Abstract
Dendritic cells (DC) are potent professional antigen-presenting cells that can activate naive T lymphocytes and initiate cellular immune responses. As adjuvants, DC may be useful in enhancing the immunogenicity of tumor antigens and mediating tumor regression. Endogenous expression of antigen by DC offers the potential advantage of allowing prolonged constitutive presentation of endogenously processed epitopes and exploitation of multiple restriction elements for the presentation of the same antigen. In this report, we show that human DC are (a) capable of infection by recombinant poxviruses encoding melanoma-associated antigen (MAA) genes and (b) capable of efficiently processing and presenting these MAA to cytotoxic T cells. In 6/6 HLA A*0201 -expressing melanoma patients tested, the virally driven expression of MART-1/Melan A MAA by DC was sufficient to generate CD8+ T lymphocytes that could recognize naturally processed epitopes on tumor cells. In most cases, specific anti-MART-1 reactivity could be detected after a single stimulation. Analysis of epitope dominance revealed that the amino acid sequence recognized by these cytotoxic T lymphocytes (CTL) corresponded to the MART-127-35 residues previously shown to be most commonly recognized by cytotoxic T lymphocytes expanded from metastatic melanoma lesions. These data show that the virally driven expression of MAA by DC can be exploited for the efficient induction of clinically relevant cytotoxic T-cell responses. This has clinical implications for active immunization therapy, and currently vaccine trials have been proposed for patients with metastatic melanoma.
Keywords: Melanoma, Dendritic cells, Melanoma-associated antigens, MART1, Viral vectors, Poxviruses, CTL
Most melanoma-associated antigens (MAA), including MART-1/Melan A, are nonmutated proteins processed intracellularly to yield peptide fragments presented by major histocompatibility complex (MHC) class I molecules and thus recognized by cytotoxic T lymphocytes (CTL) (1–4). Repetitive in vitro sensitization of peripheral blood mononuclear cells (PBMC) with synthetic peptides representing sequences derived from these epitopic determinants can induce potent CTL that recognize naturally processed antigen on the surface of melanoma cells (5,6). Furthermore, we have shown that multiple in vivo administrations of the same synthetic peptides emulsified in incomplete Freund’s adjuvant (IFA) can specifically enhance CTL precursor reactivity in the peripheral circulation (7,8). This enhanced immunologic reactivity, however, is not sufficient to induce clinical responses in most patients. Preclinical models have shown that the administration of peptide alone is not very efficient (9), and the choice of an appropriate adjuvant, including dendritic cells (DC), may be critical in enhancing antigen presentation in vivo (10).
DC are antigen-presenting cells that are useful in the initiation of cellular immune responses (11). First described by Steinman and Cohn in 1973 (12,13), DC possess a special capacity to circulate and migrate to secondary lymphoid organs, where they exert their effects by stimulating resting T lymphocytes in a MHC class I (14,15) and class II (16–20) restricted manner. Clinically, the presence of DC in tumors has been correlated with improved prognosis in a variety of human cancers (21). Previously, DC have been isolated from progenitor cells of human bone marrow (22) or cord blood (23). It was not until recently that significant quantities of DC could be generated from human peripheral blood (24). Sallusto et al. (24) have shown that monocytes derived from the peripheral circulation of humans can be activated into DC by in vitro culture with granulocyte macrophage-colony stimulating factor (GM-CSF) and interleukin-4 (IL-4).
DC can function as an efficient adjuvant for MHC class I restricted antitumor sensitization in vivo (15,18,25–30). Bone marrow–derived murine DC pulsed with MHC class I restricted soluble β-galactosidase (28), ovalbumin-derived peptides (15,27), or unfractionated acid-eluted tumor peptides (26) could induce long-lasting immunity against challenges with tumors. These studies have shown that peptide-pulsed DC are more efficient in inducing antitumor protection than immunization with peptide alone (27,28) or emulsified in IFA (29). However, exogenous loading of peptide on HLA molecules has two major disadvantages: first, the stability of the peptide binding to the HLA molecule will affect its immunogenicity (31), and second, the amino acid sequence appropriate for each HLA allele must be known. These limitations may be circumvented by a system that allows endogenous presentation of antigens, such as the infection of DC with recombinant viral vectors. Bhardwaj et al. (32) have shown that DC are infectable by influenza virus. Infected DC express viral proteins and are capable of generating anti-viral specific CD8+ CTL in humans (32). In this study, we tested whether the infection of human DC with viral vectors encoding MAA genes could induce significant expression of the relevant antigen to allow endogenous presentation in association with HLA class I molecules and induce anti-MAA CTL reactivity in vitro.
MATERIALS AND METHOD
Culture Medium (CM)
Iscove’s Media (Gibco Laboratories, Grand Island, NY, U.S.A.) supplemented with 10% heat-inactivated human AB serum, 10 mM HEPES buffer, 100 U/ml penicillin-streptomycin, 0.5 mg/ml amphotericin B, and 0.03% glutamine.
Cell Lines
Preparation of PBMC and Lymphocytes
HLA phenotypes of patients’ PBMC and cell lines were established using the Amos modified microcytotoxicity test or polymerase chain reaction (PCR) (33,34). Molecular analysis of HLA-A2 subtype was performed by high-resolution sequence-specific primer PCR as previously described (34,35). Leukaphereses were performed in HLA-A*0201 patients with metastatic melanoma referred for treatment to the Surgery Branch, National Cancer Institute. PBMC were also obtained from one HLA-A*0201 patient with metastatic renal cell cancer. PBMC were separated by Ficoll-Hypaque gradient and used for preparation of DC and T cells. CD8+ T enrichment of T cells was achieved by positive selection on biomagnetic separation beads (Dynal Corp., New York). In all experiments, the T-cell population was greater than 95% CD8+ and included <5% contamination with CD4+ cells by fluorescence-activated cell sorter (FACS) analysis.
TIL Culture and TIL Clone
1235 tumor-infiltrating lymphocytes (TIL) were generated from a metastatic lesion of an HLA-A*0201 melanoma patient. These CTL were >99% CD8+ lymphocytes. The CD8+ T-cell clone A42 was established as previously described (2,36). Both 1235 TIL and A42 recognize the immunodominant nonamer peptide MART-127-35 (AAGIGILTV) of the MART-1/Melan A human melanoma antigen (37). These established CTL cultures were propagated in CM plus 6,000 IU/ml of IL-2 (Chiron Corp., Emeryville, CA, U.S.A.).
624-MEL
The 624-MEL melanoma cell line was derived from a metastatic lesion of an HLA-A*0201 patient. The HLA-A2+/MART-1+ 624.38 and the HLA-A2−/MART-1+ 624.28 clones were generated by limiting dilution as previously described (37).
T2 Cell Line
T2 cells were used in cytokine release and cytotoxicity assays for HLA-A*0201 restricted epitopes. This cell line expresses only the HLA-A*0201 allele and is defective in endogenous processing, which enhances the effectiveness of exogenous peptide loading (38,39).
