Abstract
Graves’ disease (GD) is an autoimmune process involving the thyroid and connective tissues in the orbit and pretibial skin. Activating anti-thyrotropin receptor Abs are responsible for hyperthyroidism in GD. But neither these auto-Abs nor the receptor they are directed against have been convincingly implicated in the connective tissue manifestations. Insulin-like growth factor-1 receptor (IGF-1R)-bearing fibroblasts over-populate connective tissues in GD and when ligated with IgGs from these patients, express the T cell chemoattractants, IL-16 and RANTES. Disproportionately large fractions of peripheral blood T cells also express IGF-1R in patients with GD, and may account, at least in part, for expansion of IGF-1R+ memory T cells. We now report a similarly skewed B cell population exhibiting the IGF-1R+ phenotype from the blood, orbit and bone marrow of patients with GD. This expression profile exhibits durability in culture and is maintained or increased with CpG activation. Moreover, IGF-1R+ B cells produce pathogenic antibodies against the thyroid stimulating hormone receptor. In lymphocytes from patients with GD, IGF-1 enhanced IgG (p<0.05) production and increased B cell expansion (p<0.02) in vitro while those from control donors failed to respond. These findings suggest a potentially important role for IGF-1R display by B lymphocytes in patients with GD in supporting their expansion and abnormal immunoglobulin production.
Keywords: Thyroid associated ophthalmopathy, B lymphocyte, Graves’ disease, IGF-1 receptor, autoimmunity
Introduction
B lymphocytes play a critical role in normal immunity and the development of autoimmune disease (1-3). They initiate and support immune function through antigen presentation, expression of co-stimulatory molecules and production of Abs (1, 4-6). Moreover, B cells can develop into Ig-producing plasma cells which not only provoke host defense, but can also recognize host tissue, leading to autoimmunity (2). Auto-Ab production plays a central role in the pathogenesis of several of these diseases, such as rheumatoid arthritis and Graves’ disease (GD) (2, 7). In addition to Ig production and antigen presentation, B cells secrete many cytokines and thus they modulate immune activation. Among these cytokines are IL-6, TNF-α, and IL-10, all of which can condition T cell responses (5, 8, 9). B cell antigen presentation is robustly up-regulated through the CD40/CD40 ligand (CD154) pathway which is also essential to T cell activation (10). The concept that B cells play important roles in autoimmunity receives considerable support from the effective interruption of disease progression and activity following therapeutic B cell depletion (11, 12).
GD represents a syndrome comprising thyroid growth, the over-production of thyroid hormone, and connective tissue manifestations including orbital inflammation and expansion, processes known as thyroid-associated ophthalmopathy (TAO) (3) Aberrant production of IgGs directed at the thyrotropin receptor (TSHR) resides at the heart of the thyroid glandular component of GD (13). A portion of these Abs mimic thyrotropin in their actions, and because they are unencumbered by a normal negative feedback mechanism, result in unregulated TSHR activation and excessive hormone production (14). Patients with fully-expressed GD also display infiltration of the orbit by lymphocytes that is characteristic of TAO. Why immunocompetent cells are recruited to the orbit and the circumstances surrounding the generation of Abs directed against self in this disease remain unclear. While anti-TSHR Abs convincingly account for much of the intra-thyroidal pathology in GD, vanishingly little evidence has been generated to implicate them in the pathogenesis of TAO. Recently, we reported the production of IgGs directed against the insulin like growth factor receptor (IGF-1R) in patients with GD (GD-IgG) (15, 16). Moreover, IGF-1R has been found to be over-expressed by fibroblasts from these patients and IGF-1R activation leads to signaling which is absent in fibroblasts from control donors without autoimmune diseases. Specifically, GD-IgG/fibroblast interactions result in the production of T cell chemoattractants such as IL-16 and RANTES (15, 16). These cytokines may represent the critical signaling that leads to T cell trafficking to the orbit in TAO. Thus, multiple manifestations of GD involve B cells playing important roles in disease pathogenesis through their Ab production, antigen presentation and participation in T cell function.
IGF-1 and IGF-1R comprise an important signaling pathway that exerts far-reaching influence on growth and development (17-19). In general, IGF-1R signaling is associated with a powerful survival advantage (20, 21). Although ubiquitous, the receptor levels vary among tissues and cell-types. Moreover, IGF-1R over-expression has been linked to the malignant phenotype exhibited by certain cancers (19, 22-24). This pathway plays important roles in hematopoietic cell growth and differentiation and normal immune function (19). Peripheral blood T and B cells and monocytes from control human donors express low levels of IGF-1R in vivo (25, 26). Administration of IGF-1 increases the circulating pool of CD4+ T cells and splenic B cells in mice (27, 28), suggesting a role for this growth factor in myelopoietic cell expansion (29). It promotes T cell proliferation during early activation (30) and inhibits apoptosis of both immature and mature T cells through at least three distinct mechanisms (31, 32). With regard to B cell expansion, IGF-1 plays an essential role in the development of bone marrow CD34+ cells into pro B cells (33). In addition, IGF-1 increases expression of the IL-4-induced type II IgE receptor (FcεRII/CD23) by both human primary immune cells and established B cell lines suggesting a role in B cell activation and promoting B cell function (34).
We have recently reported that T lymphocytes from patients with GD represent a cell population skewed toward the CD3+IGF-1R+ phenotype (35). This disproportionate fraction of IGF-1R-displaying T cells can be traced to discreet subsets. In particular, the increased receptor expressing population results from an expanded memory CD45RO+ T cell population. This skew was found in peripheral blood and also in cells harvested from disease-involved orbital connective tissue (35). In contrast, the abundance of CD45RA+ IGF-1R+ naïve T cells is similar in peripheral blood from control donors and those with GD. Expression of the receptor by GD-derived T cells has functional consequences in that IGF-1 enhances BrdU incorporation and inhibits Fas-mediated apoptosis (35). Here we report that the phenotype of B cells in patients with GD is also disproportionately biased toward the CD19+IGF-1R+ phenotype. Moreover, this skewed phenotype conveys functional consequences including enhanced B cell expansion and exaggerated Ab production including those directed against the thyrotropin stimulating hormone receptor (TSHR). IGF-1 increases the production of IgG but not IgM in B cells derived from patients with GD compared to those from control donors. Thus, the underlying mechanism for aberrant IgG production that drives multiple aspects of GD may, at least in part, result from the over-representation of B cells displaying IGF-1R.
Materials and Methods
Materials
Ficoll-Hypaque was purchased from Sigma Aldrich (St. Louis, MO). FacLyse buffer, Cytofix, anti-CD19 CyChrome, anti-IGF-1Rα PE (clone 1H7), and isotype mouse IgG1 FITC, PE, APC and CyChrome were purchased from BD Biosciences (San Jose, CA). Fetal bovine serum (FBS) was supplied by Life Technologies (Grand Island, NY). IGF-1 and Des1-3 IGF-1 were from Calbiochem (San Diego, CA) and Gro-Pep (Adelaide, Australia), respectively.
