Abstract
BACKGROUND
Cytokines are key mediators of inflammation that may play important roles in prostate cancer initiation and progression. Cytokines found in cancerous prostates may provide further insight into the mechanisms of cancer initiation and progression, and facilitate the exploration of new markers of prostatic neoplasia and inflammation. We describe the cytokine profile of prostatic fluids obtained from cancerous prostate glands and correlate it to both cancer status and inflammation grade.
METHODS
Prostatic fluid was collected from fresh radical prostatectomy specimens and analyzed by human cytokine antibody microarray. Cases were selected from patients with either minimal or extensive cancer volume on final pathology. Among the cytokines with the greatest difference between the tumor volume groups, eight had their levels quantitated by ELISA and correlated with cancer status and grade of inflammation by neutrophils, macrophages and lymphocytes.
RESULTS
Among 174 cytokines analyzed, HGF was the most increased (6.57-fold), and HGF and IL18Bpa were significantly elevated in patients with extensive prostate cancer. IL17, GITR, ICAM-1, and IL-18Bpa were elevated in specimens with neutrophilic inflammation into gland lumina, and IL18Bpa, IL17, GITR, ICAM-1 were elevated in specimens with lymphocytic inflammation in prostatic stroma.
CONCLUSIONS
Prostatic fluid cytokines were identified that may be useful in early detection and prognostication efforts for prostate cancer, particularly if they can be found not only in prostatic fluids obtained ex vivo, but in expressed prostatic secretions or urine samples from patients with their prostates still in situ.
Keywords: cancer, inflammation, cytokine
INTRODUCTION
Prostate cancer is the most common cancer and the second leading cause of cancer-related death in men over 40 years of age in the United States [1]. The etiology of prostate cancer is not well understood. Chronic infection and inflammation are causes of cancer in the stomach, liver and large intestine. Data from histopathological, molecular histopathological, epidemiological and genetic epidemiological studies show that chronic inflammation might also be important in prostate carcinogenesis [2]. Proliferative inflammatory atrophy (PIA), where proliferative glandular epithelium with the morphological appearance of simple atrophy occurs in association with inflammation, is thought to be a possible precursor to prostate cancer [3]. Chronic and/or acute glandular inflammation is indeed observed in many radical prostatectomy specimens [4].
Cytokines are proteins that are expressed from immune, epithelial, and stromal cells, that can be excreted into the lumina of glands [5,6]. Cells communicate with each other by networks of interrelated cytokines. Cytokines are not only key mediators of inflammation, but may also play important roles in the initiation and progression of prostate cancer. While some cytokine analysis of prostatic fluid from expressed prostatic secretions has been performed [5,6], a comprehensive cataloguing of cytokines from the cancerous prostate has not been reported. Such a cytokine profile may provide further insight into the mechanisms of prostate cancer initiation and progression, and may facilitate the exploration of new markers of prostatic neoplasia and inflammation. In this study, we describe the cytokine profile of prostatic fluids obtained from cancerous prostate glands and correlate it to both cancer status and inflammation grade.
MATERIALS AND METHODS
Collection of Samples
Prostatic fluids were collected by squeezing ex vivo prostate glands that were freshly obtained following radical prostatectomy for prostate cancer and collecting drops of fluid from the protruding apical urethral stump. The radical prostatectomy specimens were then submitted for routine formalin fixation, sectioning, and pathologic analysis as per standard protocol [7]. Prostate glands with either minimal prostate cancer (M, n=20) or extensive prostate cancer (E, n=20) as estimated by tumor volume were chosen for this study. Specimens with minute foci of a maximum tumor area of less than 15mm2 were assigned to the M group, and specimens with a maximum tumor area of more than 80mm2 were assigned to the E group. The prostatic fluids were kept at -80° C until the cytokine determination experiments. Approval was obtained from our Institutional Review Board before initiating the study and all patients provided written informed consent.
Cytokine Antibody Array
A Raybio™ Human Cytokine Array kit (Raybiotech, Norcross, GA, USA) including 174 cytokines was used per the manufacturer’s recommendations. Briefly, membranes immobilized with capture antibodies were blocked with 5% bovine serum albumin/TBS (triethanolamine-buffered saline) for 1 hr. Membranes were then incubated with prostatic fluid samples (1 ml, in 10-fold dilution with TBS and Complete protease inhibitor cocktail tablets (Roche Diagnostics, Indianapolis, IN) for 2 hrs. at room temperature. After extensive washing with TBS/0.1% Tween 20 (3 times, 5 min each) and TBS (twice, 5 min each) to remove unbound cytokines, membranes were incubated with biotin-conjugated anticytokine antibodies. Membranes were washed and then incubated with horseradish peroxidase-conjugated streptavidin (2.5 pg/ml) for 1 hr. at room temperature. Unbound materials were washed out with TBS/0.1% Tween 20 and TBS. Finally, the signals were detected by the enhanced chemiluminescence system, followed by additional washing. Spots were visualized using enhanced chemiluminescence (ECL plus Western Blotting System, Amersham Biosciences, Pittsburgh, PA). Membranes were exposed to Kodak X-Omat radiographic film for 1 min. per image. Each film was scanned into TIFF Image files, and spots were digitized into densities with Gel-Pro-Analyzer (Media Cybernetics, Bethesda, MD). The densities were exported into Microsoft Excel, and the background intensity was subtracted prior to analysis.
