Abstract
Introduction
Möbius’ syndrome typically presents as a sporadic trait with congenital facial palsy and abduction impairment. We used high resolution magnetic resonance imaging (MRI) and genetic analysis to examine a family with features of Möbius’ syndrome.
Methods
We examined three related family members having congenital complete opthalmoplegia with ptosis and facial diplegia. Orbits were imaged in quasi-coronal and sagittal planes 2 mm thick. Subarachnoid cranial nerves were imaged in planes 1 mm thick. Linkage and mutation analysis were performed to determine if the pedigree harbored mutations in four candidate genes.
Results
In affected subjects, MRI showed marked hypoplasia of extraocular muscles and intraorbital motor nerves. In the anterior orbit, rectus extraocular muscles were less hypoplastic but markedly curved toward insertion. Oblique extraocular muscles were hypoplastic and abnormally inserted. Posterior bony orbits were hypoplastic. Optic nerves were markedly straightened. Brainstems and cranial nerves III, VI, VII, and VIII were normal bilaterally. No pathogenic mutations were detected in affected individuals.
Conclusions
Previous MRI studies have demonstrated brainstem hypoplasia and cranial nerve aplasia in Möbius’ syndrome. The current family had normal brainstems and subarachnoid portions of motor cranial nerves innervating the orbit, but marked extraocular muscle hypoplasia. These clinical and MRI findings are atypical for Möbius’ syndrome and other congenital cranial dysinnervation disorders (CCDDs). Congenital facial weakness and complete ophthalmoplegia may occur despite MRI evidence of normal brainstem anatomy. The endophenotype appears to result from a genetic defect distinct from the CCDDs defined thus far, rather than a global brainstem insult.
Introduction
Möbius’ syndrome is a heterogeneous clinical disorder whose clinical definition has evolved in the recent literature. The minimum criteria include congenital facial palsy with impairment of ocular abduction.1–3 The wide clinical spectrum and multiple areas of brainstem involvement in patients with Möbius’ syndrome have led to its early conceptualization as a developmental disorder of the brainstem rather than an isolated cranial nerve developmental disorder.3 Möbius’ syndrome has more recently been classified with other congenital cranial dysinnervation disorders (CCDDs).4 These include congenital fibrosis of the extraocular muscles (CFEOM), Duane’s retraction syndrome, congenital facial palsy, horizontal gaze palsy, and congenital ptosis.4,5 The pathogenesis of CCDDs is proposed to be the absence or misguidance of motor axons rather than a primary abnormality of extraocular muscle development.4,6 High-resolution MRI has directly demonstrated cranial nerves and extraocular muscle pathology in several CCDDs and neuropathic strabismus.7–9
In this report, we describe high-resolution MRI evidence suggesting peripheral cranial dysinnervation without brainstem dysplasia in three familial cases within the Möbius spectrum, having the rare clinical feature of complete ophthalmoplegia and bilateral facial diplegia.
Subjects and Methods
This observational case series was approved by the local Institutional Review Board and conducted in accord with the Health Insurance Portability and Accountability Act with written informed consent of participants.
We performed MRI in three related family members with features of Möbius’ syndrome, including complete ophthalmoplegia, blepharoptosis, and facial diplegia. General techniques have been described previously.7–9 Orbits were imaged with T1 weighting in quasi-coronal and sagittal planes using surface coils. Intraorbital resolution was 234–312 μm within 2 mm thick planes. Subarachnoid cranial nerves were imaged in oblique axial planes parallel to the optic chiasm using head coils and heavy T2 weighting, yielding resolution of 195 μm in planes 1 mm thick. Extraocular muscle and nerve sizes were evaluated qualitatively in relationship to a database of approximately 600 studies performed using similar technique.
High-molecular weight genomic DNA was extracted from participant blood samples using the Puregene kit (Quaigen, Valencia, CA). Segregation analysis was performed using fluorescently labeled microsatellite markers spanning the FEOM1 locus (D12S1692, D12S1067, D12S1048), FEOM2 locus (D11S4162, D11S1314, D11S1369), FEOM3 locus (D16S520, D16S498, D16S3121, D16S303), and HOXA1 locus (MT26723, MT27012, D7S516). Fluorescently labeled primers were purchased from Invitrogen (Carlsbad, CA), and amplicons were generated by 35 cycles of PCR amplification containing 20 ng of genomic DNA in 10-μl reaction volumes of Qiagen HotStar Taq DNA Polymerase (Qiagen), containing 10XPCR Buffer, 4 pmol of each fluorescent primer pair, 2.5 nmol each of dATP, dTTP, dGTP, and dCTP, and 0.1 U Taq polymerase. The products were analyzed in an Applied Biosystems (Foster City, CA) 3730 DNA Analyzer. For mutation analysis, the coding exons and intron-exon boundaries of PHOX2A, KIF21A, and HOXA1 were amplified from DNA of at least one of the affected individuals using previously reported primer sets, and each amplicon was sequenced and analyzed as previously described.10–12
Results
Clinical and MRI
Case 1 (Figure 1, individual III:4) is a 15-year-old Hispanic adolescent male with congenital total ophthalmoplegia, blepharoptosis, facial diplegia, hypotonia, and diminished deep tendon reflexes. His birth and gestational history were unremarkable.