Preparation of DC
After Ficoll-Hypaque separation, 1–3 × 108 PBMC were processed for preparation of DC according to principles previously described (24). The PBMC were cultured in 75 cm2 culture flasks for 3 h at 37°C with 200 IU/ml (Pepro Tech Inc., Rocky Hill, NJ, U.S.A.) human recombinant IL-3 (hrIL-3). The nonadherent cells were removed while the adherent cells were cultured for 5–7 days in sterile conditions in 10 ml of CM. Human recombinant GM-CSF (hrGM-CSF, 2,000 IU/ml, Pepro Tech, Inc.) and human recombinant IL-4 (hrIL-4, 2,000 IU/ml, Pepro Tech, Inc.) were added every 2–3 days from day 0.
MART-1, G9-209-2M, and Flu M1 Peptides
MART-127-35 (AAGIGILTV) was produced to GMP grade by solid-phase synthesis techniques by Peptide Technologies, Inc. (Gaithersburg, MD, U.S.A.). The other MART-1-derived peptides (the 9-mers MART-131-39, MART-132-40, MART-135-43, MART-156-64) were synthesized by a solid-phase method using a multiple peptide synthesizer and purified by high-performance liquid chromatography, as previously described (2). These alternative epitopes for the MART-1 antigen in the context of HLA-A*0201 were selected for the reasons described in the Results section. G9-209-2M peptide (IMDQVPFSV) was produced to GMP grade by solid-phase synthesis techniques by Chiron Mimotopes Peptide Systems (San Diego, CA, U.S.A.). The G9-209-2M peptide is a 9 amino acid sequence with a single substitution at position 2 (T to M) from the natural gp100 MAA–derived epitope (3). This modification has been shown to enhance binding to the HLA-A*0201 molecule and T-cell reactivity in vitro (40). This gp100-derived peptide was selected as a negative control for testing of anti-MART-1 CTL reactivity because of its high affinity for HLA-A*0201. Flu-M158-66 peptide (GILGFVFTL) from the influenza matrix protein was synthesized by Multiple Peptide Systems (San Diego, CA, U.S.A.).
Peptide-Pulsing of DC and T2
The recovered DC or T2 cells were pulsed with peptide for 2 h in 15-ml conical tubes at 37°C at a concentration of 1 × 106 cells/ml and 1 μg/ml peptide.
Flow Cytometric Analysis of Cell Population (FACS) (Fig. 1)
FIG. 1.
Representative example of phenotype analysis of dendritic cells (DC) by fluorescence-activated cell sorter analysis. Surface phenotype of peripheral blood mononuclear cells (PBMC); adherent cells (AC) prepared on 75-cm2 flasks after removal of nonadherent cells after a 3-h incubation with human recombinant interleukin-3 (hrIL-3) (200 IU/ml) at 37°C; and DC cultured with hrIL-4 (2,000 IU/ml) and human recombinant granulocyte-macrophage colony-stimulating factor (hrGM-CSF) (2,000 IU/ml) at day 5. Cell fluorescence is indicated by solid histograms and the control staining with isotype-matched IgG in blank histograms.
Approximately 105 cells were incubated in 20 μl of primary antibody for 30 min at 4°C. The cells were phenotyped on a FACScan (Becton Dickinson, San Jose, CA, U.S.A.) according to a previously described method (41).
Antibodies
For DC characterization, the following primary monoclonal antibodies (mAbs) were used for FACS: fluorescein isothiocyanate (FITC)-conjugated mouse anti-human IgG CD86, CD80, CD1a (Pharmingen, San Diego, CA) and DR, CD54, CD 14 (Becton Dickinson, San Jose, CA). For analysis of CD8+ purity FITC conjugated anti-CD4 and anti-CD8 murine mAbs were purchased from Becton Dickinson.
Viable Staining of DC and Detection of Virally Induced MAA by Immunohistochemistry and Immunofluorescence (Fig. 2)
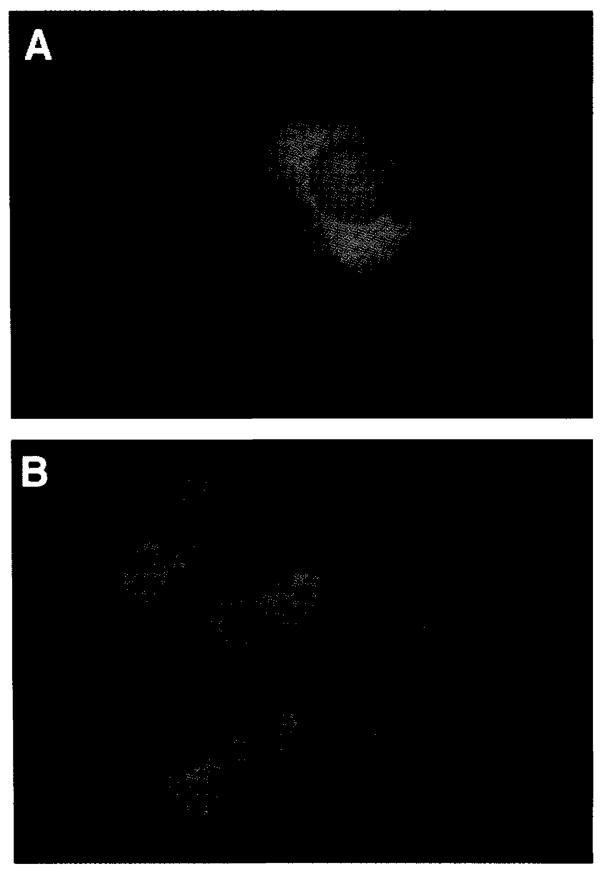
FIG. 2.
Morphologic appearance of dendritic cells (DC). Vital stain with calcein AM of DC after 5 days in culture with human recombinant interleukin-4 (hrIL-4) (2,000 IU/ml) and human recombinant granulocyte-macrophage colony-stimulating factor (hrGM-CSF) (2,000 IU/ml) (A). Viral expression of MART-1 in the same DC by immunofluorescence (B). DC were incubated with rV-MART-1 for 1 h at 37°C at 10:1 multiples of infection. After 12 h, DC were stained with anti-MART-1 mAb (42). No staining or fluorescence was detected with anti-gp100 monoclonal antibody (mAb) (HMB45) as the negative controls. Conversely, DC infected with the rV-gp100 gene were not stained by anti-MART-1 mAb but were brightly stained with HMB 45.
DC were viably stained by incubation with 15 μg/ml calcein AM (Molecular Probes, Eugene, OR, U.S.A.) for 30 min at 37°C followed by a quench reaction with 15 μl/ml ethidium bromide (One Lambda Inc., Canoga Park, CA, U.S.A.). For detection of expression of MART-1, cytospin preparations of DC were fixed in acetone and stained with anti-MART-1 murine IgG2b (M2-7C10) (42). For immunofluorescence studies, the DC were incubated with FITC-conjugated goat anti-mouse IgG (Becton Dickinson) at room temperature and visualized under a Nikon DM580 microscope. Negative controls consisted of the cross-staining of virally infected DC with the irrelevant anti-MAA mAb (anti-gp100 HMB45, BioGenex Laboratories, San Ramon, CA, U.S.A.) as well as the absence of staining by the anti-MART-1 mAb of DC infected with virus encoding for gp100 MAA or nonmelanoma tumor cell lines. Staining intensity of the slides was scored on a scale of 0–4+, and the proportion of positive cells was categorized as <25%, 25–50%, 51–75%, or >75%.
