Abstract
S-(Phenacyl)glutathione reductase (SPG-R) plays a significant role in the biotransformation of reactive α-haloketones to non-toxic acetophenones. Comparison of the apparent subunit size, amino-acid composition, and catalysis of the reduction of S-(phenacyl)glutathiones indicated that a previously described rat SPG-R (Kitada et al. (1985) J. Biol. Chem. 260,11749-11754) is homologous to the omega-class glutathione transferase GSTO1-1. The available data show that the SPG-R reaction is catalyzed by GSTO1-1 and not by other GSTs, including the closely related GSTO2-2 isoenzyme. In the proposed reaction mechanism, the active-site cysteine residue of GSTO1-1 reacts with the S-(phenacyl)glutathione substrate to give an acetophenone and a mixed disulfide with the active-site cysteine; a second thiol substrate (e.g., glutathione or 2-mercaptoethanol) reacts with the active-site disulfide to regenerate the catalytically active enzyme and to form a mixed disulfide. A new spectrophotometric assay was developed that allows the rapid determination of SPG-R activity and specific measurement of GSTO1-1 in the presence of other GSTs. This is the first specific reaction attributed to GSTO1-1, and these results demonstrate the catalytic diversity of GSTO1-1, which, in addition to SPG-R activity, catalyzes the reduction of dehydroascorbate and monomethylarsonate (V) and also possesses thioltransferase and GST activity.
Introduction
The cytosolic glutathione transferases are a large family of enzymes that play significant roles in both the detoxication and bioactivation of xenobiotics and the catabolism or synthesis of a number of important endogenous compounds (1). The recently identified omega-class glutathione transferases (GSTs) are widely distributed across a range of species from Caenorhabditis elegans to humans (2, 3). Two human omega-class genes have been characterized, and their RNA and protein (GSTO1-1 and GSTO2-2) products have been identified in multiple tissues (4). The omega-class GSTs are of considerable interest because of the discovery of their role in the reduction of methylated arsenic species and their genetic linkage to the age-at-onset of both Alzheimer's and Parkinson's diseases (5-9). An omega-class GST has also been implicated in the processing and release of the proinflammatory cytokine interlukin-1 (10). Both GSTO1-1 and GSTO2-2 have been expressed in Escherichia coli and characterized (6). The crystal structure of GSTO1-1 has been determined and revealed an active-site cysteine residue. The presence of an active-site cysteine residue differs significantly from the tyrosine or serine residue found in the active site of other mammalian cytosolic GSTs (2). Both human omega-class GSTs exhibit thioltransferase, dehydroascorbate reductase, and monomethylarsonate (V) reductase activities that are dependent on an active-site cysteine residue (3). These reactions are not catalyzed by other GST classes, and the dehydroascorbate reductase activity of GSTO2-2 is high, indicating that this may be its primary physiological function (6). Because of their involvement in novel reduction reactions and the association of the omega-class GSTs with neurological diseases that may be associated with exposure to environmental agents, we have investigated other potential substrates of physiological and toxicological interest.
α-Haloketones are important, biologically active chemicals that have a variety of uses and avenues for human exposure. Some α-haloketones have been identified as metabolites of insecticides (11), and 2-chloroacetophenone is used as a temporary incapacitating agent (tear gas) (12). α-Haloketones are also used in laboratory research as affinity labels and as active-site inhibitors of enzymes (13, 14). α-Haloketones react readily with sulfur nucleophiles, and 2-chloroacetophenone depletes glutathione concentrations in isolated rat hepatocytes (15).
Previous studies showed that the reduction of toxic α-haloketones to nontoxic acetophenones requires glutathione (11, 16-18). In the initially proposed reaction mechanism, α-haloketones react nonenzymatically with glutathione to yield S-(phenacyl)glutathiones, which are the true substrates of an enzyme that was termed S-(phenacyl)glutathione reductase (SPG-R) (18, 19). The enzyme was thought to catalyze the attack of a thiol on the sulfur atom of the S-(phenacyl)glutathione to yield a disulfide and a carbanion that is stabilized by enolization and yields the corresponding acetophenone after protonation (18) (Figure 1). SPG-R has been purified from rat hepatic cytosol and has a molecular weight of 30–37 kDa that is similar to the apparent subunit molecular weight of GSTO1 after SDS-PAGE (2, 19). Comparison of the amino acid composition of each enzyme also revealed a strong similarity.
Figure 1.
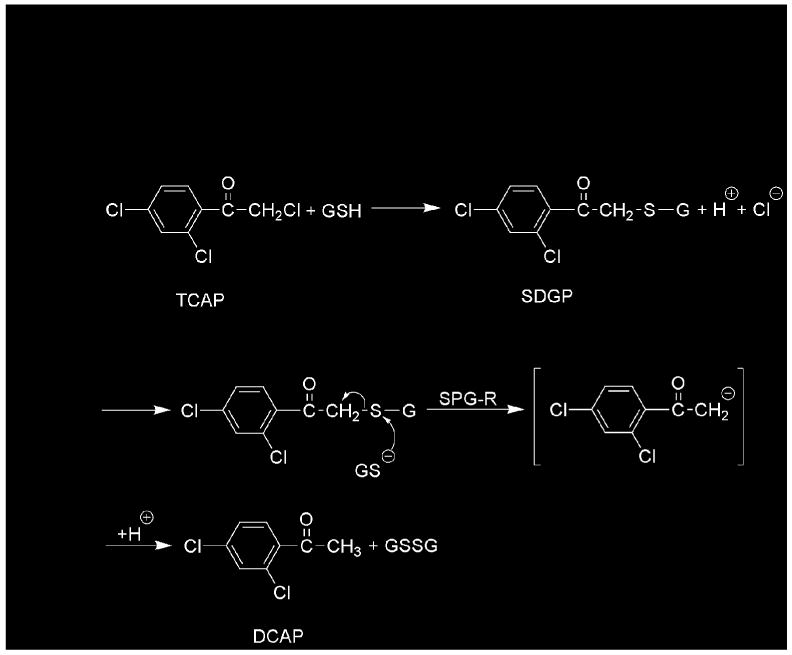
Reaction mechanism originally proposed for the glutathione-dependent, enzymatic reduction of 2,2′,4′-trichloroacetophenone (TCAP) to 2′,4′-dichloroacetophenone (DCAP). Modified from Ahktar (17) and Brundin et al. (20).