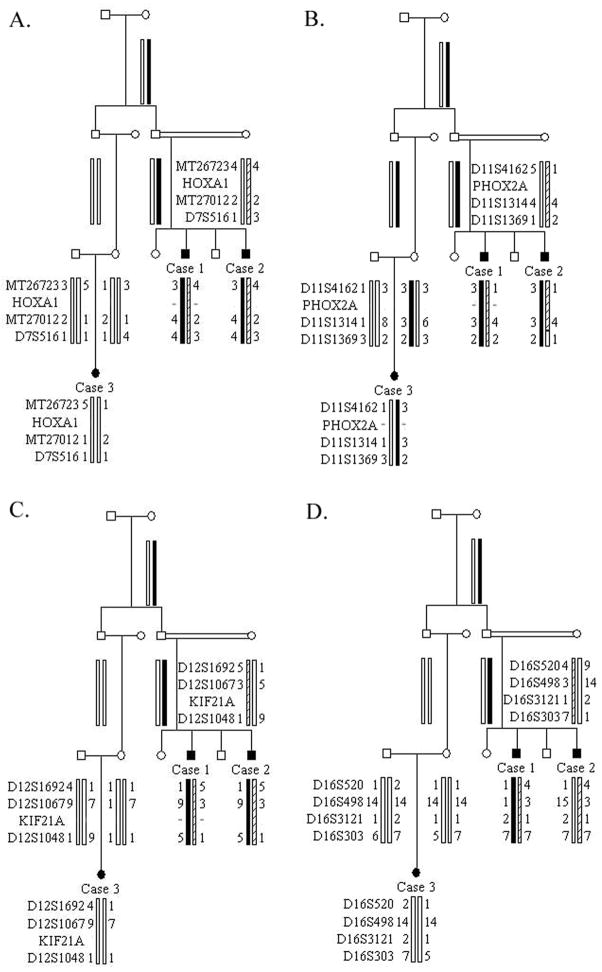
Figure 1.
Pedigree and genetic analysis. Black symbols indicate those clinically affected with Möbius’ syndrome; = indicates consanguinity. References within text refer to the generation number (roman numeral) and position within generation (arabic numeral). Genotyping data and schematic segregating haplotype bars for markers spanning the (A) HOXA1, (B) FEOM2, (C) FEOM1, and (D) FEOM3 loci are shown below the symbol for each individual. The position of the (A) HOXA1, (B) PHOX2A, and (C) KIF21A genes is indicated, and a negative sign (−) indicates the individual(s) sequenced for the specific gene. The FEOM3 disease gene has not yet been identified.
Visual acuity with low myopic correction was 20/80 in both eyes. He had moderate bilateral blepharoptosis, leaving the upper lid margins at the center of the pupils, and minimal levator function. The palpebral fissures were 8 mm vertically. Upper eyelid creases and Bell’s phenomenon were bilaterally absent, and lagophthalmos was present bilaterally (Fig. 1). There was bilateral total external ophthalmoplegia, even with the horizontal and vertical doll’s head maneuver. At near, there was an exotropia of 18Δ, and both eyes were vertically centered in the orbits. The pupils were symmetrical and reacted briskly to light but not accommodation. There was bilateral corneal scarring and nodular keratopathy characteristic of exposure, consistent with the level of corrected vision. The lenses were clear. The retinas and optic nerves were normal.
The trigeminal muscles of mastication were bilaterally weak, but sternocleidomastoid and trapezius function were normal. There was complete bilateral facial paralysis. Hearing was bilaterally normal. There was bilateral retrognathia, mandibular hypoplasia, and a high arched palate, but normal dentition.
