google Scholar]
- 4.Bi Z, Barna M, Komatsu T, Reiss CS. Vesicular stomatitis virus infection of the central nervous system activates both innate and acquired immunity. J Virol. 1995;69:6466–6472. doi: 10.1128/jvi.69.10.6466-6472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bi Z, Quandt P, Komatsu T, Barna M, Reiss CS. IL-12 promotes recovery from experimental CNS VSV infection. J Immunol. 1995;155:5684–5689. [PubMed] [Google Scholar]
- 6.Bi Z, Reiss CS. Inhibition of vesicular stomatitis virus infection by nitric oxide. J Virol. 1995;69:2208–2213. doi: 10.1128/jvi.69.4.2208-2213.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bi Z, Reiss CS. Interferon-γ-induced upregulation of type 1 nitric oxide synthase activity in neurons inhibits viral replication. In: Stamler J, Gross S, Moncada S, Higgs EA, editors. Biology of Nitric Oxide. Part 5. Portland Press; London: 1996. pp. 331–334. [Google Scholar]
- 8.Busfeld D, Nain M, Hofmann P, Gemsa D, Sprenger H. Selective induction of the monocyte-attracting chemokines MCP-1 and IP-10 in vesicular stomatitis virus-infected human monocytes. J Interferon Cytokine Res. 2000;20:615–621. doi: 10.1089/107999000414781. [DOI] [PubMed] [Google Scholar]
- 9.Cheeran MC, Hu S, Sheng WS, Rashid A, Peterson PK, Lokensgard JR. Differential responses of human brain cells to West Nile virus infection. J Neurovirol. 2005;11:512–524. doi: 10.1080/13550280500384982. [DOI] [PubMed] [Google Scholar]
- 10.Chen N, Restivo A, Reiss CS. Leukotrienes promote recovery early during experimental VSV encephalitis. J Neuroimmunol. 2001;120:94–102. doi: 10.1016/s0165-5728(01)00415-5. [DOI] [PubMed] [Google Scholar]
- 11.Chen N, Reiss CS. Innate immunity in viral encephalitis: Role of C5. Viral Immunol. 2002;15:363–370. doi: 10.1089/08828240260066288. [DOI] [PubMed] [Google Scholar]
- 12.Chesler DA, Dodard C, Lee GY, Levy D, Reiss CS. The IFN-γ-induced antiviral response to VSV infection is STAT-1-dependent. J Neurovirol. 2004;10:57–63. doi: 10.1080/13550280490261707. [DOI] [PubMed] [Google Scholar]
- 13.Chesler DA, McCutcheon JA, Reiss CS. The IFN-γ-induced antiviral response to VSV infection: Neuronal nitric oxide synthase expression is post-translationally regulated. J Interferon Cytokine Res. 2004;24:141–149. doi: 10.1089/107999004322813381. [DOI] [PubMed] [Google Scholar]
- 14.Chesler DA, Reiss CS. IL-12 while beneficial, is not essential for the host response to VSV encephalitis. J Neuroimmunol. 2002;131:92–97. doi: 10.1016/s0165-5728(02)00257-6. [DOI] [PubMed] [Google Scholar]
- 15.Chesler DA, Reiss CS. The role of IFN-γ in immune responses to viral infections of the central nervous system. Cytokine Growth Factor Rev. 2002;13:441–454. doi: 10.1016/s1359-6101(02)00044-8. [DOI] [PubMed] [Google Scholar]
- 16.Christian AY, Barna M, Bi Z, Reiss CS. Host immune response to vesicular stomatitis virus infection of the central nervous system in C57BL/6 mice. Viral Immunol. 1996;9:195–205. doi: 10.1089/vim.1996.9.195. [DOI] [PubMed] [Google Scholar]
- 17.Constantinuescu C, Frei K, Malipiero U, Rostami A, Fontana A. Astrocytes and microglia produce IL-12. Ann N Y Acad Sci. 1996;795:328–333. doi: 10.1111/j.1749-6632.1996.tb52684.x. [DOI] [PubMed] [Google Scholar]
- 18.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. IL-23, rather than IL-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 19.D’Andrea A, Rengaraju M, Valiante NM, Chehimi J, Kubin M, Aste M, Chan SH, Kobayashi M, Young D, Nick-barg E, et al. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J Exp Med. 1992;176:1387–1398. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Devi S, Laning J, Luo Y, Dorf ME. Biologic activities of β-chemokine TCA3 on neutrophils and macrophages. J Immunol. 1995;154:5376–5383. [PubMed] [Google Scholar]