Infection of DC with Vaccinia or Fowlpox Virus Containing the MART-1 Gene
Recombinant vaccinia and fowlpox viruses encoding MART-1 (rV-MART-1 and rF-MART-1) were provided by Therion Biologies (Cambridge, MA, U.S.A.). Infection of cell lines with recombinant poxviruses were done as previously described (43). DC (1 × 106) were incubated in minimal volume of media at 37°C with rV-MART-1, rV-Flu-M1, rF-MART-1. Titration experiments suggested that 1 × 107 plaque-forming units/ml, equal to 10:1 multiples of infection for 1 h were able to consistently induce expression of MART-1 in 50–75% of DC. The cells were suspended in 10 ml of fresh, warm CM overnight. DC were washed twice, tested for antigen expression by immunohistochemistry and immunofluorescence, and subsequently used as stimulators.
In Vitro Sensitization of Peripheral Blood Lymphocytes with DC
CD8+ lymphocytes cells (4–5 × 106/well) were coincubated with 1 × 106 peptide-pulsed (MART-127-35, Flu-M1 58-66) or virally infected DC (rV-MART-1, rV-Flu-M1) in 24-well plates. CD8+ T cells stimulated with rV-MART-1 were restimulated after 1 week with DC virally infected with rF-MART-1. This non-cross-reacting viral vector was used for restimulation to minimize antiviral reactivity. The stimulators were added to responding cells (1×106/well) at a stimulator:responder ratio of 1:4 or 1:5.IL-2 (300 IU/ml) was added 24 h after each stimulation and every 2–3 days thereafter. The effectors were tested for specificity after 7–9 days from the original stimulation and 7 days after the restimulation.
Assessment of CTL Reactivity: Cytokine Release Assay
Effector cells (105) were coincubated with 105 stimulator cells for 24 h at 37°C in 200 μl of CM (5 × 105 effector cells/ml). Interferon-γ (IFN-γ) levels were measured by human IFN-γ Quantikine enzyme-linked immunosorbent assay kits (R & D Systems, Minneapolis, MN, U.S.A.). Data are presented as picograms of IFN-γ released by 5 × 105 effectors/24 h.
Assessment of CTL Reactivity: Cytotoxicity Assay
Cytotoxicity was measured by a 4-h calcein AM release assay as previously described (44). For recognition of exogenous peptide, T2 cells were pulsed with 1 (μg/ml peptide for 2 h at 37°C, washed once, and used as targets. CTL were also tested against 624-MEL targets at week 2. The effector cells were plated at various effector-to-target ratios in sextets, as noted in the figure 5 legend. The trays were read automatically using a Nikon Diaphot-TMD inverted microscope controlled by Lambda Scan automated microfluorimeter hardware (One Lambda). Results could also be confirmed by sight. Percent lysis was calculated as follows: [1 − (mean experiment − mean blank)/(mean maximum − mean blank)] × 100. Data are reported as percent lysis.
FIG. 5.
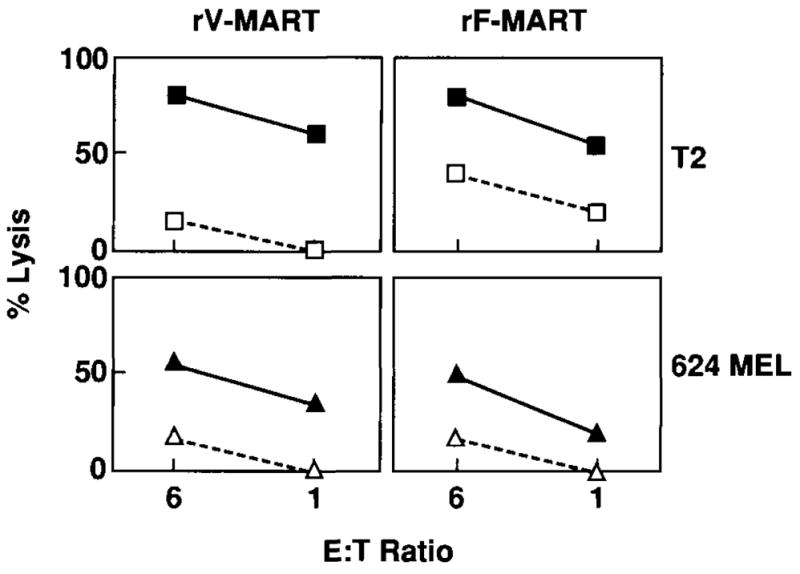
Cytotoxic activity (calcein AM release) of anti-MART-1 cytotoxic T lymphocytes (CTL) induced with recombinant virus-infected dendritic cells (DC). Anti-MART-1 CTL were induced by a primary in vitro stimulation with DC infected with rV-MART-1 followed 7 days later with a second stimulation with DC infected with rF-MART-1 virus (rV-MART-1 = left two panels). By reversing the sequence of the viral vectors used for stimulation, anti-MART-1 CTL were also induced by a primary stimulation with DC infected with rF-MART-1 virus followed 7 days later by a second stimulation with DC infected with rV-MART-1 virus (rF-MART-1 = right two panels). Recognition of the epitope MART-127-35 was tested by pulsing T2 cells with 1 μg/ml MART-127-35 (■) or 1 μg/ml irrelevant G9-209-2M (□). Recognition of naturally processed MART-1 epitopes was tested against the 624.38 (▲) HLA-A*0201+/MART-1+ and the 624.28 (△), HLA-A*0201-/MART-1 + melanoma clones.
RESULTS
DC Preparation
DC were prepared from human peripheral lymphocytes from HLA-A*0201 melanoma patients. Phenotyping of DC (Fig. 1) showed a high level of expression of HLA class I and II, co-stimulatory molecules (B7.1, B7.2, ICAM-1) and low expression of CD14 and CD1a consistent with DC phenotype. Viable staining of these cells (Fig. 2A) at days 5–7 revealed the characteristic morphologic appearance of DC with veiled edges and multiple processes (43).
Peptide-Pulsed DC Can Induce a Rapid MHC Class I Restricted CTL Reactivity
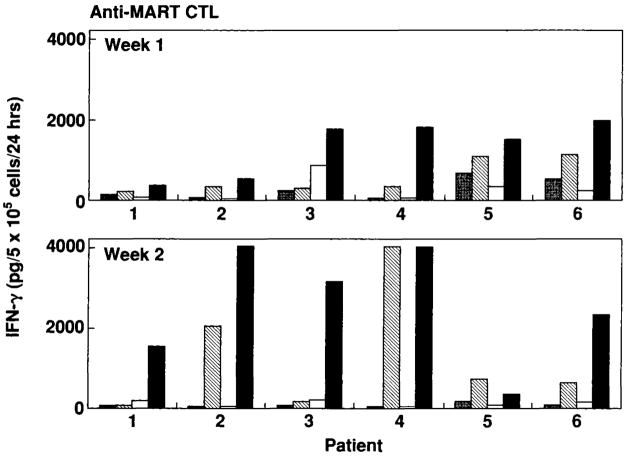
Generation of anti-MART-l27-35 or anti-Flu M158-66 CTL reactivity was attempted in five patients by in vitro stimulation with peptide-pulsed DC (Table 1, nos. 1–5). DC were pulsed with peptide for 2 h at 37°C at a concentration of 1 × 106 cells/ml and 1 μg/ml peptide and washed away. Specific reactivity was tested by IFN-γ release assay after 7–9 days in culture. Potent anti-Flu-M158-66 reactivity was detected in all patients, suggesting that peptide-pulsed DC could induce a rapid MHC class I restricted secondary in vitro sensitization of CTL. Anti-MART-127-35 reactivity, however, was detectable in only one of the five patients at week 1, a second stimulation with MART-127-35, pulsed DC enhanced CTL reactivity in that patient and allowed detection of minimal reactivity in a second patient. These findings are in agreement with previous reports in which DC could be used to consistently sensitize peripheral lymphocytes against MAA, but at least four restimulations were required (45).