Patient samples
Subjects, aged 20-65, were recruited from the patient population of Jules Stein Eye Institute and Harbor-UCLA Medical Center. Informed consent was obtained as approved by the Institutional Review Boards of the Center for Health Sciences at UCLA and Harbor-UCLA Medical Center. The study population comprised patients evaluated for GD without or with TAO. Control subjects were healthy volunteers without known autoimmune disease who presented for aesthetic or functional eyelid surgery. Individuals excluded from the study included those with non-thyroid autoimmune disease, asthma, granulomatous disease, sinusitis or HIV infection. Patients with GD comprised a clinically heterogeneous group and included those who were hyperthyroid (n=3) and euthyroid (n=27). Twenty eight of 30 patients manifested TAO. A minority of patients had active inflammatory disease (clinical activity score ≥3; n=6) while most exhibited stable TAO (CAS< 3; n=22). No association between IGF-1R display and disease duration, or degree of orbital inflammation was noted. Bone marrow samples were derived from patients with stable GD, a healthy volunteer or at necropsy within 24 hours of death. Orbital tissue was obtained from surgical waste during orbital decompression surgery in patients with TAO or from healthy individuals during cosmetic surgery. The tissue was transported on ice, homogenized, and single cell suspensions prepared. Tissue was filtered through 70 μm pores and processed for flow cytometry.
Clinical data including age, sex, medications, smoking history, physical exam and laboratory values were recorded. Careful examination of the skin failed to detect evidence of thyroid-related dermopathy in any of the study participants.
Flow cytometry
Peripheral blood (approx. 5 ml) was obtained and stored in tubes containing EDTA. Staining buffer (SB) was prepared in phosphate-buffered saline containing 4% FBS with 0.1% sodium azide (Sigma Aldrich). Staining for flow cytometry was performed within 24 h of blood collection, according to the manufacturer’s instructions (BD Biosciences, San Jose). Briefly, 100 μl whole blood or bone marrow aspirate was placed in 12 × 75 mm polypropylene tubes and fluorochrome-conjugated monoclonal antibodies were added (1 μg/106 cells). These were then incubated in the dark for 20 min at room temperature. FACSlyse solution (2 ml) was added for 10 min at room temperature to lyse RBCs. Cells were washed twice with SB, re-suspended in Cytofix (BD Biosciences) and kept in the dark at 4°C until cytometric analysis (within 24 h). Analysis was performed on a FACS Calibur flow cytometer (BD Biosciences). Mean fluorescent intensity (M.F.I.) was calculated as a ratio of mean fluorescence sample / isotype fluorescence. Percent positive expression was determined as the population of cells with increased fluorescent intensity compared to isotype.
PBMC preparation
PBMCs were prepared using a technique described previously (36). Briefly, whole blood was diluted 1:2 in PBS and layered over Ficoll Hypaque, centrifuged at 500 × g for 25 min and washed 3 times in PBS. 1 × 106 cells/ml were cultured in RPMI medium supplemented with CpG (2 μg/ml) (Integrated DNA Technologies), IGF-1 (10 ng/ml), and/or Des 1-3 IGF-1 (10 ng/ml).
Quantification of B cell number
PBMCs were cultured as described above. An aliquot of each sample was analyzed by flow cytometry and used to calculate the fractional B cell population after live cell gate. This percentage was then multiplied by the cell number to quantify B cell number. Cell counts were determined according to the manufacturer’s protocol (CyQuant; Invitrogen, Carlsbad CA). Briefly, PBMCs were cultured in 96 well plates and incubated for 24-72 hours. At the desired time, the 96 well plate was centrifuged at 500 × g for 5 minutes and the supernatant decanted by inversion. Plates were frozen at -70C until analysis. Culture solution did not contain Phenol Red since the dye can interfere with CyQuant GR dye fluorescence. Plates were thawed at room temperature and 200 μl of CyQuant GR dye/cell lysis buffer was added to each well and incubated for 5 minutes with gentle mixing. Samples were protected from light and emissions were determined using a Wallac Victor 1420 fluorometer (Perkin Elmer) at 480 nm excitation and 520 nm emission wave-lengths. They were assayed in quadruplicate and compared to a standard curve.
B cell production of IgM and IgG
PBMCs were stimulated in vitro as previously described (37). Briefly, 2.5 × 106 PBMC/200μl in 96 well plates or 5 × 106 PBMC /ml in 24 well plates were cultured in 10% heat inactivated FBS-supplemented RPMI for 12 days. Cells were stimulated with nothing, CpG 2006 (2 μg/ml) (Integrated DNA Technologies), Staphylococcus aureus Cowen Strain I (20 μg/ml) SAC; Sigma Aldrich), and pokeweed mitogen (1/100,000) in the presence or absence of IGF-1 (10 ng/ml). The chosen CpG concentration yielded sub-maximal mitogen activity. IgM and IgG concentrations were determined after 12 days in culture. Supernatants were harvested and Ig concentrations were assayed using a commercially available ELISA, according to the manufacturer’s specification (Bethyl Laboratories, Montgomery, TX). Sample concentrations were compared to a standard curve.
B cell production of anti-TSHR Abs was assayed in both CD19+ IGF-1R+ and CD19+ IGF-1R- populations after sorting by flow cytometry (38). These cells were cultured with monocytes in a 1:2 ratio and incubated with EBV-containing supernatant generated by the B95.8 marmoset cell line for 1hr at 37□C, at a concentration of 2 × 106 cells/ml. After EBV exposure, cells were washed and cultured at 1 × 106 cells/ml (RPMI, 1% L-glutamine, 1% P/S, and 10%FBS) in the presence of the monocytes. After 2 weeks, conditioned media were analyzed for Ab according to the manufacturer’s specifications (TRAK LIA kit, Brahms Diagnostics, Germany).
Statistics
Values are reported as the mean ± standard error. Statistical analysis was performed using a 2-tail Student’s t test with a confidence level >95%.