- 21.dos Santos AC, Barsante MM, Esteves Arantes RM, Bernard CC, Teixeira MM, Carvalho-Tavares J. CCL2 and CCL5 mediate leukocyte adhesion in experimental autoimmune encephalomyelitis: An intravital microscopy study. J Neuroimmunol. 2005;162:122–129. doi: 10.1016/j.jneuroim.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 22.Doyle HA, Murphy JW. Role of the C-C chemokine TCA3 in the protective anti-cryptococcal cell-mediated immune response. J Immunol. 1999;162:4824–4833. [PubMed] [Google Scholar]
- 23.Forger J, Bronson R, Huang AS, Reiss CS. Murine infection by vesicular stomatitis virus: Initial characterization of the H-2d system. J Virol. 1991;65:4950–4958. doi: 10.1128/jvi.65.9.4950-4958.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gidday JM, Gasche YG, Copin JC, Shah AR, Perez RS, Shapiro SD, Chan PH, Park TS. Leukocyte-derived matrix metalloproteinase-9 mediates blood–brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. Am J Physiol Heart Circ Physiol. 2005;289:H558–H568. doi: 10.1152/ajpheart.01275.2004. [DOI] [PubMed] [Google Scholar]
- 25.Glass WG, Chen BP, Liu MT, Lane TE. Mouse hepatitis virus infection of the central nervous system: Chemokine-mediated regulation of host defense and disease. Viral Immunol. 2002;15:261–272. doi: 10.1089/08828240260066215. [DOI] [PubMed] [Google Scholar]
- 26.Glass WG, Lim JK, Cholera R, Pletnev AG, Gao J-L, Murphy PM. Chemokine receptor CCR5 promotes leukocyte trafficking to the brain and survival in West Nile virus infection. J Exp Med. 2005;202:1087–1098. doi: 10.1084/jem.20042530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hodges JL, Ireland DDC, Reiss CS. IL-18 does not play a major role in VSV pathogenesis. Viral Immunol. 2001;14:181–191. doi: 10.1089/088282401750234556. [DOI] [PubMed] [Google Scholar]
- 28.Hoffman LM, Fife BT, Begolka WS, Miller SD, Karpus WJ. Central nervous system chemokine expression during Theiler’s virus-induced demyelinating disease. J Neurovirol. 1999;5:635–642. doi: 10.3109/13550289909021292. [DOI] [PubMed] [Google Scholar]
- 29.Huneycutt BS, Bi Z, Aoki C, Reiss CS. Central neuropathogenesis of vesicular stomatitis virus infection in immunodeficient mice. J Virol. 1993;67:6698–6706. doi: 10.1128/jvi.67.11.6698-6706.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huneycutt BS, Plakhov IV, Schusterman Z, Huang AS, Reiss CS, Aoki C. Distribution of vesicular stomatitis virus proteins in the brains of BALB/c mice: An immunohistochemical analysis. Brain Res. 1994;635:81–95. doi: 10.1016/0006-8993(94)91426-5. [DOI] [PubMed] [Google Scholar]
- 31.Inglis VI, Jones MPJ, Tse ADY, Easton AS. Neutrophils both reduce and increase permeability in a cell culture model of the blood–brain barrier. Brain Res. 2004;998:218–229. doi: 10.1016/j.brainres.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 32.Ireland DDC, Bang T, Komatsu T, Reiss CS. Delayed administration of IL-12 is efficacious in promoting recovery from lethal viral encephalitis. Viral Immunol. 1999;12:35–40. doi: 10.1089/vim.1999.12.35. [DOI] [PubMed] [Google Scholar]
- 33.Ireland DDC, Palian B, Reiss CS. IL-12Rβ1- or IL-12Rβ2-deficiency indicate that neither IL-12 nor IL-23 are essential for host recovery from viral encephalitis. Viral Immunol. 2005;18:397–402. doi: 10.1089/vim.2005.18.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ireland DDC, Reiss CS. Neuronal expression of functional IL-12 receptors. Viral Immunol. 2004;17:411–422. doi: 10.1089/vim.2004.17.411. [DOI] [PubMed] [Google Scholar]
- 35.Komatsu T, Barna M, Reiss CS. IL-12 promotes recovery from viral encephalitis. Viral Immunol. 1997;10:35–47. doi: 10.1089/vim.1997.10.35. [DOI] [PubMed] [Google Scholar]
- 36.Komatsu T, Bi Z, Reiss CS. IFN-γ-induced type 1 nitric oxide synthase activity inhibits viral replication in neurons. J Neuroimmunol. 1996;68:101–108. doi: 10.1016/0165-5728(96)00083-5. [DOI] [PubMed] [Google Scholar]