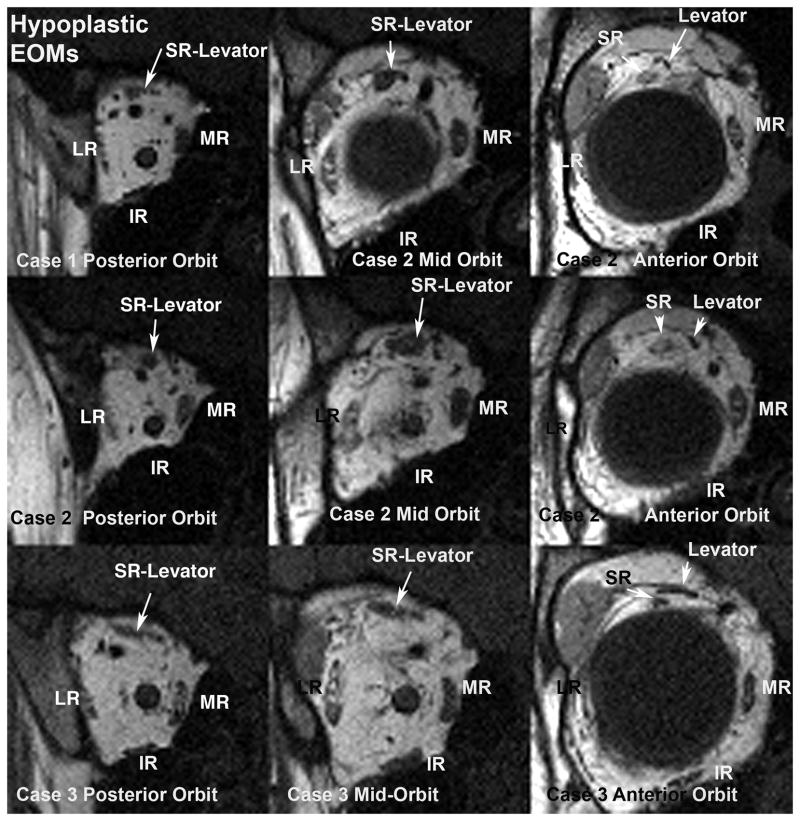
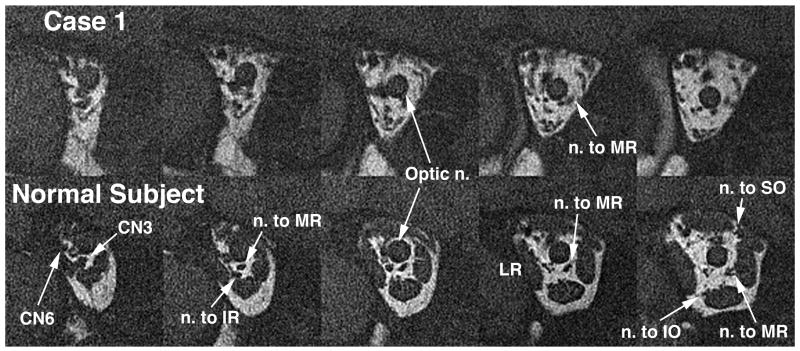
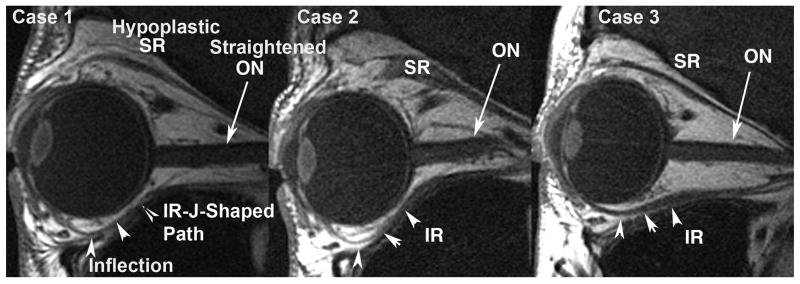
Orbital MRI demonstrated marked hypoplasia of all extraocular muscles most prominent in their posterior aspects (Fig. 3, row 1), and the posterior portions of both orbits were reduced in diameter relative to normal. There was focal sparing of a nodular area within right levator and superior rectus muscle. Rectus muscle hypoplasia was most prominent posterior to the entry point of the corresponding motor nerve, and the motor nerves in the orbit appeared qualitatively small or undetectable (Fig. 4). Lateral rectus paths were bilaterally displaced inferiorly relative to medial rectus paths. The inferior oblique muscles were anteriorly inserted bilaterally. The superior oblique tendons were nasally inserted bilaterally. The rectus extraocular muscles were sharply inflected toward the orbital walls, as was strikingly evident in a J-shaped path for the inferior rectus muscle at the location of its connective tissue pulley (Fig. 5). No gross brainstem hypoplasia was observed, and adjacent cranial nerves III, V, VI, VII, and VIII were bilaterally normal (Fig. 6). The internal auditory canal was identified bilaterally (Fig. 6).
