Abstract
Objective
The key initial step in atherogenesis is the subendothelial retention of apolipoprotein B-containing lipoproteins. Acid sphingomyelinase (acid SMase), an enzyme present extracellularly within the arterial wall, strongly enhances lipoprotein retention in model systems in vitro, and retained lipoproteins in human plaques are enriched in ceramide, an SMase product. We now sought to test a direct, causative role for acid SMase in lipoprotein retention and atherogenesis in vivo.
Methods and Results
We studied atherogenesis and lipoprotein retention in Asm−/− vs. Asm+/+ mice on the Apoe−/− and Ldlr−/− backgrounds. Asm−/−;Apoe−/− mice had a ~40–50% decrease in early foam cell aortic root lesion area compared with Asm+/+;Apoe−/− mice (P<0.05) despite no difference in plasma cholesterol or lipoproteins. To assay lipoprotein retention in vivo, the two groups of mice were injected with fluorescently labeled Apoe−/− lipoproteins. Early foam cell lesions of Asm−/−;Apoe−/− showed a striking 87% reduction in lipoprotein trapping (P<0.0001) compared with Asm+/+;Apoe−/− lesions. Similar results were obtained with Ldlr−/− mice, including an 81% reduction in lipoprotein retention within Asm−/−;Ldlr−/− lesions compared with Asm+/+;Ldlr−/− lesions (P<0.0005).
Conclusions
These findings support a causal role for acid SMase in lipoprotein retention and lesion progression and provides further support for the response-to-retention model of atherogenesis.
Keywords: Atherosclerosis-Pathophysiology, Animal models of human disease, Sphingomyelinase, Lipoprotein retention
The key initial step in early atherogenesis is the retention, or trapping, of apoB-lipoproteins within the subendothelium of focal, susceptible regions of the arterial tree.1–3 Retained and modified lipoproteins provoke a series of biologic responses that can explain all subsequent features of early atherogenesis.1,2 Lipoprotein retention within pre-lesional segments initially involves direct binding of positively charged domains on apoB to negatively charged elements of arterial matrix, chiefly proteoglycans 4,5. In later stages, lipoprotein retention can be enhanced further by size-related trapping of large lipoproteins in the subendothelium and by uptake by subendothelial macrophages (below). Moreover, lesional cells secrete additional molecules, such as sphingomyelinase and lipoprotein lipase, that are proposed to shift the molecular basis for further lipoprotein retention while also substantially accelerating retention and hence lesion progression. Thus, understanding the molecular mechanisms of subendothelial lipoprotein retention in pre-lesional and then lesional arteries is a critical goal of atherogenesis research.
Previous work has suggested a number of factors that can influence subendothelial lipoprotein retention, including (a) the concentration of circulating atherogenic lipoproteins; (b) endothelial permeability; (c) the nature and amounts of pro-retentive molecules within the subendothelial space, notably proteoglycans and lipoprotein lipase (LpL), which bridges lipoproteins to matrix; and (d) structure and composition of the lipoproteins, which can be altered by enzymatic and non-enzymatic processes within the subendothelial space.1–3
A large number of studies in vitro implicate the secretory form of acid sphingomyelinase (S-SMase) in pro-retentive modifications of atherogenic lipoproteins. S-SMase arises from the acid SMase (Asm) gene, which also gives rise to lysosomal acid SMase.6 S-SMase is secreted by cell types known to be in atherosclerotic lesions, particularly endothelial cells, where secretion is induced by atherogenic inflammatory cytokines.7,8 S-SMase hydrolyzes sphingomyelin (SM) to ceramide on the surface of atherogenic lipoproteins, which can occur at neutral pH with modified lipoproteins or in the acidic environment of lesion with native lipoproteins.9–11 The resulting increase in lipoprotein ceramide promotes lipoprotein aggregation,12 which can promote retention by increasing proteoglycan binding, impairing exit from lesions of large aggregates, and/or promoting uptake by arterial-wall macrophages, leading to foam cell formation.1,2,10,13–15 In addition, S-SMase-induced lipoprotein retention in vitro shows a remarkably robust synergy with LpL.15
Correlative support for a role of S-SMase in atherogenesis per se has been provided by several human and animal atherosclerosis studies. For example, extracellular acid SMase is present in human and murine atherosclerotic lesions,16 and aggregated lipoproteins extracted from human atheromata are specifically enriched in ceramide, indicating hydrolysis by sphingomyelinase.12 Moreover recent work demonstrated an association between high SM content in circulating lipoproteins, which enhances S-SMase-mediated hydrolysis, and increased risk for aortic atherosclerosis in mice and coronary artery disease in humans.17,18 In addition, inhibition of sphingomyelin synthesis in mice lowers lipoprotein sphingomyelin content and decreases atherogenesis.19
Nevertheless, no causal studies to examine the effect of direct genetic manipulation of acid SMase on lipoprotein retention and atherogenesis have appeared. Here we show that acid SMase deficiency in two well-studied hyperlipidemic models of murine atherosclerosis impedes lesion development and, most importantly, causes a striking decrease in arterial trapping of atherogenic lipoproteins. These findings provide important support for the pro-retentive role of acid SMase in early atherosclerosis and for the response-to-retention model of atherogenesis.
Materials and Methods
Please refer to online supplement for additional methods (http://www.ahajournals.org).
