Abstract
This is the first study of the effects of mother-infant separation (MS) on adolescent behavior of Holtzman rats. Different rat strains, such as Harlan Sprague-Dawley and Holtzman, share a common origin. However, MS may lead to hypoactive behavioral effects in Holtzman rats because of their greater susceptibility to show depressive-like responses to stress. Sixty Holtzman pups were divided into 3 groups at postnatal day 2 (P2). For 10 days, the MS group was separated 6 hours daily and the early handled (EH) group 15 min daily. A standard facility reared (SFR) group was not separated. Animals were tested for novel open-field activity (P28), defensive withdrawal in a light-dark (LD) apparatus (P29) and familiar open-field (P30). Behavioral measures were classified into general activity (ambulatory and short movement time), orienting (rearing time) and risk-taking (velocity and exposed zone time). MS rats displayed reductions in general activity and risk-taking, and increases in orienting time. In contrast, EH favored risk-taking behavior, which may be consistent with previous findings implicating early handling as beneficial in coping with stress. Sex differences in these behaviors were limited. This study suggests a genetic predisposition in Holtzman rats for predominantly hypoactive/anxiety-like behaviors when exposed to an early life stressor.
Keywords: Maternal separation, ADD, Hypoactive phenotype, Neonatal stress, Learned helplessness, Individual differences, Rats
1. Introduction
Early life stress, particularly childhood stress, may lead to behavioral dysfunctions later in life (Singh & Maki, 1968; Thoman & Arnold, 1968; McCall et al., 1969; Anisman et al., 1998; Teicher et al., 2003). Several lines of research in rats have demonstrated that mother-infant separation (MS) within the first two weeks of life is a stressful event that leads to changes in behavior that are related to the stressfulness of situations encountered later in life (Sanchez et al., 2001; Daniels et al., 2004). The common feature, regardless of separation protocol and species, is that prolonged MS is a stressful manipulation that results in immediate and long-term changes to both brain and behavior (Kuhn & Schanberg, 1998; Rosenfeld, Wetmore & Levine, 1992; Plotsky & Meaney, 1993; Braun et al., 2003).
In assessing the adverse behavioral effects of MS, an important caveat is that different separation protocols and rat strains utilized by various researchers have led to discrepancies in the relevant literature. Two opposite behavioral phenotypes may result from similar neonatal MS protocols. One is characterized by hyperactive/impulsive behaviors in the open-field and defensive withdrawal tests (Arnold & Siviy, 2002; Braun et al., 2003; Colorado et al, 2006; Kaneko et al.,1994; von Hoersten et al., 1993). The other phenotype expresses hypoactive/anxiety-like behaviors in open-field, defensive withdrawal, and elevated plus-maze tests (Daniels et al., 2004; Huot et al. 2001; Janus et al., 1987; Matthews et al., 2003).
These opposite behavioral phenotypes are similar to those seen in the Naples High- and Low- Excitability strains of selectively bred rats (Cerbone et al., 1993). The High Excitability rats are presumed to model the hyperactive/impulsive type of attention deficit disorder (ADD+), whereas the Low Excitability rats model the predominantly hypoactive/inattentive type (ADD−) (Gonzalez-Lima and Sadile, 2000). Thus, depending on genetic background, different strains of rats could show diverging ADD-like phenotypes. These opposite phenotypes are similar to those induced by MS protocols, and may derive from use of different genetic strains or differences in the MS protocols. We hypothesized that use of the same MS protocol in strains with subtle genetic differences could be sufficient to produce two opposite behavioral phenotypes. To test this hypothesis we treated Holtzman (HO) rats with the same separation protocol that we previously treated Sprague-Dawley (SD) rats.
Our laboratory has previously characterized the adolescent behavior of SD rats (Colorado et al., 2006) after maternal separation (MS), early handling (EH), and standard facility rearing (SFR) protocols in males. The MS manipulation is a known stressor, whose effects in SD male rats included hyperactive and impulsive behavior in open-field and defensive withdrawal tests (Colorado et al., 2006). The present study extends this manipulation to the HO rat strain; using both male and female rats to also investigate potential sex differences. Different strains of albino rats, such as Harlan SD and HO, share a common origin and likely share a majority of their genes. However, we hypothesized that our MS protocol may lead to hypoactive behavioral effects in HO rats because of their greater susceptibility to show depressive-like behavioral responses to stress in comparison to SD rats in the learned helplessness paradigm (Wieland et al., 1986). These behavioral differences may be related to reported strain differences in regulatory systems, including the reproductive system and the hypothalamic-pituitary-adrenal (HPA) axis (Matthys et al., 1998).
Therefore, the objective of this study was to investigate whether HO rats would display a hypoactive behavioral profile later in life in response to the stressful nature of the MS intervention.
