Abstract
Purpose
To develop and validate a free-breathing cardiac cine acquisition, with potential to simplify cardiac MR studies, provide registered slices and increase spatial resolution.
Materials and Methods
A 2D free-breathing (FB) navigator-gated cine radial acquisition for cardiac function was developed which used two navigators (one placed prior to the QRS, and another 500 ms after the QRS complex, after systole) to provide complete motion-compensated assessment of systole, without loss of end-diastole. Eleven subjects were studied.
Results
The 2D FB method provided results visually and quantitatively similar to the 2D breath-hold (BH) methods. Comparison of volumes measured with the free-breathing to those measured by standard 2D BH cine resulted in mean bias ± 2 standard deviations of 1.0 ml ± 13.7 ml, 1.1 ml ± 7.6 ml, 3.0 g ± 18.8 g, and 0.3 %± 2.5%, for end-diastolic volume, end-systolic volume, and left-ventricular mass, and ejection fraction, respectively. Slice misregistration was identified in 4 (36%) of the BH studies, but none (0%) of the FB studies. In subjects with slice misregistration, there was greater discordance in LV volume measurements (P<0.05 for end-diastolic mass).
Conclusion
The free-breathing cine acquisition provided results qualitatively and quantitatively similar to 2D breath-hold methods with improved slice registration.
Keywords: Myocardial function, Free-breathing, Navigator-gating, balanced SSFP, radial imaging, projection reconstruction
INTRODUCTION
Free-breathing (FB) cine 2D cardiac magnetic resonance (CMR) imaging of myocardial function is an important goal as an alternative to the standard breath-held (BH) approach, to eliminate repeated breath-holding and to minimize slice misregistration. Such an approach has many applications, including dobutamine stress CMR (1), since repeated breath-hold commands increase the complexity of this imaging test. Furthermore, imaging with multiple BHs commonly leads to slice misregistration, due to breath-hold variability, resulting in unevaluated (i.e. missed) and over-evaluated segments of the myocardium(2,3). Breath-hold variability leads to through-plane and in-plane centroid shifts with a standard deviation of about 3 mm and 4 mm respectively(3), for the short axis orientation. For basal slices, misregistration can effect volumetric measurements (e.g. if a basal slice, which contributes significantly to volumes and mass, is not acquired or is reacquired). Therefore, we sought to develop a FB cine CMR method that provides registered slices.
Many alternatives to the standard clinical 2D BH cine technique have been proposed. These include real-time 2D methods(4) and self-gating(5). At the same time, 3D approaches, mostly using breath-holds, have been studied(6-11). 3D imaging is attractive because of the potential for higher signal to noise ratio (SNR) due to signal averaging, and because it provides registered slices, but has drawbacks including loss of inflow enhancement and the excitation of a large volume of magnetization, causing greater possibility of ”dark band” flow artifacts(12,13).
Here we present an alternative approach using 2D balanced steady state free precession (SSFP) and FB navigator-gated (NAV-gated) imaging for reducing respiratory motion artifacts. NAV-tracked BH cine has been described previously(2) for improving slice registration between BHs. In that study the navigator was placed after each QRS, so that the initial image was delayed and did not represent end-diastole, resulting in smaller end-diastolic volumes. Use of NAVs is challenging for balanced SSFP cine, because the NAVs interrupt steady-state, and create about 25 ms of ”dead-time” during which the cardiac function is not monitored. However, based on recent work using two NAVs (14-16) for phase-contrast, we have developed an efficient reliable NAV-gated method, which does not interfere with imaging of systolic function. By placing the NAVs immediately prior to the QRS, and midway through the RR interval, enddiastole and all of systole can be imaged. The method allows for assessment of global and regional systolic function, including wall motion abnormalities which are confined to the systolic phase. However, the loss of a frame at mid diastole is limitation for assessment of diastolic function. We hypothesized that this NAV-gated FB 2D approach would provide well-registered slices, because the registration is not reliant on the subject's breath-holding capability, and have high NAV efficiency due to the short interval between the two NAVs.
MATERIALS AND METHODS
Eleven subjects (9 healthy subjects and 2 patients (one with atrial fibrillation); 9 F, age 26±14 years) were studied on a 1.5 T Philips Achieva (Philips Medical Systems, Best, NL). All subjects provided written informed consent. In all subjects, both the conventional 2D BH cine and the 2D FB cine balanced SSFP methods were used to image the entire left ventricle in the short-axis orientation.