Mice
Asm+/− mice20 were crossed onto either the Apoe−/− or Ldlr−/− background (Jackson Laboratory, Bar Harbor, ME). All of these mice were of the C57BL6/J strain, and none of the alleles were linked. The resulting Asm+/−;Apoe−/− mice were mated to obtain the Asm+/+;Apoe−/− and Asm−/−;Apoe−/− mice used for this study. The pups were weaned at 21 days and fed on standard mouse chow until 10 weeks of age, at which point Apoe−/− mice have developed atherosclerotic lesions. Similarly, Asm+/−; Ldlr−/− were bred to obtain the Asm+/+; Ldlr−/− and Asm−/−; Ldlr−/− mice used for this study. These pups were weaned at 21 days and, starting at six weeks of age, the mice were fed on the “Western” diet (21% anhydrous milk fat, 0.15% cholesterol; cat. #TD88137, Harlan Teklad, Madison, WI) for 12 weeks. The maximum age of Asm−/− in this study, 18 weeks, does not allow the development of neurologic or other complications.20
Quantification of Subendothelial Lipoprotein Retention
Approximately 600 µg fluorescently-labeled lipoproteins were injected into the tail vein of each mouse. Eighteen hours after injection, the mice were anesthetized, the cardiac cavity was exposed, and the heart was extensively perfusion-fixed with 4% paraformaldehyde in PBS. The 18-h timepoint assesses lipoprotein retention, because it allows lipoprotein entry into the arterial wall, but then sufficient time for any untrapped lipoproteins to diffuse back out.21 The proximal and the thoracic aortas were removed, and frozen sections were prepared as above. The sections were placed on a slide, immersed in polyvinyl alcohol mounting medium with 1,4-diazabicyclo[2.2.2]octane (DABCO; Sigma–Aldrich, St. Louis, MO), sealed with a coverslip and nail polish, and stored at 4°C until analysis. Images were obtained with a Zeiss LSM-510 Meta scanning confocal microscope using a 40x objective. For fluorescence imaging, 543-nm excitation and an LP560 emission filters were used. Transmitted light images were collected using a DIC/brightfield detector. Images of each area were captured at the same laser intensity, gain, and offset to ensure consistency between sections from different mice. One-micron optical sections were obtained for each fluorescent lesional area. The fluorescence intensity and the lesional area were quantitated using ImageJ version 1.38x (NIH; http://rsb.info.nih.gov/ij/). For each optical section, the subendothelial space (with overlying endothelium) was defined as the region of interest (ROI). Brightfield images within the ROI were used to quantify lesion area, and fluorescence images within the ROI were used to quantify intensity from trapped, fluorescently labeled lipoproteins. For the latter quantification, we used both unweighted and weighted protocols. For the unweighted analysis, the number of pixels falling within the 40–255 gray value range for each optical section was determined and added together to give overall area of fluorescence. This value was then divided by the lesional area. The weighted analysis was performed by first determining the area of fluorescence within the ROI of each optical section for five fluorescence intensity value ranges: 40–84, 85–129, 130–174, 175–219, 220–255. These five area measurements were then multiplied by 1, 2, 3, 4, or 5, respectively, to give greater weight to areas of highest intensity. These weighted values were then summed for each optical section and divided by the lesional area.
Results
Reduced Aortic Early Foam Cell Lesion Area in Acid SMase-Deficient Apoe−/− Mice
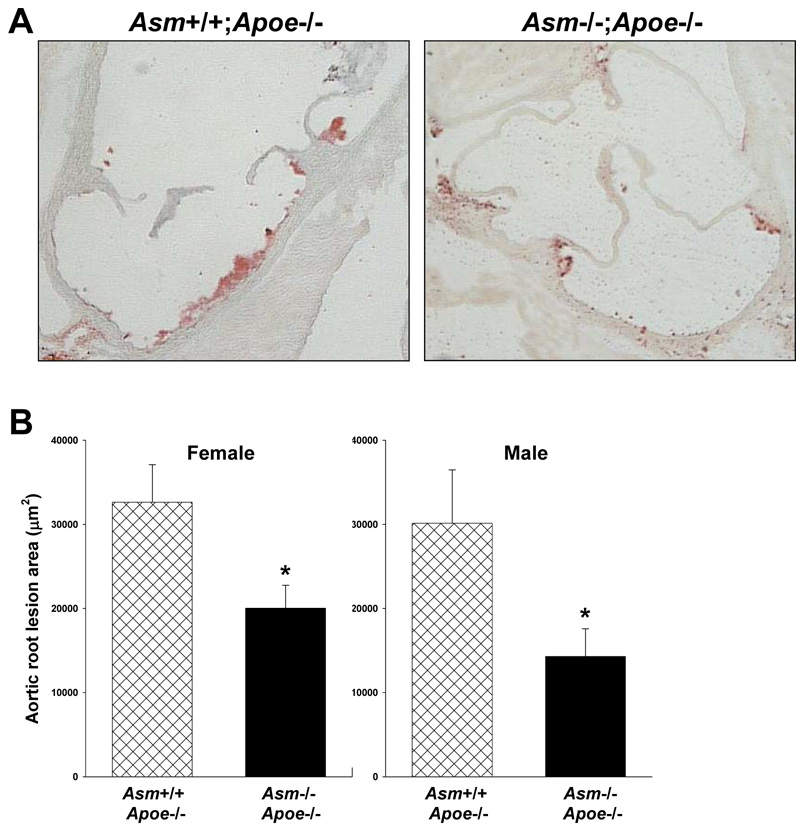
As expected, macrophages from Asm−/− mice have close to undetectable acid SMase activity, whereas those from wild-type mice have readily detectable activity (Supplementary Figure I). In preparation for atherosclerosis studies, we measured the plasma lipoproteins of 10-wk-old chow-fed Asm+/+;Apoe−/− and Asm−/−;Apoe−/− mice. As shown in Supplementary Figure IIA–B, total plasma cholesterol and lipoprotein-cholesterol profiles were indistinguishable between the two groups of mice, although the FPLC method used cannot distinguish between size differences in the largest particles in the void volume. There general appearance, behavior, and weights between the two groups were similar (Supplementary Figure IIC). These similarities allowed us to assess the hypothesized atherogenic role of acid SMase at the level of the arterial wall. As expected for chow-fed Apoe−/− mice of this age,22,23 the aortic root lesions of our Asm+/+;Apoe−/− showed small but distinct Oil Red O-positive foci in the immediate sub-endothelial area, consistent with very early foam cell lesions (Figure 1A, left image). In contrast, the aortic roots of the Asm−/−;Apoe−/− mice showed substantially smaller Oil Red O-positive areas (Figure 1A, right image). Quantitative area data from a large number of mice are shown in Figure 1B. There was a statistically significant 40–50% decrease in foam cell lesion area in both female and male Asm−/−;Apoe−/− mice compared with their sex-matched Asm+/+;Apoe−/− littermates. Thus, acid SMase deficiency is associated with a decrease in very early foam cell area. The effect of acid SMase deficiency on more advanced lesions in another model is described below.