Results
A greater proportion of B cells derived from patients with GD express IGF-1R compared to those from control donors
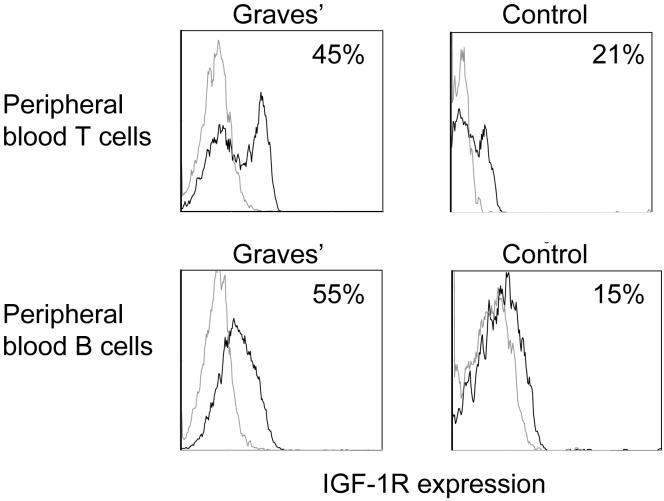
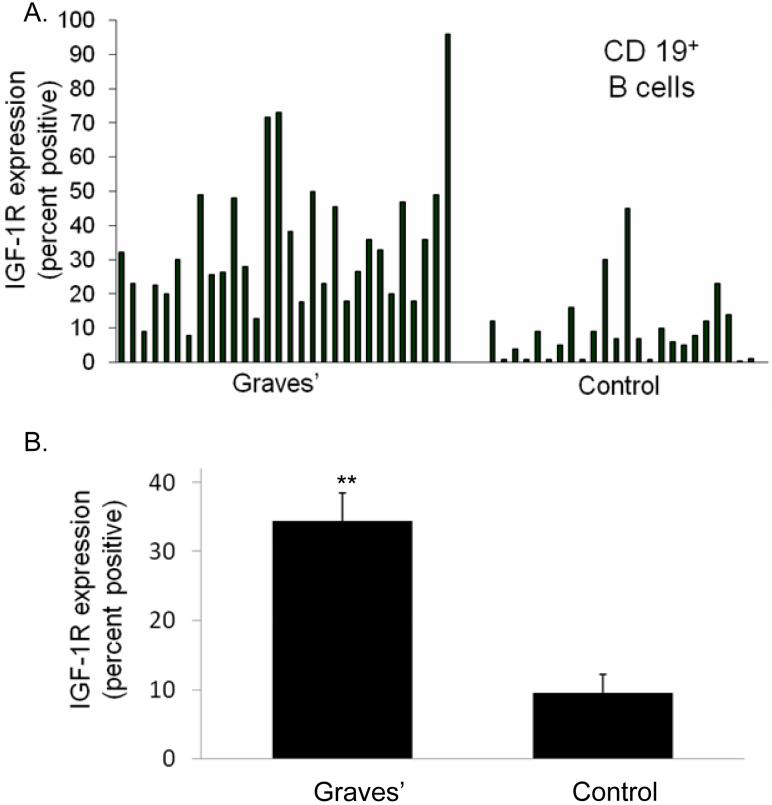
We have previously demonstrated that a disproportionate fraction of peripheral blood T cells from patients with GD express IGF-1R (35). CD4+CD45RO+ and CD8+CD45RO+ memory T cells from these patients displayed the greatest divergence from controls with regard to the fractional expression of IGF-1R. Given the prominent role for B cell function in the pathogenesis of GD, we have now investigated IGF-1R display by these lymphocytes in the peripheral blood and a cohort of control donors using multi-parameter flow cytometry. Analogous to their T cells, B cells (CD19+, IGF-1R+) from donors with GD exhibit increased fractional expression of the receptor, as demonstrated in a representative study shown in Figure 1. The disparity between control lymphocytes and those from the patients was similar to that found in their T cells. As shown in Figure 2, cumulative data from 30 different patients with GD demonstrate that 34 ± 4% (mean ± SE) of B cells express IGF-1R while the receptor was detected in 9 ± 3% cells from control donors (n=24; p<10-6, GD vs controls). The range of IGF-1R expression was considerably broader among patient-derived B cells (7% to 98% CD19+ IGF-1R+) compared to controls (1% to 45%). The fraction of IGF-1R+ B cells appears durable since longitudinal examination of lymphocytes obtained serially from four different patients revealed that their abundance was invariant over a one-year period (data not shown). Moreover, the frequency of IGF-1R+ B cells remained stable following treatment with oral prednisone in a single patient and during clinical remission in two others over 6 months of follow up. The fraction of IGF-1R+ B cells from patients with GD does not decline as a function of time in culture (data not shown), exhibiting similar phenotypic stability to that found previously in T cells (35).
Figure 1.
Increased fraction of peripheral blood T and B cells from a patient with GD display IGF-1R compared to those from a control donor. PBMCs were stained with anti-CD3, anti-CD19 and anti-IGF-1R Abs as described in “Methods” and subjected to multi-parameter flow cytometry. The grey open histograms represent staining with isotype control Abs.
Figure 2.
A disproportionate fraction of peripheral blood B cells from 30 patients with GD express IGF-1R compared with that found in 24 control donors (A) individual data sets demonstrating fractional IGF-1R+ B cells. (B) Analysis of IGF-1R display in B cells as an aggregate of multiple patients with GD vs control donors. (34 ± 4% IGF-1R+ B cells (mean ± SE, n=30) vs 9 ± 3% IGF-1R+ control B cells (n=24)). Data are expressed as mean ± standard error (**p< 1 × 10-6).
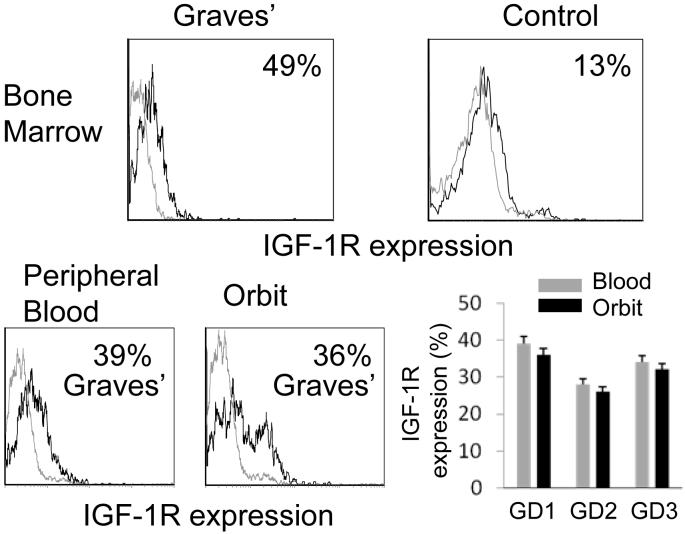
We next investigated whether the skewed CD19+IGF-1R+ B cell phenotype found in peripheral blood from patients with GD was also present in their bone marrow and orbit. As demonstrated in Figure 3 (upper panels), 49% of marrow-derived CD19+ CD3- CD45+ CD15- CD14- B cells from a single patient with GD express IGF-1R compared to 13% in cells from a control donor, representative of 2 control subjects (13% and 21%). Figure 3 (lower panels) contains data demonstrating that orbital and peripheral blood B lymphocytes, isolated from matched common donors with GD, exhibit similarly skewed IGF-1R display (peripheral B cells, 34% ± 6%; orbital B cells, 31% ± 5%, n=3). Thus, the increased relative abundance of IGF-1R+ B cells from patients with GD is similar in lymphocytes harvested from peripheral blood and orbit.
Figure 3.
Increased fraction of bone marrow- and orbit-derived B cells from patients with GD express IGF-1R. Bone marrow aspirate (upper panels) from a patient with GD demonstrates increased fraction of IGF-1R+ B cells compared to a control aspirate. B cells isolated from the orbit and peripheral blood of a patient with GD (lower panels) display increased fraction of IGF-1+ B cells (representative flow cytometry histograms of three patients). Isotype Ab staining is shown as an open grey histogram. (lower right) IGF-1R expression by B cells from the peripheral blood and orbit, each sample pair obtained from one of 3 separate patients. .
Impact of IGF-1 on B cell phenotype prior to and following stimulation with CpG
Since IGF-1 can influence metabolic activity and proliferation of B cells (27, 39), we investigated whether it altered the expression of co-stimulatory molecules or cell-surface signatures associated with lymphocyte activation. As shown in Table I, basal expression of CD23, CD80, and CD86 is similar on B cells from donors with GD and their controls. As expected, CpG stimulation increased B cell expression of all three markers. In contrast, IGF-1 failed to influence levels of any of these surface molecules after 24, 48 and 72 hours (48 hour shown in Table I). The vast majority of B cells expressing CD23, CD80, or CD86 fail to display IGF-1R. These cells remain IGF-1R-, regardless of whether they remain untreated or are exposed to CpG, either in the absence or presence of IGF-1.