2D free-breathing cine with NAV-gating
Free-breathing cardiac function data were acquired with a 2D balanced SSFP undersampled radial acquisition. Navigators (25 ms in duration each), placed on the right hemi-diaphragm with signals acquired just prior to the QRS, and 500 ms after the QRS, were used (Fig. 1). Data collected during a heart beat were accepted if these two NAVs were both within the 5 mm acceptance window. The NAVs were surrounded by α/2-TR/2 store/restore sequences (17). The first cardiac phase was prepared using a series of 5−8 ramped flip angles, resulting in a 15 to 25 ms delay in data acquisition(18). The scan parameters were: TR/TE/θ= 3.1ms/1.5ms/40−45°. The flip angle was less than the standard flip angle of 60° used in BH cine (see below), in order to lower the specific absorption rate (SAR) during the ∼3−4 minute scan. Sixteen cardiac phases were acquired with prospective ECG-gating (with dead time at the end of each RR interval, see Fig. 1), with approximately 20 views per segment acquired, providing a 50 ms temporal resolution. The method requires about 60 heart-beats with navigator measurements within the 5mm acceptance window. With typical navigator efficiency of 20 to 40%, the actual scan time was 2.5 to 5 minutes. Spatial resolution was 2 × 2 × 8 mm (2mm gaps), using 160 × 80 Np (acquired with UNFOLD interleaving which alternated with each cardiac phase(19)); 320 cm FOV; 9−12 slices acquired dependent on the chamber size. FB images were reconstructed offline, using gridding reconstruction, FFT and UNFOLD (19) processing. The UNFOLD filter was a low pass filter applied in the temporal frequency domain(10).
1.
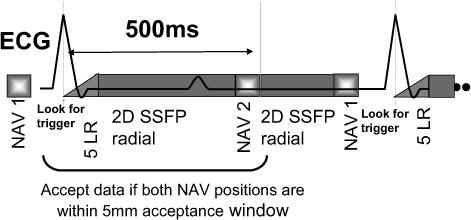
Pulse sequence schematic for the 2D free-breathing NAV-gated cardiac function pulse sequence. The sequence is based on an ECG-triggered segmented multiphase balanced SSFP acquisition. The 1st respiratory navigator (NAV) is placed prior to the QRS, followed by time waiting for the QRS trigger. When the trigger is detected, a 5 pulse linear ramp (5LR) start up sequence is performed, after which data are acquired for multiple phases. The 2nd NAV is performed 500 ms later (after systole). The data from a heart-beat are used, if the 1st and 2nd NAV measurements are within the acceptance window.
2D breath-hold Cine
For comparison, conventional Cartesian 2D BH cine imaging was performed in analogous short-axis orientation using the following sequence: balanced SSFP, TR/TE/θ= 3.1ms/1.5ms/60°, 30 cardiac phases reconstructed using retrospective ECG-gating, with about 12 views per segment acquired, 12 heart-beats per slice, 2 × 2 × 8 mm (2 mm gaps) spatial resolution, 160 × 160 Ny, 320 cm FOV, 9−12 slices in 9−12 breath-holds.
Measurements
SNR was measured from a single basal end-systolic slice in each subject. Noise was estimated as the standard deviation of an air space ROI (approximately 40 pixels), anterior to the chest wall, with an attempt to avoid regions with artifacts. The left ventricular (LV) end-diastolic (EDV) and end-systolic (ESV) volumes were measured, from the short-axis data, using ROIs and computer aided planimetry with custom tools (Matlab 7.1, Mathworks, Natick, MA). End-diastolic myocardial volumes (EDMV) were measured, and converted to end-diastolic masses (EDM) by multiplication by 1.05 g/ml. End-systole was defined as the mid-ventricular frame with the smallest LV cavity, and end-diastole was defined as the first cardiac phase in the dataset. The 2D BH and FB data sets were reformatted in a long-axis view (using Matlab) to visualize slice misregistration. Slice misregistration was classified as large if the septal wall was observed to shift by a distance comparable to its width, otherwise small.
Statistics
All data are presented as mean ± standard deviation. The results from the 2D FB method and 2D BH method were compared using a two tailed Student's T-test. A p<0.05 was considered significant. The limits of agreement between the two techniques were estimated as the mean difference ± 2SD, following the Bland-Altman analysis.