Enzyme-Linked Immunosorbent Assay (ELISA)
Eight cytokines in prostatic fluids were measured by ELISA. A human ELISA kit (Raybiotech) was used to detect hepatocyte growth factor (HGF), interleukin 12p70 (IL12), glucocorticoid-induced tumor necrosis factor receptor (GITR), intercellular adhesion molecule 1 (ICAM-1), and neurotrophin-3 (NT-3). A Quantikine human immunoassay kit (R&D Systems, Minneapolis, MN) was used to detect interleukin 17 (IL17), and epithelial-neutrophil activating peptide (ENA78). DuoSet ELISA development system (R&D Systems) was used to detect interleukin 18 binding protein a (IL18Bpa). Each cytokines was measured based on the manufacturer’s recommendations. For examples, to measure HGF, IL12, GITR, ICAM-1 and NT-3 prostatic fluids were diluted accordingly. Samples were added (100μl/well) in duplicate for incubation for 2.5 hrs. at room temperature. Biotinylated antibodies were subsequently added (100μl/well)) and incubated for 1 hr. at room temperature. Incubation with streptavidin- horseradish-peroxidase (for 15 min.) was followed by detection with 3,3V,5,5V-tetramethylbenzidine (TMB) for 30 min. The reaction was stopped by the addition of 1.5 M H2SO4. Plates were read using a wavelength of 450 nm on a microplate reader (PHERA star, BMG LABTECH, Durham, NC).
Histological Analysis
Hematoxylin and eosin stained sections were used to assess the inflammatory status of the prostate. For each case, two sections were chosen from right posterior, left posterior, right anterior, and left anterior prostate at apex and middle (8 sections total) and were examined by light microscopy for the presence of neutrophils, macrophages and lymphocytes. An inflammation grade of 1 for neutrophils or macrophages (low-grade inflammation) was assigned to specimens in which neutrophils or macrophages were observed only in prostatic gland lumina, with no epithelial disruption, or in which neutrophils were not observed at all. A grade of 2 (high-grade inflammation) was assigned to specimens in which the gland lumina were filled with immune cells and/or pus and more than 10 neutrophils or macrophages were found in the epithelial lining under 40x magnification, or in which these immune cells were found in the interstitium with associated epithelial destruction. A grade of 1 for lymphocytes was assigned to the specimens in which confluent sheets of inflammatory cells with nodule/follicle formation were observed focally or multifocally in the stroma (less than 50% of area), while a grade of 2 was assigned to specimens in which those were observed diffusely in the stroma (more than 50% of area) in at least one section [8].
Data Analysis and Statistics
Positive control signals on each membrane were used to normalize cytokine signal intensities from cytokine antibody arrays. Then, the data was normalized to PSA levels in each prostatic fluid sample to account for differential yields of fluid actually of prostatic origin. Total PSA levels in each prostatic fluid sample were measured by Hybritech PSA assay on the Beckman Coulter Access Immunoassay System (Beckman Coulter, Inc., Fullerton, CA). The normalized intensity value of cytokines in each group (M or E) was converted into the relative n-fold change between groups. Data from ELISA in prostatic fluids were also normalized to the average PSA levels in each prostatic fluid sample. Data from prostatic fluids was analyzed as categorized by tumor volume (M and E), Gleason score (6, and ≥7), or inflammation grade (1 or 2). Statistical analyses were done using GraphPad Prizm 4.0 for Windows. Mann-Whitney tests were used to analyze the difference of 2 categories. Chi-square tests were used to analyze the correlations between tumor volume and inflammation grade. Spearman’s correlations were used to analyze the correlations of 2 cytokines and that of cytokines and tissue weights or age. Statistical significance was defined as a p value < 0.05.
RESULTS
Cytokine Profile of Prostatic Fluid by Cytokine Array
The normalized intensity values of cytokines from group E (extensive volume prostate cancer) were divided by those from group M (minimal volume prostate cancer) to calculate the relative n-fold change. The ranked cytokine profile of the relative n-fold change obtained by cytokine array is listed in Table 1; for a comprehensive listing see Appendix 1. Among 174 cytokines analyzed, HGF was the most increased cytokine in group E (6.57-fold).
Table 1.
Cytokine Profile of Prostatic Fluid
| Cytokine | Ratio (E/M) | Average signal of E group (SD) | Average signal of M group (SD) |
|---|---|---|---|
| HGF | 6.57 | 118.24 (164.50) | 18 (14.90) |
| IL18Bpa | 2.58 | 4.37 (6.67) | 1.69 (2.60) |
| ICAM-1 | 2.41 | 53.34 (68.50) | 22.15 (23.09) |
| IL17 | 2.34 | 1.74 (2.78) | 0.74 (1.15) |
| NT3 | 2.32 | 1.79 (2.38) | 0.77 (1.31) |
| IL12p70 | 2.32 | 4.41 (5.92) | 1.90 (1.48) |
| GITR | 1.99 | 3.80 (4.56) | 1.91 (1.69) |
| ENA78 | 1.74 | 20.93 (28.61) | 11.99 (18.71) |
E = Extensive prostate cancer, M = Minimal prostate cancer.
Full cytokine profiling is shown in Appendix.