Figure 3.
Quasi-coronal MRI of posterior right orbit (left column), mid-orbit (middle column), and anterior orbit (right column) for Case1 (top row), Case 2 (middle row), and Case 3 (bottom row). As seen in the left column, the extraocular muscles are hypoplastic in the posterior orbit and motor nerves are barely detectable. There is relative sparing of the medial rectus (MR) muscles, which appear larger than the inferior (IR), lateral (LR), and superior rectus (SR) muscles in the posterior orbit. Note larger rectus muscle cross sections in the mid-orbit (middle column) and anterior orbit (right column), although the levator muscle remains attenuated in the anterior orbit (right column). Note infraplacement of the lateral rectus (LR) relative to the medial rectus (MR) in cases 1 and 2.
Figure 4.
Quasi-coronal MRI of deep posterior right orbit of Case 1 (top row) and a normal control subject (bottom row), illustrating hypoplasia of the deep portions of the extraocular muscles and hypoplasia or absence of the motor nerve branches to them in the affected subject. Images are in contiguous 2 mm thick planes. CN3: inferior division of the oculomotor nerve; CN6: abducens nerve; IO: inferior oblique muscle; IR: inferior rectus muscle; LR: lateral rectus muscle; MR: medial rectus muscle; SO: superior oblique muscle.
Figure 5.
Quasi-saggital MRI of the right orbits of Cases 1–3 showing hypoplastic superior rectus (SR) and inferior rectus (IR) muscles. An unusual J-shaped course of the inferior rectus from the narrow posterior orbit is observed (arrowheads). ON: optic nerve.
Figure 6.
Oblique axial T2 weighted MRI of brainstem from three related subjects with Möbius syndrome and ophthalmoplegia. Case 1 (top row), Case 2 (middle row), and Case 3 (bottom row) MRI panels show noncontiguous representative sections of the midbrain (left column), pons (second column), and pontine medullary junction (third and fourth columns). Cranial nerves 3, 6, 7, 8 (CN 3, CN6, CN7, CN8), and internal auditory canal (IAC) are normal and are identifed bilaterally in all subjects.
Case 2, the brother of Case 1 (Figure 1, III:6), is a 13-year-old adolescent male with congenital total ophthalmoplegia, blepharoptosis, and bilateral facial weakness. His birth and gestational history are unremarkable. His eyes reportedly remain open during sleep.
Visual acuity was 20/50 in the right eye and 20/400 in the left eye, with low myopia and moderate astigmatism greater in the right than left eye. Upper eyelid creases were absent. Both palpebral fissures measured 7 mm vertically, leaving the right upper eyelid margin at and the left margin slightly below the pupillary center; both upper eyelid margins moved 3 mm during blinks. Corneal sensation was bilaterally normal. Both pupils were 4 mm in dim light and reacted equally and briskly to light but not to accommodation. There was mild conjunctival injection, superficial keratopathy with anterior stromal thinning on the left, and superficial white corneal nodules on the right. The corneal changes were consistent with the level of vision. Intraocular pressures were normal. The lenses were clear. Although both eyes were vertically centered in the orbits, there was complete bilateral ophthalmoplegia, 10Δ left hypertropia and 18Δ exotropia in central gaze at near. No reflexive eye movements could be evoked by forced eyelid closure or rapid head rotations. The optic nerves were pink and a cup to disk ratio of 0.6 bilaterally. The retinas appeared normal.
There was complete bilateral facial weakness partially sparing the corrugator supraciliaris, and eyelid closure was bilaterally weak. Cutaneous sensation in the face was bilaterally normal. Hearing was grossly normal bilaterally. Sternocleidomastoid and trapezius strength were bilaterally normal. There was a high arched palate and supranumary molar teeth, with mild retrognathia. The tongue showed normal bulk and was in the midline.