Table I. Exogenous IGF-1 does not alter surface expression of CD80, CD86 or CD23 by GD or control B cells.
| Graves’ n=5 | Control n=5 | |||||||
|---|---|---|---|---|---|---|---|---|
| CD19+ B cells | hours in culture | Total (%) | CD221+ (%) | CD221- (%) | Total (%) | CD221+ (%) | CD221- (%) | |
| CD80+ | 0 | 6 ± 3 | 1 ± 1 | 5± 2 | 6 ± 3 | 1 ± | 6 ± 3 | |
| control | 48 | 8 ± 4 | 3 ± 2 | 5 ± 3 | 14 ± 7 | 4 ± 2 | 10 ± 5 | |
| CpG | 48 | 16 ± 5 | 4 ± 2 | 11 ± 4 | 21 ±12 | 8 ± 4 | 20 ±12 | |
| IGF-1 | 48 | 8 ± 3 | 3 ± 2 | 8 ± 3 | 8 ±4 | 2 ± 2 | 6 ± 4 | |
| CpG + IGF-1 | 48 | 19 ± 7 | 4 ± 2 | 15 ± 6 | 15 ± 6 | 3 ± 2 | 12 ± 5 | |
| CD86+ | 0 | 2 ± 2 | na | na | 3 ± 1 | na | na | |
| control | 48 | 31 ± 7 | 9 ± 8 | 25 ± 8 | 21 ± 7 | 5 ± 1 | 17 ± 6 | |
| CpG | 48 | 65 ± 13 | 15 ± 6 | 50 ± 14 | 48 ± 13 | 8 ± 3 | 40 ± 9 | |
| IGF-1 | 48 | 36 ± 16 | 7 ± 4 | 29 ± 16 | 19 ± 8 | 4 ± 1 | 15 ± 7 | |
| CpG + IGF-1 | 48 | 63 ± 19 | 7 ± 4 | 55 ± 17 | 50 ± 14 | 5 ± 2 | 45 ± 9 | |
| CD23+ | 0 | 17 ± 6 | 4 ± 3 | 14 ± 7 | 23 ± 11 | 6 ± 3 | 18 ± 8 | |
| control | 48 | 19 ± 9 | 5 ± 2 | 17 ± 9 | 11 ± 5 | 3 ± 2 | 9 ± 6 | |
| CpG | 48 | 52 ± 14 | 12 ± 5 | 37 ± 10 | 54 ± 19 | 8 ± 2 | 45 ± 18 | |
| IGF-1 | 48 | 21 ± 11 | 4 ± 2 | 19 ± 9 | 13 ± 7 | 3 ± 2 | 11 ± 5 | |
| CpG + IGF-1 | 48 | 52 ± 18 | 5 ± 2 | 47 ± 14 | 65 ± 21 | 8 ± 3 | 57 ±19 | |
PBMC were isolated and cultured with or without CpG (2 μg/ml) and IGF-1 (10 ng/ml) for 48 hours and assayed for expression of CD80, CD86, CD23
Each datum point represents percent positive-staining CD19+ B cells ± S.E.M.
All comparisons of treatments compared to their respective controls were not significant
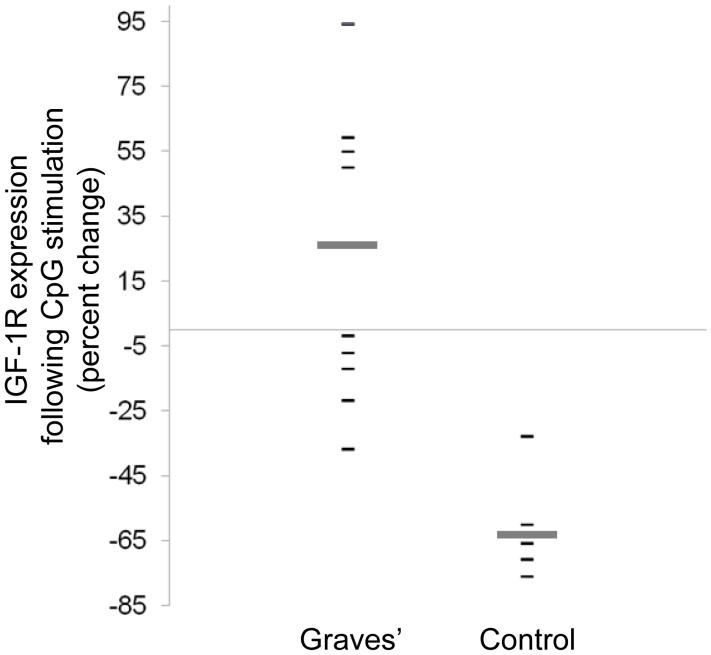
We have previously reported that expression of IGF-1R on T cells from patients with GD is aberrantly up-regulated following cell activation (35). We therefore investigated whether B cell activation was also associated with increased IGF-1R levels. CpG-provoked lymphocyte activation resulted in divergent patterns of IGF-1R display in cells from patients and their controls. This treatment either maintained or increased B cell expression of IGF-1R in most patients with GD (Figure 4; n=9; mean increase, 25 ± 21 %). In contrast, identical treatment reduced the abundance of IGF-1R displaying B cells from control donors (n=5, mean decrease 64 ± 16 %, p<0.002 vs GD). The MFI for CD19+ IGF-1R+ B cells derived from patients and control donors was similar at all time points assayed.
Figure 4.
Abundance of IGF-1R+ B cells from patients with GD is maintained or increased with CpG activation compared to those from control donors. PBMCs were isolated and cultured in the presence of CpG (2 μg/ml). IGF-1R display was assessed by flow cytometry at 24 h and is expressed as percent change from un-stimulated cultures. Each datum point represents a single patient’s sample. Broad horizontal bars indicate mean values for each group (GD vs control, p< 0.002).
IGF-1 promotes expansion of GD-derived B cells
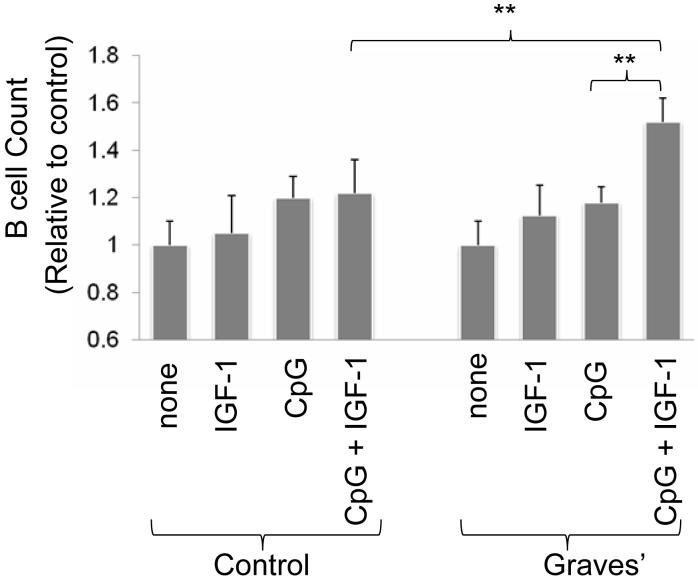
IGF-1R activation enhances myeloma proliferation and splenic B cell maturation in vivo but these effects in vitro have been found to be variable (40-42). We next investigated whether the increased fraction of circulating IGF-1R+ B lymphocytes found in GD conveys a functionally different cell phenotype. PBMCs or purified B cells (>95% purity) were sub-maximally stimulated with CpG (2 μg/ml) in the absence or presence of IGF-1 (10 nM). IGF-1 significantly enhances B cell number in cultures derived from patients with GD when added in combination with CpG compared to lymphocytes receiving CpG or IGF-1 alone for 24h, 48h and 72h (p<0.02; Figure 5). Moreover, Des 1-3 IGF-1 (10 nM), an analogue that binds to and selectively activates IGF-1R (43) also enhances the actions of CpG (data not shown; 1.4 ± 0.5 fold increase compared to CpG alone). In contrast, treatment with CpG, IGF-1 or Des 1-3 IGF-1, either as single agents or in combination, fail to expand B cells from control donors.