We report herein that GSTO1-1 has significant SPG-R activity and that the active-site Cys32 plays a critical role in the reaction. SPG-R activity was also characterized with a new, selective spectrophotometric assay. The data show that GSTO1-1 is likely homologous to the SPG-R that was previously purified from rat liver (19).
Materials and Methods
Enzyme purification
Human GSTO1-1 was expressed in E. coli (M15-rep4) from the plasmid pQE30 (QIAGEN, Hilden, Germany), as previously described (3). This expression vector generates recombinant enzymes with 6 histidine residues at the N-terminus to facilitate purification. The enzyme was purified by chromatography on Ni-agarose. After elution with imidazole, the purified enzyme was dialyzed against 20 mM Tris-HCl, 60 mM NaCl, pH 8, and concentrated to 2 mg mL-1 before storage at −20 °C. GSTO2-2 was also expressed in E. coli from a pQE30 plasmid. GSTO2-2 is prone to the formation of inclusion bodies in E coli and precipitation during purification. Soluble GSTO2-2 was successfully generated by the use of a low-temperature bacterial expression protocol and the application of a cyclic urea gradient refolding strategy (6).
Instrumental analyses
ESI-MS analyses were performed by direct injection with an Agilent LC/MSD ion-trap mass spectrometer (Agilent Technologies) with an electrospray interface operated in the positive-ion mode: dry temp, 325 °C; nebulizer, 10 psi; drying gas, 4 L min-1, skim, 40.5 V; capillary exit, -3000 V; and trap drive, 32.5. 1H NMR spectra were recorded with a Brüker 400 MHz spectrometer; samples were dissolved in [2H6]DMSO, and chemical shifts are reported in ppm downfield from TMS.
Synthesis of S-(phenacyl)glutathiones
S-(Phenacyl)glutathiones (Figure 2) were prepared by the method of Vince et al. (20) by reaction of glutathione (Sigma) with 2-chloroacetophenone 1a (Fluka), 2,2′,4′-trichloroacetophenone 2a, 2-chloro-4′-fluoroacetophenone 3a, or 3-chloropropiophenone 4a (Aldrich). The precipitated products were washed extensively with ice-cold water to remove residual glutathione, dried, and stored at −20 °C. The removal of residual glutathione was confirmed by the absence of a reaction of the S-(phenacyl)glutathiones with 5,5′-dithiobis-(2-nitrobenzoic acid). Before use, the S-(phenacyl)glutathiones were solubilized briefly in 1M NaOH before rapid neutralization with Tris-HCl.
Figure 2.
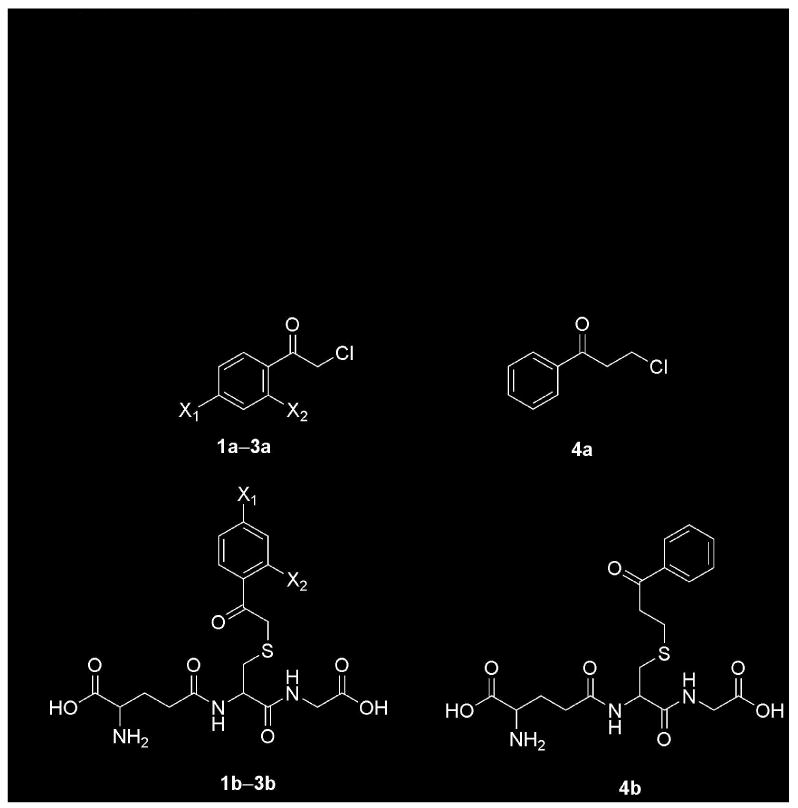
Structures of precursor α- and β-haloketones, S-(phenacyl)glutathiones, and S-(2-benzoylethyl)glutathione. 1a, 2-chloroacetophenone, X1 = X2 = H; 1b, S-(phenacyl)glutathione, X1 = X2 = H; 2a, 2,2′4′-trichloroacetophenone, X1 = X2 = Cl; 2b, S-(2′,4′-dichlorophenacyl)glutathione, X1 = X2 = Cl; 3a, 2-chloro-4′-fluoroacetophenone, X1 = F, X2 = H; 3b, S-(4′-fluorophenacyl)glutathione, X1 = F, X2 = H; 4a, 3-chloropropiophenone; 4b, S-(2-benzoylethyl)glutathione.