TABLE 1.
Specific interferon-γ (IFN-γ) release by CD8+ T lymphocytes from HLA-A*0201 patients with metastatic melanoma
| CTL culture | Anti-Flu M1 | Anti-MART-1 (week 1) | Anti-MART-1 (week 2) |
|---|---|---|---|
| 1 | >4,000(104) | 72(140) | 356 (48) |
| 2 | 1,221(36) | 0(0) | 8(48) |
| 3 | >4,000 (0) | 344 (0) | >4,000 (0) |
| 4 | >4,000 (260) | 188(296) | 0(0) |
| 5 | >4,000 (888) | 0(0) | 0(0) |
T cells were sensitized in vitro by the co-incubation of 1 × 106 dendritic cells (DC) pulsed with Flu-M1 58-66 or MART-127-35 and 5 × 106 CD8+ T lymphocytes. T-cell reactivity was tested by IFN-γ release assay 7–9 days after the initial stimulation (week 1). To enhance the specificity of anti-MART-1 cytotoxic T lymphocytes (CTL), a second stimulation was done with peptide-pulsed DC and tested 7 days later (week 2). For the IFN-γ release assay, 105 effector cells were co incubated with peptide-pulsed T2 cells at 1 μg/ml for 24 h at 37°C in 200 μl of culture media. Data are presented as picograms of lFN-γ released by 5 × 105 effectors/24 h. CTL tested against peptide-pulsed T2 cells with the irrelevant peptide (G9-209-2M) are shown in parentheses. In these experiments, the peripheral blood mononuclear cells were cultured in the presence of relevant peptide (1 μg/ml) in addition to peptide-pulsed DC. Similar experiments in which DC were not added to the cultures did not elicit significant anti-MART-1 reactivity (not shown).
We previously noted that DC pulsed with 1–10 μg/ml MART-127-35 peptide were less efficient than T2 cells as stimulators of IFN-γ secretion by anti-MART-127-35 specific CTL. The same DC, however, were as efficient as T2 cells in presenting the Flu M158-66 peptide (data not shown). These data suggested that perhaps the lower stability of the MART-127-35,/HLA-A*0201 complex could be responsible for the poor immunogenicity of pulsed DC, because the MART-127-35 peptide is poorly soluble in aqueous medium and has a low-to-intermediate affinity for HLA-A*0201 (5). Therefore, we reasoned that virally driven expression of MART-1 could enhance the efficiency of CTL induction in vitro by amplifying the antigenic stimulation through prolonged viral replication (46).
Endogenous Processing and Presentation of MAA by DC
To investigate whether DC were permissive to poxvirus-driven expression of MAA, DC were infected with viral vectors (rV-MART-1, rF-MART-1, rV-Flu-M1), and cytoplasmic MAA expression was analyzed with anti-MART-1 (M2-7C10) or anti-gp100 (HMB45) mAb by immunofluorescent staining procedures as previously described (42) (Fig. 2B). Approximately 50–75% of DC infected with rV-MART-1 or rF-MART-1 were strongly and consistently fluorescent. To confirm endogenous processing and presentation of the MART-127-35/HLA-A*0201 complex, we analyzed the pattern of cytokine release elicited by the virally infected DC on A42 (anti-MART-127-35) T-cell clone (Fig. 3). A42 recognized endogenously processed MART-127-35 as readily as the exogenous peptide provided at concentrations of 1 or 10 μg/ml.
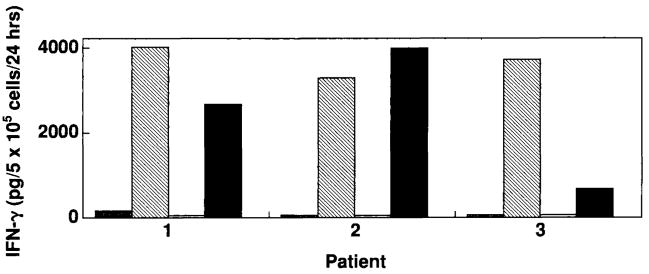
FIG. 3.
Recognition by the anti-MART-127-35 A42 cytotoxic T lymphocyte clone of endogenously processed MART-1 protein by rV-MART-1-infected dendritic cells (DC). Recognition is compared with that of MART-127-35 (1 μg/ml) pulsed DC. 105 rV-MART-1 DC (black bars) or peptide-pulsed DC (hatched bars) from three melanoma patients were coincubated with 105 A42 cells for 24 h at 37°C in 200 ml of culture medium. The efficacy of endogenous presentation by these DC was assessed by the induction of interferon-γ (IFN-γ) (pg/ml) release by A42. Negative stimulator cell controls included DC infected with an irrelevant rV encoding the sequence for the gp100 melanoma-associated antigens (white) or pulsed with the G9-209-2M peptide (gray bars). Similar results were obtained with rF-MART-1-infected DC.
Generation of CTL Reactivity by DC Expressing Endogenous MAA or Flu M1
Anti-Flu and anti-MART-1 CTL cultures were generated from PBMC obtained from four and six melanoma patients, respectively. CTL raised with virally infected DC or peptide-pulsed DC were tested against peptide-pulsed T2 cells 7–9 days after the initial stimulation. After this single stimulation, anti-Flu-M1 reactivity was strongly positive in all but one CTL when stimulated with peptide-pulsed or virally infected DC (Fig. 4). Anti-MART-127-35 reactivity was detectable after a single stimulation in all six patients, although to variable degrees and to a lesser extent than anti-Flu M1 reactivity (data not shown). To enhance and confirm specificity of the anti-MART-1 CTL cultures, a second stimulation was performed with rF-MART-1-infected DC. This vector was used to avoid potentiation of antiviral (antivaccinia) reactivity in the CTL cultures. Seven days after this second stimulation, the effector cells were retested for specific reactivity against MART-127-35 (1 μg/ml) pulsed T2 cells (data not shown). All CTL cultures generated with rV-MART-1-infected DC as stimulators were highly specific for MART-1, although one culture (no. 5) had lost some of the reactivity noted at week 1. Five of six cultures showed stronger reactivity compared with CTL generated with peptide-pulsed DC. We have previously shown that anti-MART-1 reactivity can be more readily generated in vitro in patients with metastatic melanoma than in non-melanoma-bearing hosts (47). Therefore, we tested whether virally infected DC could efficiently stimulate anti-MART-1 reactivity in a nonmelanoma cancer patient with metastatic renal cell cancer. Although anti-MART-127-35 CTL reactivity with peptide-pulsed DC was not detected in this patient, reactivity was detected after one stimulation with rV-MART-1-infected DC (IFN-γ secretion by 5 × 105 effector cells/24 h = 1,216 pg against MART-127-35 pulsed T2 cells versus 200 pg against G9-209-M pulsed T2 cells).