Figure 1. Acid SMase deficiency is associated with smaller foam cell lesions in chow-fed Apoe−/− mice.
A, Oil Red O-stained aortic root sections from female Asm+/+;Apoe−/− and Asm−/−;Apoe−/− mice. B, Aortic root cross-sectional lesion quantification. *P<0.05.
Marked Decrease in Lipoprotein Retention within Aortic Root Lesions of Acid SMase-Deficient Apoe−/− Mice
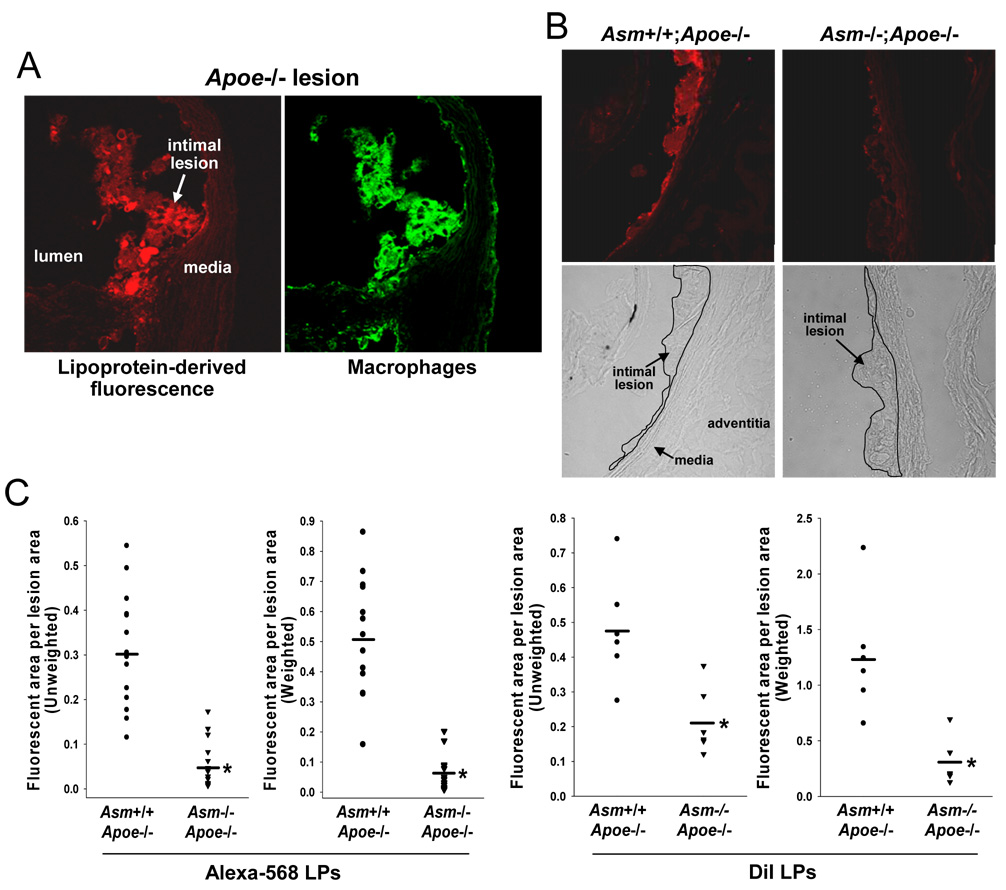
Previous mechanistic data with S-SMase, a product of the Asm gene, suggested a specific and unique mechanism that could account for the decreased lesion area in Asm−/−;Apoe−/− mice, namely, a decrease in lipoprotein retention.1,2,15 To directly test this hypothesis, mice from each group were injected with Alexa Fluor 568-labeled d<1.063 Apoe−/− lipoproteins, and then the lesions were examined 18 h later for accumulation of fluorescence.24 In preliminary experiments with a small number of Asm+/+;Apoe−/− mice, we analyzed the lesions by confocal microscopy for both Alexa Fluor 568 fluorescence (lipoproteins; red-orange emission) and for macrophages using an anti-Mac3 primary antibody and an Alexa Fluor 488 secondary antibody (green emission). As exemplified in Figure 2A, most of the red-orange fluorescence co-localized with green fluorescence, indicative of lipoprotein trapping either on extracellular matrix closely associated with macrophages, directly on the macrophage cell surface, or after phagocytosis of these lipoproteins by macrophages.
Figure 2. Acid SMase deficiency is associated with less subendothelial lipoprotein retention in Apoe−/− mice.