S-(Phenacyl)glutathione 1b
ESI-MS, m/z 448 [M + Na]+, m/z 470 [M + 2Na − H]+, m/z 510 [M + 3Na −2]+. NMR, 1.93 (2H, pentet (apparent), J = 6.8 Hz); 2.34 (2H, t, J = 7.2 Hz); 2.52 (2H, s); 2.69 (1H, dd, J = 9.6 Hz); 2.94 (1H, dd, J = 13.6); 3.39 (1H, t, J = 6.8 Hz); 3.72 (2H, m); 4.06 (1H, d, J = 15.2 Hz); 4.15 (1H, d, J = 15.2 Hz); 4.52 (1H, m); 7.54 (1H, t, J = 7.6 Hz); 7.66 (1H, t, J = 7.2 Hz); 7.99 (1H, d, J = 7.2 Hz); 8.46 (1H, d, J = 8.8 Hz); 8.62 (1H, t, J = 5.6 Hz).
S-(2′,4′-Dichlorophenacyl)glutathione 2b
ESI-MS, m/z 516 [M + Na]+, m/z 538 [M + 2Na − H]+, m/z 560 [M + 3Na − 2H]+. 1H NMR, 1.90 (2H, m); 2.32 (2H, m); 2.52 (2H, s); 2.67 (1H, dd, J = 13.6 Hz); 2.94 (1H, dd, J = 13.6 Hz); 3.4 (obscured by water peak); 3.72 (2H, s); 3.96 (2H, d, J = 15.6 Hz); 4.02 (d, J = 15.6 Hz), 4.48 (1H, m); 7.58 (1H, d, J = 6.4 Hz); 7.78, (1H, m); 8.70 (1H, t, J = 5.6 Hz).
S-(4-Fluorophenacyl)glutathione 3b
ESI-MS, m/z 466 [M + Na]+, m/z 488 [M + 2Na − H]+, m/z 510 [M + 3 Na − 2H]+. 1H NMR, 1.90 (2H, m); 2.32 (2H, m); 2.68 (1H, dd, J = 13.6, 9.6 Hz); 2.94 (1H, dd, J = 13.6, 4.4 Hz); 3.34 (1H, t, J = 7.0 Hz); 3.72 (2H, m); 4.05 (1H, d, J = 15.2 Hz); 4.14 (1H, J = 15.0 Hz); 4.50 (1H, m); 7.38 (1H, t, J = 7.6 Hz); 8.08 (1H, m); 8.44 (1H, d, J = 8.4 Hz); 8.66 (1H, m).
S-(2-Benzoylethyl)glutathione 4b
ESI-MS, m/z 462 [M + Na]+, m/z 484 [M + 2Na − H]+, m/z 506 [M + 3Na − 2H]+. 1H NMR, 1.92 (2H, m); 2.34 (2H, m); 2.68 (1H, dd, J = 14.0, 9.6 Hz); 2.83 (1H, t, J = 7.2 Hz); 2.83 (1H, t, J = 7.2 Hz); 2.96 (1H, dd, J = 14.0, 4.8 Hz); 3.36 (1H, m); 3.36 (2H, m); 3.72, (2H, s); 4.48 (1H, m); 7.54 (1H, m); 7.65 (1H, m); 7.99 (1H, J = 7.6 Hz), 8.42 (2H, d, J = 8.4 Hz); 8.69 (1H, J = 5.6 Hz).
Measurement of enzyme activities
Assay I. This spectrophotometric method relies on the glutathione reductase-catalyzed reduction of glutathione disulfide and the concomitant oxidation of NADPH; glutathione disulfide is formed by the GSTO1-1-catalyzed reaction of glutathione with an S-(phenacyl)glutathione. The reaction mixtures contained 1 mM 2,2′,4′-trichloroacetophenone 2a, 10 mM glutathione, 0.3 mM NADPH, 1.5 mM EDTA acid, 1 unit of glutathione reductase (Sigma), 100 mM potassium phosphate buffer, pH 7.4, and purified recombinant GSTO1-1 or GSTO2-2 in a final volume of 1 mL. The reaction mixture was incubated at 37 °C, and the rate of oxidation of NADPH was recorded at 340 nm. A mM absorptivity of 6.22 was used to calculate the reaction rate, which is expressed as μmol min-1 mg protein-1. In some experiments, other α-haloketones were used as substrates. Control reaction mixtures lacked GSTO1-1 or GSTO2-2.
Assay II. This spectrophotometric method records the reduction of the S-(phenacyl)glutathione substrate directly. Typically, the reaction mixtures contained 100 mM Tris, pH 8.0, 1.5 mM EDTA, 10 mM 2-mercaptoethanol or glutathione, 0.5 mM S-(phenacyl)glutathione, and purified recombinant GSTO1-1 or GST02-2. The reaction mixtures were incubated at 37 °C, and the absorbance was recorded from 250 to 400 nm. The wavelength of the maximum absorbance change for each S-(phenacyl)glutathione substrate was determined by inspection of the recorded spectra (Figure 3). A mM absorptivity was calculated by relating the absorbance change to the concentration of substrate determined independently by allowing Assay I to proceed to completion in the presence of GSTO1-1. The mM absorptivity represents the absorbance change of a 1mM solution of substrate with a 1cm light path. The wavelength of maximum absorbance and the mM absorptivities for each substrate were found to be; S-(Phenacyl)glutathione 1b, 271nm, -1.89 OD units; S-(2′,4′-dichlorophenacyl)glutathione 2b, 276nm, -1.48 OD units; S-(4′-fluorophenacyl)glutathione 3b, 266nm, -2.43 OD units.
Figure 3.

hGSTO1-1 catalyzed reduction of S-(phenacyl)glutathione 1b. S-(Phenacyl)glutathione 1b and 2-mercaptoethanol were incubated at 37 °C, and spectra were recorded at 1 min intervals, as described in Assay II (see Materials and Methods).
The pH dependency of the reaction was determined with a series of buffers used previously for the characterization of glucose 6-phosphate dehydrogenase variants (21). For the determination of kinetic constants, the concentration of S-(phenacyl)glutathione was varied between 1 and 0.03 mM. The inhibition of the reaction by glutathione was studied by varying the concentration of glutathione between 0 and 10 mM and varying the concentration of S-(phenacyl)glutathione between 1 and 0.03 mM; the 2-mercaptoethanol concentration was kept constant at 10 mM.