RESULTS
Figure 2 compares a 2D stack of short-axis slices with breath-holds and with free-breathing, showing similar image quality from apex to base. Figure 3 compares images from the FB and BH methods for the 1st, 2nd and systolic cardiac phases. For the FB scans, the quality of the initial cardiac phase (Fig. 3) was visually acceptable using dummy RF pulses and a linear ramp to catalyze the transition to steady state. For the FB scans, the epicardial border delineation improved somewhat in later phases, especially the contrast between myocardium and epicardial fat. Total scan time was approximately 10 minutes (including resting periods) with the BH method and 3.7 minutes (including a NAV efficiency of 30±9.8%) with the FB method. The average blood and myocardial SNR were 70±16 and 23±7 for 2D BH, and 51±15, and 30±9 for 2D FB respectively. Blood-myocardium contrast to noise ratio (CNR) was 47±14 and 21±10 for the BH and FB respectively. The lower flip angle used for FB method to reduce SAR (40°−45° vs. 60° for BH) theoretically results in higher myocardial signal and thus lower CNR between blood and myocardium signal, as observed.
2.
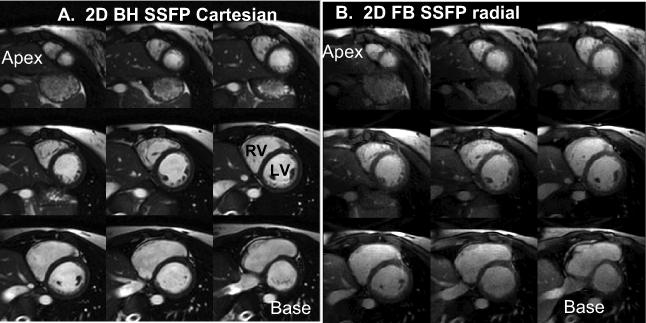
Comparison of standard 2D breath-hold cine imaging with 2D free-breathing cine in one subject, showing all slices from base to apex at endsystole.
3.
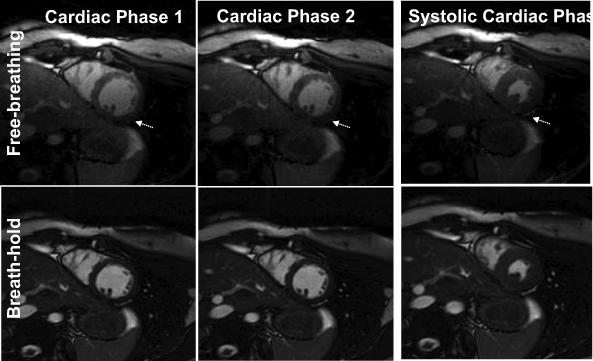
Comparison of standard 2D breath-hold cine with 2D free-breathing cine in one subject, showing the 1st two phases and the systolic phase for a mid-wall slice. For the FB method, the 1st and 2nd phase are of similar quality, demonstrating an acceptable transition to steady state. Some improvement in epicardial border delineation is observed in subsequent cardiac phases for the FB method (arrows).
Slice misregistration was visible in the long-axis reformats of all 2D BH stacks, to a greater or lesser extent, with much less misregistration present in the 2D FB scans (Fig. 4). Of the 11 studies, the slice misregistration was large in 4 (36%) of the 2D BH short-axis stacks, vs. 0 (0%) of the 2D FB stacks. Figure 5A plots LV volumes (end-diastolic and end-systolic) and end-diastolic myocardial volume of 2D FB vs. 2D BH. The correlation coefficients were R=0.97, 0.97 and 0.85 for LVEDV, LVESV, and LVEDM, respectively. The data are also shown in a Bland-Altman plot in Figure 5B. Table 1 provides the results of comparison of LVESV, LVEDV, EDM, and LV ejection fraction (LVEF), including the Bland-Altman limits of agreement. The differences between the BH and FB measurements were not significant. Figure 5C compares discordance between BH and FB measures of LVEDV, LVESV, and LVEDMV, showing greater discordance for studies with large slice misregistration vs. small misregistration, which was significant only for LVEDM (p = 0.01 for EDMV, p = 0.35 for EDV, p = 0.30 for ESV).
4.
