Abstract
Recent evidence suggests that amiloride, a potent and nonselective blocker of acid sensing ion channels, suppresses generalized seizures induced by maximal electroshock and pentylenetrazole. Here I further determined and quantified the effects of amiloride on the occurrence of limbic seizures and status epilepticus-induced by intraperitoneal administration of pilocarpine, a muscarinic acetylcholine receptor agonist. Pretreatment with various doses (5, 10, 30, 100, 200 mg/kg) of amiloride significantly delayed the onset of the first episode of limbic seizures and the occurrence status epilepticus following administration of pilocarpine (380 mg/kg). At the dose of 100 and 200 mg/kg, amiloride suppressed limbic seizures in 33% of pilocarpine-treated animals and significantly reduced the seizure severity score in 67% of the remaining animals. These findings suggest that amiloride may modulate seizure generation and propagation, probably via mechanisms involving acid sensing ion channels in the pilocarpine model of temporal lobe epilepsy.
Keywords: amiloride, acid sensing ion channel, pilocarpine, seizures, status epilepticus
1. Introduction
Epilepsies are diseases of neuronal excitability that affect about 1-2% of the world population. Based on the mechanisms of neuronal hyperexcitability that leads to seizures, pharmacological approaches of epilepsy have been primarily focused on modulation of voltage-gated ion channels, enhancement of GABAergic inhibition, and reduction of glutamatergic excitation (Rogawski and Meldrum 2004). Protons (H+) are potent intrinsic neuromodulators in the brain, and evidence suggests that acid-sensing ion channels (ASIC), the proton-gated channels, are activated by increase in acidification (Akaike and Ueno 1994). Since both acute extracellular and intracellular acidification suppresses seizure activity, ASIC are thought to play an important role in the control of seizures (Xiong and Stringer 2000; Xiong et al. 2000). Alternatively, chronic extracellular and/or intracellular acidification may play a role in the generation of seizures. A growing body of evidence suggests that amiloride, a potent and nonselective ASIC blocker, exhibits anticonvulsant effects in models of acute generalized seizures induced by pentylenetetrazole (PTZ) and maximal electroshock (Ali et al. 2004, 2005). Similarly, amiloride also delayed the development of kindling induced by PTZ thereby suggesting an important role of ASIC in this epileptogenesis (Ali et al., 2005). In the pilocarpine model of temporal lobe epilepsy, the levels of expression of mRNA encoding for ASIC2b and ASIC1a subunits were decreased in the hippocampus, indicating an involvement of ASIC in this epileptogenesis (Biagini et al., 2001). Given the promising potential of amiloride against the development of seizures, we sought to further determine and quantify amiloride’s effects against pilocarpine-induced seizures and status epilepticus in rats.