Glutathione transferase activity with 1-chloro-2,4-dinitrobenzene (CDNB) and glutathione as substrates was determined by a previously described spectrophotometric method (22).
Protein determinations
Protein concentrations were measured by the method of Bradford (23) with bovine serum albumin as the standard.
Results
GSTO1-1 has SPG-R activity
We first noted that the reported subunit molecular size of rat liver SPG-R after SDS-PAGE was 30–37 kDa (19), which is similar to the apparent subunit size of human GSTO1-1 (31 kDa) determined under similar conditions (2). Further comparison revealed that the amino-acid composition of rat SPG-R was similar to the amino-acid composition of rat GSTO1 deduced from a cDNA clone (24), but differed from the composition of other GSTs (25) (Table 1). There were some minor differences in the His, Ile, Pro and Arg content but these variations may be attributed to experimental error in the determination of the rat SPG-R values. Because glutathione is a substrate for rat SPG-R and because of the physical similarities of SPG-R with GSTO1-1, we postulated that SPG-R and GSTO1-1 may be identical. To test this hypothesis, we tested recombinant human GSTO1-1 for SPG-R activity with Assay I (see Materials and Methods). In an initial experiment, significant activity was detected when 2,2′,4′-trichloroacetophenone 2a and glutathione were used as substrates (Table 2). Further studies with additional α-haloketones showed that whereas 2-chloroacetophenone 1a and 2-chloro-4′-fluoroacetophenone 3a were also substrates, no activity was observed with 3-chloropropiophenone 4a as the substrate, indicating that the reaction is selective for α-haloketones (Table 2). The previously described rat SPG-R exhibited similar substrate selectivity (19). Several previously identified polymorphic variants of hGSTO1-1 were tested, including variants with a deletion of Glu155. All the naturally occurring isoforms of hGSTO1-1 showed similar specific activities in Assay I (Table 3). In contrast, a C32A mutant, which lacked the active-site Cys32, was inactive.
Table 1. Comparison of the Amino-acid Composition of Rat Hepatic S-Phenacylglutathione Reductase, Rat GSTO1-1, and Human GSTZ1a.
| Residue | S-PGR | rGSTO1 |
|---|---|---|
| Ala | 17.8 | 16 |
| Cys | 4 | 5 |
| Asp | 21.3 | 11 (19) |
| Glu | 29.5 | 22 (29) |
| Phe | 16.8 | 14 |
| Gly | 15 | 12 |
| His | 8.9 | 5 |
| Ilu | 8.2 | 13 |
| Lys | 22.6 | 21 |
| Leu | 20.5 | 24 |
| Met | 5.6 | 8 |
| Asn | - | 8 |
| Pro | 13 | 17 |
| Gln | - | 7 |
| Arg | 13.1 | 10 |
| Ser | 16 | 16 |
| Thr | 10.6 | 10 |
| Val | 7.3 | 9 |
| Trp | - | 4 |
| Tyr | 9.9 | 9 |
Table 2. Substrate Selectivity of the S-(Phenacyl)glutathione Reductase Activity of GSTO1-1a.
| Substrate | Activity
(μmol min-1 mg protein-1) |
|---|---|
| 2-Chloroacetophenone 1a | 2.96 ± 0.13 |
| 2,2′,4′-Trichloroacetophenone 2a | 0.82 ± 0.03 |
| 2-Chloro-4′-fluoroacetophenone 3a | 2.67 ± 0.13 |
| 3-Chloropropiophenone 4a | <0.01 |
Data are shown as means ± SD of at least 3 observations. Rates were determined with Assay I.
Table 3. S-(2′,4′-Dichlorophenacyl)glutathione Reductase Activity of Human GSTO1-1 Variantsa.
| Enzyme | Specific activity
(μmol GSSG formed min-1 mg protein-1) |
|---|---|
| hGSTO1-1 | 1.3 ± 0.1 |
| hGSTO1-1 ΔE155, E208 | 1.3 ± 0.05 |
| hGSTO1-1 ΔE155, K208 | 1.1 ± 0.03 |
| hGSTO1-1 K208 | 1.1 ± 0.07 |
| hGSTO1-1 D140 | 1.1 ± 0.05 |
| hGSTO1-1 A32 | <0.01 |
| rat SPG-R | 1.0 ± 0.03 |
The substrate was 2,2′,4′-trichloroacetophenone 2a; reaction rates were determined with Assay I (Materials and Methods). The data are expressed as means ± SD. Reported rat hepatic SPG-R activity (19) is shown for comparison.
Development of Assay II
The previous study of rat SPG-R noted that if S-(phenacyl)glutathiones were used as the primary substrate, other thiols, such as 2-mercaptoethanol or cysteine, could replace glutathione. To determine if this was also true for the reaction catalyzed by GSTO1-1, we developed a new direct spectrophotometric method that does not depend on the formation of glutathione disulfide; a reaction was detected in the absence of glutathione. We used the reagents listed in Assay II (see Materials and Methods) and recorded the absorbance spectra from 240 to 400 nm at timed intervals. This experiment revealed a time-dependent decrease in absorbance at 271 nm (Figure 3). In the absence of hGSTO1-1, little change in absorbance at 271 nm over the same time period was observed, indicating that the nonenzymatic reaction is negligible. The absorbance minimum and absorptivity were determined for S-(phenacyl)glutathione 1b, S-(2,4-dichlorophenacyl)glutathione 2b, and S-(4-fluorophenacyl)glutathione 3b (see methods).
Glutathione is not essential for the SPG-R reaction
When 2-mercaptoethanol was substituted for glutathione in Assay II, hGSTO1-1 showed SPG-R activity; indeed, the specific activity was significantly increased compared with glutathione (Table 4). The increase in specific activity was highest (15-fold) with S-(2,4-dichlorophenacyl)glutathione 2b as the primary substrate. Further kinetic analysis of the two reactions revealed a 5-fold increase in the kcat when 2-mercaptoethanol was used in the place of glutathione in reactions with S-(phenacyl)glutathione 1b as a substrate (Table 5). The kinetic analysis of the reaction was complicated by inhibition of activity when the S-(phenacyl)glutathione 1b concentration was 1 mM or higher. The Km for glutathione in Assay II was high (>30 mM) and could not be accurately determined because of difficulty in saturating the enzyme. These results are similar to the previous observation that thiols, such as 2-mercaptoethanol and cysteine, can replace glutathione in the reduction of S-(phenacyl)glutathiones by rat liver SPG-R (19). This finding supports the view that the previously described rat SPG-R is probably an omega-class GST.