MRI findings in orbit and brainstem were generally similar to Case 1. The posterior bony orbits were narrowed to about half of normal diameter. There was severe, bilateral hypoplasia of the posterior bellies of all extraocular muscles, with attenuation of the right but not left superior oblique tendon, and markedly anterior insertion of the inferior oblique muscles, both of which were hypoplastic. The medial and lateral rectus muscles appeared qualitatively hypoplastic only posterior to the sites of normal motor nerve entries into corresponding normal muscles. Motor nerves to the extraocular muscles appeared qualitatively small. The rectus muscle paths were strikingly curved anteriorly before thinning and straightening posteriorly (Fig. 5). Motor nerves to the extraocular muscles were small. The optic nerves were of normal size, and the retinas were normal. Brainstem, internal auditory canals, and the subarachnoid portions of cranial nerves III, VI, VII, and VIII were bilaterally normal (Fig. 6).
Case 3 (Figure 1, IV:1) is a 4-year-old second cousin of Case 1 and 2, who was first examined at age 6 months after normal gestation and delivery, when she presented with resolved infantile hypotonia, complete congenital ophthalmoplegia, and facial diplegia but no blepharoptosis. At that time, she had moderate weakness of eyelid closure with slow, incomplete blinks. There was no Bell’s phenomenon. At first presentation, the child was orthotropic and had no significant refractive error. By age 4 years, there was mild bilateral blepharoptosis with lagophthalmos. Uncorrected visual acuity was 20/32 bilaterally. There was complete bilateral ophthalmoplegia, alignment ranging from between orthotropia and 10Δ exotropia. No eye movements were evoked by optokinetic or vestibular stimulation. There were moderate corneal exposure changes bilaterally with limbal vascularization and other changes consistent with exposure keratopathy. The corneal changes were consistent with the level of vision. The ocular examination was otherwise normal, with pink optic nerves free of cupping and normal retinas. There was marked bilateral facial weakness with only a weak grin (Fig. 2).
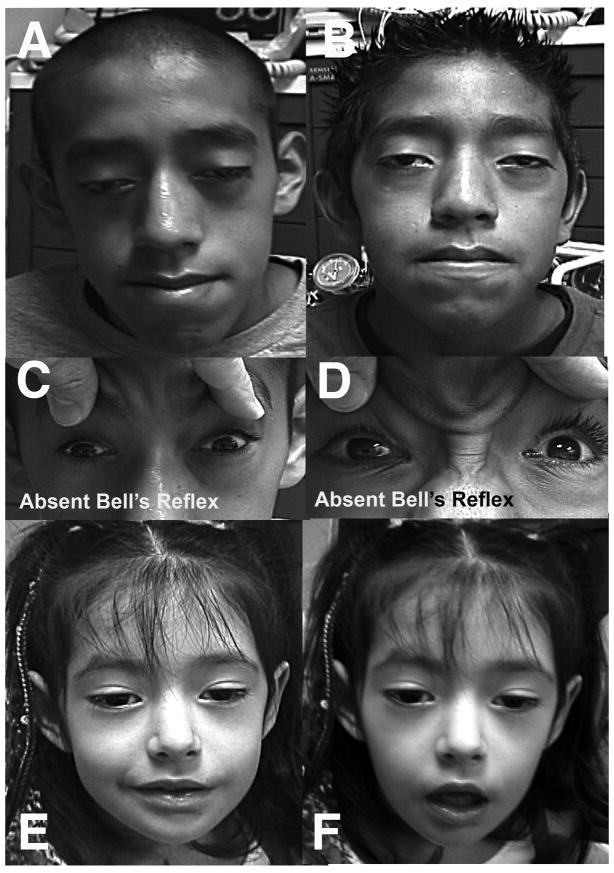
Figure 2.
External photos of three affected subjects. Cases 1 (A) and 2 (B) demonstrating ptosis, facial diplegia, and complete ophthlmoplegia. All subjects lacked Bell’s phenomenon, as seen for Cases 1 (C) and 2 (D) during attempted eyelid closure. Lower facial weekness in Case 3 is demonstrated during attempted smile (E) and facial relaxation (F).
Brain MRI was normal, but orbital MRI demonstrated marked hypoplasia of the posterior bellies of the rectus extraocular muscles, and of all portions of the superior oblique muscle bellies, with moderate hypoplasia of the inferior oblique bellies. There was relative preservation of the levator muscles bilaterally. The diameters of the posterior bony orbits were bilaterally reduced. Motor nerve branches in the orbit were small. Brainstem, internal auditory canals, and the subarachnoid portions of cranial nerves III, VI, VII, and VIII were bilaterally normal (Fig. 5).