Figure 5.
IGF-1 potentiates B cell expansion when added together with a concentration of CpG yielding a sub-maximal response. B cell number was assessed as described in “Methods” after 5 days in culture with CpG (2 μg/ml), IGF-1 (10ng.ml) as single agents or in combination. Data derive from 5 independent experiments (mean ± SE, ** denotes differences at p<0.02).
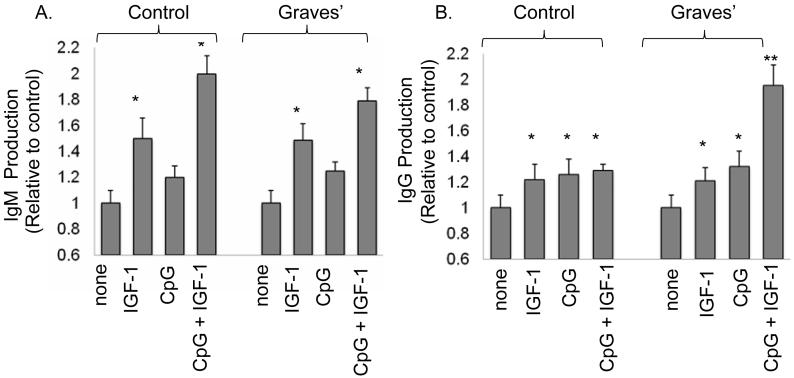
IGF-1 synergistically enhances the production of IgG by B cells from patients with GD but not those from control donors
Because both auto-Ab production and action play central roles in the pathogenesis of GD, we next investigated whether the increased fraction of IGF-1R+ B cells in these patients might enhance Ab production. PBMCs from patients with GD and control donors were sub-maximally stimulated with CpG (2 μg/ml) in the absence or presence of IGF-1 (10 nM) for 12 days and the conditioned medium was then assayed for IgM and IgG. IGF-1 significantly enhances production of IgM when added alone or in combination with CpG, in both GD-derived and control B cells (Figure 6A; *p<0.05 treatments vs untreated). The magnitude of effect imposed by IGF-1 was similar in cells from both sources. In contrast, while IgG production is increased with IGF-1 and CpG when added as single agents in both control and disease-derived cells (Figure 6B; *p<0.05, CpG or IGF-1 compare to un-stimulated controls), CpG and IGF-1 appear to act synergistically only in GD-derived B cells, since the combination of these agents enhances IgG production more than either added alone (Figure 6B; **p<0.01 CpG plus IGF-1 compared to IGF-1 or CpG alone). To assess the potential for IGF-1R+ B cells to produce pathogenic anti-TSHR Abs, B cell populations were sorted by flow cytometry and transformed with EBV (38, 44). The IGF-1R+ B cells consistently produced these Abs, while production by the IGF-1R- B cell population was variable. Production of anti-TSHR Abs by the respective B cell populations was assessed in three patients. In the first, (GD1), IGF-1R+ cells produced 20 IU/100 μl, while IGF-1R- cells produced 10 IU/100μl. In subject GD2, IGF-1R+ produced 12 IU/100μl, while production in IGF-1R- was 0 IU/100μl; and in subject GD3, IGF-1R+ cells generated 2 IU/100μl while those with the IGF-1R- phenotype anti-TSHR Ab production was undetectable (0 IU/100μl)(IGF-1R+ vs. IGF-R-, p≤0.06). Thus, the expanded IGF-1R+ B cell population found circulating in patients with GD may generate increased levels of pathogenic Abs.
Figure 6.
IGF-1 potentiates IgM and IgG production, both in B cells from patients with GD and in those from control donors. IgM and IgG were assayed after 12 days in culture stimulated with CpG and/or IGF-1. (A) IGF-1 (10 ng/ml) alone or in combination with CpG (2 μg/ml), significantly increase IgM production by GD and control B cells. (B) CpG and IGF-1, used as single agents enhanced IgG production by B cells from both sources (*p<0.05 vs US). The combination of CpG and IGF-1 significantly increases IgG production only in B cells from patients with GD compared with treatment with either single agent (**p<0.01). Results are expressed as the mean ± SE of 5 independent experiments.
Discussion
Here we report for the first time that B cells from patients with GD are over-represented by those exhibiting the IGF-1R+ phenotype. This mirrors earlier findings by us that T cells (35) and fibroblasts (16) from these same patients also exhibit a strongly skewed phenotype with regard to cell surface IGF-1R display. Thus over-expression of this putative self-antigen appears to be more widely spread in GD than initially thought. The over-abundance of IGF-1R+ B cells is not limited to the peripheral circulation but can also be demonstrated in affected orbital tissues from patients with TAO (Figure 3). This receptor display conveys several functional attributes to B cell behavior in that IGF-1 enhances both survival and Ab production in vitro. Moreover, IGF-1R+ B cells can produce anti-TSHR Abs.
B cells play diverse roles in immune function by virtue of their further differentiation into Ig secreting plasma cells and their importance in antigen presentation. A critical component of B lymphocyte development concerns how committed precursor cells from the hematopoietic lineage undergo immunoglobulin heavy chain gene rearrangement. This results in light chain production and leads to the release of mature B cells from the bone marrow (45). A number of factors, including IGF-1 emanating from marrow stroma, support early B cell proliferation and drive their differentiation (42, 46, 47). When administered in vivo, IGF-1 expands intra-splenic B cells through increased proliferation of mature cells (28, 48). In marrow, B cells become more abundant following IGF-1 administration in normal adult mice and also in those receiving lethal irradiation followed by reconstitution with syngeneic bone marrow (29). In addition, IGF-1 enhances IL-7-dependent B cell proliferation in concert with kit ligand (39). Studies conducted in vitro disclose that the differentiation of human CD34+ marrow cells is impeded by down-regulating the IGF-1 contributed by co-cultured MS-5 cells (46). These studies implicate IGFBP-6 as a necessary component of IGF-1-dependent B cell differentiation while IGFBP-3 may act as an inhibitor. Our examination of the bone marrow from a patient with GD discloses the ubiquitous nature of IGF-1R+ B cells within this tissue and suggests the importance of the receptor and the strong survival signals it appears to convey.