2. Materials and methods
2.1. Subjects
Subjects were 60 Holtzman albino rat pups born to 6 timed-pregnant mothers bred in the colony of the Animal Resources Center at the University of Texas at Austin. Pregnant mothers were singly housed and maintained on a 12h/12h light/dark photoperiod, with lights on at 0600 h and lights off at 1800 h. Food and water were available ad libitum. The day of birth was marked as postnatal day (P) 0. On P2, litters were culled to as close to ten pups as possible, always consisting of an equal male-to-female ratio. The litters were divided into three groups using the same procedures described before (Colorado et al., 2006): Maternal Separation (MS), whose separation consisted of 6 hours; Early Handled (EH), whose separation consisted of 15 minutes; and Standard Facility Reared (SFR), who were not handled, except for the culling on P2 and biweekly cage changes by facility staff. All procedures were conducted in accordance with the guidelines of the National Institutes of Health in an animal facility accredited by the American Association for the Accreditation of Laboratory Animal Care, and the protocol was approved by the Institutional Animal Care and Use Committee.
2.2. Maternal separation protocol
The MS protocol consisted of daily separation from P2 through P6, no separation from P7 to P8, and daily separation from P9 through P13, as in our previous study (Colorado et al., 2006). Maternal separation began at 0730 h and ended at 1330 h daily, while early handling occurred from 1300 h to 1315 h daily. The dams were removed from the home cage, and all pups were removed and placed as a litter into a bedding-lined holding cage, and dams were returned to the home cage for the duration of the separation. Pups in holding cages were then placed in an incubator (30–34° C) to maintain thermoregulation for the duration of the separation period. Upon return to the home cage, dams were again removed from the home cage, pups were returned to the cage, and the dams were returned to the pups.
Following the final separation period on P13, pups were not handled again until weaning at P21. At this time, the mothers were removed from the cages. On P23, daily handling and weighing of the pups began. Pups were handled for 5 minutes daily in order to habituate them to the experimenters. Separation of the sexes occurred on P28; however, no females had reached the day of vaginal opening at this time. Thus, anogenital distance was used to identify the sexes for separation. Males and females received identical treatment from birth through day 30.
2.3. Apparatus
All tests were conducted in an open-field activity chamber (43 × 43 × 30.5 cm) (Med Associates, St. Albans, VT). The four lateral sides of the chamber were made of clear plastic, with a white fiberglass bottom. Activity was detected by three sets of aligned arrays of infrared light beam motion detectors (16 × 16, 2.5 cm apart) on each side of the chamber, thus creating a detection grid. Two pairs of arrays were located 1 cm above the floor, to measure X and Y coordinates in the open-field; another array located 6 cm above the floor was in place to measure the Z coordinate of each subject, to detect vertical behavior. The chambers were controlled by the Activity Monitor program, version 5.10 (Med Associates), which recorded various parameters related to the time course of the subject’s behavior (e.g., distance traveled).
The defensive withdrawal test, also known as light-dark test (Takahashi et al., 1989), used a modified setup of the open-field activity chamber. A dark compartment that covered half of the total area of the chamber was inserted in the chamber, dividing its total area into two compartments: an illuminated side and a dark side. The chamber included a small hole that allowed the animals to move between the dark and light compartments of the chamber. The chambers were washed with a diluted Bio-clean solution between each session.
2.4. Behavioral testing
All animals were tested for open-field activity during the first day of behavioral testing (P28), followed by testing in the defensive withdrawal apparatus on the next day (P29). They were tested a second time in the open-field on P30. In the open-field test (OFT), each animal was initially placed in the same corner of an open-field activity chamber and ambulatory behavior was recorded for 10 minutes. In the light-dark (LD) test, the animals were placed in one of the corners of the illuminated compartment and ambulatory behavior was recorded for 10 minutes.
Subjects were tested on two different occasions in the OFT in order to determine whether repeated exposures to the chamber would produce different behavioral results. The first day of testing (P28) was designated the novel open-field test, while the third day of testing (P30) was designated the familiar open-field test, as in our previous behavioral profiles (Colorado et al., 2006, Shumake et al., 2005).
2.5. Behavioral measures
The Activity Monitor (Med Associates, version 5.10) program recorded various behavioral parameters over the three 10-minute sessions, including data related to ambulatory, rearing, short movements, resting, and vertical behavior during OFT and LD tests. The parameters measured by the program included the following behaviors, classified into orienting, risk-taking, and general activity as in our previous MS behavioral study (Colorado et al., 2006).
2.5.1. General activity
General activity was assessed by measuring ambulatory and non-ambulatory (stereotypic short movement) time in the OFT and LD settings. Both novel and familiar open-field tests were performed because some effects on behavior may be a result of novelty (Colorado et al., 2006; Shumake et al., 2005; Kaneko et al., 1994; Matthews, Wilkinson & Robbins, 1996; Brake et al., 2004). Measures of ambulatory activity were of interest given our laboratory’s previous findings relating MS to hyperactivity in the open-field (Colorado et al., 2006). Measured parameters related to general activity were:
Ambulatory time
Total time (sec) spent in ambulatory movement.
Short movement time
Total time (sec) spent moving without ambulatory displacement, within an area of 2 × 2 horizontal beams. This included movements that do not require the rat to ambulate such as licking, grooming, turning and head movements.