A, Confocal fluorescence images of an aortic root sections from an 8.5-wk/old Asm+/+;Apoe−/− mouse 18 h after injection with Alexa Fluor 568 (orange-red)-labeled d<1.063 lipoproteins from Apoe−/− mice. The sections were stained for macrophages using a green fluorescent secondary antibody. B, Confocal fluorescence and brightfield images of aortic root sections from an 8.5-wk/old Asm+/+;Apoe−/− mouse and a 12-wk/old Asm−/−;Apoe−/− mouse. C, Quantification of Alexa Fluor 568 and DiI fluorescence intensity. For the DiI study, mice were injected with DiI-labeled d<1.063 lipoproteins from Apoe−/− mice. *P<10−7 for Alexa and <0.005 for DiI‥
We next quantified total lipoprotein retention by analyzing Alexa Fluor 568 fluorescence in lesions from Asm+/+;Apoe−/− versus Asm−/−;Apoe−/− mice. The fact that atheromata develop properties that amplify subsequent lipoprotein retention25,26 presented a possible confounding factor. Thus, the smaller size of Asm−/−;Apoe−/− lesions (above) might by itself reduce lipoprotein retention. To overcome this potential bias, we conducted our experiment under conditions in which the two groups of mice had similar lesion areas. We accomplished this goal by comparing Asm−/−;Apoe−/− mice with slightly younger Asm+/+;Apoe−/− mice. In particular, we found that the average lesion area of 12-week-old Asm−/−;Apoe−/− mice was statistically identical to that of 8.5-week-old Asm+/+;Apoe−/−. We therefore compared aortic root fluorescence 18 h after injection of Alexa Fluor 568-labeled lipoproteins into these two groups of mice.
Similar to what was shown in Figure 2A, the aortic root lesions of 8.5-week-old Asm+/+;Apoe−/− mice accumulated red/orange fluorescence in subendothelial areas that corresponded to nascent foam cell lesions (Figure 2B). In striking contrast, similar-sized lesions of 12-week-old Asm−/−;Apoe−/− mice accumulated very little fluorescence. For each genotype, 15 separate lesional sites were analyzed, which represents 3 areas per aorta from five Asm+/+;Apoe−/− mice and 2–3 areas per aorta from six Asm−/−;Apoe−/− mice. We used two methods to quantify the fluorescence data (Figure 2C). In the first method (left panel), "unweighted" fluorescence encompassing the entire 40–255 gray value range of intensities was quantified as a single endpoint. In the second method ("weighted"; right panel), fluorescence of each image was divided into five intensity categories, and a greater score was given for the higher levels of fluorescence (see Materials and Methods for details). Both methods showed a ~80% decrease in fluorescence in the aortic root of Asm−/−;Apoe−/− mice compared with Asm+/+;Apoe−/− controls (P<0.0001).
Although SDS-PAGE of the Alexa-labeled lipoproteins showed no alteration in apoprotein profile compared with unlabeled lipoproteins (Supplementary Figure IIIA), these Alexa-labeled lipoproteins showed a slight increase in electronegativity as assessed by agarose gel electrophoresis (Supplementary Figure IIIB). We therefore repeated the in-vivo retention experiment using d<1.063 Apoe−/− lipoproteins labeled with DiI, which tags lipoprotein lipid instead of apolipoproteins, the component labeled by Alexa Fluor 568. As shown in the supplementary figure, this method of labeling did not alter the electrophoretic mobility of the lipoproteins. Using DiI-labeled lipoproteins, we found similar results to that obtained above with Alexa-labeled material: 76% decrease in the retained lipoproteins using the weighted method, P=0.004 (Figure 2D).
To evaluate the specificity of these striking differences in lipoprotein retention within aortic root lesions, we carried out a series of additional analyses and experiments. To assess focality, we examined the thoracic aorta, which is resistant to foam cell lesions in these young, chow-fed mice.22,23 We found that there was no detectable fluorescence in either group in this site (data not shown). Similarly, we found no difference in fluorescence accumulation in the spleens of the two groups of mice (ratio of fluorescence area:total imaged area was 0.37 ± 0.11 for Asm+/+;Apoe−/− spleen and 0.41 ± 0.10 for Asm−/−;Apoe−/− spleen; P=0.40). Thus, the site of differential lipoprotein retention correlated with the focal site of atherogenesis. We next considered the unlikely possibility that the fluorescent lipoproteins in the Asm−/−;Apoe−/− mice were being rapidly and extensively removed from the plasma soon after injection, e.g., into other organs or excretory routes, before having access to the aortic root. To evaluate this possibility, we measured plasma fluorescence at various intervals from the time of injection until the time of lesion analysis. We found no significant difference in the removal of fluorescence from plasma between the two groups of mice (data not shown). Thus, the data in Figure 2 indicate a true decrease in subendothelial lipoprotein retention in aortic root lesions of Asm−/−;Apoe−/− mice.