2. Results
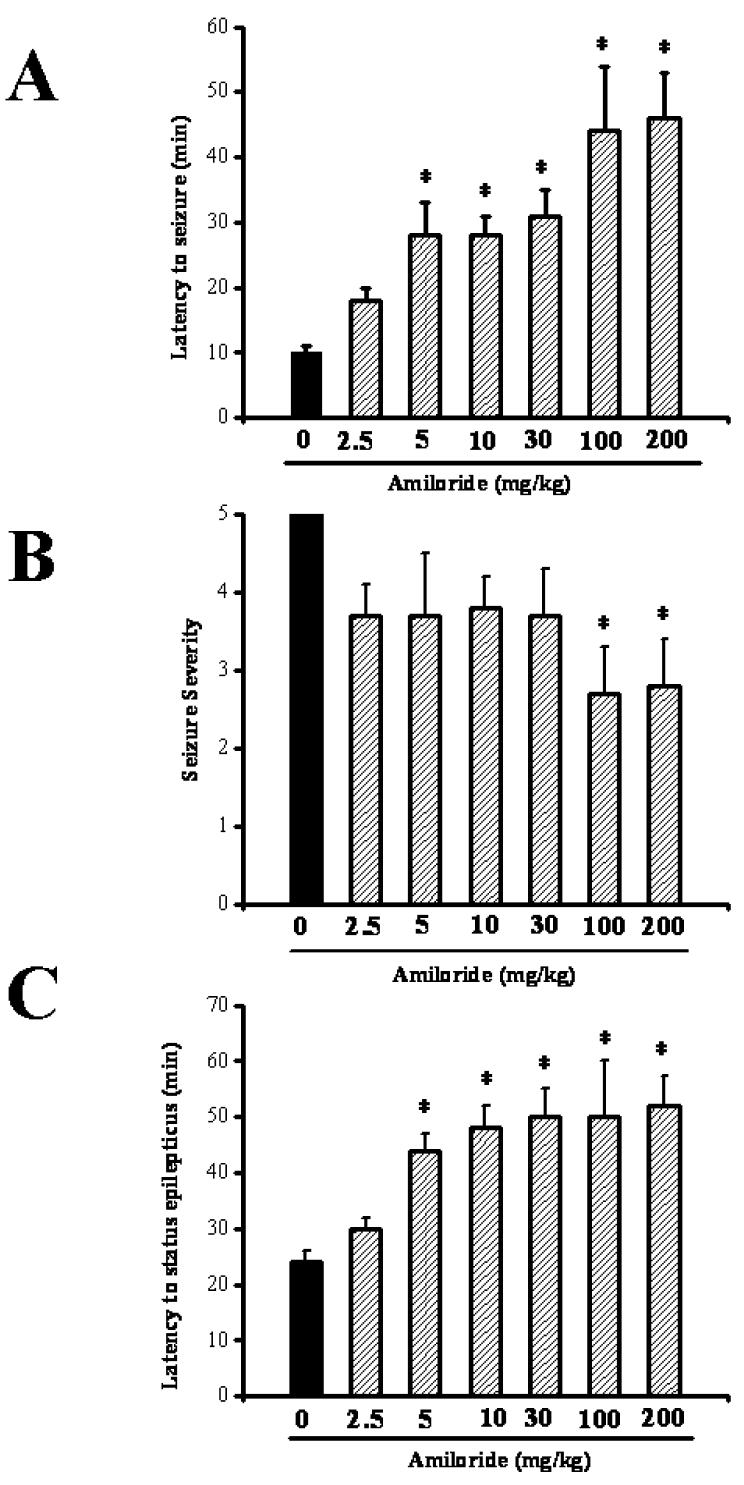
In the control group, all rats exhibited generalized limbic seizures following administration of pilocarpine (380 mg/kg; i.p.). Latency to the first episode of limbic seizures was 10.1±0.9 min (n=9); the mean seizure severity score was 5±0 (n=9). Pretreatment with various doses of amiloride did not affect the incidence of pilocarpine-induced limbic seizures. However, amiloride significantly delayed the onset of pilocarpine-induced limbic seizures (F=10.4, P<0.001); this effect was observed with doses of 5 (28±5 min, n= 6), 10 (28±3 min, n=6), 30 (31±4 min, n=6), 100 (44±10 min, n=6) and 200 (46±7 min, n=6) mg/kg compared to controls (10±1 min, n=9; Fig. 1A) but not with 2.5 mg/kg (18±2 min, n=6). The mean seizure severity score was significantly (F=15.1; P <0.02) reduced with amiloride only at the doses of 100 mg/kg (2.7±0.6, n=4) and 200 mg/kg (2.8±0.6, n=4) compared to controls (5.0±0, n=9; Fig 1B).
Figure 1.
Various doses of amiloride (2.5, 5, 10, 30, 100, 200 mg/kg; p.o.) were administered 90 min before pilocarpine (380 mg/kg; i.p.). A,B. Amiloride delays the onset and reduces the severity of pilocarpine-induced seizures. C. Amiloride delays the onset of pilocarpine-induce status epilepticus. Data represent mean ± S.E.M. *P <0.05.
Limbic seizures developed into status epilepticus in 23±2 min after pilocarpine administration in all tested control rats (n=9). Pretreament with amiloride at the doses of 30, 100 and 200 mg/kg suppressed the occurrence of pilocarpine-induced status epilepticus in 33% of tested rats. In the remaining rats in which continuous seizures were not suppressed, amiloride significantly delayed the onset of status epilepticus compared to controls (30 mg/kg: 50±9 min, n=4; 100 mg/kg: 50±5 min, n=4; 200 mg/kg: 52±8 min, n=4; controls: 23±2 min, n=9; Fig. 1C). Such delayed onset of status epilepticus was also observed with amiloride at doses that did not suppress status epilepticus (5 mg/kg: 44±3 min, n=6; 10 mg/kg: 48±4 min, n=6; controls: 23±2 min; Fig. 1C). No significant change in the onset of status epilepticus was observed with amiloride at the dose of 2.5 mg/kg (29±2 min, n=6) compared to controls.