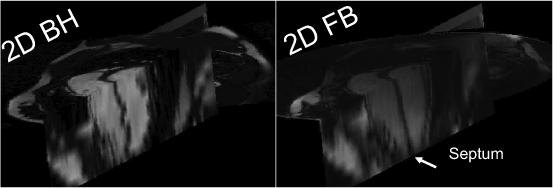
Comparison in one subject of long-axis reformats of a short-axis stack of slices for 2D BH and FB studies, showing the slice misregistration of the BH study, which is readily observed in the septal wall.
5.
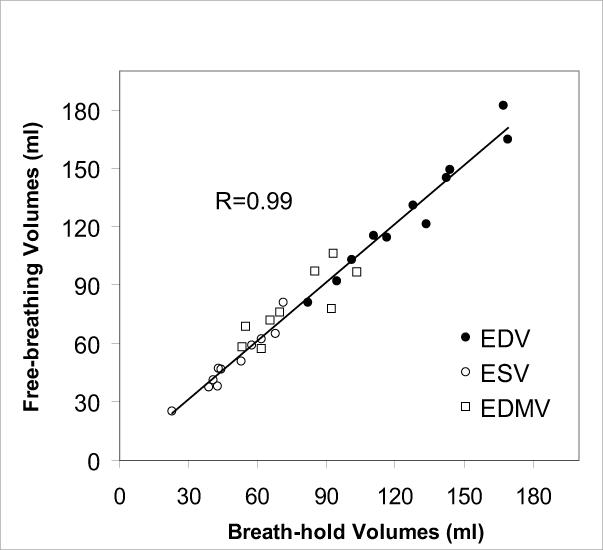
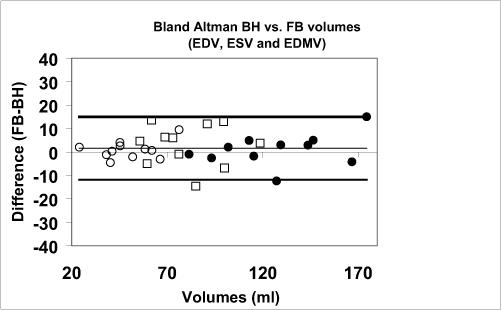
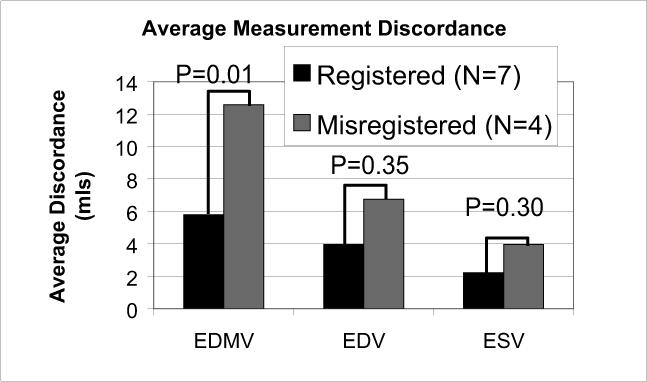
A) Direct comparison of end-diastolic LV volume (EDV), end-systolic LV volume (ESV), and end-diastolic myocardial volume (EDMV) for the 2D free-breathing and 2D BH methods, in all 11 subjects. B) Bland Altman analysis, showing bias and ± 2 standard deviations. C) The extent of disagreement between the 2D BH and FB methods, plotted for studies with registered vs. misregistered BH images. Greater disagreement was observed for studies with misregistration, but significance was only found for myocardial volume.
Table 1.
Bland Altman comparison of 2D breath-hold and free-breathing measurements of left ventricular function.
| 2D FB | 2D BH | Bias | 2 SD | p-value | |
|---|---|---|---|---|---|
| EDV (ml) | 127.2 | 126.2 | 1.0 | 13.7 | 0.64 |
| ESV (ml) | 50.3 | 49.5 | 1.1 | 7.6 | 0.49 |
| EDM (g) | 86.4 | 83.4 | 3.0 | 18.8 | 0.31 |
| EF (%) | 59.1 | 58.8 | 0.3 | 2.5 | 0.59 |
SD = standard deviation, FB = free-breathing, BH = breath-hold, EDV = end-diastolic volume, ESV = end-systolic volume, EDM = end-diastolic mass, EF = ejection fraction.