Table 4. Substrate Selectivity of the S-Phenacylglutathione Reductase Activity of hGSTO1-1a.
| Substrate | Glutathione
(μmol mg protein-1 min-1) |
2-Mercaptoethanol
(μmol mg protein-1 min-1) |
|---|---|---|
| S-(Phenacyl)glutathione 1b | 3.9 ± 0.33 | 11.1 ± 0.1 |
| S-(2′,4′-Dichlorophenacyl)glutathione 2b | 1.2 ± 0.01 | 15.3 ± 0.26 |
| S-(4-Fluorophenacyl)glutathione 3b | 3.6 ± 0.36 | 7.4 ± 0.15 |
| S-(Propiophenacylglutathione 4b | <0.01 | <0.01 |
Reaction rates were determined with Assay II (Materials and Methods). Data are shown as means ± SD of at least 3 observations.
Table 5. Kinetic Parameters for GSTO1-1 with S-(Phenacyl)glutathione 1b and Glutathione and 2-Mercaptoethanol as Thiol Substratesa.
| Glutathione | 2-Mercaptoethanol | |||||
|---|---|---|---|---|---|---|
|
kcat (S-1) |
KmSPG (mM) |
kcat/Km (M-1 S-1) |
kcat (S-1) |
KmSPG (mM) |
kcat/Km (M-1 S-1) |
|
| GSTO1-1 | 0.8 ± 0.4 | 0.062 ± 0.01 | 1.3 × 104 | 5.8 ± 0.47 | 0.33 ± 0.05 | 1.76 × 104 |
Reaction rates were determined with Assay II (Materials and Methods). Data are shown as means ± SD of at least 3 observations.
Cys32 is required for the SPG-R activity of GSTO1-1
Previous crystallographic and mutagenic studies demonstrated that Cys32 is located in the active site of hGSTO1-1 and is essential for its thioltransferase and monomethylarsonate reductase activities. We found that a C32A mutant of GSTO1-1 lacked SPG-R activity, thereby confirming that Cys32 is the primary active-site residue (Table 3).
Proposed reaction mechanism
The proposed reaction mechanism shown in (Figure 4) indicates that the enzyme binds S-(phenacyl)glutathiones at the glutathione binding site and that the active-site Cys32 thiol attacks the thiol of the S-(phenacyl)glutathione substrate to give the enolate of acetophenone 5 and a mixed disulfide with the glutathione and Cys32 of GSTO1-1. In the second step, a thiol, such as glutathione (in vivo) or 2-mercaptoethanol in Assay II, reacts with the active-site disulfide formed by reaction of a S-(phenacyl)glutathione with Cys32 and forms a disulfide with the displaced glutathione. The enolate of acetophenone is readily protonated to give the observed acetophenone 6 (26). Previous studies demonstrated that the acetophenone formed in the reaction is labeled with a proton from the medium and that glutathione disulfide is formed when glutathione is incubated with 2,2′,4′-trichloroacetophenone 2a (18). Furthermore, there is chemical precedent for the reduction of α-carbonyl sulfides, e.g., S-(phenacyl)glutathiones, by thiolates (27)
Figure 4.

Proposed reaction mechanism for the hGSTO1-1-catalyzed reduction of S-(phenacyl)glutathiones to acetophenones. 1b, S-(phenacyl)glutathione, 2b, S-(2′,4′-dichlorophenacyl)glutathione, 3b, S-(4′-fluorophenacyl)glutathione, 5, keto-enol forms of product acetophenones, 6, product acetophenones.
The possibility that glutathione or the glutathione-2-mercaptoethanol mixed disulfide product of the reaction in Assay II acts as an inhibitor of the reaction by nonproductively competing with S-(phenacyl)glutathione 1b for the glutathione binding site on GSTO1-1 was considered. Such inhibition could explain the low SPG-R activity when glutathione was used as a cosubstrate. Similarly, the increased formation of glutathione disulfide might also explain the inhibition of Assay II by high concentrations of S-(phenacyl)glutathione 1b. When glutathione was added to Assay II along with 2-mercaptoethanol, glutathione was a weak mixed inhibitor (Ki ≈ 25mM). This degree of inhibition is probably insufficient to explain the relatively low activities observed when glutathione is used as the thiol cosubstrate.
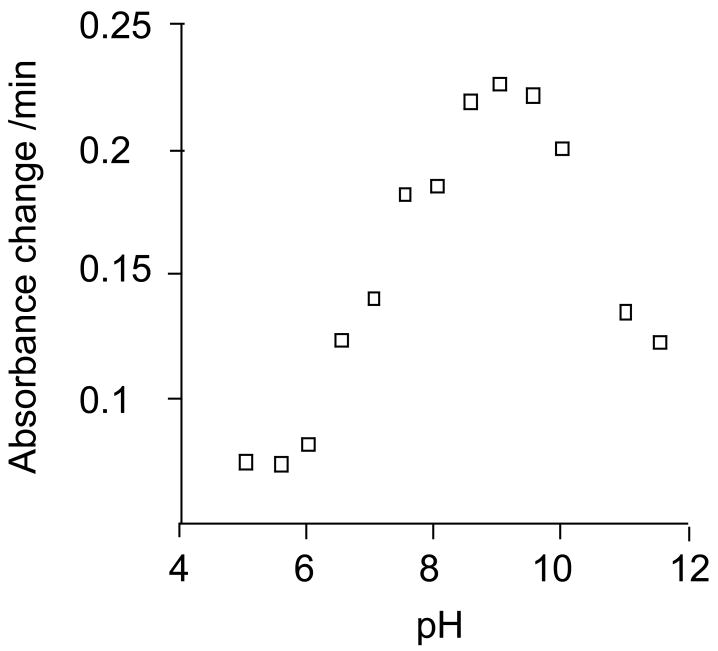
Determination of the pH optimum of the reaction
A range of buffers was used to determine the SPG-R activity of GSTO1-1 from pH 5 to pH 11.5. The data in Figure 5 show that the optimum activity was found at approximately pH 9, which is consistent with the pKa of glutathione in solution of 8.63 and is also consistent with the proposal that the reduction of the Cys32-glutathione mixed disulfide (Figure 4) by glutathione or 2-mercaptoethanol may be the rate limiting step in the reaction. It is likely that the decreasing activity at higher pH results from conformational changes and increasing instability of the enzyme.