FIG. 4.
Specific interferon-γ (IFN-γ) release by CD8+ T lymphocytes from six HLA-A*0201 patients with metastatic melanoma. Anti-MART-1 cytotoxic T lymphocytes (CTL) were generated by stimulation with dendritic cells (DC) pulsed with MART-127-35 peptide or infected with rV-MART-1 and tested 7–9 days after stimulation (week 1). After 7 days, anti-MART-1 CTL stimulated with peptide-pulsed DC were restimulated with peptide-pulsed DC while cultures stimulated with rV-MART-1 DC were restimulated with DC infected with non-cross-reacting rF-MART-1. These CTL cultures were retested 7 days after the restimulation (week 2). Effectors were tested against T2 cells pulsed with 1 μg/ml peptide. Anti-MART-1 CTL cultures generated with peptide-pulsed DC were tested against T2 cells pulsed with 1 μg/ml of MART-127-35 (hatched bars) and G9-209-2M (gray bars) peptides. Anti-MART-1 CTL cultures generated with rV-MART-1 DC were tested against T2 cells pulsed with 1 μg/ml MART-127-35 (black bars) and G9-209-2M (white bars) peptides.
Lytic activity of CTL from a melanoma patient was tested after two stimulations using calcein AM cytotoxicity assay against peptide-pulsed T2 and 624 MEL cell lines (Fig. 5). Although residual nonspecific LAK lysis could be noted in these early cultures, the lysis of relevant targets was consistently higher than the lysis of the irrelevant targets. In some experiments, the sequence of recombinant virus stimulation was inverted by stimulating CD8+ T cells with rF-MART-1-infected DC first, followed by a secondary stimulation with rV-MART-1-infected DC. Recombinant fowlpox virus was as effective as recombinant vaccinia virus in sensitizing CTL (Fig. 5).
CTL Induced by rV-MART-1-Infected DC Recognize Immunodominant Epitopes Naturally Processed by Melanoma Cells
Cytokine release assays showed that anti-MART-1 CD8+ T cells generated by in vitro sensitization with rV-MART-1-infected DC could recognize naturally processed MART-1 epitopes on HLA-A*0201 tumor targets (624.38 MEL) (Table 2). The amount of secretion of IFN-γ by these CTL cultures was less than the amount secreted by the A42 clone and 1235 TIL, but this is compatible with the early stage of these CTL lines. To identify the epitope predominantly recognized by the anti-MART-1 CTL cultures, we analyzed the pattern of IFN-γ secretion in response to stimulation with five candidate peptides (MART-127-35, MART-129-37, MART-132-40, MART-135-43, MART-156-64) pulsed on T2 cells. These peptides were selected based on the known binding motif for HLA-A*0201. MART-127-35 epitope is most commonly recognized by TILs (2). MART-132-40 epitope eluted from melanoma cells (48) has been shown to be capable of inducing specific HLA-A*0201-restricted CTL reactivity in vitro (5). MART-129-37 and MART-156-64 were recently described candidate epitopes with binding motifs for HLA-A*0201 but one demonstrating poor (MART-129-37) and one good (MART-156-64) stability when complexed to the HLA-A*0201 molecule (49). All six CTL bulk cultures showed preferential reactivity against MART-127-35, providing further evidence that this epitope is dominant in the HLA-A*0201 system.
TABLE 2.
Specific reactivity of virally induced anti-MART-1 cytotoxic T lymphocytes (CTL) against various HLA-A*0201 compatible MART-1-derived peptides and naturally processed MART-1 epitopes on tumor cells
| T2 + MART-1 peptide
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Effector | 27–35 | 31–39 | 32–40 | 35–43 | 56–64 | T2 + G9-209-2M | 624.38-Mel | 624.28-Mel |
| CTL-1 | 1,724 | 0 | 0 | 0 | 54 | 34 | 815 | 0 |
| CTL-2 | 666 | 184 | 204 | 195 | 160 | 156 | 209 | 60 |
| CTL-3 | 683 | 64 | 49 | 56 | 124 | 93 | 137 | 35 |
| CTL-4 | 438 | 91 | 99 | 114 | 7 | 0 | 58 | 0 |
| CTL-5 | 3,924 | 392 | 396 | 424 | 404 | 340 | 1,416 | 147 |
| CTL-6 | 844 | 392 | 356 | 344 | 348 | 376 | 44 | 0 |
| A42 anti-MART-1 27-35 clone | >4,000 | 0 | 54 | 0 | 0 | 0 | 2,559 | 13 |
| 1235 TIL | >4,000 | 5 | 155 | 0 | 53 | 12 | 3,704 | 30 |
| Anti-G9 209-2M CTL | 0 | 0 | 0 | 0 | 0 | >4,000 | 1,154 | 0 |
CTL 1–6 were raised by stimulation with dendritic cells (DC) infected with rV-MART-1 and restimulated after 7 days with DC infected with rF-MART-1. Interferon-γ release (pg/5 × 105 CD8− cells/24 h) was analyzed against T2 cells pulsed with peptide (2 μg/ml) or against the HLA-A*0201+/MART-1 + 624.38 MEL or the HLA-A*0201 –/MART-1 + 624.28 MEL melanoma clones (37). As effector controls, anti-MART-127-35 A42 CTL clone and 1235 tumor-infiltrating lymphocytes and anti-G9-209-2M CTL were used.
DISCUSSION
DC are specialized antigen-presenting cells that are critical in initiating cellular immune responses (11). Recently, they have been shown to be capable of sensitizing T cells in an MHC class I-restricted manner through presentation of endogenously processed antigen (14) or by exogenous loading of relevant peptides (15). Data from preclinical models have suggested that DC could represent an ideal adjuvant for immunization against cancer involving the administration of known (15,18,25,27–30) or unknown (26) antigenic determinants. The strategies to induce HLA class I-restricted antigen presentation by DC, however, are still under investigation. Preclinical models (50) have shown that antitumor protection could be enhanced by the administration of peptide-pulsed DC (27–29).
The strategy of loading DC with exogenously administered peptides, however, has two main disadvantages: It requires the knowledge of the amino acid sequence of the epitopic determinant specific for each HLA allomorph, and it is dependent on the stability of the peptide/HLA complex. This stability, in turn, can be affected by the solubility of the peptide in aqueous medium and by its binding affinity in the same conditions. Furthermore, peptide binding is rapid and reversible and as the peptide is removed from the culture medium, a dissociation constant distinctive of the ligand-receptor interaction will determine the amount of peptide/HLA molecule present on the cell surface over time (51). This could be particularly significant for those peptides with low or intermediate binding affinity for a specific HLA allele such as MART-127-35 for the HLA-A*0201 allele (5). The overall stability of the peptide/HLA complex will ultimately determine its immunogenicity (31).