DISCUSSION
In this study, we demonstrated that 2D free-breathing cardiac function imaging is possible using NAVs for respiratory-compensation. The two NAVs provide efficient respiratory motion compensation for complete assesment of the first half of the cardiac cycle, i.e. systolic function. Quantitative comparisons of LV volumes with the 2D gold-standard show excellent correlation, with limits of agreement similar to that of reported inter and intra-observer variability of 2D BH SSFP(20).
Discordance betweem BH and FB measurements was greatest for myocardial mass (EDM) (Table 1), although the difference was not significant (p=0.31) in this small study. The data show that this was caused by larger end-diastolic epicardial contours using 2D FB radial. Potentially, this may relate to steady state effects in the FB method, which reduce the contrast between myocardium and epicardial fat in the first cardiac phase (see Fig. 3, arrow).
The free-breathing method provides registered slices and high NAV efficiency, both important for improving assesment of myocardial function, for example during a stress exam. This investigation showed that the common presence of slice misregistration resulted in less agreement between 2D BH and 2D FB measures of LV function, which reached statistical significance for LV mass. This important finding suggests that the 2D FB method may be more accurate than the 2D BH method.
The SNR measurements demonstrate that 2D SNR is sufficient, and the benefits of 3D for SNR are not required at this spatial resolution, or even greatly increased spatial resolution. Furthermore, past studies show that the expected SNR increases due to averaging have been offset by reduced inflow signal with the 3D approach(8,10). Since SNR and scan time are sufficient, increased spatial resolution is possible with this 2D FB cine method. This would contribute to visualization of valves, right ventricular wall motion and trabeculation.
Limitations
This study compared 2D BH and FB methods. However, there were other differences, including different temporal resolution (50 ms vs. 36 ms), use of radial with undersampling, and a smaller flip angle for FB (40−45° vs. 60°). The differences in total scan time (10 minutes for BH vs. 4 minutes for FB) were due to radial undersampling by a factor of 2 and a temporal resolution which was 1.4 times reduced for the FB method. Parallel imaging, which is used clinically at our center for cine scans, can reduce the scan time of the BH cine study by about 2, but was not used here in order to permit simple SNR measurements. Our employment of the radial technique for the FB study was motivated by its well-known superior motion blurring and ghosting properties. However, the conclusions that NAV-gated FB cardiac function is feasible, and that it reduces the common occurrence of slice misregistration which may result in inaccurate volumetric measurements, is independent of our choice of trajectory and temporal resolution.
In conclusion, we have developed a 2D FB dual navigator-gated method using two NAVs, permitting complete assessment of systole. This method provided results qualitatively and quantitatively similar to 2D BH methods with improved slice registration, and may facilitate acquisition of images with higher spatial resolution.
Acknowledgments
Grant Support: This work was supported in part by grants from the American Heart Association (AHA SDG 0530061N) and the National Institutes of Health (NIBIB K01 EB004434-01A1).
REFERENCES
- 1.Kwong RY, Schussheim AE, Rekhraj S, et al. Detecting acute coronary syndrome in the emergency department with cardiac magnetic resonance imaging. Circulation. 2003;107(4):531–537. doi: 10.1161/01.cir.0000047527.11221.29. [DOI] [PubMed] [Google Scholar]
- 2.Chuang ML, Chen MH, Khasgiwala VC, McConnell MV, Edelman RR, Manning WJ. Adaptive correction of imaging plane position in segmented k-space cine cardiac MRI. J Magn Reson Imaging. 1997;7(5):811–814. doi: 10.1002/jmri.1880070507. [DOI] [PubMed] [Google Scholar]