2.5.2. Orienting behavior
Orienting was defined as time standing on hind legs (rearing or vertical time) and thus breaking the upper beams in the apparatus after stopping ambulation and non-ambulatory short movements. This is of interest because the duration of rearing is associated with orienting and non-selective attentive behavior; where longer vs. shorter rearing time indicates more vs. less non-selective attention, respectively (Aspide et al., 1998; Gallo et al., 2002; Gonzalez-Lima, 2005). Measured parameter related to orienting:
Vertical time
Total time (sec) breaking upper beams.
2.5.3. Risk-taking or impulsive behavior
The risk-taking indices are meant to assess an ADHD-like impulsive profile, by characterizing impulsivity in the form of bursts of fast velocity of ambulation as well as increased exposed-zone time in both OFT and LD tests (Colorado et al., 2006). Exposed-zone time included the relative time a rat spent in the center as opposed to the periphery of the open-field chamber. More time in the periphery (thigmotaxic time) is related to anxiety-like behavior whereas more time in the center places the rat at risk (Clement et al., 1995). The time spent in the light compartment of the LD chamber provided another index of risk-taking since rats prefer the covered dark compartment to the exposed lighted compartment. Characteristics of the impulsive-hyperactive type of ADHD include hyper-reactivity to spatial novelty, faster locomotion and impulsivity (Gonzalez-Lima, 2005). Measured parameters related to impulsive behavior:
Average velocity
Mean velocity (cm/sec), averaged per minute, of ambulatory movement.
Exposed-zone time
On P28 and P30, total time (sec) spent in the center of the open-field, where the center/surround border is defined as 68%:32% of the total area. On P29, total time (sec) spent in the light zone of the light/dark test. These measures involved leaving a defensive zone to enter a more exposed zone.
2.6. Statistical analysis
Data analysis was performed using the SPSS for Windows program, version 11.5. An omnibus, four-way repeated measures analysis of variance (ANOVA) was used to measure both group and sex effects in the various behavioral parameters. Two within-subject factors were used in this analysis: Minutes (10 min per session) and Session (3 sessions: P28, P29, P30). Two between-subject factors were also used: Group (3 levels: MS, EH, and SFR) and Sex (2 levels: male and female). Significance was set at p < .05. In the case of significant interactions, subsequent simple effects tests were corrected with a Bonferroni procedure. Significant group effects were followed by individual group comparisons with Scheffe post hoc tests.
3. Results
3.1. Group effects
Generally, as compared to both the EH or SFR groups, the MS group showed more orienting behavior in the familiar open-field, less risk-taking in the light-dark test, and less ambulatory and short movement activity across the three days of testing (P28, novel open-field; P29, light-dark test; P30, familiar open-field).
3.1.1. General activity
An omnibus four-way repeated measures ANOVA (Group × Sex × Session × Minutes) revealed no main effects of sex in any of the behavioral variables measured. Therefore, a three-way repeated measures ANOVA (Group × Session × Minutes) was used to evaluate the group effects in the novel open-field, light-dark, and familiar open-field test, across 3 sessions and 10 minutes for each behavior measured by the motion-detecting beams in the open-field chambers.
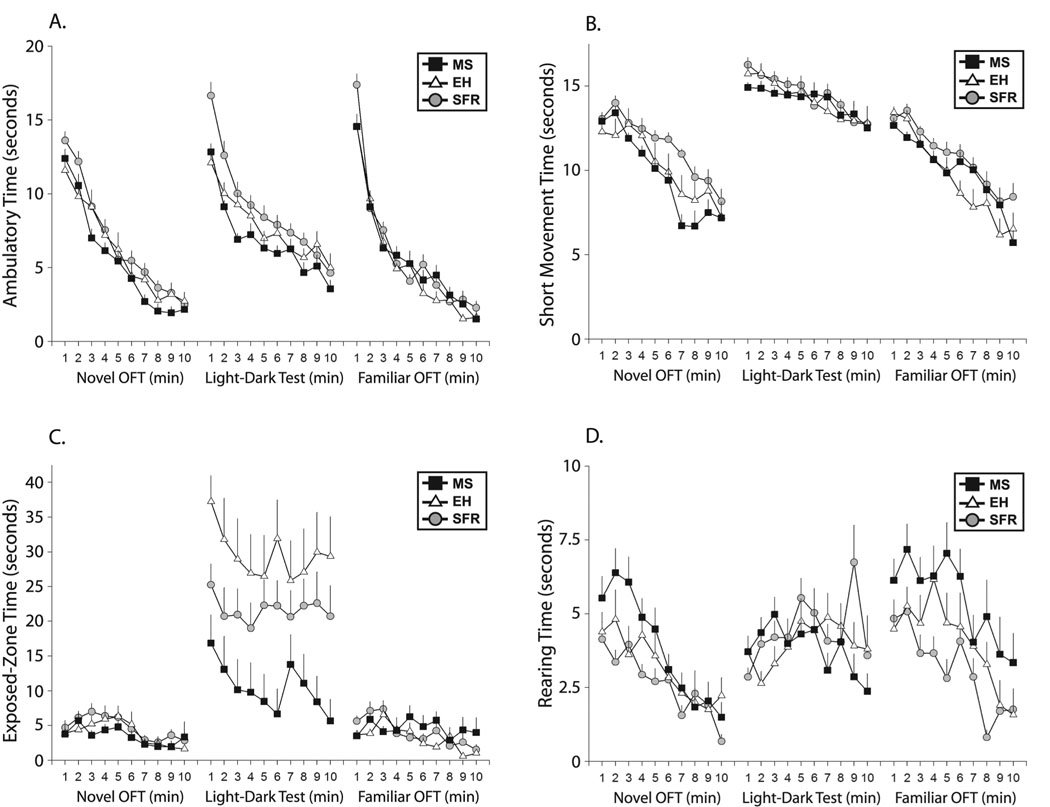
The three-way repeated measures ANOVA (Group × Session × Minutes) showed a significant main effect of group for both ambulatory time (F(2,53) = 3.56, p = 0.035) and short movement time (F(2,53) = 4.384, p = 0.017). The maternal-separation group showed less activity, particularly in the first two sessions, as shown for ambulatory time (Fig. 1A) and short movement time (Fig. 1B).