Various strategies have been reported to obviate some of these problems. The use of unfractionated acid-eluted tumor peptides (26) has been proposed as a strategy to eliminate the limitations related to HLA restriction and possibly allow presentation of unknown antigenic determinants. This method, although attractive in situations in which the antigenic determinant is not known, may have the potential disadvantage of discriminating among the eluted peptides according to biochemical characteristics not necessarily correlated with biological significance. Other strategies have taken advantage of the ability of DC to incorporate exogenous particles and present them to T cells in an MHC class I–restricted fashion (14). Bhardwaj et al. (32) have shown that DC are infectable by the influenza virus and, while they are permissive for the expression of viral products, they resist their cytopathic effect and can function as potent presenters of viral antigens to CD8+ T cells. We, therefore, reasoned that DC similarly infected with viral constructs encoding for melanoma-associated antigens may efficiently express epitopic determinants not only specific for the viral vector used but also for the MAA. The insertion of genes encoding tumor-associated antigens into viral vectors has been extensively analyzed by our group and has been shown to have several advantages over the direct administration of tumor-associated antigens (52–58). The infection of DC with such potent recombinant immunogens could have several advantages for immunization against MAA including: rapid and abundant expression of exogenous proteins in the majority of infected cells, endogenous processing and presentation of antigen by the appropriate restriction element and, finally, continuous production and transport of endogenously processed peptide/HLA complexes to the cell surface, allowing prolonged exposure of possible effector cells to the relevant stimulus.
We have investigated this hypothesis in a well-characterized in vitro model consisting of the sensitization of cytotoxic T cells against the human melanoma antigen MART-1 in the context of HLA-A*0201 restriction (2,5,36,47). We have previously shown that HLA class I-restricted anti-MART-1 CTL reactivity can be generated by in vitro sensitization of PBMC with HLA class 1-restricted epitopes in the presence of IL-2 (5). With this method, it was possible to generate anti-MART-1 reactivity with 2 of 12 9-mer peptides selected according to the known binding motif for HLA-A*0201. However, of the two CTL populations generated with this method, only anti-MART-127-35 CTL were capable of recognizing MART-1/HLA-A*0201-expressing tumor targets, suggesting that the other peptide (MART-135-43 : VILGVLLLI) was not naturally processed by melanoma cells. Castelli et al. (48) have used mild acid elution of melanoma cells and mass spectrometry to identify a naturally processed MART-1-derived peptide (MART-132-40: ILTVILGVL) that could be recognized by CTL clones obtained by limiting dilution from the peripheral blood of HLA-A2+ melanoma patients. We could not, however, detect CTL reactivity against this peptide in bulk cultures of TILs (2) nor induce in vitro reactivity against this epitope by PBMC stimulation (5). By analyzing CTL reactivity generated by endogenous presentation of MART-1 by DC, we could assess whether a preferential sensitization against such epitopes could be detected. In all cases, the anti-MART-1 CTL generated in this fashion could recognize preferentially the MART-127-35 epitope as well as naturally processed epitopes by tumor cells. The “hierarchical” recognition of MART-1 epitopes suggests immunodominance of the MART-127-35 peptide either in relation to preferential cleavage of this epitope (59) or in relation to the epitope mimicry phenomena recently described by Loftus et al. (60). Evidence is growing that most epitopes are silent under physiological conditions, not because of a “hole” in the T-cell repertoire but rather because they are expressed at a density too low to induce CTL (59,61–65). These data suggest that, although individual T-cell repertoires allow in vitro sensitization of CTL by exogenous presentation of various MART-1-derived peptides (5), only a few peptides may be naturally processed and presented in sufficient quantities to generate efficiently CTL reactivity. These data, however, do not exclude the possibility that a more sensitive analysis based on cloning of the bulk populations generated with this system could have detect CTL reactivity against the other epitopes.
Previous reports have shown that peptide-pulsed DC can be used to generate anti-MART-1 reactivity in vitro (45). However, this method required multiple in vitro restimulations and did not seem to have any benefit compared with the previously reported induction of CTL reactivity using whole PBMC populations (5). Our findings confirmed that DC pulsed with MART-1 peptide were not as efficient in achieving in vitro sensitization as DC pulsed with Flu-derived peptides, and we attributed this lower efficiency to the lower stability of the MART-127-35/HLA-A*0201 peptide complex. We originally noted reduced secretion of IFN-γ in comparable conditions by CTL exposed to DC pulsed with the MART-1 epitope compared with DC pulsed with G9-209-2M or Flu-M158-66. These same CTL could efficiently recognize the MART-127-35/HLA-A0201 complex on the surface of the endogenous transport defective T2 cells and secrete IFN-γcomparable to that of Flu-M1 and gp100. By introducing a virally driven expression of MART-1, it seemed that a more efficient induction of T-cell reactivity could be achieved. This result could be attributed to the prolonged exposure of the CD8+ effectors to the MART-1 epitope by the continued expression of viral products, as recently illustrated in some murine models (46). This interpretation, however, is speculative, and other mechanisms might have been responsible for this enhancement of the T-cell response.
It is interesting to note that a significant proportion of the PBMC tested for this analysis were obtained from older patients who had previously received vaccination against smallpox. Yet, in these patients, we could generate anti-MART-1 reactivity despite our original concern that the previous antiviral sensitization could overdrive the melanoma-specific stimulus.
In summary, we report a new strategy of in vitro sensitization of CD8+ lymphocytes with virally infected autologous DC encoding melanoma-associated antigens. This strategy has several potential advantages including (a) independence from HLA-driven selection of epitopes, (b) amplified and prolonged production of virally driven MAA gene products, and (c) potential presentation of more than one epitopic determinant. This strategy could be useful for in vivo immunization of a population with high HLA polymorphism and for in vitro identification of relevant epitopes specific for various HLA alleles.