Genetic analysis
Linkage analysis of this small pedigree reveals that the affected individuals do not share common haplotypes and do not reduce to homozygosity at the recessive FEOM2 and HOXA1 loci (Fig. 1A–B). Simlarly, the three affected children do not share a common allele at the dominant FEOM1 and FEOM3 loci (Fig. 1C–D). Because it is possible that the cousin does not share the same genetic etiology as the two brothers, sequence analysis was also conduced and no mutations were detected (Fig. 1A–D).
Discussion
Moebius syndrome is characterized by a congenital facial palsy with impairment of ocular abduction.1,2,3 Other cranial nerves, orofacial malformations, limb malformations, and gross motor disturbance are also often present.1,3 The current three cases fulfill criteria for Moebius syndrome. However, the additional features of complete ophthalmoplegia and familial transmission are rare in published series.2,3,13–15 MacDermot et al2 reviewed 26 reports of familial transmission within the Moebius spectrum and concluded that external ophthalmoplegia and the absence of skeletal defects may be the primary predictive factors for familial recurrence. This conclusion was based on an earlier report by Legum et al16 of a family in which the primary phenotype in 10 affected members was complete ophthalmoplegia and bilateral facial nerve palsy. MacDermot2 also reported a case of mother to son transmission in which the clinical phenotype featured bilateral ptosis, horizontal gaze palsy, and facial nerve palsy. The cases presented here share the similar rare phenotype of complete ophthalmoplegia and familial transmission with the cases reported by Legum and MacDermot. Tischfield et al11 reported horizontal gaze palsy and facial weakness in consanguineous patients with recessive HOXA1 mutations.17 These patients differed from the current report in that most also had sensorineural deafness and a subset had hypoventilation and developmental deficits. The pedigree reported here supports the conclusion that complete ophthalmoplegia and bilateral facial palsy without global brainstem and skeletal abnormalities are associated with familial transmission.
In this pedigree, no mutations were detected in the CCDD genes PHOX2A, HOXA1, and KIF21A. PHOX2A is mutated in autosomal recessive CFEOM2, and, similar to the patients described here, affected individuals have bilateral ptosis, ophthalmoplegia with exotropia, and sluggishly reactive pupils. Facial weakness and additional dysmorphisms, however, are not reported.10 Patients with homozygous HOXA1 mutations may have facial weakness; however, unlike this pedigree they are typically accompanied by deafness, abnormal inner ear anatomy, developmental delays, vascular anomalies, horizontal gaze palsy with globe retraction, and intact vertical ocular rotations.11,17 Mutations in KIF21A were less likely, as they occur in autosomal dominant CFEOM in which patients’ eyes are fixed downward and facial weakness is minimal to absent.12,18 Given the absence of mutations in candidate CCDD genes, further genetic insight may be gained through a genome-wide screen of this consanguineous family for homozygosity, assuming autosomal recessive inheritance and a founder mutation.
While Möbius’ syndrome typically involves horizontal gaze and limitation of abduction, complete ophthalmoplegia has been rarely reported to occur within the Moebius spectrum.2–4,16 In a study of 37 Dutch patients with Möbius’ syndrome, two unrelated patients were characterized by having facial palsy, vertical and horizontal ophthalmoplegia, and bilateral ptosis.3 Verzijl et al3 classified the cases as CFEOM. Traboulsi reported two patients with total external ophthalmoplegia associated with Moebius syndrome. Based on the rarity of total ophthalmoplegia, he concluded that the phenotypic similarities with CFEOM support the view that some cases of Möbius’ syndrome are genetic.4
Previous radiological studies in patients with sporadic Möbius’ syndrome have identified brainstem hypoplasia and the universal finding of complete absence of facial nerves.3,19–21 Verzijl et al21 concluded that these abnormalities support the idea that Moebius syndrome is part of a global anomaly of the posterior fossa. In all three cases herein, MRI of the brainstems and contiguous cranial nerves III, VI, VII, and VIII exiting the brainstem were normal. All motor nerve branches, however, were abnormally small in the orbit. The marked extraocular muscle hypoplasia supports a pathogenesis distinct from common cases of sporadic Möbius’ syndrome.
Sporadic cases of Möbius’ syndrome have been postulated to result from birth trauma, vascular insufficiency, or teratogenic insult.3,22–24 Towfighi et al24 described pathological observations from 14 cases of Moebius syndrome and grouped them according to etiology. Group I resulted from hypoplasia or atrophy of cranial nerve nuclei, Group II from primary peripheral cranial nerve involvement, Group III with focal necrosis of brainstem nuclei, and Group IV due to myopathy. Although we found normal brainstems and contiguous cranial nerves III, VI, VII, VIII, the cases described here exhibited marked hypoplasia of the extraocular muscles and of the intraorbital motor nerves innervating them. Such findings could also result from a genetic defect in the developing extraocular muscle lower motor neuron system rather than from a primary myopathy. The mechanism might be similar to the defect in the developmental kinesin KIF21A, an axonal transport protein whose mutation in CFEOM1 produces a primary cranial neuropathy with secondary extraocular muscle myopathy.12,25 An analogous mechanism might result in the profound EOM hypoplasia seen in this study with sparing of the brainstem.