Correlation of HGF and IL18BPa with Cancer Status by ELISA
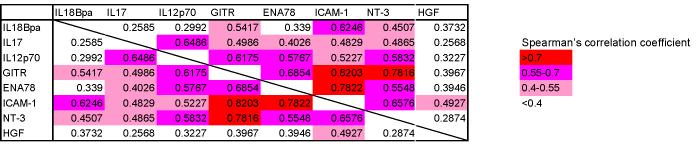
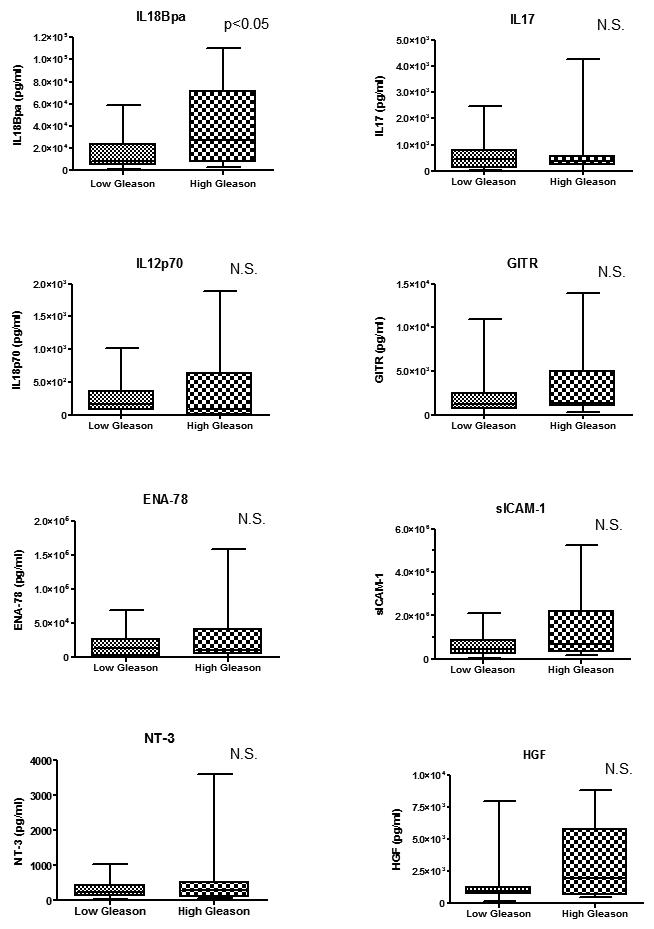
Among the cytokines with the greatest difference between groups E and M, we selected eight cytokines for further study (HGF, IL18Bpa, ICAM-1, IL17, NT-3, IL12, GITR, and ENA78) and confirmed their levels in prostatic fluids qualitatively by ELISA. Each of these cytokines was elevated in group E; the HGF and IL18Bpa elevations were statistically significant compared to group M (Fig. 1). In an analysis based on Gleason score, only IL18Bpa was significantly elevated in specimens with high Gleason grade (≥7). Strong correlations were noted between some cytokines, especially between ICAM-1 and GITR (Spearman’s correlation coefficient r = 0.820), ICAM-1 and ENA78 (r=0.782), and NT3 and GITR (r=0.782) (Table 2). No correlation was found between each cytokine and specimen weight. Weak correlations were found between increasing age and IL12 (r=0.3480) and increasing age and NT3 (r=0.4001).
Fig. 1.
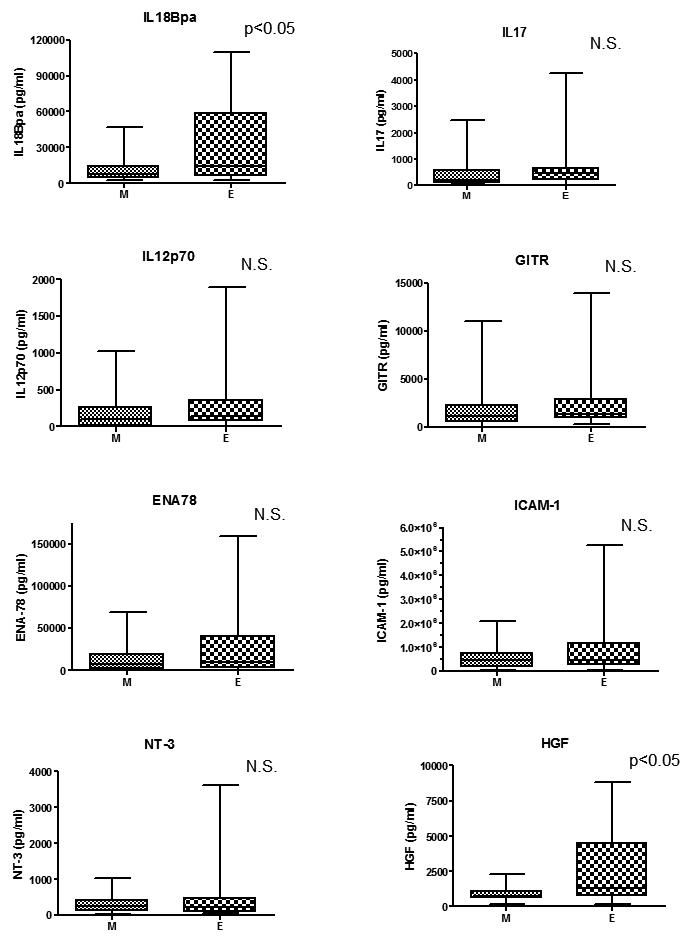
Correlation of cytokines with cancer status. Each cytokine levels measured by ELISA was analyzed stratified by cancer status. The HGF and IL18Bpa elevations were statistically significant compared to group M. (M: minimal prostate cancer (n=20), E: extensive prostate cancer (n=20))
Table 2.