Marked Decrease in Lipoprotein Retention in Aortic Root Lesions of Acid SMase-Deficient Ldlr−/− Mice
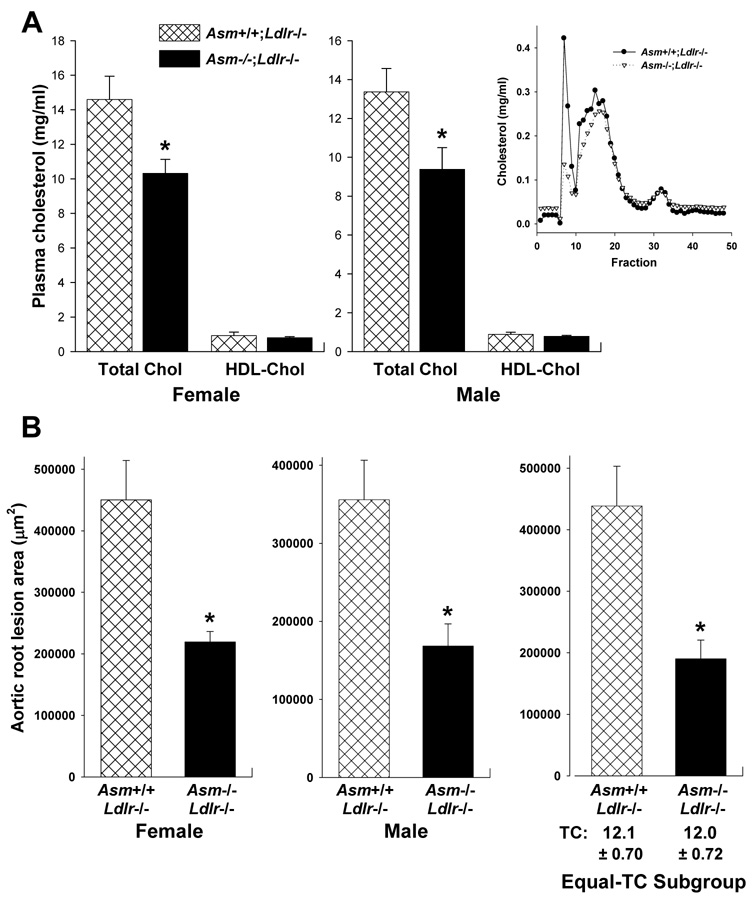
To determine whether acid SMase deficiency results in decreased lipoprotein retention in another model of early atherogenesis, we undertook a similar study in the LDL receptor-deficient model of murine atherosclerosis. For this study, Asm+/+;Ldlr−/− and Asm−/−;Ldlr−/− mice were fed on a Western-type diet for 12 wks. There were no differences in general appearance or behavior between the two groups, and the weights of the mice were not statistically different (Supplementary Figure IV). Total plasma cholesterol was approximately 25% lower in the Asm−/−;Ldlr−/− mice (Figure 3A). As can be seen from the lipoprotein profile (inset in Figure 3A), the difference in the cholesterol values between the two groups was due mostly to differences in a peak that corresponds to large lipoproteins, i.e., VLDL or chylomicrons. Note that the levels of LDL and HDL appeared similar in the two groups of mice. The SM content of d<1.063 plasma lipoproteins from the two groups of mice were not statistically different: 0.04 ± 0.01 vs. 0.06 ± 0.02 µg SM/µg cholesterol in Asm+/+;Ldlr−/− and Asm−/−;Ldlr−/− mice, respectively (P = 0.17). This finding is consistent with the concept that acid SMase hydrolyzes lipoproteins in the arterial wall, where subendothelial lipoprotein modification and acidic pH likely promote lipoprotein-SM hydrolysis.12 Moreover, analysis of apolipoproteins by SDS-PAGE and of lipoprotein charge by native gel electrophoresis of d<1.063 plasma lipoproteins from the two groups of mice revealed no marked differences (Supplementary Figure V).
Figure 3. Acid SMase deficiency is associated with smaller aortic root lesions in Western diet-fed Ldlr−/− mice.
A, Total plasma and HDL cholesterol concentrations for littermate female Asm+/+;Ldlr−/− and Asm−/−; Ldlr−/− mice and male Asm+/+;Ldlr−/− and Asm−/−; Ldlr−/− mice (n = 6, 9, 9, and 8 mice respectively). *P<0.05. Inset, FPLC lipoprotein profile for male mice. B, The left (female) and middle (male) graphs show the average aortic root lesion areas in Asm+/+;Ldlr−/− and Asm−/−; Ldlr−/− mice (n = 16 per genotype). The right graph shows a subgroup analysis of lesion area in male and female mice with statistically identical mean values of cholesterol (n = 8 per genotype). *P<0.001.
Aortic root lesion area was approximately 50% lower in Asm−/−;Ldlr−/− mice of both sexes (Figure 3B, first two graphs). We considered the possibility that this difference could be due to the lower plasma cholesterol in the Asm−/−;Ldlr−/− mice. Close inspection of cholesterol and lesion values for individual mice, however, revealed a poor correlation between variations in plasma cholesterol and variation in lesion area within each genotype, i.e., individual mice at the higher end of the plasma cholesterol distribution within each genotype did not necessarily have the largest lesions, nor vice versa. Moreover, a number of mice in the Asm−/−;Ldlr−/− group had plasma cholesterol values that were similar to those in the Asm+/+;Ldlr−/− group. We therefore conducted a subgroup analysis of lesion area in mice with statistically identical mean values of cholesterol (12.1 ± 0.70 in Asm+/+ vs. 12.0 ± 0.72 in Asm−/−, n = 8 per genotype). As shown in the right graph of Figure 3B, the lesion area of the Asm−/−;Ldlr−/− mice in this subgroup was 55% smaller than that of the Asm+/+;Ldlr−/− mice (P=0.001). This analysis suggests that the smaller lesion area in Asm−/−;Ldlr−/− mice cannot be explained at all by lower plasma cholesterol in these mice. Moreover, because the lesions in this experiment were approximately ten-fold larger than those in the previous experiment with young, chow-fed Apoe−/− mice, the data indicate that acid SMase plays a role in lesion development beyond the very early foam cell stage.