In the present cases, MRI disclosed several clinically unsuspected features of the endophenotype. These include hypoplasia of the posterior bony orbit, displaced rectus muscle paths, and abnormal insertions of the superior and inferior oblique muscles. The extraocular muscle hypoplasia observed here was predominantly in the posterior orbit, with sparing of anterior musculotendinous structures. Such anterior extraocular muscle sparing is also typical of CFEOM1, hereditary Duane syndrome linked to chromosome 2, and congenital and acquired oculomotor palsy.7–9,26,27 Sparing of anterior orbital structures in this pedigree and in other CCDDs suggests that differing factors, under different genetic control, may be responsible for development of the anterior vs. posterior orbit, and orbital contents.
Acknowledgments
Support: Supported by U.S. Public Health Service, National Eye Institute: grants EY13583, EY08313, and EY00331. J. Demer is Leonard Apt Professor of Ophthalmology. S. Dumars received the Leonard Apt Fellowship and Adeline Stein Fellowship in Pediatric Ophthalmology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kumar D. Moebius syndrome. J Med Genet. 1990;27:122–6. [PMC free article] [PubMed] [Google Scholar]
- 2.MacDermot KD, Winter RM, Taylor D, Baraitser M. Oculofacialbulbar palsy in mother and son: Review of 26 reports of familial transmission within the ‘Mobius spectrum of defects’. J Med Genet. 1991;28:18–26. doi: 10.1136/jmg.28.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verzijl HT, van der Zwaag B, Cruysberg JR, Padberg GW. Mobius syndrome redefined: A syndrome of rhombencephalic maldevelopment. Neurology. 2003;61:327–33. doi: 10.1212/01.wnl.0000076484.91275.cd. [DOI] [PubMed] [Google Scholar]
- 4.Traboulsi EI. Congenital abnormalities of cranial nerve development: Overview, molecular mechanisms, and further evidence of heterogeneity and complexity of syndromes with congenital limitation of eye movements. Trans Am Ophthalmol Soc. 2004;102:373–89. [PMC free article] [PubMed] [Google Scholar]
- 5.Engle EC. Oculomotility disorders arising from disruptions in brainstem motor neuron development. Arch Neurol. 2007;64:633–7. doi: 10.1001/archneur.64.5.633. [DOI] [PubMed] [Google Scholar]
- 6.Miller NR. Strabismus syndromes: The congenital cranial dysinnervation disorders (CCDDs) In: Hoyt DTaC., editor. Pediatric Ophthalmology and Strabismus. 3. New York (NY): Elsevier Saunders; 2005. pp. 933–42. [Google Scholar]
- 7.Demer JL, Ortube MC, Engle EC, Thacker N. High-resolution magnetic resonance imaging demonstrates abnormalities of motor nerves and extraocular muscles in patients with neuropathic strabismus. J AAPOS. 2006;10:135–42. doi: 10.1016/j.jaapos.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demer JL, Clark RA, Engle EC. Magnetic resonance imaging evidence for widespread orbital dysinnervation in congenital fibrosis of extraocular muscles due to mutations in KIF21A. Invest Ophthalmol Vis Sci. 2005;46:530–9. doi: 10.1167/iovs.04-1125. [DOI] [PubMed] [Google Scholar]
- 9.Demer JL, Clark RA, Lim KH, Engle EC. Magnetic resonance imaging evidence for widespread orbital dysinnervation in dominant Duane’s retraction syndrome linked to the DURS2 locus. Invest Ophthalmol Vis Sci. 2007;48:194–202. doi: 10.1167/iovs.06-0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakano M, Yamada K, Fain J, et al. Homozygous mutations in ARIX(PHOX2A) result in congenital fibrosis of the extraocular muscles type 2. Nat Genet. 2001;29:315–20. doi: 10.1038/ng744. [DOI] [PubMed] [Google Scholar]