 |
Relationship between Cytokine Levels and Prostatic Inflammation
Routine histochemical analysis demonstrated that neutrophils and macrophages were present in prostatic glandular lumina (grade 1 inflammation, Fig. 2) and in the lining of the prostate epithelium (grade 2 inflammation). Isolated lymphocytes aggregated in the stroma surrounding ducts (grade 1 cases) and lymphoid follicles were occasionally noted (grade 2). There was no statistical correlation between the inflammation grade by each immune cell type assessed (neutrophil, macrophage, and lymphocyte) and tumor volume (M and E) (Chi-square test). Data pertaining to eight cytokines found in prostatic fluids were analyzed according to inflammation grade in the radical prostatectomy specimens. In cases stratified by neutrophil inflammation, IL17, GITR and ICAM-1 were significantly associated with increasing (grade 2) inflammation (p<0.05), and ENA78 (p=0.0594) and NT-3 (p=0.0554) levels were close to reaching statistical significance (Fig. 3). In cases stratified by macrophage inflammation, none of these cytokines was significantly elevated in grade 2 vs. grade 1 infiltrates (Fig. 4). In cases stratified by lymphocyte inflammation, IL18Bpa, IL17, GITR and ICAM-1 were significantly elevated in grade 2 lymphocytic infiltration (p<0.05) (Fig. 5).
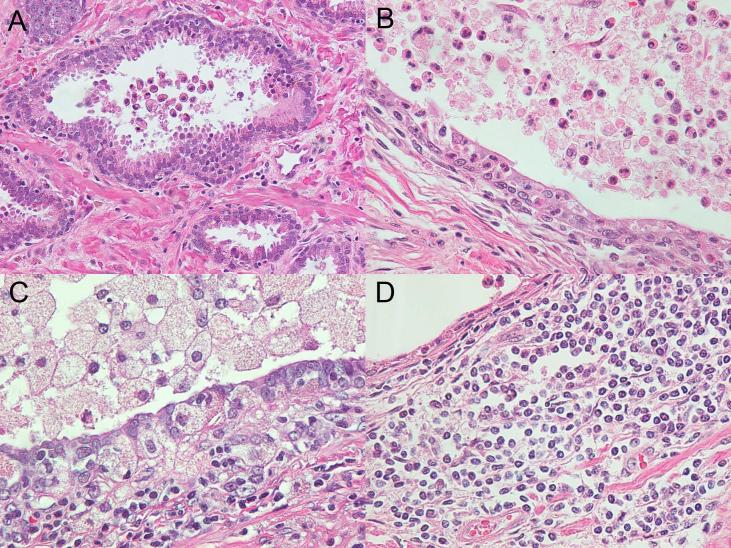
Fig. 2.
Inflammation of prostate by neutrophils, macrophages or lymphocytes. A: Some neutrophils or macrophages were observed only in gland lumina, with no epithelial destruction. B: A gland lumen filled with neutrophils and pus, with neutrophils noted within the epithelial lining (inflammation grade 2). C: A gland lumen filled with macrophages and pus, with macrophages noted within the epithelial lining (inflammation grade 2). In grade 2 lymphocytic inflammation, confluent sheets of inflammatory cells with nodule/follicle formation. D: were observed diffusely in the stroma (more than 50% of the area) in at least one section.
Fig. 3.
Relationship between cytokine levels and neutrophil inflammation. IL17, GITR and ICAM-1 were significantly associated with grade 2 inflammation (p<0.05).
Fig. 4.
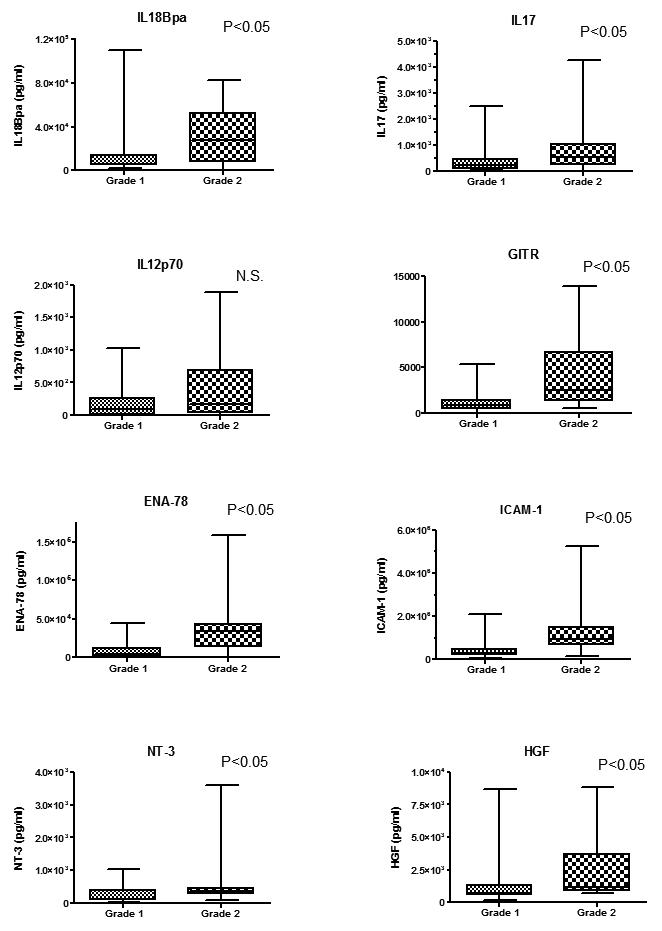
Relationship between cytokine levels and macrophage inflammation. No cytokines was significantly elevated in grade 2 vs. grade 1 inflammation.