- 37.Komatsu T, Ireland DDC, Chang N, Dore A, Yoder M, Reiss CS. Regulation of the blood brain barrier during viral encephalitis: Roles of IL-12 and NOS. Nitric Oxide Biol Chem. 1999;3:327–339. doi: 10.1006/niox.1999.0237. [DOI] [PubMed] [Google Scholar]
- 38.Komatsu T, Ireland DDC, Chen N, Reiss CS. Neuronal expression of NOS-1 is required for host recovery from viral encephalitis. Virology. 1999;258:389–395. doi: 10.1006/viro.1999.9734. [DOI] [PubMed] [Google Scholar]
- 39.Komatsu T, Ireland DDC, Reiss CS. IL-12 and viral infections. Cytokine Growth Factor Rev. 1999;9:277–285. doi: 10.1016/S1359-6101(98)00017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Komatsu T, Reiss CS. IFN-γ is important but not required for the IL-12-response to vesicular stomatitis virus infection of the olfactory bulb. J Immunol. 1997;159:3444–3452. [PubMed] [Google Scholar]
- 41.Komatsu T, Srivastava N, Revzin M, Ireland DDC, Chesler D, Reiss CS. Mechanisms of NO inhibition of viral replication. Virology. 1999;259:334–341. doi: 10.1006/viro.1999.9801. [DOI] [PubMed] [Google Scholar]
- 42.Minagar A, Shapshak P, Fujinami R, Ownby R, Heyes M, Eisdorfer C. The role of macrophage/microglia in the pathogenesis of three neurological disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. J Neurol Sci. 2002;202:13–23. doi: 10.1016/s0022-510x(02)00207-1. [DOI] [PubMed] [Google Scholar]
- 43.Murphy CA, Hoek RM, Wiekowski MT, Lira SA, Sedgwick JD. Interactions between hematopoietically derived TNF and central nervous system-resident glial chemokines underlie initiation of autoimmune inflammation in the brain. J Immunol. 2002;169:7054–7062. doi: 10.4049/jimmunol.169.12.7054. [DOI] [PubMed] [Google Scholar]
- 44.Nansen A, Marker O, Bartholdy C, Thomsen AR. CCR2+ and CCR5+ CD8+ T cells increase during viral infection and migrate to sites of infection. Eur J Immunol. 2000;30:1797–1806. doi: 10.1002/1521-4141(200007)30:7<1797::AID-IMMU1797>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 45.Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through TLRs. J Immunol. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- 46.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M, Gorman D, Wagner J, Zurawski S, Liu Y, Abrams JS, Moore KW, Rennick D, de Waal-Malefyt R, Hannum C, Bazan JF, Kastelein RA. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 47.Plakhov IV, Aoki C, Arlund E, Reiss CS. The earliest events in vesicular stomatitis virus infection of the murine olfactory neuroepithelium and entry of the central nervous system. Virology. 1995;209:257–262. doi: 10.1006/viro.1995.1252. [DOI] [PubMed] [Google Scholar]
- 48.Plakhov IV, Aoki C, Reiss CS, Huang AS. Pathogenesis of murine encephalitis is limited by defective interfering particles: An immunohistochemical study. J Neurovirol. 1995;1:207–218. doi: 10.3109/13550289509113967. [DOI] [PubMed] [Google Scholar]
- 49.Plakhov IV, Bi Z, Aoki C, Reiss CS. The earliest events in vesicular stomatitis virus infection of the murine olfactory neuroepithelium and invasion of the central nervous system. ACLAD Newslett. 1995;16:7–8. doi: 10.1006/viro.1995.1252. [DOI] [PubMed] [Google Scholar]
- 50.Raivich G, Banati R. Brain microglia and blood-derived macrophages: Molecular profiles and functional roles in multiple sclerosis and animal models of autoimmune demyelinating disease. Brain Res Brain Res Rev. 2004;46:261–281. doi: 10.1016/j.brainresrev.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 51.Reiss CS, Aoki C. Immunity to vesicular stomatitis virus. Rev Med Virol. 1994;4:129–140. [Google Scholar]
- 52.Reiss CS, Komatsu T, Barna M, Bi Z. Interleukin-12 promotes enhanced recovery from viral infection of the central nervous system. Ann N Y Acad Sci. 1996;795:257–265. doi: 10.1111/j.1749-6632.1996.tb52675.x. [DOI] [PubMed] [Google Scholar]