- 11.Tischfield MA, Bosley TM, Salih MA, et al. Homozygous HOXA1 mutations disrupt human brainstem, inner ear, cardiovascular and cognitive development. Nat Genet. 2005;37:1035–7. doi: 10.1038/ng1636. [DOI] [PubMed] [Google Scholar]
- 12.Yamada K, Andrews C, Chan WM, et al. Heterozygous mutations of the kinesin KIF21A in congenital fibrosis of the extraocular muscles type 1 (CFEOM1) Nat Genet. 2003;35:318–21. doi: 10.1038/ng1261. [DOI] [PubMed] [Google Scholar]
- 13.Cronemberger MF, de Castro Moreira JB, Brunoni D, et al. Ocular and clinical manifestations of Mobius’ syndrome. J Pediatr Ophthalmol Strabismus. 2001;38:156–62. doi: 10.3928/0191-3913-20010501-09. [DOI] [PubMed] [Google Scholar]
- 14.Miller MT, Stromland K. The mobius sequence: A relook. J AAPOS. 1999;3:199–208. doi: 10.1016/s1091-8531(99)70003-0. [DOI] [PubMed] [Google Scholar]
- 15.Miller MT, Ray V, Owens P, Chen F. Mobius and Mobius-like syndromes (TTV-OFM, OMLH) J Pediatr Ophthalmol Strabismus. 1989;26:176–88. doi: 10.3928/0191-3913-19890701-07. [DOI] [PubMed] [Google Scholar]
- 16.Legum C, Godel V, Nemet P. Heterogeneity and pleiotropism in the Moebius syndrome. Clin Genet. 1981;20:254–9. doi: 10.1111/j.1399-0004.1981.tb01030.x. [DOI] [PubMed] [Google Scholar]
- 17.Bosley TM, Salih MA, Alorainy IA, et al. Clinical characterization of the HOXA1 syndrome BSAS variant. Neurology. 2007;69:1245–53. doi: 10.1212/01.wnl.0000276947.59704.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engle EC, Goumnerov BC, McKeown CA, et al. Oculomotor nerve and muscle abnormalities in congenital fibrosis of the extraocular muscles. Ann Neurol. 1997;41:314–25. doi: 10.1002/ana.410410306. [DOI] [PubMed] [Google Scholar]
- 19.Ouanounou S, Saigal G, Birchansky S. Mobius syndrome. AJNR Am J Neuroradiol. 2005;26:430–2. [PMC free article] [PubMed] [Google Scholar]
- 20.Pedraza S, Gamez J, Rovira A, et al. MRI findings in Mobius syndrome: Correlation with clinical features. Neurology. 2000;55:1058–60. doi: 10.1212/wnl.55.7.1058. [DOI] [PubMed] [Google Scholar]
- 21.Verzijl HT, Valk J, de Vries R, Padberg GW. Radiologic evidence for absence of the facial nerve in Mobius syndrome. Neurology. 2005;64:849–55. doi: 10.1212/01.WNL.0000152980.92436.D9. [DOI] [PubMed] [Google Scholar]
- 22.D’Cruz OF, Swisher CN, Jaradeh S, Tang T, Konkol RJ. Mobius syndrome: Evidence for a vascular etiology. J Child Neurol. 1993;8:260–5. doi: 10.1177/088307389300800310. [DOI] [PubMed] [Google Scholar]
- 23.Pastuszak AL, Schuler L, Speck-Martins CE, et al. Use of misoprostol during pregnancy and Mobius’ syndrome in infants. N Engl J Med. 1998;338:1881–5. doi: 10.1056/NEJM199806253382604. [DOI] [PubMed] [Google Scholar]
- 24.Towfighi J, Marks K, Palmer E, Vannucci R. Mobius syndrome. Neuropathologic observations Acta Neuropathol (Berl) 1979;48:11–7. doi: 10.1007/BF00691785. [DOI] [PubMed] [Google Scholar]
- 25.Engle EC. The genetic basis of complex strabismus. Pediatr Res. 2006;59:343–8. doi: 10.1203/01.pdr.0000200797.91630.08. [DOI] [PubMed] [Google Scholar]
- 26.Lim KH, Engle EC, Demer JL. Abnormalities of the oculomotor nerve in congenital fibrosis of the extraocular muscles and congenital oculomotor palsy. Invest Ophthalmol Vis Sci. 2007;48:1601–6. doi: 10.1167/iovs.06-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu TE, Isenberg SJ, Demer JL. Magnetic resonance imaging demonstrates neuropathology in congenital inferior division oculomotor palsy. J AAPOS. 2006;10:473–5. doi: 10.1016/j.jaapos.2006.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]