Fig. 5.
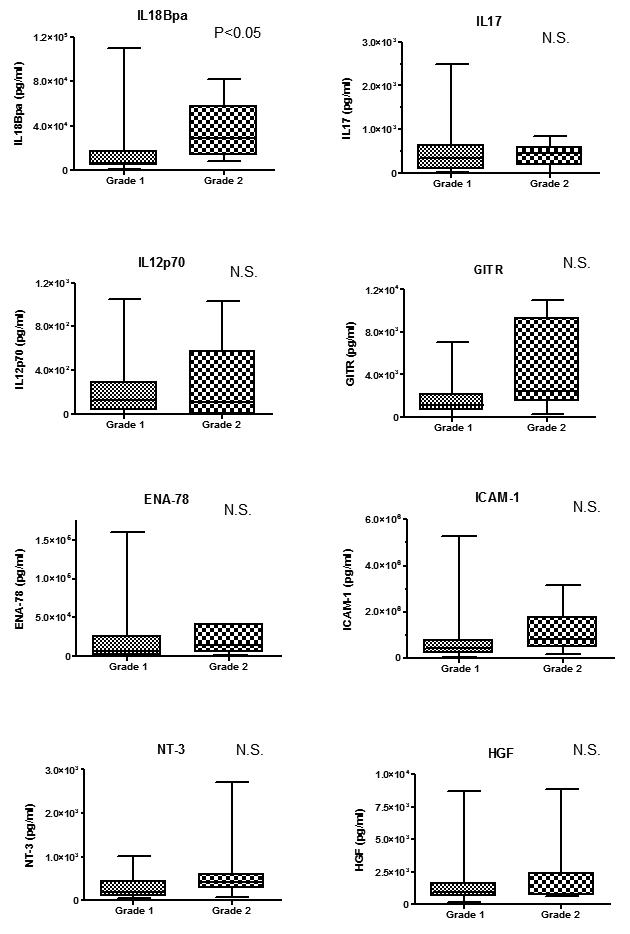
Relationship between cytokine levels and lymphocyte inflammation. IL18Bpa, IL17, GITR and ICAM-1 were significantly elevated in grade 2 lymphocytic inflammation (p<0.05).
DISCUSSION
The prostate gland secretes many substances, including citric acid, polyamines, zinc, and cytokines. Cytokines are secreted from lymphocytes, macrophages, and mast cells, and also from prostatic epithelial and stromal cells [9-11]. Recently, cytokines have been shown to play important roles in prostatic inflammation, carcinogenesis and cancer progression [9,12,13]. In this study, we for the first time describe the cytokine profile of prostatic fluid from cancerous prostates. A better knowledge of the cytokines present in prostate fluid may aid in understanding the implications of the prostatic cytokine network on inflammation, carcinogenesis, and prostate cancer progression, and may lead to novel cancer detection strategies.
We initially studied prostatic cytokines by array, and catalogued the most prevalent cytokines noted from fluids obtained from prostate specimens with extensive cancer as compared to those from prostates with minimal cancer. Among the most up-regulated cytokines in cases with extensive disease, we selected HGF, IL18Bpa, ICAM-1, IL17, NT-3, IL12, GITR, and ENA78, for more quantitative assessment by ELISA. These cytokines were selected from the groups of cytokines that were elevated because of their known roles in cancer and inflammation-related pathways.
HGF has been shown to be important in prostate cancer progression, invasion and metastasis[14]. IL18Bpa and IL12 are involved in the Th1 immune response[15,16]. IL-12 is the major cytokine responsible for the differentiation of T helper 1 cells, which are in turn potent producers of IFN-γ [15]. IL18Bpa is a potent inhibitor of IL-18, which is a central player in inflammation and in the immune response, and which has antineoplastic properties. ICAM-1 is expressed by leukocytes, epithelial cells, endothelial cells and tumor, stimulates neovascularization [17], and is elevated in the serum of patients with cancer [18]. IL-17 is a pro-inflammatory cytokine, that plays a crucial role in the development of autoimmunity and allergic reactions, and the expression of IL17 from Th17 is stimulated by IL23, which promotes tumor incidence and growth [19]. NT-3 is a member of the neurotrophins; it is expressed by prostate epithelial cells and stromal cells from prostates with cancer, but not by benign prostatic tissue [10]. GITR is expressed by T regulatory cells (Treg) as well as activated T cells and NK cells. ENA-78 produced by monocytes, macrophages, fibroblasts, endothelial cells, and several types of epithelial cells is a member of the CXC family of chemokines, and acts as a potent chemoattractant and activator of neutrophil function as well as an angiogenic factor in cancer [20,21]
In the cytokine antibody array portion of our study, HGF in prostatic fluid was the cytokine most increased in extensive disease cases, a finding that was confirmed statistically by ELISA. HGF, which can be derived from a variety of tissues, is known to be elevated in the serum of men with metastatic prostate cancer [22]. In the prostate, stromal cells secrete HGF, which acts locally on prostate epithelial cells expressing its receptor, the tyrosine kinase c-Met. Prostate cancer can also express HGF via stimulation by IL-1β, PDGF, bFGF, VEGF, and EGF derived from stromal cells [23]. The intracellular cascade that ensues secondary to c-Met phosphorylation appears to be responsible for most of the effects of HGF, including its pro-mitogenic and anti-apoptotic properties, and its effects on developmental cell migration. Alterations of HGF or c-Met levels can affect these and other biological pathways associated with cancer progression [14].