Fig. 1. Group effects on behavioral measures as a function of time (min) in the novel open-field test (OFT), light-dark test, and familiar OFT for maternally separated (MS), early handled (EH), and standard facility reared (SFR) groups.
A: Ambulatory time (mean plus standard error). B: Short movement time (mean plus standard error). The MS group showed less ambulatory (p = 0.035) and short movement activity (p = 0.017), particularly in the first two sessions. C: The MS group showed less exposed-zone time (mean plus standard error) on P29 (p = 0.013); i.e., remained in the safe dark compartment. The EH group showed the opposite effect during this test. D: The MS group showed greater rearing time (mean plus standard error), but only significantly (p = 0.001) on the familiar OFT.
3.1.2. Risk-taking behavior
Another three-way repeated measures ANOVA (Group × Session × Minutes) tested thigmotaxic behavior (the preference for a safer zone vs. an exposed zone). For the novel and familiar open-field tests, exposed-zone time was measured as time spent in the center of the open-field, away from the walls; for the light-dark test, exposed-zone time was measured as time spent in the light zone (and outside the dark compartment) of the open-field.
The three-way repeated measures ANOVA (Group × Session × Minutes) showed a significant main effect of group for time spent in the exposed zone (F(2, 53) = 4.701, p = 0.013). The effect of MS was particularly pronounced on P29, in which the MS group clearly preferred to remain in the dark compartment during the light-dark test (Fig. 1C). The EH group showed the opposite effect during the light-dark test, spending significantly more time in the light compartment than the other groups.
3.1.3. Orienting behavior
Rearing time was used as an index of orienting behavior as in our previous behavioral study of SD rats (Colorado et al. 2006). Rearing time measurements showed a trend for a group effect in the omnibus three-way repeated measures ANOVA (F(2,53) = 2.769, p = 0.072), and a significant main effect of group in a two-way repeated measures ANOVA (Group × Minutes) during the familiar open-field test on day P30 (F(2,53) = 8.428, p = 0.001). Whereas the effects of MS on general activity and impulsive behavior were more pronounced in the first two sessions of the open-field, orienting behavior shows the opposite effect: the MS group showed significantly greater rearing time, but only in the familiar open-field (Fig. 1D).
3.2. Sex differences
Because the omnibus four-way repeated measures ANOVA (Group × Sex × Session × Minutes) revealed no main effects of sex in any of the behaviors measured, a two-way repeated measures ANOVA (Sex × Minutes) was used to evaluate sex differences within the open-field sessions which showed trends for a main effect of sex. No significant sex differences were observed for orienting behavior during any session; however, measures of general activity and risk-taking behavior revealed significant sex differences during the novel open-field test, with males showing both greater activity and risk-taking than females.
3.2.1. General activity
A two-way repeated measures ANOVA (Sex × Minutes) showed a significant main effect of sex on short movement time during the novel open-field session (F(1,54) = 5.080, p = 0.028). Male subjects showed significantly more short movement behavior during this session, as shown in Fig. 2A.
Fig. 2. Sex differences in behavior.
A: Short movement time (mean plus standard error) as a function of time (min) in the novel open-field test. Males showed more short movement activity than females (p = 0.028). Males also showed more impulsivity (B: ambulatory velocity) (p = 0.017) and risk-taking (C: exposed zone-time) (p = 0.002) than females in the novel open field.
3.2.2. Risk-taking behavior
The two-way repeated measures ANOVA (Sex × Minutes) showed significant main effects of sex on two parameters related to impulsivity and risk-taking: ambulatory velocity (F(1,54) = 6.026, p = 0.017) and exposed-zone time (F(1,54) = 10.740, p = 0.002). Like the sex difference in short movement time, males showed more risk-taking behaviors. Both effects were seen only in the novel open-field session on P28. The sex differences are shown in Fig. 2B: ambulatory velocity; and 2C: exposed-zone time.