- 53.Reiss CS, Plakhov IV, Komatsu T. Viral replication in olfactory receptor neurons and entry into the olfactory bulb and brain. Ann N Y Acad Sci. 1998;855:751–761. doi: 10.1111/j.1749-6632.1998.tb10655.x. [DOI] [PubMed] [Google Scholar]
- 54.Reiss CS, Komatsu T. Does nitric oxide play a critical role in viral infections? Mini-review. J Virol. 1998;72:4547–4551. doi: 10.1128/jvi.72.6.4547-4551.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reiss CS, Ireland DDC, Chesler DA, Plakhov IV, Chen N, Hodges JL, Samma MA, Komatsu T. Pathogenesis of vesicular stomatitis virus encephalitis. In: Pandalai G, editor. Recent Research Developments in Virology. Vol. 1. Transworld Research Network; Trivandrum, Kerala, India: 1999. pp. 703–716. [Google Scholar]
- 56.Reiss CS, Chesler DA, Hodges J, Ireland DDC, Chen N. Innate immune responses in viral encephalitis. Curr Top Microbiol Immunol. 2002;265:63–94. doi: 10.1007/978-3-662-09525-6_4. [DOI] [PubMed] [Google Scholar]
- 57.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 58.Segal BM. CNS chemokines, cytokines, and dendritic cells in autoimmune demyelination. J Neurol Sci. 2005;228:210–214. doi: 10.1016/j.jns.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 59.Stalder AK, Pagenstecher A, Yu NC, Kincaid C, Chiang CS, Hobbs MV, Bloom FE, Campbell IL. Lipopolysaccharide-induced IL-12 expression in the central nervous system and cultured astrocytes and microglia. J Immunol. 1997;159:1344–1351. [PubMed] [Google Scholar]
- 60.Suzumura A, Sawada M, Takayanagi T. Production of interleukin-12 and expression of its receptors by murine microglia. Brain Res. 1998;787:139–142. doi: 10.1016/s0006-8993(97)01166-9. [DOI] [PubMed] [Google Scholar]
- 61.Taoufik Y, de Goer de Herve MG, Giron-Michel J, Durali D, Cazes E, Tardieu M, Azzarone B, Delfraissy JF. Human microglial cells express a functional IL-12 receptor and produce IL-12 following IL-12 stimulation. Eur J Immunol. 2001;31:3228–3239. doi: 10.1002/1521-4141(200111)31:11<3228::aid-immu3228>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 62.Tran EH, Price EN, Owens T. IFN-γ shapes immune invasion of the central nervous system via regulation of chemokines. J Immunol. 2000;164:2759–2768. doi: 10.4049/jimmunol.164.5.2759. [DOI] [PubMed] [Google Scholar]
- 63.Trinchieri G, Wysocka M, D’Andrea A, Rengaraju M, Aste-Amezaga M, Kubin M, Valiante NM, Chehimi J. Natural killer cell stimulatory factor (NKSF) or interleukin-12 is a key regulator of immune response and inflammation. Prog Growth Factor Res. 1992;4:355–368. doi: 10.1016/0955-2235(92)90016-b. [DOI] [PubMed] [Google Scholar]
- 64.Trottier MD, Palian BM, Reiss CS. VSV replication in neurons is inhibited by type I IFN at multiple stages of infection. Virology. 2005;333:215–225. doi: 10.1016/j.virol.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 65.Trottier MD, Poliquin L, Yang J, Lyles DS, Reiss CS. Differential expression of type 1 interferon in the periphery and central nervous system of mice in response to vesicular stomatitis virus infection. Submitted. [Google Scholar]
- 66.Tsunoda I, Lane TE, Blackett J, Fujinami RS. Distinct roles for IP-10/CXCL10 in three animal models: Theilers’ virus infection, EAE, and MHV infection, for multiple sclerosis—implication of differing roles of IP-10. Mult Scler. 2004;10:26–34. doi: 10.1191/1352458504ms982oa. [DOI] [PubMed] [Google Scholar]
- 67.Ubogu EE, Cossoy MB, Ransohoff RM. The expression and function of chemokines involved in CNS inflammation. Trends Pharmacol Sci. 2006;27:48–55. doi: 10.1016/j.tips.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 68.Ure DR, Lane TE, Liu MT, Rodriguez M. Neutralization of chemokines RANTES and MIG increases virus antigen expression and spinal cord pathology during Theiler’s virus infection. Int Immunol. 2005;17:569–579. doi: 10.1093/intimm/dxh236. [DOI] [PMC free article] [PubMed] [Google Scholar]