3. Discussion
The present study demonstrates that pretreatment with various doses of amiloride significantly delays the onset of pilocarpine-induced limbic seizures and status epilepticus. The enhanced latency to seizures is associated with reduction of seizure severity at relatively higher doses of amiloride. Together these findings suggest that amiloride modulates the occurrence of pilocarpine-induced limbic seizures and status epilepticus. Recent evidence has showed that amiloride exerts anticonvulsant effect against acute generalized seizures induced by maximal electroshock and PTZ, as well as PTZ kindled seizures (Ali et al. 2004, 2005). Interestingly, amiloride also delayed the development of PTZ kindling (Ali et al. 2005). Consistent with these results, we found that amiloride delays the onset of pilocarpine-induced limbic seizures and reduces the seizure severity. These findings suggest that amiloride may have both antiepiletogenic and anticonvulsant effects. However, the mechanisms underlying the potential antiepileptogenic and anticonvulsant effects of amiloride are not yet fully understood. Amiloride appears to block various ion channels including ASIC, voltage-gated sodium channels, and low threshold (T-type) voltage-gated calcium channels (Hinton and Eaton 1989). Evidence suggests that ethosuximide, a clinically used anticonvulsant and potent blocker of T-type calcium channels failed to prevent the occurrence of pilocarpine-induced seizures (Coulter et al. 1989a, b; Turski et al. 1987). Similarly, phenytoin and carbamazepine, clinically used anticonvulsant thought to block sodium channels, also failed to prevent pilocarpine-induced seizures (Leite and Cavalheiro, 1995; Turski et al. 1989). Thus, it is likely that amiloride suppresses the occurrence of pilocarpine-induced seizures and status epilepticus via modulation of ASIC.
In conclusion, amiloride may have antiepileptogenic and anticonvulsant potential in the pilocarpine model of temporal lobe epilepsy. Understanding the mechanisms contributing to the effects of amiloride against the development of limbic epilepsy can provide a frame work for the development of clinically useful ASIC blockers.
4. Experimental procedure
Sprague-Dawley rats, (male, 150-220g, Taconic, Germantown MD) were used. All experiments were performed with the approval of the Georgetown Animal Care and Used Committee. Effort was made to minimize the number of animals used as well as their suffering. Pilocarpine hydrochloride (380 mg/kg Sigma Chemical, St Louis, MO) was dissolved in 0.9% saline and intraperitoneally injected to induce seizures. Methyl scopolamine (1 mg/kg in 0.9% saline) was intraperitoneally administered 30 min before pilocarpine administration to minimize the peripheral effects of pilocarpine (Turski et al 1983). Rats were closely observed for the occurrence of limbic seizures and status epilepticus during 120 min following pilocarpine administration. The convulsive behavior of seizures were classified as follows (Racine 1972, modified): class I, hypoactivity, mouth and facial automatism; class II, head nodding and mastication; class III, forelimb clonus without rearing; class IV bilateral forelimb clonus and rearing, class V rearing and loss of posture. Status epilepticus was defined as an uninterrupted seizure class 5 for 30 min. Amiloride (2.5, 5, 10, 30, 100 and 200 mg/kg; Tocris MA) was dissolved in water and orally administered 90 min before pilocarpine. The initial dose (2.5 mg/kg) of amiloride was chosen because of its anticonvulsant effect against PTZ-induced generalized clonic seizures (Ali et al 2004). Subsequent doses of amiloride was then progressively increased by 2-3 fold until significant protection against pilocarpine-induced seizures was observed. The 90 min time interval was found to be the most effective in our preliminary tests (data not shown). Animals that did not display class 2 seizures within the 2-hour observation period were considered protected. For each group, the incidence of seizure and status epilepticus was recorded. Time from the end of pilocarpine injection to appearance of the first episode of convulsive behaviors (forelimb clonus), referred to as the seizure latency, was recorded. Data analysis of the latency to seizures and status epilepticus were performed using one-way ANOVA with Dunnett’s post hoc test. Comparison of the seizure severity score was done with Kruskal-Walis ANOVA and Dunnett’s post hoc test. Before using ANOVA, data were submitted to the normality test (Shapiro-Wilk test) and the test for homogeneity of variances (Levene’s test). The incidence of seizures and status epilepticus were analyzed using Chi square test. A significant statistical difference was determined by value of at least p<0.05.