Figure 5.
Effect of pH on the SPG-R activity of hGSTO1-1. SPG-R activity was determined with S-(phenacyl)glutathione 1b and 2-mercaptoethanol as substrates in Assay II. Details of the buffers used have been described (21).
SPG-R activity of other cytosolic GSTs
It was of interest to determine whether other cytosolic GSTs exhibited SPG-R activity. GSTO2-2 has 64 % amino acid identity to GSTO1-1 including an active-site Cys residue. No SPG-R activity could be detected with recombinant hGSTO2-2 (data not shown). Furthermore, chloride intracellular channel protein 2 (CLIC-2), which is related to the omega-class GSTs and has a Cys residue in an analogous active-site region (28), was studied; again, this protein lacked detectable SPG-R activity (data not shown). Other GST isoenzymes that have tyrosine or serine in their active site were predicted to lack SPG-R activity. This prediction was confirmed by the observation of the lack of SPG-R activity for hGSTA1-1, hGSTA3-3, hGSTA4-4, hGSTM1-1, hGSTM2-2, hGSTM3-3, hGSTT2-2, and hGSTZ1-1(data not shown).
SPG-R activity in tissue extracts
The apparent selectivity of the SPG-R reaction catalyzed by GSTO1-1 indicated that S-(phenacyl)glutathiones may be useful as selective substrates for the measurement of GSTO1-1 activity in the presence of other GSTs. Hence, SPG-R activity was quantified with Assay II and S-(2′,4′-dichlorophenacyl)glutathione 2b as the substrate in soluble extracts of the T47-D breast cancer cell line. Unlike other breast cancer cell lines, T47-D cells do not express GSTO1-1 (29). SPG-R activities were compared in T47-D cells that had been transfected with pcDNA3 (control) and in T47-D cells that had been transfected with GSTO1 cDNA in the pcDNA3 expression vector. As shown in Figure 6, T47-D control cells showed no immunologically detectable GSTO1 protein and lacked detectable SPG-R activity. In contrast, the Western blot shows the presence of GSTO1-1 expression, and significant SPG-R activity was observed in cells transfected with GSTO1 cDNA. The bulk GST activity with CDNB as the substrate represents the combined activity of alpha-, mu-, and pi-class GSTs present in T47-D cells. Despite the presence of other GSTs that catalyze the reaction with CDNB, only cells that express GSTO1-1 show SPG-R activity.
Figure 6.
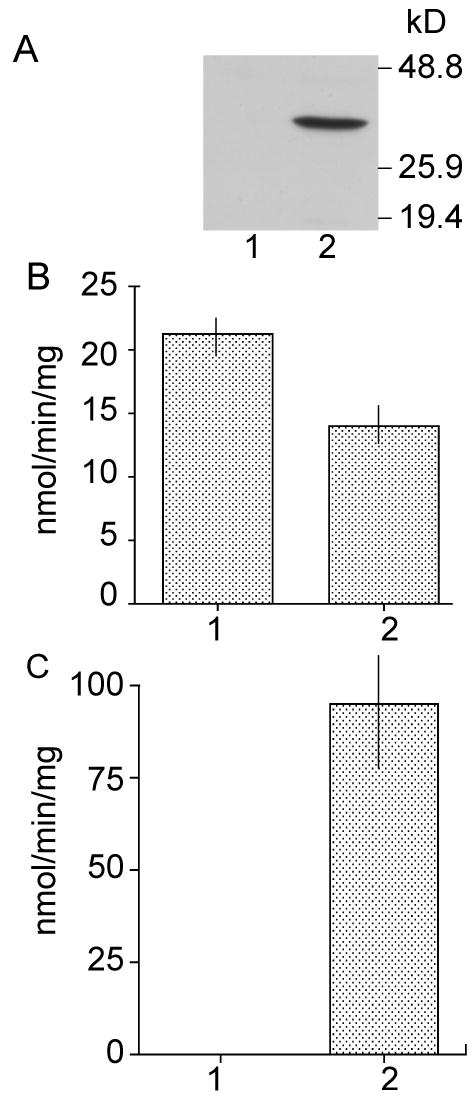
Expression of hGSTO1-1 in T47-D breast cancer cells. Panel A, Western blot of cell extracts probed with antiserum to human recombinant hGSTO1-1. Panel B, GST activity of cell extracts with CDNB as the substrate. Panel C, SPG-R activity of cell extracts determined with Assay II. 1, T47-D cells transfected with pcDNA3. 2, T47-D cells transfected with hGSTO1cDNA cloned in pcDNA3. Data are shown as means ± SD.
Previous studies of several mouse tissues indicated that GSTO1 mRNA is relatively abundant in the liver and kidney (3). When SPG-R activity was measured in liver and kidney extracts from BALBc mice with S-(2′,4′-dichlorophenacyl)glutathione 2b as the substrate, activities that ranged from 15 to 30 nmol min-1 mg protein-1 were observed. This level of SPG-R activity is at the limit of reliable detection with crude unpurified samples. Thus, although this method appears to be selective for SPG-R activity, it is not sufficiently sensitive to determine the activity of endogenous GSTO1-1 in crude tissue extracts.