3.3. Litter effects
To search for any effects across the 6 litters, each pair of litters for each of the 3 groups were compared for all 18 behaviors showing group differences. On average we found 9 out of 18 means to be greater in one litter compared with the other in the pair. This observed value matches the 50% expected value predicted by chance.
4. Discussion
After the MS treatment, adolescent HO rats showed less activity and risk-taking in the novel and light-dark open-field tests. They were more hesitant, and less likely to venture into the light compartment during the light-dark test. Specifically, during the familiar open-field, MS subjects showed greater orienting behavior in the form of rearing time. The stress of MS, in this strain of rats, may evoke a less active and more fearful behavioral profile, a hypoactive phenotype similar to that seen in Naples Low-Excitability rats (Cerbone et al., 1993).
Additionally, in the largest effect of the present study, subjects in the EH group showed more risk-taking, the opposite effect from our MS group in the light-dark test, supporting data from other studies which suggest that early handling has the effect of attenuating the physiological response to stress in adulthood (Meerlo et al., 1999). Early handling made the rats in this group less fearful, less stressed, and more risk-takers in the light-dark test, as evidenced by more ambulatory behavior in the light area of this arena. This finding corroborates other published studies in which a 15-minute separation period results in attenuated anxiety-like behavior and increased risk-taking in an open-field (Cannizzaro et al., 2006, Madruga et al., 2006) as well as reductions in conditioned fear responding (Meerlo et al., 1999). MS had the exact opposite effect, as seen in the light-dark test on P29, which differentiated between the three groups more than any other behavior measure in any other session of this study (P28, P29, P30).
Compared to the group effects, sex differences were minor, and limited to the novel open-field session on P28. Males showed increased activity and risk-taking behavior during this session, but not during the light-dark or familiar open-field sessions. This is consistent with the diminished sex differences previously reported in the HO strain of rats (Terner et al., 2003).
Our lab’s previous behavioral profile of the effects of MS on orienting behavior, risk-taking/impulsivity, and general activity (Colorado et al., 2006) utilized male rats of the SD strain. The current study, testing both sexes of the HO strain of rats, found distinctly different results. In terms of general activity, whereas Colorado et al. (2006) found increased ambulation and short movement counts as a result of the MS treatment, this study revealed an opposite pattern of decreased ambulation and short movement counts in the MS group. In terms of risk-taking and impulsive behavior, Colorado et al. (2006) found significantly greater exposed-zone time in the MS group in both the novel and familiar open-field, but not in the light-dark test; this study found significantly reduced exposed-zone time in the MS group, particularly in the light-dark test. In terms of orienting behavior, Colorado et al. (2006) found significantly decreased rearing in the MS group in SD rats, while this study found increased rearing in the MS group in the HO strain.
Different responses in HO as compared to SD rats have also been found with other behavioral manipulations and in response to drug treatments. HO rats showed baseline differences in conditioned bar-pressing for water, responding to a temporally-based response schedule more frequently but receiving fewer reinforcements than SD rats (Balcells-Olivero et al., 1998). In the same study, administration of antidepressant drugs increased the amount of reinforcements received by HO rats but had no effect on SD rats. Another study reported that HO differed from Wistar and SD strains for conditioned avoidance and that diazepam affected the avoidance behavior of HO rats differently than SD rats – low levels of diazepam inhibited the avoidance response in HO rats while enhancing it in SD rats (Kuribara et al., 1976). Similarly, chemical lesions of the catecholaminergic system resulted in differing ethanol consumption rates – no change in HO rats, but decreased consumption in SD rats (Melchior et al., 1976). It appears that the subtle genetic differences between SD and HO strains extend into both neural organization (such as that of drug responsivity) and subsequent behavioral responses to stress.
The opposite behavioral profile seen in HO and SD rats subjected to the same MS protocol may seem paradoxical, until one considers the interactions between genetic predisposition and early experience, and how they might differ between two strains of rats. While SD and HO rats are distinct, selectively bred, and genetically unique strains, it is likely that they share the vast majority of their genes, because of their common ancestry. Indeed, the HO strain was made of rats derived from the SD strain and they are often referred to as Holtzman-SD (Matthys et al., 1998).
But the MS paradigm occurs very early in postnatal development, when environmental influences can have far more profound effects than they can later in life. In fact, small genetic differences, present at birth, may be evoked or augmented by early experience, before critical periods during youth and adolescence. The traumatic event of MS has been documented to cause changes in behavior during adolescence, such as increased responsivity to novelty (Colorado et al., 2006; Marin and Planeta, 2004). These changes, observed in adolescence (approximately P28–P70 in rats), may be differentially initiated by the diverging genetic profiles of these strains, then potentiated by the early nature of the MS treatment.
Based on our findings, the MS paradigm may represent a unique approach to the study of genetic/environmental, “nature/nurture” interactions, if subtle genetic predispositions can be enhanced by this early environmental influence. This methodology could also yield practical benefits, particularly in the development of selectively-bred animal models of various disorders, which could be facilitated with this paradigm.