- 3.Swingen C, Seethamraju RT, Jerosch-Herold M. An approach to the three-dimensional display of left ventricular function and viability using MRI. Int J Cardiovasc Imaging. 2003;19(4):325–336. doi: 10.1023/a:1025450211508. [DOI] [PubMed] [Google Scholar]
- 4.Plein S, Smith WH, Ridgway JP, et al. Qualitative and quantitative analysis of regional left ventricular wall dynamics using real-time magnetic resonance imaging: comparison with conventional breath-hold gradient echo acquisition in volunteers and patients. J Magn Reson Imaging. 2001;14(1):23–30. doi: 10.1002/jmri.1146. [DOI] [PubMed] [Google Scholar]
- 5.Larson AC, Kellman P, Arai A, et al. Preliminary investigation of respiratory self-gating for free-breathing segmented cine MRI. Magn Reson Med. 2005;53(1):159–168. doi: 10.1002/mrm.20331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alley MT, Napel S, Amano Y, et al. Fast 3D cardiac cine MR imaging. J Magn Reson Imaging. 1999;9(5):751–755. doi: 10.1002/(sici)1522-2586(199905)9:5<751::aid-jmri21>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 7.Barger AV, Grist TM, Block WF, Mistretta CA. Single breath-hold 3D contrast-enhanced method for assessment of cardiac function. Magn Reson Med. 2000;44(6):821–824. doi: 10.1002/1522-2594(200012)44:6<821::aid-mrm1>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 8.Jung BA, Hennig J, Scheffler K. Single-breathhold 3D-trueFISP cine cardiac imaging. Magn Reson Med. 2002;48(5):921–925. doi: 10.1002/mrm.10280. [DOI] [PubMed] [Google Scholar]
- 9.Kozerke S, Tsao J, Razavi R, Boesiger P. Accelerating cardiac cine 3D imaging using k-t BLAST. Magn Reson Med. 2004;52(1):19–26. doi: 10.1002/mrm.20145. [DOI] [PubMed] [Google Scholar]
- 10.Peters DC, Ennis DB, Rohatgi P, Syed MA, McVeigh ER, Arai AE. 3D breath-held cardiac function with projection reconstruction in steady state free precession validated using 2D cine MRI. J Magn Reson Imaging. 2004;20(3):411–416. doi: 10.1002/jmri.20145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uribe S, Muthurangu V, Boubertakh R, et al. Whole-heart cine MRI using real-time respiratory self-gating. Magn Reson Med. 2007;57(3):606–613. doi: 10.1002/mrm.21156. [DOI] [PubMed] [Google Scholar]
- 12.Storey P, Li W, Chen Q, Edelman RR. Flow artifacts in steady-state free precession cine imaging. Magn Reson Med. 2004;51(1):115–122. doi: 10.1002/mrm.10665. [DOI] [PubMed] [Google Scholar]
- 13.Markl M, Alley MT, Elkins CJ, Pelc NJ. Flow effects in balanced steady state free precession imaging. Magn Reson Med. 2003;50(5):892–903. doi: 10.1002/mrm.10631. [DOI] [PubMed] [Google Scholar]
- 14.Jung B, Zaitsev M, Hennig J, Markl M. Navigator gated high temporal resolution tissue phase mapping of myocardial motion. Magn Reson Med. 2006;55(4):937–942. doi: 10.1002/mrm.20808. [DOI] [PubMed] [Google Scholar]
- 15.Nezafat R, Stehning C, Gharib A, et al. Improved spatial-temporal resolution MR coronary blood flow imaging at 3T. J Cardiovasc MR. 2005;7(1):424. [Google Scholar]
- 16.Stehning C, Nezafat R, Gharib A, et al. Dual Navigators for Time-resolved MR Coronary Blood Flow. Fla; Miami Beach: 2005. p. 1616. [Google Scholar]
- 17.Scheffler K, Heid O, Hennig J. Magnetization preparation during the steady state: fat-saturated 3D TrueFISP. Magn Reson Med. 2001;45(6):1075–1080. doi: 10.1002/mrm.1142. [DOI] [PubMed] [Google Scholar]
- 18.Deshpande VS, Shea SM, Laub G, Simonetti OP, Finn JP, Li D. 3D magnetization-prepared true-FISP: a new technique for imaging coronary arteries. Magn Reson Med. 2001;46(3):494–502. doi: 10.1002/mrm.1219. [DOI] [PubMed] [Google Scholar]
- 19.Madore B, Glover GH, Pelc NJ. Unaliasing by fourier-encoding the overlaps using the temporal dimension (UNFOLD), applied to cardiac imaging and fMRI. Magn Reson Med. 1999;42(5):813–828. doi: 10.1002/(sici)1522-2594(199911)42:5<813::aid-mrm1>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 20.Alfakih K, Plein S, Thiele H, Jones T, Ridgway JP, Sivananthan MU. Normal human left and right ventricular dimensions for MRI as assessed by turbo gradient echo and steady-state free precession imaging sequences. J Magn Reson Imaging. 2003;17(3):323–329. doi: 10.1002/jmri.10262. [DOI] [PubMed] [Google Scholar]