While prostatic fluid HGF and IL-18Bpa levels were related to tumor volume, prostatic fluid IL-18BPa, IL17, GITR, and ICAM-1 levels were correlated with inflammation. These results indicate that the above cytokines may be regulated or released by specific immune cells in the gland lumina (neutrophils) or in the epithelial lining or stroma (lymphocytes). In fact, IL17 is expressed by Th17, a distinct T cell subset that stimulates the production of cytokines that attract neutrophils to the site of inflammation [24]. Neutrophils use ICAM-1 on epithelial cells to migrate across the epithelial lining [25], and ICAM-1 is also one of the cytokines induced by IL17 [24]. Among these cytokines, IL18Bpa and GITR may be new markers of prostatic inflammation that is associated with cancer initiation or progression. Importantly, IL18Bpa was correlated with both cancer and inflammation status in our study. IL18 plays an important role in host defenses against various infectious microbes, but overproduction of IL18 causes autoimmune diseases and inflammatory tissue damage [26]. The excretion of IL18Bpa from monocytes and NK cells is induced by IL12 and interferon gamma, and IL18Bpa limits the inflammatory response induced by IL18 [27]. IL18Bpa is also secreted by colon cancer cell lines after interferon gamma stimulation [28], which suggests that prostate cancer cells themselves may also secrete IL18Bpa upon stimulation by lymphocyte-derived cytokines with the background of inflammation. Since IL18Bpa inhibits the anti-tumor cytokine IL-18, the finding of IL18Bpa in malignant prostates suggests an attempt by the cancer to escape immune surveillance and may be correlated with poor prognosis.
GITR has been shown to co-stimulate T cells and abrogate suppression of Treg [29], and to diminish NK cell antitumor immunity [30]. GITR also correlates with neutrophilic infiltration. In GITR-/- mice, neutrophil infiltration into arthritic areas was significantly less than in GITR+/+ mice [31]. Whereas GITR-expressing Treg help limit collateral tissue damage caused by vigorous antimicrobial immune response in normal tissues [32], Treg cells are increased in human solid tumors and an increased number of Treg cells correlates with poor prognosis [33]. The increase of GITR induced by the inflammation may be associated with the increase of Treg which suppress the anti-tumor immunity.
We found strong correlations between the expression levels of certain cytokines: ICAM-1 and GITR, ICAM-1 and ENA78, and GITR and NT-3. ENA-78 strongly attracts neutrophils, and the adhesion of neutrophils to vessel walls or epithelial cells in an area of inflammation occurs via ICAM-1 [34]. It is plausible that GITR-expressing cells, such as regulatory T cells or NK cells, stimulate the expression of NT-3 or ICAM-1 on prostate cancer cells; it may also be that GITR-expressing cells also express ICAM-1 and/or NT-3. Elucidating the reasons for the correlation between these cytokine pairs will require further studies.
A limitation of our study is that we did not assess the cytokine profile of prostatic fluid derived from prostates that were completely benign. The reason for this is that radical prostatectomy (complete removal of the prostate) is not performed on patients without prostate cancer. One consideration was to analyze the cytokine profile of prostatic fluid derived from radical cystoprostatectomy cases in men shown pathologically not to have prostate cancer. However, these men by definition have high grade and/or muscle-invasive bladder cancer neighboring the prostate, which might result in a cytokine profile difficult to discriminate from that associated with urothelial cancer, (which can also reside in the prostatic urethra). Rather than selecting such patients, we chose to make our comparisons between cases with minimal (or “clinically insignificant”) prostate cancer (M) and those with prostate cancers of significant volume (E). Interestingly, one case in the M group was diagnosed with prostate cancer by biopsy, but had no cancer found in the radical prostatectomy specimen despite intensive re-sectioning. While this case cannot be considered a completely negative control for prostate cancer, analysis of prostatic fluids from this case by ELISA did not demonstrate any significant differences in comparison with the average data derived from the other M group cases. In addition, when we controlled for specimen weight as a surrogate of BPH, we did not note any significant differences in cytokine levels across all cases. Thus, we feel that the cytokine profiles we have described delineate important differences between early cancers and late cancers and regarding the related inflammatory status of the prostate.