IGF-1 binds peripheral human B cells selectively (49), an association which is both saturable and can be displaced with unlabelled IGF-1 and insulin. Administration of IGF-1 to mice results in elevated circulating Ab levels (28, 47) Baudler and colleagues (50) reconstituted Rag2-deficient C57BL/6 mice with fetal liver cells from IGF-1R-/- mice. T cell independent humoral responses to the Type 2 antigen, 4-hydroxy-3-nitrophenyl acetyl Ficoll are substantially diminished while those against the T cell-dependent antigen, 4-hydroxy-3-nitrophenyl acetyl-chicken globulin appear normal. B cell development remains normal in IGF-1R deficient chimeras as does T cell differentiation. In these animals, IGF-1 enhanced immunoglobulin production, an effect that proved independent of B cell proliferation (50). These earlier findings appear congruent with our own observations where incubation of B cells with IGF-1 results in a substantial increase in IgG production without concomitant changes in IgM abundance. Furthermore, Kimata et al demonstrated that IGF-1 can enhance IgG subtype production in both tonsillar and peripheral B cells (40, 41). This effect was dependent at least in part on CD40 ligation. Thus, our results demonstrating relative over-production of IgG by IGF-1R-displaying B cells from patients with GD is consistent with previous studies. We also demonstrate that IGF-1R-displaying B cells produce activating anti-TSHR Abs and thus may participate specifically in disease pathogenesis. IGF-1R activation results in the recruitment of docking proteins including insulin receptor substrate (IRS) (43). This common pathway is shared by several hormone and cytokine receptors including IL-4R which play critical roles in B cell Ab production (51). IRS proteins mediate IGF-1 and IL-4 stimulated activation via the phosphatidylinositol 3-kinase (PI3 kinase) signaling pathway (52). PI3 kinase signaling positively regulates immunoglobulin production in vivo (53, 54), and thus increased expression of the IGF-1R may promote Ig production through this mechanism. Given the established role of IL-4R in this process, including class switching, and the intersecting signaling pathways shared with IGF-1R, it is possible that these receptors may promote Ig production through a shared mechanism.
IGF-1 enhances DNA synthesis in myeloma cell lines in vitro in the absence of IL-6 (55). Both IGF-I and IGF-II enhance the effects of IL-6 on plasma cell proliferation. The effects are absent in normal B cells, suggesting actions of IGF-1 may diverge in normal and malignant cells. Both IGF-IR and insulin receptors are expressed at higher levels in myeloma cell lines than in B lymphoblastoid lines (56). IGF-1 may also target plasma cell metabolism since RPMI 8226 cells express high affinity binding sites for both insulin and IGF-1 (57) and the latter increases receptor phosphorylation and PI3 kinase activation, enhances DNA synthesis, and up-regulates lactate production in these cells. Thus there exists substantial precedent for IGF-1, its receptor and the pathways it utilizes in cell signaling for playing an important regulatory role in B cells and their plasma cell derivatives.
We have previously reported that circulating GD-IgG is directed against IGF-1R and can be detected in almost all patients with the disease, but in very few control donors (15, 58, 59). When treated with IGF-1 or with GD-IgG, cultured orbital fibroblasts from patients with GD, but not their controls, synthesize high levels of IL-16 and RANTES, two powerful T cell chemoattractants (15). In addition, orbital fibroblasts from these patients synthesize increased levels of hyaluronan when treated with GD-IgG, an action mediated by IGF-1R (59). We have also reported previously that IGF-1R is expressed by a disproportionately large fraction of fibroblasts from patients with GD (15). We now demonstrate over-representation of B cells displaying the IGF-1R+ phenotype in patients with GD. Activation of these cells through thi receptor appears to promote B cell survival and antibody production, including those potentially providing a mechanistic link between orbital and thyroid processes. Taken in aggregate, our findings suggest that IGF-1R may play an important role in the pathogenesis of GD. This receptor must now be examined in a broader context of human autoimmunity. It remains possible that the relative abundance of IGF-1R+ B cells may represent a spectrum within the general population and that those individuals with higher levels might be more susceptible to GD and its allied diseases. Clearly our current findings, coupled with those reported previously concerning T cells suggests the need to examine other autoimmune diseases for similar alterations in lymphocyte phenotypes(35).
Acknowledgments
The authors are grateful to Ms. Debbie Hanaya for her expert assistance in the preparation of this manuscript and to Robert Goldberg MD for patient recruitment. We gratefully acknowledge the invaluable assistance of the Harbor-UCLA GCRC staff in their efforts in facilitating these studies.
Footnotes
This work was supported in part by National Institutes of Health grants EY008976, EY011708, DK063121, EY016339, RR00425, an unrestricted grant from Research to Prevent Blindness, a Research to Prevent Blindness Career Development Award, and the Bell Charitable Foundation.
References
- 1.Dorner T, Lipsky PE. Signalling pathways in B cells: implications for autoimmunity. Curr Top Microbiol Immunol. 2006;305:213–240. doi: 10.1007/3-540-29714-6_11. [DOI] [PubMed] [Google Scholar]
- 2.Martinez-Gamboa L, Brezinschek HP, Burmester GR, Dorner T. Immunopathologic role of B lymphocytes in rheumatoid arthritis: rationale of B cell-directed therapy. Autoimmun Rev. 2006;5:437–442. doi: 10.1016/j.autrev.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Prabhakar BS, Bahn RS, Smith TJ. Current perspective on the pathogenesis of Graves’ disease and ophthalmopathy. Endocr Rev. 2003;24:802–835. doi: 10.1210/er.2002-0020. [DOI] [PubMed] [Google Scholar]
- 4.Tandon N, Metcalfe RA, Barnett D, Weetman AP. Expression of the costimulatory molecule B7/BB1 in autoimmune thyroid disease. Quart J Med. 1994;87:231–236. [PubMed] [Google Scholar]
- 5.Sato S, Fujimoto M, Hasegawa M, Takehara K, Tedder TF. Altered B lymphocyte function induces systemic autoimmunity in systemic sclerosis. Mol Immunol. 2004;41:1123–1133. doi: 10.1016/j.molimm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 6.Bour-Jordan H, Salomon BL, Thompson HL, Santos R, Abbas AK, Bluestone JA. Constitutive Expression of B7-1 on B Cells Uncovers Autoimmunity toward the B Cell Compartment in the Nonobese Diabetic Mouse. J Immunol. 2007;179:1004–1012. doi: 10.4049/jimmunol.179.2.1004. [DOI] [PubMed] [Google Scholar]