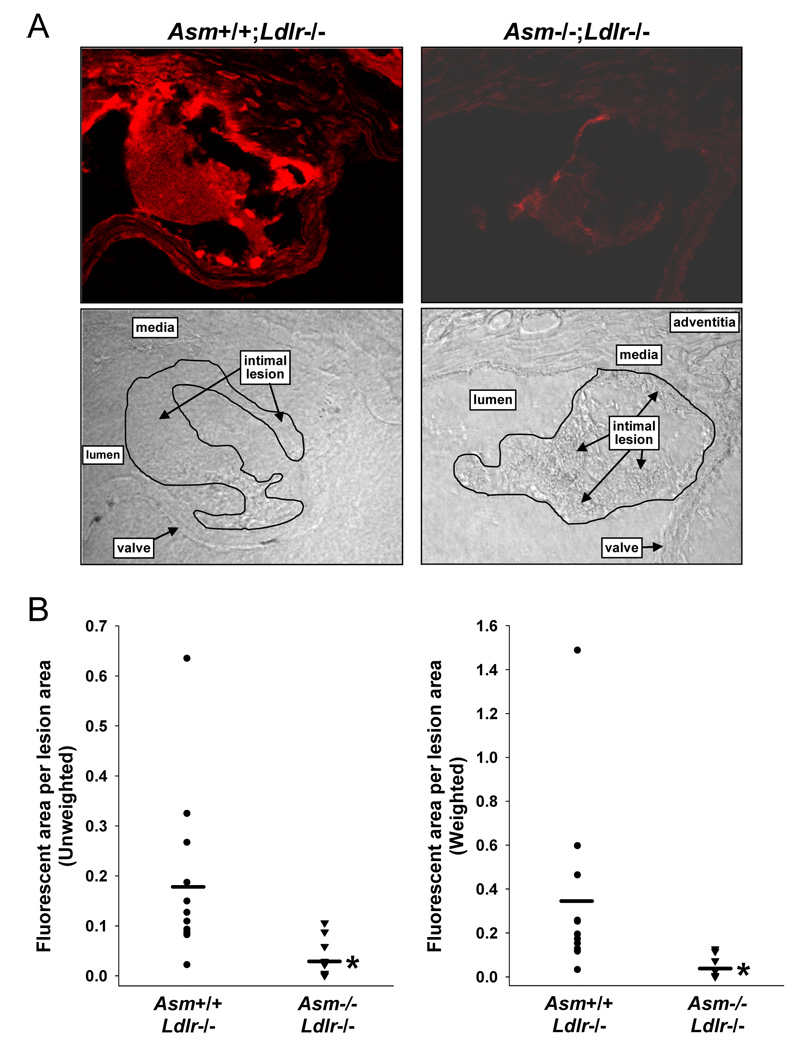
To assess lipoprotein retention in the Ldlr−/− model, we again focused on very early lesions and on lesions of similar size between the two genotypes to avoid the potential confounding issue of amplified lipoprotein retention within advanced lesions (above). Thus, Alexa Fluor 568-labeled d<1.063 lipoproteins from Ldlr−/− mice were injected into Asm+/+;Ldlr−/− mice after three weeks on the Western diet and into Asm−/−;Ldlr−/− mice after six weeks on the Western diet. This protocol produced lesions of similar size between the two genotypes, and mean lesion area in these mice was similar to that in the Apoe−/− study (above). As shown by representative confocal image in the left panels of Figure 4A, Ldlr−/− lesions showed ample retention of the Alexa Fluor568-labeled LDL. When these lesions were co-stained for perlecan, an arterial-wall proteoglycan implicated in lipoprotein retention 1–3, there was approximately 20% co-localization between the LDL (red) and perlecan (green) fluorescence (Supplementary Figure VI). Most importantly, analysis of Asm−/−;Ldlr−/− lesions for labeled LDL fluorescence showed a striking reduction compared with that seen in Asm+/+;Ldlr−/− lesions (right panels of Figure 4A). The unweighted and weighted quantified data revealed a 81% reduction in the acid SMase-deficient lesions (P<0.0005 for weighted analysis) (Figure 4B). The percent LDL-perlecan co-localization was similar in the two groups of mice (data not shown), and so the absolute amount of perlecan-associated labeled LDL was also ~80% reduced in the Asm−/−;Ldlr−/− lesions (see Discussion). Thus, acid SMase deficiency is associated with a striking reduction in lipoprotein retention within early lesions in both the Apoe−/− and diet-fed Ldlr−/− models of atherosclerosis.
Figure 4. Acid SMase deficiency is associated with less subendothelial lipoprotein retention in Western diet-fed Ldlr−/− mice.
A, Confocal fluorescence and brightfield images of aortic root sections from an Asm+/+;Apoe−/− mouse fed the Western diet for 3 wks and from an Asm−/−;Apoe−/− mouse fed the diet for 6 wks. B, Quantification of Alexa Fluor 568 fluorescence intensity in the lesions described in (A). *P≤0.001.
Discussion
Given the critical role of subendothelial lipoprotein retention in the initiation of atherogenesis,1,2 identifying individual molecules that affect this process in vivo is an important goal in atherosclerosis research. Borén and colleagues5 showed decreased lipoprotein retention in early lesions of mice expressing an apolipoprotein B100 transgene in which a proteoglycan-binding region was mutated by genetic engineering. This mode of LDL retention appears to be enhanced in the setting of elevated levels of angiotensin II, which increases the arterial content of pro-retentive proteoglycans and promotes atherogenesis.27 Importantly, Borén and colleagues5 also showed that lipoprotein retention was decreased in advanced lesions in apolipoprotein B100 transgenic mice lacking lipoprotein lipase, which can non-enzymatically mediate the binding of lipoproteins to subendothelial matrix molecules.26 These data suggest a model in which lipoprotein retention in pre-lesional, susceptible sites of the arterial wall is dominated by a specific apolipoprotein B-proteoglycan interaction, whereas LpL bridging becomes a more dominant process in lipoprotein retention once lesions start to become established. LpL is secreted by macrophages, which likely explains the role of LpL in retention in established lesions, i.e., after macrophage foam cells accumulate in the sub-endothelial space. In this regard, early atherosclerotic lesions also develop activated endothelium, which is an important source of S-SMase.7 Thus, in view of the role of acid SMase in lipoprotein retention in foam cell lesions shown here and our previous work showing LpL—S-SMase synergy in lipoprotein-matrix interaction and lipoprotein uptake by macrophages,15 the combined appearance of LpL and S-SMase once lesions develop may provide a molecular explanation for the fact that lipoprotein retention is greatly accelerated in lesions vs. susceptible pre-lesional sites.21
Previous work in vitro has suggested plausible hypotheses on the possible mechanisms by which lipoprotein retention and atherogenesis are decreased in Asm−/− lesions. In particular, secretory acid SMase induces lipoprotein aggregation, which can promote subendothelial retention by enhanced uptake by macrophages and by decreased arterial-wall exit of large lipoprotein aggregates.10,14,15 Moreover, sphingomyelinase-treated lipoproteins acquire increased affinity for subendothelial matrix.14 Regarding this latter point, we estimate that there was ~80% less LDL associated with perlecan in Asm−/−;Ldlr−/− lesions compared with Asm+/+;Ldlr−/−lesions based on combining the overall LDL retention data with the LDL-perlecan co-localization data. However, a substantial portion of the labeled LDL did not co-localize with perlecan, suggesting association with other matrix molecules and/or uptake by macrophages. The latter scenario is consistent with our data showing close association between labeled LDL and macrophages in the Apoe−/− lesions. Finally, it is formally possible that the absence of lysosomal acid SMase in our model could have contributed to the decrease in atherogenesis. However, it might have actually dampened our results, because the absence of lysosomal acid SMase in cholesterol-loaded macrophages decreases cholesterol efflux from these cells.28