CONCLUSIONS
In conclusion, we hope that our cytokine data provide information that is helpful to researchers studying cytokine networks, paracrine stimulation pathways, and oncogenesis in the prostate. HGF and IL18Bpa were elevated in prostatic fluid from patients with extensive prostate cancers. IL17, GITR, ICAM-1, and IL-18Bpa were elevated in prostatic fluid from specimens with neutrophil inflammation in gland lumina, and IL18Bpa, IL17, GITR, ICAM-1 were elevated in fluid from specimens with lymphocytic inflammation in stroma. These and other cytokines may perhaps be useful in early detection and prognostication efforts if they are found not only in prostatic fluid obtained ex vivo, but in expressed prostatic secretions or post-DRE urine samples from patients with their prostates till in situ.
Supplementary Material
Fig. 6.
ACKNOWLEDGEMENTS
This study was funded by NIH/NIDDK grant 1K23DK071262, Department of Defense grant PC041214 and NIH/NCI grant U24 CA115102.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57(1):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.De Marzo AM, Platz EA, Sutcliffe S, Xu J, Gronberg H, Drake CG, Nakai Y, Isaacs WB, Nelson WG. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7(4):256–269. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Marzo AM, Marchi VL, Epstein JI, Nelson WG. Proliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesis. Am J Pathol. 1999;155(6):1985–1992. doi: 10.1016/S0002-9440(10)65517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen RJ, Shannon BA, McNeal JE, Shannon T, Garrett KL. Propionibacterium acnes associated with inflammation in radical prostatectomy specimens: a possible link to cancer evolution? J Urol. 2005;173(6):1969–1974. doi: 10.1097/01.ju.0000158161.15277.78. [DOI] [PubMed] [Google Scholar]
- 5.Hochreiter WW, Nadler RB, Koch AE, Campbell PL, Ludwig M, Weidner W, Schaeffer AJ. Evaluation of the cytokines interleukin 8 and epithelial neutrophil activating peptide 78 as indicators of inflammation in prostatic secretions. Urology. 2000;56(6):1025–1029. doi: 10.1016/s0090-4295(00)00844-x. [DOI] [PubMed] [Google Scholar]
- 6.Gann PH, Klein KG, Chatterton RT, Ellman AE, Grayhack JT, Nadler RB, Lee C. Growth factors in expressed prostatic fluid from men with prostate cancer, BPH, and clinically normal prostates. Prostate. 1999;40(4):248–255. doi: 10.1002/(sici)1097-0045(19990901)40:4<248::aid-pros6>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 7.Allan RW, Sanderson H, Epstein JI. Correlation of minute (0.5 MM or less) focus of prostate adenocarcinoma on needle biopsy with radical prostatectomy specimen: role of prostate specific antigen density. J Urol. 2003;170(2 Pt 1):370–372. doi: 10.1097/01.ju.0000074747.72993.cb. [DOI] [PubMed] [Google Scholar]
- 8.Nickel JC, True LD, Krieger JN, Berger RE, Boag AH, Young ID. Consensus development of a histopathological classification system for chronic prostatic inflammation. BJU Int. 2001;87(9):797–805. doi: 10.1046/j.1464-410x.2001.02193.x. [DOI] [PubMed] [Google Scholar]
- 9.Nishimura K, Kitamura M, Miura H, Nonomura N, Takada S, Takahara S, Matsumoto K, Nakamura T, Matsumiya K. Prostate stromal cell-derived hepatocyte growth factor induces invasion of prostate cancer cell line DU145 through tumor-stromal interaction. Prostate. 1999;41(3):145–153. doi: 10.1002/(sici)1097-0045(19991101)41:3<145::aid-pros1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 10.Weeraratna AT, Arnold JT, George DJ, DeMarzo A, Isaacs JT. Rational basis for Trk inhibition therapy for prostate cancer. Prostate. 2000;45(2):140–148. doi: 10.1002/1097-0045(20001001)45:2<140::aid-pros8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 11.Lu S, Dong Z. Characterization of TGF-beta-regulated interleukin-8 expression in human prostate cancer cells. Prostate. 2006;66(9):996–1004. doi: 10.1002/pros.20424. [DOI] [PubMed] [Google Scholar]
- 12.Steiner GE, Djavan B, Kramer G, Handisurya A, Newman M, Lee C, Marberger M. The picture of the prostatic lymphokine network is becoming increasingly complex. Rev Urol. 2002;4(4):171–177. [PMC free article] [PubMed] [Google Scholar]
- 13.Ao M, Franco OE, Park D, Raman D, Williams K, Hayward SW. Cross-talk between paracrine-acting cytokine and chemokine pathways promotes malignancy in benign human prostatic epithelium. Cancer Res. 2007;67(9):4244–4253. doi: 10.1158/0008-5472.CAN-06-3946. [DOI] [PubMed] [Google Scholar]
- 14.Hurle RA, Davies G, Parr C, Mason MD, Jenkins SA, Kynaston HG, Jiang WG. Hepatocyte growth factor/scatter factor and prostate cancer: a review. Histol Histopathol. 2005;20(4):1339–1349. doi: 10.14670/HH-20.1339. [DOI] [PubMed] [Google Scholar]