- 7.El Fassi D, Nielsen CH, Hasselbalch HC, Hegedus L. The rationale for B lymphocyte depletion in Graves’ disease. Monoclonal anti-CD20 antibody therapy as a novel treatment option. Eur J Endocrinol. 2006;154:623–632. doi: 10.1530/eje.1.02140. [DOI] [PubMed] [Google Scholar]
- 8.Aggarwal BB, Shishodia S, Takada Y, Jackson-Bernitsas D, Ahn KS, Sethi G, Ichikawa H. TNF blockade: an inflammatory issue. Ernst Schering Res Found Workshop. 2006:161–186. doi: 10.1007/3-540-37673-9_10. [DOI] [PubMed] [Google Scholar]
- 9.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 10.Salmaso C, Olive D, Pesce G, Bagnasco M. Costimulatory molecules and autoimmune thyroid diseases. Autoimmunity. 2002;35:159–167. doi: 10.1080/08916930290013441. [DOI] [PubMed] [Google Scholar]
- 11.Yanaba K, Hamaguchi Y, Venturi GM, Steeber DA, Clair EW, St, Tedder TF. B cell depletion delays collagen-induced arthritis in mice: arthritis induction requires synergy between humoral and cell-mediated immunity. J Immunol. 2007;179:1369–1380. doi: 10.4049/jimmunol.179.2.1369. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen CH, El Fassi D, Hasselbalch HC, Bendtzen K, Hegedus L. B-cell depletion with rituximab in the treatment of autoimmune diseases. Graves’ ophthalmopathy the latest addition to an expanding family. Expert Opin Biol Ther. 2007;7:1061–1078. doi: 10.1517/14712598.7.7.1061. [DOI] [PubMed] [Google Scholar]
- 13.Chen CR, Pichurin P, Nagayama Y, Latrofa F, Rapoport B, McLachlan SM. The thyrotropin receptor autoantigen in Graves disease is the culprit as well as the victim. Journal of Clin Invest. 1897;111:1897–1904. doi: 10.1172/JCI17069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akamizu T. Monoclonal antibodies to thyroid specific autoantigens. Autoimmunity. 2003;36:361–366. doi: 10.1080/08916930310001603055. [DOI] [PubMed] [Google Scholar]
- 15.Pritchard J, Han R, Horst N, Cruikshank WW, Smith TJ. Immunoglobulin activation of T cell chemoattractant expression in fibroblasts from patients with Graves’ disease is mediated through the insulin-like growth factor I receptor pathway. J Immunol. 2003;170:6348–6354. doi: 10.4049/jimmunol.170.12.6348. [DOI] [PubMed] [Google Scholar]
- 16.Pritchard J, Horst N, Cruikshank W, Smith TJ. Igs from patients with Graves’ disease induce the expression of T cell chemoattractants in their fibroblasts. J Immunol. 2002;168:942–950. doi: 10.4049/jimmunol.168.2.942. [DOI] [PubMed] [Google Scholar]
- 17.Bateman JM, McNeill H. Insulin/IGF signalling in neurogenesis. Cell Mol Life Sci. 2006;63:1701–1705. doi: 10.1007/s00018-006-6036-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Binz K, Joller P, Froesch P, Binz H, Zapf J, Froesch ER. Repopulation of the atrophied thymus in diabetic rats by insulin-like growth factor I. Proc Natl Acad Sci U S A. 1990;87:3690–3694. doi: 10.1073/pnas.87.10.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zumkeller W. The insulin-like growth factor system in hematopoietic cells. Leuk Lymphoma. 2002;43:487–491. doi: 10.1080/10428190290011958. [DOI] [PubMed] [Google Scholar]
- 20.Reiss K, Porcu P, Sell C, Pietrzkowski Z, Baserga R. The insulin-like growth factor 1 receptor is required for the proliferation of hemopoietic cells. Oncogene. 1992;7:2243–2248. [PubMed] [Google Scholar]
- 21.Roldan A, Charreau E, Schillaci R, Eugui EM, Allison AC. Insulin-like growth factor-1 increases the mitogenic response of human peripheral blood lymphocytes to phytohemagglutinin. Immunol Lett. 1989;20:5–8. doi: 10.1016/0165-2478(89)90060-6. [DOI] [PubMed] [Google Scholar]
- 22.Kurmasheva RT, Houghton PJ. IGF-I mediated survival pathways in normal and malignant cells. Biochim Biophys Acta. 2006;1766:1–22. doi: 10.1016/j.bbcan.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Ahlen J, Wejde J, Brosjo O, von Rosen A, Weng WH, Girnita L, Larsson O, Larsson C. Insulin-like growth factor type 1 receptor expression correlates to good prognosis in highly malignant soft tissue sarcoma. Clin Cancer Res. 2005;11:206–216. [PubMed] [Google Scholar]
- 24.Zadik Z, Estrov Z, Karov Y, Hahn T, Barak Y. The effect of growth hormone and IGF-I on clonogenic growth of hematopoietic cells in leukemic patients during active disease and during remission--a preliminary report. J Pediatr Endocrinol. 1993;6:79–83. doi: 10.1515/jpem.1993.6.1.79. [DOI] [PubMed] [Google Scholar]
- 25.Kooijman R, Willems M, De Haas CJ, Rijkers GT, Schuurmans AL, Van Buul-Offers SC, Heijnen CJ, Zegers BJ. Expression of type I insulin-like growth factor receptors on human peripheral blood mononuclear cells. Endocrinology. 1992;131:2244–2250. doi: 10.1210/endo.131.5.1425423. [DOI] [PubMed] [Google Scholar]
- 26.Kooijman RK, Scholtens LE, Rijkers GT, Zegers BJ. Differential expression of type I insulin-like growth factor receptors in different stages of human T cells. Eur J Immunol. 1995;25:931–935. doi: 10.1002/eji.1830250411. [DOI] [PubMed] [Google Scholar]
- 27.Clark R, Strasser J, McCabe S, Robbins K, Jardieu P. Insulin-like growth factor-1 stimulation of lymphopoiesis. J Clin Invest. 1993;92:540–548. doi: 10.1172/JCI116621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jardieu P, Clark R, Mortensen D, Dorshkind K. In vivo administration of insulin-like growth factor-I stimulates primary B lymphopoiesis and enhances lymphocyte recovery after bone marrow transplantation. J Immunol. 1994;152:4320–4327. [PubMed] [Google Scholar]
- 29.Alpdogan O, Muriglan SJ, Kappel BJ, Doubrovina E, Schmaltz C, Schiro R, Eng JM, Greenberg AS, Willis LM, Rotolo JA, O’Reilly RJ, van den Brink MR. Insulin-like growth factor-I enhances lymphoid and myeloid reconstitution after allogeneic bone marrow transplantation. Transplantation. 2003;75:1977–1983. doi: 10.1097/01.TP.0000070167.81584.A2. [DOI] [PubMed] [Google Scholar]
- 30.Hettmer S, Dannecker L, Foell J, Elmlinger MW, Dannecker GE. Effects of insulin-like growth factors and insulin-like growth factor binding protein-2 on the in vitro proliferation of peripheral blood mononuclear cells. Hum Immunol. 2005;66:95–103. doi: 10.1016/j.humimm.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 31.Liu E, Law HK, Lau YL. Insulin-like growth factor I promotes maturation and inhibits apoptosis of immature cord blood monocyte-derived dendritic cells through MEK and PI 3-kinase pathways. Pediatr Res. 2003;54:919–925. doi: 10.1203/01.PDR.0000088067.04673.1B. [DOI] [PubMed] [Google Scholar]
- 32.Navarro M, Baserga R. Limited redundancy of survival signals from the type 1 insulin-like growth factor receptor. Endocrinology. 2001;142:1073–1081. doi: 10.1210/endo.142.3.7991. [DOI] [PubMed] [Google Scholar]