References
- 1.van der Bruggen P, Traversari C, Chomez P, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–7. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 2.Kawakami Y, Eliyahu S, Sakaguchi K, et al. Identification of the immunodominant peptides of the MART-1 human melanoma antigen recognized by the majority of HLA-A2-restricted tumor infiltrating lymphocytes. J Exp Med. 1994;180:347–52. doi: 10.1084/jem.180.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawakami Y, Eliyahu S, Jennings C, et al. Recognition of multiple epitopes in the human melanoma antigen gp100 by tumor-infiltrating T lymphocytes associated with in vivo tumor regression. J Immunol. 1995;154:3961–8. [PubMed] [Google Scholar]
- 4.Wolfel T, Van Pel A, Brichard V, et al. Two tyrosinase nonapeptides recognized on HLA-A2 melanomas by autologous cytolytic T lymphocytes. Eur J Immunol. 1994;24:759–64. doi: 10.1002/eji.1830240340. [DOI] [PubMed] [Google Scholar]
- 5.Rivoltini L, Kawakami Y, Sakaguchi K, et al. Induction of tumor reactive CTL from peripheral blood and tumor infiltrating lymphocytes of melanoma patients by in vitro stimulation with an immunodominant peptide of the human melanoma antigen MART-1. J Immunol. 1995;154:2257–65. [PubMed] [Google Scholar]
- 6.Salgaller ML, Afshar A, Marincola FM, Rivoltini L, Kawakami Y, Rosenberg SA. Recognition of multiple epitopes in the human melanoma antigen gp100 by peripheral blood lymphocytes stimulated in vitro with synthetic peptides. Cancer Res. 1995;55:4972–9. [PubMed] [Google Scholar]
- 7.Cormier JN, Salgaller ML, Prevette T, et al. Enhancement of cellular immunity in melanoma patients immunized with a peptide from MART-1/Melan A. Cancer J Sci Am. 1997;3:37–14. [PMC free article] [PubMed] [Google Scholar]
- 8.Salgaller ML, Marincola FM, Cormier JN, Rosenberg SA. Immunization against epitopes in the human melanoma antigen gp100 following patient immunization with synthetic peptides. Cancer Res. 1996;56:4749–57. [PubMed] [Google Scholar]
- 9.Vitiello A, Ishioka G, Grey HM, et al. Development of a lipopeptide-based therapeutic vaccine to treat chronic HBV infection. I. Induction of a primary cytotoxic T lymphocyte response in humans. J Clin Invest. 1995;95:341–9. doi: 10.1172/JCI117662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–96. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 11.Grabbe S, Beissert S, Schwarz T, Granstein RD. Dendritic cells as initiators of tumor immune responses: a possible strategy for tumor immunotherapy? [see comments] Immunol Today. 1995;16:117–21. doi: 10.1016/0167-5699(95)80125-1. [DOI] [PubMed] [Google Scholar]
- 12.Steinman RM, Lustig DS, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. 3. Functional properties in vivo. J Exp Med. 1974;139:1431–5. doi: 10.1084/jem.139.6.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142–62. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang AY, Golumbek P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science. 1994;264:961–5. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- 15.Porgador A, Gilboa E. Bone marrow-generated dendritic cells pulsed with a class I-restricted peptide are potent inducers of cytotoxic T lymphocytes. J Exp Med. 1995;182:255–60. doi: 10.1084/jem.182.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Constant S, Sant’Angelo D, Pasqualini T, et al. Peptide and protein antigens require distinct antigen-presenting cell subsets for the priming of CD4+ T cells. J Immunol. 1995;154:4915–23. [PubMed] [Google Scholar]
- 17.Levin D, Constant S, Pasqualini T, Flavell R, Bottomly K. Role of dendritic cells in the priming of CD4+ T lymphocytes to peptide antigen in vivo. J Immunol. 1993;151:6742–50. [PubMed] [Google Scholar]
- 18.Inaba K, Metlay JP, Crowley MT, Steinman RM. Dendritic cells pulsed with protein antigens in vitro can prime antigen-specific, MHC-restricted T cells in situ [published erratum appears in J Exp Med 1990;172:1275] J Exp Med. 1990;172:631–40. doi: 10.1084/jem.172.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products [see comments] J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caux C, Massacrier C, Dezutter-Dambuyant C, et al. Human dendritic Langerhans cells generated in vitro from CD34+ progenitors can prime naive CD4+ T cells and process soluble antigen. J Immunol. 1995;155:5427–35. [PubMed] [Google Scholar]
- 21.Becker Y. Dendritic cell activity against primary tumors: an overview. In Vivo. 1993;7:187–91. [PubMed] [Google Scholar]
- 22.Reid CD, Stackpoole A, Tikerpae J. TNF and GM-CSF dependent growth of an early progenitor of dendritic Langerhans cells in human bone marrow. Adv Exp Med Biol. 1993;329:257–62. doi: 10.1007/978-1-4615-2930-9_43. [DOI] [PubMed] [Google Scholar]
- 23.Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J. GM-CSF and TNF-alpha cooperate in the generation of dendritic Langerhans cells. Nature. 1992;360:258–61. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- 24.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Young JW, Inaba K. Dendritic cells as adjuvants for class I major histocompatibility complex-restricted antitumor immunity [comment] J Exp Med. 1996;183:7–11. doi: 10.1084/jem.183.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zitvogel L, Mayordomo JI, Tjandrawan T, et al. Therapy of murine tumors with tumor peptide-pulsed dendritic cells: dependence on T cells, B7 costimulation, and T helper cell 1-associated cytokines [see comments] J Exp Med. 1996;183:87–97. doi: 10.1084/jem.183.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Celluzzi CM, Mayordomo JI, Storkus WJ, Lotze MT, Falo LD., Jr Peptide-pulsed dendritic cells induce antigen-specific CTL-mediated protective tumor immunity [see comments] J Exp Med. 1996;183:283–7. doi: 10.1084/jem.183.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paglia P, Chiodoni C, Rodolfo M, Colombo MP. Murine dendritic cells loaded in vitro with soluble protein prime cytotoxic T lymphocytes against tumor antigen in vivo [see comments] J Exp Med. 1996;183:317–22. doi: 10.1084/jem.183.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mayordomo JI, Zorina T, Storkus WJ, et al. Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nat Med. 1995;1:1297–302. doi: 10.1038/nm1295-1297. [DOI] [PubMed] [Google Scholar]
- 30.Paglia P, Chiodoni C, Rodolfo M, Colombo MP. Murine dendritic cells loaded in vitro with soluble protein prime cytotoxic T lymphocytes against tumor antigen in vivo [see comments] J Exp Med. 1996;183:317–22. doi: 10.1084/jem.183.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Burg SH, Visseren MJ, Brandt RM, Kast WM, Melief CJ. Immunogenicity of peptides bound to MHC class I molecules depends on the MHC-peptide complex stability. J Immunol. 1996;156:3308–14. [PubMed] [Google Scholar]
- 32.Bhardwaj N, Bender A, Gonzalez N, Bui LK, Garrett MC, Steinman RM. Influenza virus-infected dendritic cells stimulate strong proliferative and cytolytic responses from human CD8+ T cells. J Clin Invest. 1994;94:797–807. doi: 10.1172/JCI117399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.von Rohr A, Ghosh AK, Thatcher N, Stern PL. Immunomodulation during prolonged treatment with combined interleukin-2 and interferon-alpha in patients with advanced malignancy. Br J Cancer. 1993;67:163–71. doi: 10.1038/bjc.1993.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rivoltini L, Loftus DJ, Barracchini K, et al. Binding and presentation of peptides derived from melanoma antigens MART-1 and gp100 by HLA-A2 subtypes: implications for peptide-based immunotherapy. J Immunol. 1996;156:3882–91. [PubMed] [Google Scholar]