Discussion
Comparison of subunit size and amino-acid composition as well as their capacity to catalyze the SPG-R reaction strongly indicate that the enzyme previously described as SPG-R and GSTO1-1 are the same. Specifically, the activity of human GSTO1-1 with 2,2′,4′-trichloroacetophenone 2a and glutathione as substrates (0.82 units/mg) is similar to that reported for rat SPG-R (1.0 units/mg) (19). In addition, both enzymes can use a thiol other than glutathione in reactions with S-(phenacyl)glutathiones, indicating a similar reaction mechanism. Unfortunately, purified rat enzyme and antiserum to rat SPG-R are no longer available to enable an immunological comparison.
This study has revealed a novel enzymatic activity for GSTO1-1 that appears to be highly selective and distinct from reactions catalyzed by other members of the cytosolic GST structural family. Even GSTO2-2, a member of the same evolutionary class and the most closely related GST that also shares a similar active-site Cys residue, was devoid of SPG-R activity. Although knowledge of this reaction expands the range of detoxification reactions that are catalyzed by GSTs, it is not immediately clear if there is an endogenous substrate that utilizes this reaction. It is also possible that thiols other than glutathione could react with α-haloketones to form other S-phenacyl compounds however it is unlikely that they would be substrates for GSTO1-1 as they would probably not bind in the glutathione binding site.
The proposed reaction mechanism (Figure 4), which involves the formation of a mixed disulfide with the active-site cysteine32 residue and its regeneration by an unbound thiol in a thiol-disulfide exchange reaction, appears to be novel within the GST family. The selectivity of the SPG-R reaction catalyzed by GSTO1-1 is demonstrated by the absence of activity in GSTO1-1 deficient T47-D cells despite the presence of other GSTs that catalyze conjugation reaction with CDNB, and the failure of a range of recombinant GSTs from several different classes to catalyze the SPG-R reaction. This is the first specific reaction attributed to GSTO1-1. Previous studies have shown that GSTO1-1 catalyzes thioltransferase, dehydroascorbate reductase, and monomethylarsonate (V) reductase activities, but these reactions are also catalyzed to varying degrees by other enzymes (3). Thus the SPG-R reaction may be of use in determining GSTO1-1 activity in the presence of a complex mixture of other GSTs.
It has previously been proposed that the reaction of α-haloketones with glutathione and their subsequent conversion to acetophenones via SPG-R activity affords protection against the toxic properties of those compounds. Individual variation in GSTO1-1 activity could alter an individual's capacity to detoxify α-haloketones. Because we have previously reported several genetic variants of GSTO1 with some different biochemical properties (4, 6), we tested their specific SPG-R activities. Although all of the variants studied had specific activities that were similar to the wild-type enzyme, our previous studies have shown that the relatively rare deletion of Glu155 results in decreased stability, indicating that individuals with this allele may exhibit a relative SPG-R deficiency.
In summary, the observation of SPG-R activity is the first specific reaction attributed to GSTO1-1 and further demonstrates its catalytic diversity.
Acknowledgments
This research was supported by grant 366731 from the National Health and Medical Research Council of Australia (P.G.B.) and by grant ES03127 from the National Institute of Environmental Health Sciences (M.W.A.). The authors thank Ms Jean Cappello for technical assistance and Leif Olson and Jalil Shojaie for their assistance in recording and interpreting the NMR and mass spectra.
Abbreviations used
- SPG-R
S-(phenacyl)glutathione reductase
References
- 1.Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- 2.Board PG, Coggan M, Chelvanayagam G, Easteal S, Jermiin LS, Schulte GK, Danley DE, Hoth LR, Griffor MC, Kamath AV, Rosner MH, Chrunyk BA, Perregaux DE, Gabel CA, Geoghegan KF, Pandit J. Identification, Characterization and Crystal structure of the Omega Class Glutathione Transferases. J Biol Chem. 2000;275:24798–24806. doi: 10.1074/jbc.M001706200. [DOI] [PubMed] [Google Scholar]
- 3.Whitbread AK, Masoumi A, Tetlow N, Schmuck E, Coggan M, Board PG. Characterization of the omega class of glutathione transferases. Methods Enzymol. 2005;401:78–99. doi: 10.1016/S0076-6879(05)01005-0. [DOI] [PubMed] [Google Scholar]
- 4.Whitbread AK, Tetlow N, Eyre HJ, Sutherland GR, Board PG. Characterization of the human Omega class glutathione transferase genes and associated polymorphisms. Pharmacogenetics. 2003;13:131–144. doi: 10.1097/00008571-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Zakharyan RA, Sampayo-Reyes A, Healy SM, Tsaprailis G, Board PG, Liebler DC, Aposhian HV. Human monomethylarsonic acid (MMA(V)) reductase is a member of the glutathione-S-transferase superfamily. Chem Res Toxicol. 2001;14:1051–1057. doi: 10.1021/tx010052h. [DOI] [PubMed] [Google Scholar]
- 6.Schmuck EM, Board PG, Whitbread AK, Tetlow N, Cavanaugh JA, Blackburn AC, Masoumi A. Characterization of the monomethylarsonate reductase and dehydroascorbate reductase activities of Omega class glutathione transferase variants: implications for arsenic metabolism and the age-at-onset of Alzheimer's and Parkinson's diseases. Pharmacogenet Genomics. 2005;15:493–501. doi: 10.1097/01.fpc.0000165725.81559.e3. [DOI] [PubMed] [Google Scholar]