For example, the congenitally helpless strain of rat, previously characterized by our lab in terms of both brain activity and behavior (Shumake et al., 2001, 2002, 2003, 2004, 2005; Wrubel et al., 2006), was selectively bred to show a depressive-like phenotype, similar to that seen in the MS subjects in this study’s HO strain of rats. The MS paradigm may benefit the development of animal models such as this, by providing an early indicator of a baseline difference in genetic makeup, a way to screen not just individuals but entire strains, for a predisposition that may be evoked by a traumatic event. Based on the behavioral profile of the HO strain seen here, these subjects may represent better candidates for selective breeding for a depressive-like phenotype than the Harlan SD rats seen in our previous study (Colorado et al., 2006).
The divergent phenotypes induced by our same MS protocol in SD and HO strains are also remarkably similar to the phenotypes of the Naples High and Low Excitability strains, which have been proposed as putative animal models of ADD+ and ADD− subtypes, respectively (Aspide et al., 1998). Sadile and his group in Naples, Italy, developed these strains from selectively bred SD rats using mazes such as the Lat maze, hexagonal tunnel maze, and asymmetric radial maze. Rats that were vulnerable to exhibit high or low exploratory behavior to spatial novelty tasks were selectively bred together. Thus, the different reactivity to novelty was the selection trait so that Naples High and Low Excitability rat strains are hyper-reactive and hypo-reactive to spatial novelty, respectively, as compared to randomly-bred rats. Naples High Excitability rats resemble SD rats exposed to our MS protocol, as both show increased locomotion and risk-taking, but the duration of their rearing behavior is reduced. Conversely, Naples Low Excitability rats are like HO rats exposed to our MS protocol, as they similarly show decreased locomotion, but the duration of rearing lasted longer. These strains show alterations in non-selective attention as measured by the duration of rearing episodes, which is reduced in the High Excitability strain, and increased in the Low Excitability strain, as compared to the random-bred level (Aspide et al., 1998).
The Naples High Excitability phenotype is presumed to model the ADD+ variant (hyperactive/impulsive type) where hyper-reactivity and rapid attention shifts (reduced orienting time) prevail, whereas the Naples Low Excitability phenotype models the ADD− variant (predominantly hypoactive/inattentive type) with hypo-reactivity and sluggish attention (prolonged orienting time) (Gallo, Gonzalez-Lima and Sadile, 2002). There is mounting evidence that heredity plays an important role in the predisposition to behavioral traits that qualify for ADD diagnoses in patients (Levy et al., 1997). Genetic predispositions are expressed in interaction with the environment, and animal models suggest that MS during early postnatal development may result in both hyperactive and hypoactive phenotypes depending on genetic background, which may resemble ADD+ and ADD− phenotypes seen in children (Gonzalez-Lima, 2005).
5. Conclusion
Maternally separated HO rats displayed reductions in general activity and risk-taking, and increases in orienting time. In contrast, early handling favored risk-taking behavior, which may be consistent with previous findings implicating early handling as beneficial in coping with stress. Sex differences in these behaviors were limited, supporting existing literature. This study expands the literature by showing a possible genetic predisposition in HO rats for hypoactive behavior when exposed to MS as an early life stressor, and is the first to study long-term effects of mother-infant separation in the HO strain of rats.
Acknowledgements
Supported in part by NIH grant R01 MH076847 and Texas Consortium in Behavioral Neuroscience training grant T32 MH65728 directed by FGL. This work was conducted in partial fulfillment of the requirements for a Ph.D. degree at the University of Texas at Austin by JMS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anisman H, Zaharia M, Meaney MJ, Merali A. Do early-life events permanently alter behavioral and hormonal responses to stressors? Int. J. Dev. Neurosci. 1998;16:149–164. doi: 10.1016/s0736-5748(98)00025-2. [DOI] [PubMed] [Google Scholar]
- Arnold J, Siviy S. Effects of neonatal handling and maternal separation on rough-and-tumble play in the rat. Dev. Psychobiol. 2002;41:205–215. doi: 10.1002/dev.10069. [DOI] [PubMed] [Google Scholar]
- Aspide R, Gironi Carnevale UA, Sergeant JA, Sadile AG. Non-selective attention and nitric oxide in putative animal models of Attention deficit Hyperactivity Disorder. Behav. Brain. Res. 1998;95:123–133. doi: 10.1016/s0166-4328(97)00217-9. [DOI] [PubMed] [Google Scholar]