- 15.Colombo MP, Trinchieri G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13(2):155–168. doi: 10.1016/s1359-6101(01)00032-6. [DOI] [PubMed] [Google Scholar]
- 16.Vidal-Vanaclocha F, Mendoza L, Telleria N, Salado C, Valcarcel M, Gallot N, Carrascal T, Egilegor E, Beaskoetxea J, Dinarello CA. Clinical and experimental approaches to the pathophysiology of interleukin-18 in cancer progression. Cancer Metastasis Rev. 2006;25(3):417–434. doi: 10.1007/s10555-006-9013-3. [DOI] [PubMed] [Google Scholar]
- 17.Gho YS, Kleinman HK, Sosne G. Angiogenic activity of human soluble intercellular adhesion molecule-1. Cancer Res. 1999;59(20):5128–5132. [PubMed] [Google Scholar]
- 18.Lynch DF, Jr., Hassen W, Clements MA, Schellhammer PF, Wright GL., Jr. Serum levels of endothelial and neural cell adhesion molecules in prostate cancer. Prostate. 1997;32(3):214–220. doi: 10.1002/(sici)1097-0045(19970801)32:3<214::aid-pros8>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 19.Langowski JL, Zhang X, Wu L, Mattson JD, Chen T, Smith K, Basham B, McClanahan T, Kastelein RA, Oft M. IL-23 promotes tumour incidence and growth. Nature. 2006;442(7101):461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- 20.Arenberg DA, Keane MP, DiGiovine B, Kunkel SL, Morris SB, Xue YY, Burdick MD, Glass MC, Iannettoni MD, Strieter RM. Epithelial-neutrophil activating peptide (ENA-78) is an important angiogenic factor in non-small cell lung cancer. J Clin Invest. 1998;102(3):465–472. doi: 10.1172/JCI3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walz A, Schmutz P, Mueller C, Schnyder-Candrian S. Regulation and function of the CXC chemokine ENA-78 in monocytes and its role in disease. J Leukoc Biol. 1997;62(5):604–611. doi: 10.1002/jlb.62.5.604. [DOI] [PubMed] [Google Scholar]
- 22.Naughton M, Picus J, Zhu X, Catalona WJ, Vollmer RT, Humphrey PA. Scatter factor-hepatocyte growth factor elevation in the serum of patients with prostate cancer. J Urol. 2001;165(4):1325–1328. [PubMed] [Google Scholar]
- 23.Zhu X, Humphrey PA. Overexpression and regulation of expression of scatter factor/hepatocyte growth factor in prostatic carcinoma. Urology. 2000;56(6):1071–1074. doi: 10.1016/s0090-4295(00)00795-0. [DOI] [PubMed] [Google Scholar]
- 24.Witowski J, Ksiazek K, Jorres A. Interleukin-17: a mediator of inflammatory responses. Cell Mol Life Sci. 2004;61(5):567–579. doi: 10.1007/s00018-003-3228-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zen K, Parkos CA. Leukocyte-epithelial interactions. Curr Opin Cell Biol. 2003;15(5):557–564. doi: 10.1016/s0955-0674(03)00103-0. [DOI] [PubMed] [Google Scholar]
- 26.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol. 2001;19:423–474. doi: 10.1146/annurev.immunol.19.1.423. [DOI] [PubMed] [Google Scholar]
- 27.Veenstra KG, Jonak ZL, Trulli S, Gollob JA. IL-12 induces monocyte IL-18 binding protein expression via IFN-gamma. J Immunol. 2002;168(5):2282–2287. doi: 10.4049/jimmunol.168.5.2282. [DOI] [PubMed] [Google Scholar]
- 28.Paulukat J, Bosmann M, Nold M, Garkisch S, Kampfer H, Frank S, Raedle J, Zeuzem S, Pfeilschifter J, Muhl H. Expression and release of IL-18 binding protein in response to IFN-gamma. J Immunol. 2001;167(12):7038–7043. doi: 10.4049/jimmunol.167.12.7038. [DOI] [PubMed] [Google Scholar]
- 29.Ji HB, Liao G, Faubion WA, Abadia-Molina AC, Cozzo C, Laroux FS, Caton A, Terhorst C. Cutting edge: the natural ligand for glucocorticoid-induced TNF receptor-related protein abrogates regulatory T cell suppression. J Immunol. 2004;172(10):5823–5827. doi: 10.4049/jimmunol.172.10.5823. [DOI] [PubMed] [Google Scholar]
- 30.Baltz KM, Krusch M, Bringmann A, Brossart P, Mayer F, Kloss M, Baessler T, Kumbier I, Peterfi A, Kupka S, Kroeber S, Menzel D, Radsak MP, Rammensee HG, Salih HR. Cancer immunoediting by GITR (glucocorticoid-induced TNF-related protein) ligand in humans: NK cell/tumor cell interactions. Faseb J. 2007 doi: 10.1096/fj.06-7724com. [DOI] [PubMed] [Google Scholar]
- 31.Cuzzocrea S, Ayroldi E, Di Paola R, Agostini M, Mazzon E, Bruscoli S, Genovese T, Ronchetti S, Caputi AP, Riccardi C. Role of glucocorticoid-induced TNF receptor family gene (GITR) in collagen-induced arthritis. Faseb J. 2005;19(10):1253–1265. doi: 10.1096/fj.04-3556com. [DOI] [PubMed] [Google Scholar]
- 32.Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol. 2005;6(4):353–360. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 33.Beyer M, Schultze JL. Regulatory T cells in cancer. Blood. 2006;108(3):804–811. doi: 10.1182/blood-2006-02-002774. [DOI] [PubMed] [Google Scholar]
- 34.Albelda SM, Smith CW, Ward PA. Adhesion molecules and inflammatory injury. Faseb J. 1994;8(8):504–512. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.