- 35.Krausa P, Brywka M, Savage D, et al. Genetic polymorphism within HLA-A*02: significant allelic variation revealed in different populations. Tissue Antigens. 1995;45:223–31. doi: 10.1111/j.1399-0039.1995.tb02444.x. [DOI] [PubMed] [Google Scholar]
- 36.Kawakami Y, Eliyahu S, Delgado CH, et al. Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc Natl Acad Sci USA. 1994;91:3515–9. doi: 10.1073/pnas.91.9.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rivoltini L, Baracchini KC, Viggiano V, et al. Quantitative correlation between HLA Class I allele expression and recognition of melanoma cells by antigen specific cytotoxic T lymphocytes. Cancer Res. 1995;55:3149–57. [PMC free article] [PubMed] [Google Scholar]
- 38.Cerundolo V, Alexander J, Anderson K, et al. Presentation of viral antigen controlled by a gene in the major histocompatibility complex. Nature. 1990;345:449–52. doi: 10.1038/345449a0. [DOI] [PubMed] [Google Scholar]
- 39.Wei ML, Cresswell P. HLA-A2 molecules in an antigen-processing mutant cell contain signal sequence-derived peptides [see comments] Nature. 1992;356:443–6. doi: 10.1038/356443a0. [DOI] [PubMed] [Google Scholar]
- 40.Parkhurst MR, Salgaller ML, Southwood S, et al. Improved induction of melanoma reactive CTL with peptides from the melanoma antigen gp100 modified at HLA-A*0201 binding residues. J Immunol. 1996;157:2539–48. [PubMed] [Google Scholar]
- 41.Marincola FM, Shamamian P, Alexander RB, et al. Loss of HLA haplotype and B locus down-regulation in melanoma cell lines. J Immunol. 1994;153:1225–37. [PubMed] [Google Scholar]
- 42.Marincola FM, Hijazi YM, Fetsch P, et al. Analysis of expression of the melanoma associated antigens MART-1 and gp100 in metastatic melanoma cell lines and in in situ lesions. J Immunother. 1996;19:192–205. doi: 10.1097/00002371-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 43.O’Neil BH, Kawakami Y, Restifo NP, Bennink JR, Yewdell JW, Rosenberg SA. Detection of shared MHC-restricted human melanoma antigens after vaccinia virus-mediated transduction of genes coding for HLA. J Immunol. 1993;151:1410–8. [PMC free article] [PubMed] [Google Scholar]
- 44.Wang XM, Terasaki PI, Rankin GW, Jr, Chia D, Zhong HP, Hardy S. A new microcellular cytotoxicity test based on calcein AM release. Hum Immunol. 1993;37:264–70. doi: 10.1016/0198-8859(93)90510-8. [DOI] [PubMed] [Google Scholar]
- 45.Bakker AB, Marland G, de Boer AJ, et al. Generation of antimelanoma cytotoxic T lymphocytes from healthy donors after presentation of melanoma-associated antigen-derived epitopes by dendritic cells in vitro. Cancer Res. 1995;55:5330–4. [PubMed] [Google Scholar]
- 46.Kundig TM, Shahinian A, Kawai K, et al. Duration of TCR stimulation determines costimulatory requirement of T cells. Immunity. 1996;5:41–52. doi: 10.1016/s1074-7613(00)80308-8. [DOI] [PubMed] [Google Scholar]
- 47.Marincola FM, Rivoltini L, Salgaller ML, Player M, Rosenberg SA. Differential anti-MART-1/MelanA CTL activity in peripheral blood of HLA-A2 melanoma patients in comparison to healthy donors: evidence for in vivo priming by tumor cells. J Immunother. 1996;19:266–77. doi: 10.1097/00002371-199607000-00003. [DOI] [PubMed] [Google Scholar]
- 48.Castelli C, Storkus WJ, Maeurer MJ, et al. Mass spectrometric identification of a naturally processed melanoma peptide recognized by CD8+ cytotoxic T lymphocytes. J Exp Med. 1995;181:363–8. doi: 10.1084/jem.181.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Elsas A, van der Burg SH, van der Minne CE, Borghi M, Melief CJ, Schrier PI. Peptide-pulsed dendritic cells induce tumoricidal cytotoxic T lymphocytes from healthy donors against stably HLA-A*0201-binding peptides from the Melan-A/MART-1 self antigen. Eur J Immunol. 1996;26:1683–9. doi: 10.1002/eji.1830260803. [DOI] [PubMed] [Google Scholar]
- 50.Hsu FJ, Benike C, Fagnoni F, et al. Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nat Med. 1996;2:52–8. doi: 10.1038/nm0196-52. [DOI] [PubMed] [Google Scholar]
- 51.Christinck ER, Luscher MA, Barber BH, Williams DB. Peptide binding to class I MHC on living cells and quantitation of complexes required for CTL lysis. Nature. 1991;352:67–70. doi: 10.1038/352067a0. [DOI] [PubMed] [Google Scholar]
- 52.Restifo NP. Recombinant anti-cancer vaccines. Cancer J Sci Am. 1996;2:16–8. [PubMed] [Google Scholar]
- 53.Sheil JM, Bevan MJ, Lefrancois L. Characterization of dual-reactive H-2Kb-restricted anti-vesicular stomatitus virus and alloreactive cytotoxic T cells. J Immunol. 1987;138:3654–60. [PubMed] [Google Scholar]
- 54.Chen PW, Wang M, Bronte V, Zhai Y, Rosenberg SA, Restifo NP. Therapeutic antitumor response after immunization with a recombinant adenovirus encoding a model tumor-associated antigen. J Immunol. 1996;156:224–31. [PMC free article] [PubMed] [Google Scholar]
- 55.McCabe BJ, Irvine KR, Nishimura MI, et al. Minimal determinant expressed by a recombinant vaccinia virus elicits therapeutic antitumor cytolytic T lymphocyte responses. Cancer Res. 1995;55:1741–7. [PMC free article] [PubMed] [Google Scholar]
- 56.Bronte V, Tsung K, Rao JB, et al. IL-2 enhances the function of recombinant poxvirus-based vaccines in the treatment of established pulmonary metastases. J Immunol. 1995;154:5282–92. [PMC free article] [PubMed] [Google Scholar]
- 57.Wang M, Bronte V, Chen PW, et al. Active immunotherapy of cancer with a nonreplicating recombinant fowlpox virus encoding a model tumor-associated antigen. J Immunol. 1995;154:4685–92. [PMC free article] [PubMed] [Google Scholar]
- 58.Irvine KR, McCabe BJ, Rosenberg SA, Restifo NP. Synthetic oligonucleotide expressed by a recombinant vaccinia virus elicits therapeutic CTL. J Immunol. 1995;154:4651–7. [PMC free article] [PubMed] [Google Scholar]
- 59.Niedermann G, Butz S, Ihlenfeldt HG, et al. Contribution of proteasome-mediated proteolysis to the hierarchy of epitopes presented by major histocompatibility complex class I molecules. Immunity. 1995;2:289–99. doi: 10.1016/1074-7613(95)90053-5. [DOI] [PubMed] [Google Scholar]
- 60.Loftus DJ, Castelli C, Clay TM, et al. Identification of epitope mimics recognized by CTL reactive to the melanoma/melanocyte derived peptide MART-1(27-35) J Exp Med. 1996;184:647–57. doi: 10.1084/jem.184.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schild H, Rotzschke O, Kalbacher H, Rammensee HG. Limit of T cell tolerance to self proteins by peptide presentation. Science. 1990;247:1587–9. doi: 10.1126/science.2321019. [DOI] [PubMed] [Google Scholar]
- 62.Sheil JM, Shepherd SE, Klimo GF, Paterson Y. Identification of an autologous insulin B chain peptide as a target antigen for H-2Kb-restricted cytotoxic T lymphocytes. J Exp Med. 1992;175:545–52. doi: 10.1084/jem.175.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Feltkamp MC, Smits HL, Vierboom MP, et al. Vaccination with cytotoxic T lymphocyte epitope-containing peptide protects against a tumor induced by human papillomavirus type 16-transformed cells. Eur J Immunol. 1993;23:2242–9. doi: 10.1002/eji.1830230929. [DOI] [PubMed] [Google Scholar]
- 64.Houbiers JG, Nijman HW, van der Burg SH, et al. In vitro induction of human cytotoxic T lymphocyte responses against peptides of mutant and wild-type p53. Eur J Immunol. 1993;23:2072–7. doi: 10.1002/eji.1830230905. [DOI] [PubMed] [Google Scholar]
- 65.Oukka M, Riche N, Kosmatopoulos K. A nonimmunodominant nucleoprotein-derived peptide is presented by influenza A virus-infected H-2b cells. J Immunol. 1994;152:4843–51. [PubMed] [Google Scholar]