- Balcells-Olivero M, Cousins MS, Seiden LS. Holtzman and Harlan Sprague-Dawley Rats: Differences in DRL 72-sec performance and 8-hydroxy-di-propylamino tetralin-induced hypothermia. J. Pharmacol. Exp. Ther. 1998;286:742–752. [PubMed] [Google Scholar]
- Brake WG, Zhang TY, Diorio J, Meaney MJ, Gratton A. Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. Eur. J. Neurosci. 2004;19:1863–1874. doi: 10.1111/j.1460-9568.2004.03286.x. [DOI] [PubMed] [Google Scholar]
- Braun K, Kremz P, Wetzel W, Wagner T, Poeggel G. Influence of parental deprivation on the behavioral development in Octodon Degus: Modulation by maternal vocalizations. Dev. Psychobiol. 2003;42:237–245. doi: 10.1002/dev.10096. [DOI] [PubMed] [Google Scholar]
- Cannizzaro C, Plescia F, Martire M, Gagliano M, Cannizzaro G, Mantia G, Cannizzaro E. Single, intense prenatal stress decreases emotionality and enhances learning performance in the adolescent rat offspring: Interaction with a brief, daily maternal separation. Behav. Brain Res. 2006;169:128–136. doi: 10.1016/j.bbr.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Cerbone A, Pellicano MP, Sadile AG. Evidence for and against the Naples high- and low- excitability rats as genetic model to study hippocampal functions. Neurosci. Biobehav. Rev. 1993;17:295–303. doi: 10.1016/s0149-7634(05)80013-2. [DOI] [PubMed] [Google Scholar]
- Clement Y, Martin B, Venault P, Chapouthier G. Involvement of regions of the 4th and 7th chromosomes in the open-fiend activity of mice. Behav. Brain Res. 1995;70:51–57. doi: 10.1016/0166-4328(94)00177-h. [DOI] [PubMed] [Google Scholar]
- Colorado RA, Shumake J, Conejo NM, Gonzalez-Pardo H, Gonzalez-Lima F. Effects of maternal separation, early handling, and standard facility rearing on orienting and impulsive behavior of adolescent rats. Behav. Processes. 2006;71:51–58. doi: 10.1016/j.beproc.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Daniels WMU, Pietersen CY, Carstens ME, Stein DJ. Maternal separation in rats leads to anxiety-like behavior and a blunted ACTH response and altered neurotransmitter levels in response to a subsequent stressor. Metab. Brain Dis. 2004;19:3–14. doi: 10.1023/b:mebr.0000027412.19664.b3. [DOI] [PubMed] [Google Scholar]
- Gallo A, Gonzalez-Lima F, Sadile AG. Impaired metabolic capacity in the perirhinal and posterior parietal cortex lead to dissociation between attentional, motivational and spatial components of exploration in the Naples High-Excitability rat. Behav. Brain Res. 2002;130:133–140. doi: 10.1016/s0166-4328(01)00427-2. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Lima F, Sadile AG. Network operations revealed by brain metabolic mapping in a genetic model of hyperactivity and attention deficit: the Naples high- and low-excitability rats. Neurosci. Biobehav. Rev. 2000;24:157–160. doi: 10.1016/s0149-7634(99)00049-4. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Lima F. Cortical and limbic systems mediating the predisposition to attention deficit and hyperactivity. In: Larimer MP, editor. Attention Deficit Hyperactivity Disorder Research. Nova Science Publishers; 2005. pp. 1–18. [Google Scholar]
- Huot RL, Thrivikraman KV, Meaney MJ, Plotsky PM. Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacology (Berl.) 2001;158:366–373. doi: 10.1007/s002130100701. [DOI] [PubMed] [Google Scholar]
- Janus K. Effects of early separation of young rats from the mother on their open-field behavior. Physiol. Behav. 1987;40:711–715. doi: 10.1016/0031-9384(87)90272-1. [DOI] [PubMed] [Google Scholar]
- Kaneko W, Riley E, Ehlers CL. Behavioral and electrophysiological effects of early repeated maternal separation. Depression. 1994;2:43–53. [Google Scholar]
- Kuhn C, Schanberg S. Responses to maternal separation: mechanisms and mediators. Int. J. Dev. Neurosci. 1998;16:261–270. doi: 10.1016/s0736-5748(98)00034-3. [DOI] [PubMed] [Google Scholar]
- Kuribara H, Ohashi K, Tadokoro S. Rat strain differences in the acquisition of conditioned avoidance responses and in the effects of diazepam. Jpn. J. Pharmacol. 1976;26:725–735. doi: 10.1254/jjp.26.725. [DOI] [PubMed] [Google Scholar]
- Levy F, Hay D, McStephen M, Wood C, Waldman I. Attention deficit hyperactivity disorder: A category or a continuum? J. Am. Acad. Child. Adolesc. Psychiatry. 1997;36:737–744. doi: 10.1097/00004583-199706000-00009. [DOI] [PubMed] [Google Scholar]
- Madruga C, Xavier LL, Achaval M, Sanvitto GL, Lucion AB. Early handling, but not maternal separation, decreases emotional responses in two paradigms of fear without changes in mesolimbic dopamine. Behav. Brain Res. 2006;166:241–246. doi: 10.1016/j.bbr.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Marin MT, Planeta CS. Maternal separation affects cocaine-induced locomotion and response to novelty in adolescent, but not in adult rats. Brain Res. 2004;1013:83–90. doi: 10.1016/j.brainres.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Matthews K, Wilkinson LS, Robbins TW. Repeated maternal separation of preweanling rats attenuates behavioral responses to primary and conditioned incentives in adulthood. Physiol. Behav. 1996;59:99–107. doi: 10.1016/0031-9384(95)02069-1. [DOI] [PubMed] [Google Scholar]