- 33.Landreth KS, Narayanan R, Dorshkind K. Insulin-like growth factor-I regulates pro-B cell differentiation. Blood. 1992;80:1207–1212. [PubMed] [Google Scholar]
- 34.Kim JH, Park HH, Lee CE. IGF-1 potentiation of IL-4-induced CD23/Fc(epsilon)RII expression in human B cells. Mol Cells. 2003;15:307–312. [PubMed] [Google Scholar]
- 35.Douglas RS, Gianoukakis AG, Kamat S, Smith TJ. Aberrant expression of the insulin-like growth factor-1 receptor by T cells from patients with graves’ disease may carry functional consequences for disease pathogenesis. J Immunol. 2007;178:3281–3287. doi: 10.4049/jimmunol.178.5.3281. [DOI] [PubMed] [Google Scholar]
- 36.Coligan J, Kruisbeek A, Marguiles D, Shevach E, Strober W, editors. Current Protocols in Immunology. John Wiley and Sons; 2006. [Google Scholar]
- 37.Ryan EP, Pollock SJ, Murant TI, Bernstein SH, Felgar RE, Phipps RP. Activated human B lymphocytes express cyclooxygenase-2 and cyclooxygenase inhibitors attenuate antibody production. J Immunol. 2005;174:2619–2626. doi: 10.4049/jimmunol.174.5.2619. [DOI] [PubMed] [Google Scholar]
- 38.Fan JL, Desai RK, Dallas JS, Wagle NM, Seetharamaiah GS, Prabhakar BS. High frequency of B cells capable of producing anti-thyrotropin receptor antibodies in patients with Graves’ disease. Clin Immunol Immunopathol. 1994;71:69–74. doi: 10.1006/clin.1994.1053. [DOI] [PubMed] [Google Scholar]
- 39.Gibson LF, Piktel D, Landreth KS. Insulin-like growth factor-1 potentiates expansion of interleukin-7-dependent pro-B cells. Blood. 1993;82:3005–3011. [PubMed] [Google Scholar]
- 40.Kimata H, Fujimoto M. Growth hormone and insulin-like growth factor I induce immunoglobulin (Ig)E and IgG4 production by human B cells. J Exp Med. 1994;180:727–732. doi: 10.1084/jem.180.2.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kimata H, Yoshida A. Differential effect of growth hormone and insulin-like growth factor-I, insulin-like growth factor-II, and insulin on Ig production and growth in human plasma cells. Blood. 1994;83:1569–1574. [PubMed] [Google Scholar]
- 42.Kimata H, Yoshida A. Effect of growth hormone and insulin-like growth factor-I on immunoglobulin production by and growth of human B cells. J Clin Endocrinol Metab. 1994;78:635–641. doi: 10.1210/jcem.78.3.8126135. [DOI] [PubMed] [Google Scholar]
- 43.De Meyts P, Palsgaard J, Sajid W, Theede AM, Aladdin H. Structural biology of insulin and IGF-1 receptors. Novartis Found Symp. 2004;262:160–171. discussion 171-166, 265-168. [PubMed] [Google Scholar]
- 44.Traggiai E, Becker S, Subbarao K, Kolesnikova L, Uematsu Y, Gismondo MR, Murphy BR, Rappuoli R, Lanzavecchia A. An efficient method to make human monoclonal antibodies from memory B cells: potent neutralization of SARS coronavirus. Nat Med. 2004;10:871–875. doi: 10.1038/nm1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paul W, editor. Fundamental Immunology. Fifth Edition Lippincott Williams and Wilkins; Philadelphia: 2003. [Google Scholar]
- 46.Taguchi T, Takenouchi H, Matsui J, Tang WR, Itagaki M, Shiozawa Y, Suzuki K, Sakaguchi S, Ktagiri YU, Takahashi T, Okita H, Fujimoto J, Kiyokawa N. Involvement of insulin-like growth factor-I and insulin-like growth factor binding proteins in pro-B-cell development. Exp Hematol. 2006;34:508–518. doi: 10.1016/j.exphem.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 47.Robbins K, McCabe S, Scheiner T, Strasser J, Clark R, Jardieu P. Immunological effects of insulin-like growth factor-I--enhancement of immunoglobulin synthesis. Clin Exp Immunol. 1994;95:337–342. doi: 10.1111/j.1365-2249.1994.tb06534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hinton PS, Peterson CA, Dahly EM, Ney DM. IGF-I alters lymphocyte survival and regeneration in thymus and spleen after dexamethasone treatment. Am J Physiol. 1998;274:R912–920. doi: 10.1152/ajpregu.1998.274.4.R912. [DOI] [PubMed] [Google Scholar]
- 49.Stuart CA, Meehan RT, Neale LS, Cintron NM, Furlanetto RW. Insulin-like growth factor-I binds selectively to human peripheral blood monocytes and B-lymphocytes. J Clin Endocrinol Metab. 1991;72:1117–1122. doi: 10.1210/jcem-72-5-1117. [DOI] [PubMed] [Google Scholar]
- 50.Baudler S, Baumgartl J, Hampel B, Buch T, Waisman A, Snapper CM, Krone W, Bruning JC. Insulin-like growth factor-1 controls type 2 T cell-independent B cell response. J Immunol. 2005;174:5516–5525. doi: 10.4049/jimmunol.174.9.5516. [DOI] [PubMed] [Google Scholar]
- 51.Keegan AD, Nelms K, White M, Wang LM, Pierce JH, Paul WE. An IL-4 receptor region containing an insulin receptor motif is important for IL-4-mediated IRS-1 phosphorylation and cell growth. Cell. 1994;76:811–820. doi: 10.1016/0092-8674(94)90356-5. [DOI] [PubMed] [Google Scholar]
- 52.Keegan AD, Nelms K, Wang LM, Pierce JH, Paul WE. Interleukin 4 receptor: signaling mechanisms. Immunol Today. 1994;15:423–432. doi: 10.1016/0167-5699(94)90272-0. [DOI] [PubMed] [Google Scholar]
- 53.Clayton E, Bardi G, Bell SE, Chantry D, Downes CP, Gray A, Humphries LA, Rawlings D, Reynolds H, Vigorito E, Turner M. A crucial role for the p110delta subunit of phosphatidylinositol 3-kinase in B cell development and activation. J Exp Med. 2002;196:753–763. doi: 10.1084/jem.20020805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Omori SA, Rickert RC. Phosphatidylinositol 3-kinase (PI3K) signaling and regulation of the antibody response. Cell Cycle. 2007;6:397–402. doi: 10.4161/cc.6.4.3837. [DOI] [PubMed] [Google Scholar]
- 55.Jelinek DF, Witzig TE, Arendt BK. A role for insulin-like growth factor in the regulation of IL-6-responsive human myeloma cell line growth. J Immunol. 1997;159:487–496. [PubMed] [Google Scholar]
- 56.Freund GG, Kulas DT, Way BA, Mooney RA. Functional insulin and insulin-like growth factor-1 receptors are preferentially expressed in multiple myeloma cell lines as compared to B-lymphoblastoid cell lines. Cancer Res. 1994;54:3179–3185. [PubMed] [Google Scholar]
- 57.Freund GG, Kulas DT, Mooney RA. Insulin and IGF-1 increase mitogenesis and glucose metabolism in the multiple myeloma cell line, RPMI 8226. J Immunol. 1993;151:1811–1820. [PubMed] [Google Scholar]
- 58.Smith TJ. The putative role of fibroblasts in the pathogenesis of Graves’ disease: evidence for the involvement of the insulin-like growth factor-1 receptor in fibroblast activation. Autoimmunity. 2003;36:409–415. doi: 10.1080/08916930310001603000. [DOI] [PubMed] [Google Scholar]
- 59.Smith TJ, Hoa N. Immunoglobulins from patients with Graves’ disease induce hyaluronan synthesis in their orbital fibroblasts through the self-antigen, insulin-like growth factor-I receptor. J Clin Endocrinol Metab. 2004;89:5076–5080. doi: 10.1210/jc.2004-0716. [DOI] [PubMed] [Google Scholar]