The results herein provide the first molecular genetic causation evidence in support of a growing body of literature implicating acid SMase and sphingomyelin in atherogenesis and coronary artery disease in animal models and humans.9,12,16–19. Translation of this information into therapy, however, would have to take into account the fact that acid SMase deficiency in humans, which affects both the secreted and lysosomal forms of the enzyme, is associated with low HDL and elevated LDL in the plasma.29 Assuming this phenomenon reflects the effect of acid SMase deficiency in one or more non-arterial wall sites, therapy would have to be based on focal inhibition of the enzyme in the arterial wall, but presumably only its secreted form. Another approach would be to the follow the lead of a number of reports showing that treatment of mice with an inhibitor of sphingomyelin synthesis, which decreases the SM content of lipoproteins and thus their susceptibility to acid SMase-mediated hydrolysis, suppresses lesion development.19 Finally, to the extent that the study here adds support to the link between lipoprotein retention and atherogenesis, there may be promise for other therapeutic strategies directed against the interaction of apoB-containing lipoproteins with subendothelial matrix molecules.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by NIH/NHLBI Atherosclerosis SCOR grant HL-56984 (I.T.; K.J.W.), NHLBI grant HL-57560 (I.T.), NHLBI grant HL-73898 (K.J.W.), and NIDDK grant HD-28607 (E.H.S.). Confocal imaging was supported by NIH Grants S10-RR10506, S10-RR13701, P30-CA13696, and the Lieber Foundation. .
Footnotes
Disclosures
Drs. Tabas and Williams are co-inventors of patents on S-SMase. Dr. Schuchman is an inventor on patents claiming the acid SMase gene, recombinant acid SMase protein, and the diagnosis and treatment of acid SMase-deficiency.
References
- 1.Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arteriosclerosis, Thrombosis, and Vascular Biology. 1995;15:551–561. doi: 10.1161/01.atv.15.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116:1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 3.Gustafsson M, Flood C, Jirholt P, Borén J. Retention of atherogenic lipoproteins in atherogenesis. Cell Mol Life Sci. 2004;61:4–9. doi: 10.1007/s00018-003-3262-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hurt-Camejo E, Camejo G, Rosengren B, López F, Wiklund O, Bondjers G. Differential uptake of proteoglycan-selected subfractions of low density lipoprotein by human macrophages. Journal of Lipid Research. 1990;31:1387–1398. [PubMed] [Google Scholar]
- 5.Skålén K, Gustafsson M, Rydberg EK, Hulten LM, Wiklund O, Innerarity TL, Borén J. Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature. 2002;414:750–754. doi: 10.1038/nature00804. [DOI] [PubMed] [Google Scholar]
- 6.Schissel SL, Keesler GA, Schuchman EH, Williams KJ, Tabas I. The cellular trafficking and zinc dependence of secretory and lysosomal sphingomyelinase, two products of the acid sphingomyelinase gene. Journal of Biological Chemistry. 1998;273:18250–18259. doi: 10.1074/jbc.273.29.18250. [DOI] [PubMed] [Google Scholar]
- 7.Marathe S, Schissel SL, Yellin MJ, Beatini N, Mintzer R, Williams KJ, Tabas I. Human vascular endothelial cells are a rich and regulatable source of secretory sphingomyelinase. Implications for early atherogenesis and ceramide-mediated cell signaling. Journal of Biological Chemistry. 1998;273:4081–4088. doi: 10.1074/jbc.273.7.4081. [DOI] [PubMed] [Google Scholar]
- 8.Wong ML, Xie B, Beatini N, Phu P, Marathe S, Johns A, Gold PW, Hirsch E, Williams KJ, Licinio J, Tabas I. Acute systemic inflammation up-regulates secretory sphingomyelinase in vivo: a possible link between inflammatory cytokines and atherogenesis. Proc Natl Acad Sci U S A. 2000;97:8681–8686. doi: 10.1073/pnas.150098097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schissel SL, Jiang XC, Tweedie-Hardman J, Jeong TS, Camejo EH, Najib J, Rapp JH, Williams KJ, Tabas I. Secretory sphingomyelinase, a product of the acid sphingomyelinase gene, can hydrolyze atherogenic lipoproteins at neutral pH. Implications for atherosclerotic lesion development. Journal of Biological Chemistry. 1998;273:2738–2746. doi: 10.1074/jbc.273.5.2738. [DOI] [PubMed] [Google Scholar]
- 10.Marathe S, Choi Y, Leventhal AR, Tabas I. Sphingomyelinase converts lipoproteins from apolipoprotein E knockout mice into potent inducers of macrophage foam cell formation. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000;20:2607–2613. doi: 10.1161/01.atv.20.12.2607. [DOI] [PubMed] [Google Scholar]
- 11.Oorni K, Kovanen PT. Enhanced extracellular lipid accumulation in acidic environments. Current Opinion in Lipidology. 2006;17:534–540. doi: 10.1097/01.mol.0000245259.63505.c2. [DOI] [PubMed] [Google Scholar]