- Matthews K, Robbins TW. Early experience as a determinant of adult behavioural responses to reward: the effects of repeated maternal separation in the rat. Neurosci. Biobehav. Rev. 2003;27:45–55. doi: 10.1016/s0149-7634(03)00008-3. [DOI] [PubMed] [Google Scholar]
- Matthys L, Castello R, Zilz A, Widmaier EP. Differential sensitivity to ACTH, but not stress, in two sources of outbred Sprague-Dawley rats. Neuroendocrinology. 1998;67:403–411. doi: 10.1159/000054339. [DOI] [PubMed] [Google Scholar]
- McCall RB, Lester ML, Dolan CG. Differential rearing and the exploration of stimuli in the open field. Dev. Psychol. 1969;16:750–762. [Google Scholar]
- Meerlo P, Horvath K, Nagy G, Bohus B, Koolhaas J. The influence of postnatal handling on adult neuroendocrine and behavioural stress reactivity. J. Neuroendocrinol. 1999;11:925–933. doi: 10.1046/j.1365-2826.1999.00409.x. [DOI] [PubMed] [Google Scholar]
- Melchior C, Meyers L. Genetic differences in ethanol drinking of the rat following injection of 6-OHDA, 5,6-DHT or 5,7-DHT into the cerebral ventricles. Pharmacol. Biochem. Behav. 1976;5:63–72. doi: 10.1016/0091-3057(76)90289-6. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res. Mol. Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Rosenfeld P, Wetmore J, Levine S. Effects of repeated maternal separations on the adrenocortical response to stress of preweanling rats. Physiol. Behav. 1992;52:787–791. doi: 10.1016/0031-9384(92)90415-x. [DOI] [PubMed] [Google Scholar]
- Sanchez M, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: Evidence from rodent and primate models. Dev. Psychopathol. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Shumake J, Edwards E, Gonzalez-Lima F. Hypermetabolism of paraventricular hypothalamus in the congenitally helpless rat. Neurosci. Lett. 2001;311:45–48. doi: 10.1016/s0304-3940(01)02142-5. [DOI] [PubMed] [Google Scholar]
- Shumake J, Edwards E, Gonzalez-Lima F. Dissociation of septo-hippocampal metabolism in the congenitally helpless rat. Neuroscience. 2002;114:373–377. doi: 10.1016/s0306-4522(02)00297-x. [DOI] [PubMed] [Google Scholar]
- Shumake J, Edwards E, Gonzalez-Lima F. Opposite metabolic changes in the habenula and ventral tegmental area of a genetic model of helpless behavior. Brain Res. 2003;963:274–281. doi: 10.1016/s0006-8993(02)04048-9. [DOI] [PubMed] [Google Scholar]
- Shumake J, Conejo-Jimenez N, Gonzalez-Pardo H, Gonzalez-Lima F. Brain differences in newborn rats predisposed to helpless and depressive behavior. Brain Res. 2004;1030:267–276. doi: 10.1016/j.brainres.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Shumake J, Barrett D, Gonzalez-Lima F. Behavioral characteristics of rats predisposed to learned helplessness: Reduced reward sensitivity, increased novelty seeking, and persistent fear memories. Behav. Brain Res. 2005;164:222–230. doi: 10.1016/j.bbr.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Singh D, Maki WS. Effects of postweaning rearing conditions on emotionality and social-seeking behavior in the rat. Psychon. Sci. 1968;13:163–164. [Google Scholar]
- Takahashi LK, Kalin NH, Vanden Burgt JA, Sherman JE. Corticotropin-releasing factor modulates defensive-withdrawal and exploratory behavior in rats. Behav. Neurosci. 1989;103:648–654. doi: 10.1037//0735-7044.103.3.648. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neurosci. Biobehav. Rev. 2003;27:33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Terner JM, Lomas LM, Smith ES, Barrett AC, Picker MJ. Pharmacogenetic analysis of sex differences in opioid antinociception in rats. Pain. 2003;106:381–391. doi: 10.1016/j.pain.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Thoman EB, Arnold WJ. Effects of incubator rearing with social deprivation in rats. J. Comp. Physiol. Psychol. 1968;65:441–446. doi: 10.1037/h0025821. [DOI] [PubMed] [Google Scholar]
- von Hoersten S, Dimitrijevic M, Markovic B, Jankovic B. Effect of early experience on behavior and immune response in the rat. Physiol. Behav. 1993;54:931–940. doi: 10.1016/0031-9384(93)90305-y. [DOI] [PubMed] [Google Scholar]
- Wieland S, Boren JL, Consroe PF, Martin A. Stock differences in the susceptibility of rats to learned helplessness training. Life Sci. 1986;39:937–944. doi: 10.1016/0024-3205(86)90376-0. [DOI] [PubMed] [Google Scholar]
- Wrubel KM, Barrett D, Shumake J, Johnson SE, Gonzalez-Lima F. Methylene blue facilitates the extinction of fear in an animal model of susceptibility to learned helplessness. Neurobiol. Learn. Mem. 2007;87:209–217. doi: 10.1016/j.nlm.2006.08.009. [DOI] [PubMed] [Google Scholar]