- 7.Li YJ, Oliveira SA, Xu P, Martin ER, Stenger JE, Scherzer CR, Hauser MA, Scott WK, Small GW, Nance MA, Watts RL, Hubble JP, Koller WC, Pahwa R, Stern MB, Hiner BC, Jankovic J, Goetz CG, Mastaglia F, Middleton LT, Roses AD, Saunders AM, Schmechel DE, Gullans SR, Haines JL, Gilbert JR, Vance JM, Pericak-Vance MA. Glutathione S-transferase omega-1 modifiesage-at-onset of Alzheimer disease and Parkinson disease. Hum Mol Genet. 2003;12:3259–3267. doi: 10.1093/hmg/ddg357. [DOI] [PubMed] [Google Scholar]
- 8.Whitbread AK, Mellick GD, Silburn PA, Le Couteur DG, Board PG. Glutathione transferase Omega class polymorphisms in Parkinson disease. Neurology. 2004;62:1910–1911. doi: 10.1212/01.wnl.0000125282.09308.b1. [DOI] [PubMed] [Google Scholar]
- 9.Kolsch H, Linnebank M, Lutjohann D, Jessen F, Wullner U, Harbrecht U, Thelen KM, Kreis M, Hentschel F, Schulz A, von Bergmann K, Maier W, Heun R. Polymorphisms in glutathione S-transferase omega-1 and AD, vascular dementia, and stroke. Neurology. 2004;63:2255–2260. doi: 10.1212/01.wnl.0000147294.29309.47. [DOI] [PubMed] [Google Scholar]
- 10.Laliberte RE, Perregaux DG, Hoth LR, Rosner PJ, Jordan CK, Peese KM, Eggler JF, Dombroski MA, Geoghegan KF, Gabel CA. Glutathione s-transferase omega 1-1 is a target of cytokine release inhibitory drugs and may be responsible for their effect on interleukin-1beta posttranslational processing. J Biol Chem. 2003;278:16567–16578. doi: 10.1074/jbc.M211596200. [DOI] [PubMed] [Google Scholar]
- 11.Crawford MJ, Hutson DH, King PA. Metabolic demethylation of the insecticide dimethylvinphos in rats, in dogs, and in vitro. Xenobiotica. 1976;6:745–762. doi: 10.3109/00498257609151391. [DOI] [PubMed] [Google Scholar]
- 12.Ballantyne B, Swanston DW. The comparative acute mammalian toxicity of 1-chloroacetophenone (CN) and 2-chlorobenzylidene malononitrile (CS) Arch Toxicol. 1978;40:75–95. doi: 10.1007/BF01891962. [DOI] [PubMed] [Google Scholar]
- 13.McCray JW, Weil R. Inactivation of interferons: halomethyl ketone derivatives of phenylalanine as affinity labels. Proc Natl Acad Sci U S A. 1982;79:4829–4833. doi: 10.1073/pnas.79.16.4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Navarro J, Abdel Ghany M, Racker E. Inhibition of tyrosine protein kinases by halomethyl ketones. Biochemistry. 1982;21:6138–6144. doi: 10.1021/bi00267a018. [DOI] [PubMed] [Google Scholar]
- 15.Summer KH, Klein D, Lichtmannegger J, Wolff T. 2-Chloroacetophenone is an effective glutathione depletor in isolated rat hepatocytes. Arch Toxicol. 1996;71:127–129. [PubMed] [Google Scholar]
- 16.Hutson DH, Holmes DS, Crawford MJ. The involvement of glutathione in the reductive dechlorination of a phenacyl halide. Chemosphere. 1976;5:79–84. [Google Scholar]
- 17.Akhtar MH. Sequential participation of glutathione and sulph-hydryl(s) in reductive dechlorination of 2,4-di-, and 2,4,5-trichloro phenacyl chlorides by soluble fraction (105,000 × g) of chicken liver homogenate. J Environ Sci Health B. 1979;14:53–71. doi: 10.1080/03601237909372114. [DOI] [PubMed] [Google Scholar]
- 18.Brundin A, Ratnayake JH, Sunram JM, Anders MW. Glutathione-dependent reductive dehalogenation of 2,2′,4′-trichloroacetophenone to 2′,4′-dichloroacetophenone. Biochem Pharmacol. 1982;31:3885–3890. doi: 10.1016/0006-2952(82)90306-9. [DOI] [PubMed] [Google Scholar]
- 19.Kitada M, McLenithan JC, Anders MW. Purification and characterization of S-phenacylglutathione reductase from rat liver. J Biol Chem. 1985;260:11749–11754. [PubMed] [Google Scholar]
- 20.Vince R, Daluge S, Wadd WB. Studies on the inhibition of glyoxalase I by S-substituted glutathiones. J Med Chem. 1971;14:402–404. doi: 10.1021/jm00287a006. [DOI] [PubMed] [Google Scholar]
- 21.Beutler E, Mathai CK, Smith JE. Biochemical variants of glucose-6-phosphate dehydrogenase giving rise to congenital nonspherocytic hemolytic disease. Blood. 1968;31:131–150. [PubMed] [Google Scholar]
- 22.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 23.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 24.Ishikawa T, Casini AF, Nishikimi M. Molecular cloning and functional expression of rat liver glutathione- dependent dehydroascorbate reductase. J Biol Chem. 1998;273:28708–28712. doi: 10.1074/jbc.273.44.28708. [DOI] [PubMed] [Google Scholar]
- 25.Board PG, Baker RT, Chelvanayagam G, Jermiin LS. Zeta, a novel class of glutathione transferases in a range of species from plants to humans. Biochem J. 1997;328:929–935. doi: 10.1042/bj3280929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gero A. Enol titration II. Enol contents of some ketones and esters in the presence of methanol. J Org Chem. 1954;19:1960–1970. [Google Scholar]
- 27.Oki M, Funakoshi W, Nakamura A. Reaction of a-carbonyl sulfides with bases. I. Reaction between a-carbonyl sulfides with thiolates. Bull Chem Soc Japan. 1971;44:828–832. [Google Scholar]
- 28.Dulhunty A, Gage P, Curtis S, Chelvanayagam G, Board P. The Glutathione Transferase Structural Family Includes a Nuclear Chloride Channel and a Ryanodine Receptor Calcium Release Channel Modulator. J Biol Chem. 2001;276:3319–3323. doi: 10.1074/jbc.M007874200. [DOI] [PubMed] [Google Scholar]
- 29.Adam GC, Sorensen EJ, Cravatt BF. Proteomic profiling of mechanistically distinct enzyme classes using a common chemotype. Nat Biotechnol. 2002;20:805–809. doi: 10.1038/nbt714. [DOI] [PubMed] [Google Scholar]