Acknowledgments
This publication was made possible by Public Health Service grant NS047193 from the National Institutes of Health (NIH) and its contents are responsibility of the authors and do not necessarily represent the official views of NIH. The author is grateful to Dr. Einsley M. Janowski for a critical review of the manuscript.
Footnotes
Section: Disease-Related Neuroscience
Theme J: Disorders of the Nervous System
Topic: Epilepsy: anticonvulsant
References
- Akaike N, Ueno S. Proton-induced current in neuronal cell. Prog. Neurobiol. 1994;43:73–83. doi: 10.1016/0301-0082(94)90016-7. [DOI] [PubMed] [Google Scholar]
- Ali A, Ahmad FJ, Pillai KK, Vohora D. Evidence of the antiepileptic potential of amiloride with neuropharmcological benefits in rodent models of epilepsy and behavior. Epilepsy Behav. 2004;5:322–328. doi: 10.1016/j.yebeh.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Ali A, Ahmad FJ, Pillai KK, Vohora D. Amiloride protects against pentylenetetrazole-induced kindling in mice. Br. J. Pharmacol. 2005;145:880–884. doi: 10.1038/sj.bjp.0706291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagini G, Babinski K, Avoli M, Marcinkiewicz M, Seguela P. Regional and subunit-specific downregulation of acid-sensing ion channels in the pilocarpine model of epilepsy. Neurobiol. Dis. 2001;8:45–58. doi: 10.1006/nbdi.2000.0331. [DOI] [PubMed] [Google Scholar]
- Coulter DA, Huguenard JR, Prince DA. Characterization of ethosuximide reduction of low threshold calcium current in thalamic neurons. Ann. Neurol. 1989a;25:582–593. doi: 10.1002/ana.410250610. [DOI] [PubMed] [Google Scholar]
- Coulter DA, Huguenard JR, Prince DA. Specific petit mal anticonvulsant reduce calcium current in thalamic neurons. Neurosci. Lett. 1989b;98:74–78. doi: 10.1016/0304-3940(89)90376-5. [DOI] [PubMed] [Google Scholar]
- Hinton CF, Eaton DC. Expression of amiloride-blockade sodium channels in Xenopus. Am. J. Physiol. 1989;257:C825–829. doi: 10.1152/ajpcell.1989.257.4.C825. [DOI] [PubMed] [Google Scholar]
- Leite JP, Cavalheiro EA. Effect of conventional antiepileptic drugs in a model of spontaneous recurrent seizures in rats. Epilepsy Res. 1995;20:93–104. doi: 10.1016/0920-1211(94)00070-d. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II Motor seizure. Electroencephalogr. Clin. Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Rogawski MA, Meldrum BS. Neurobiology of antiepileptic drugs. Nat. Rev. Neurosci. 2004;5:553–564. doi: 10.1038/nrn1430. [DOI] [PubMed] [Google Scholar]
- Turski WA, Cavalheiro EA, Coimbra C, da Penha Berzaghi M, Ikonomodou-Turski C, Turski L. Only certain antiepilepstic drugs prevent seizures induced by pilocarpine. Brain Res. 1987;434:281–305. doi: 10.1016/0165-0173(87)90002-6. [DOI] [PubMed] [Google Scholar]
- Turski WA, Cavalheiro EA, Schwarz M, Czuczwar SJ, Kleinrok Z, Turski L. Limbic seizures produced by pilocarpine in rats: behavioral, electroencephalographic and neuropathological study. Behav. Brain Res. 1983;9:315–335. doi: 10.1016/0166-4328(83)90136-5. [DOI] [PubMed] [Google Scholar]
- Turski L, Ikonomodou C, Turski WA, Bortolotto ZA, Cavalheiro EA. Review: cholinergic mechanisms and epileptogenesis. The seizures induced by pilocarpine: a novel experimental model of intractable epilepsy. Synapse. 1989;3:154–171. doi: 10.1002/syn.890030207. [DOI] [PubMed] [Google Scholar]
- Xiong ZQ, Stringer JL. Extracellular pH responses in CA1 and the dentate gyrus during electrical stimulation, seizure discharges and spreading depression. J. Neurophysiol. 2000;83:3519–3524. doi: 10.1152/jn.2000.83.6.3519. [DOI] [PubMed] [Google Scholar]
- Xiong ZQ, Saggau P, Stringer JL. Activity-dependent intracellular acidification correlates with the duration of seizures activity. J. Neurosci. 2000;20:1290–1296. doi: 10.1523/JNEUROSCI.20-04-01290.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]