- 12.Schissel SL, Tweedie-Hardman J, Rapp JH, Graham G, Williams KJ, Tabas I. Rabbit aorta and human atherosclerotic lesions hydrolyze the sphingomyelin of retained low-density lipoprotein. Proposed role for arterial-wall sphingomyelinase in subendothelial retention and aggregation of atherogenic lipoproteins. Journal of Clinical Investigation. 1996;98:1455–1464. doi: 10.1172/JCI118934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nordestgaard BG, Wootton R, Lewis B. Selective retention of VLDL, IDL, and LDL in the arterial intima of genetically hyperlipidemic rabbits in vivo. Molecular size as a determinant of fractional loss from the intima-inner media. Arterioscler Thromb Biol. 1995;15:534–542. doi: 10.1161/01.atv.15.4.534. [DOI] [PubMed] [Google Scholar]
- 14.Oorni K, Posio P, Ala-Korpela M, Jauhiainen M, Kovanen PT. Sphingomyelinase induces aggregation and fusion of small very low-density lipoprotein and intermediate-density lipoprotein particles and increases their retention to human arterial proteoglycans. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25:1678–1683. doi: 10.1161/01.ATV.0000168912.42941.60. [DOI] [PubMed] [Google Scholar]
- 15.Tabas I, Li Y, Brocia RW, Wu SW, Swenson TL, Williams KJ. Lipoprotein lipase and sphingomyelinase synergistically enhance the association of atherogenic lipoproteins with smooth muscle cells and extracellular matrix. A possible mechanism for low density lipoprotein and lipoprotein(a) retention and macrophage foam cell formation. Journal of Biological Chemistry. 1993;268:20419–20432. [PubMed] [Google Scholar]
- 16.Marathe S, Kuriakose G, Williams KJ, Tabas I. Sphingomyelinase, an enzyme implicated in atherogenesis, is present in atherosclerotic lesions and binds to extracellular matrix. Arteriosclerosis, Thrombosis, and Vascular Biology. 1999;19:2648–2658. doi: 10.1161/01.atv.19.11.2648. [DOI] [PubMed] [Google Scholar]
- 17.Jiang XC, Paultre F, Pearson TA, Reed RG, Francis CK, Lin M, Berglund L, Tall AR. Plasma sphingomyelin level as a risk factor for coronary artery disease. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000;20:2614–2618. doi: 10.1161/01.atv.20.12.2614. [DOI] [PubMed] [Google Scholar]
- 18.Nelson JC, Jiang XC, Tabas I, Tall A, Shea S. Plasma sphingomyelin and subclinical atherosclerosis: findings from the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2006;163:903–912. doi: 10.1093/aje/kwj140. [DOI] [PubMed] [Google Scholar]
- 19.Hojjati MR, Li Z, Zhou H, Tang S, Huan C, Ooi E, Lu S, Jiang XC. Effect of myriocin on plasma sphingolipid metabolism and atherosclerosis in apoE-deficient mice. Journal of Biological Chemistry. 2005;280:10284–10289. doi: 10.1074/jbc.M412348200. [DOI] [PubMed] [Google Scholar]
- 20.Horinouchi K, Erlich S, Perl DP, Ferlinz K, Bisgaier CL, Sandhoff K, Desnick RJ, Stewart CL, Schuchman EH. Acid sphingomyelinase deficient mice: a model of types A and B Niemann-Pick disease. Nat Genet. 1995;10:288–293. doi: 10.1038/ng0795-288. [DOI] [PubMed] [Google Scholar]
- 21.Schwenke DC, Carew TE. Initiation of atherosclerotic lesions in cholesterol-fed rabbits: I. Focal increases in arterial LDL concentrations precede development of fatty streak lesions. Arteriosclerosis. 1989;9:895–907. doi: 10.1161/01.atv.9.6.895. [DOI] [PubMed] [Google Scholar]
- 22.Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb. 1994;14:133–140. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- 23.Reddick RL, Zhang SH, Maeda N. Atherosclerosis in mice lacking apo E. Evaluation of lesional development and progression. Arterioscler Thromb. 1994;14:141–147. doi: 10.1161/01.atv.14.1.141. [DOI] [PubMed] [Google Scholar]
- 24.Seo T, Qi K, Chang C, Liu Y, Worgall TS, Ramakrishnan R, Deckelbaum RJ. Saturated fatrich diet enhances selective uptake of LDL cholesteryl esters in the arterial wall. J Clin Invest. 2005;115:2214–2222. doi: 10.1172/JCI24327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwenke DC, Carew TE. Initiation of atherosclerotic lesions in cholesterol-fed rabbits. II. Selective retention of LDL vs. selective increases in LDL permeability in susceptible sites of arteries. Arteriosclerosis. 1989;9:908–918. doi: 10.1161/01.atv.9.6.908. [DOI] [PubMed] [Google Scholar]
- 26.Gustafsson M, Levin M, Skalen K, Perman J, Friden V, Jirholt P, Olofsson SO, Fazio S, Linton MF, Semenkovich CF, Olivecrona G, Boren J. Retention of low-density lipoprotein in atherosclerotic lesions of the mouse: evidence for a role of lipoprotein lipase. Circulation Research. 2007;101:777–783. doi: 10.1161/CIRCRESAHA.107.149666. [DOI] [PubMed] [Google Scholar]
- 27.Huang F, Thompson JC, Wilson PG, Aung HH, Rutledge JC, Tannock LR. Angiotensin II increases vascular proteoglycan content preceding and contributing to atherosclerosis development. Journal of Lipid Research. 2008;49:521–530. doi: 10.1194/jlr.M700329-JLR200. [DOI] [PubMed] [Google Scholar]
- 28.Leventhal AR, Chen W, Tall AR, Tabas I. Acid sphingomyelinase-deficient macrophages have defective cholesterol trafficking and efflux. Journal of Biological Chemistry. 2001;276:44976–44983. doi: 10.1074/jbc.M106455200. [DOI] [PubMed] [Google Scholar]
- 29.McGovern MM, Pohl-Worgall T, Deckelbaum RJ, Simpson W, Mendelson D, Desnick RJ, Schuchman EH, Wasserstein MP. Lipid abnormalities in children with types A and B Niemann Pick disease. J Pediatr. 2004;145:77–81. doi: 10.1016/j.jpeds.2004.02.048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.