Abstract
Adding synthetic oligodeoxynucleotides (ODN) containing unmethylated CpG motifs to Anthrax Vaccine Adsorbed (AVA, the licensed human vaccine) increases the speed and magnitude of the resultant Ab response. Ab titers persist in the protective range for >1 year, significantly longer than in animals vaccinated with AVA alone. Unexpectedly, a majority of mice immunized with CpG-adjuvanted AVA maintained resistance to anthrax infection even after their Ab titers had declined into the sub-protective range. The survival of these animals was mediated by the de novo production of protective Abs by high affinity memory B cells re-stimulated immediately after challenge. Thus, a previously unrecognized benefit of CpG ODN adjuvants is their ability to expand the long-lived memory B cell population. Current findings demonstrate that CpG-adjuvanted AVA mediates protection both by stimulating a strong/persistent serum Ab response and by generating a high-affinity long-lived pool of memory B cells.
Keywords: CpG oligonucleotide, anthrax, vaccine, adjuvant
INTRODUCTION
Bacillus anthracis is an aerobic gram-positive bacterium found naturally in wild and domesticated animals (1). B. anthracis spores are highly resistant to environmental degradation, and upon germination produce a tripartite toxin that reduces the ability of the host’s immune system to eliminate the pathogen (1). Antibodies (Ab) against protective antigen (PA) can neutralize the toxin, inhibit spore germination, and improve the phagocytosis/killing of spores by macrophages (2–5). Thus, vaccines targeting PA provide an effective and relatively inexpensive means of reducing susceptibility to anthrax (6).
Anthrax Vaccine Adsorbed (AVA) is the sole vaccine licensed to prevent human anthrax in the US. It is prepared by adsorbing the culture filtrate of an attenuated toxinogenic non-encapsulated strain of B. anthracis (V770-NP1-R) onto aluminum hydroxide (7). AVA induces a strong anti-PA response through a series of 6 immunizations over 18 months followed by yearly boosters (8). Repeated vaccination with AVA has been linked to a variety of adverse events (9–11).
Anthrax spores designed for aerosol delivery were released in the US by bioterrorists in 2001, causing morbidity, mortality, and widespread panic (12). This event underscored the need for a vaccine that induced protective immunity more rapidly than AVA and maintained protection without repeated boosts (12). One strategy to achieve these goals involved adding synthetic oligodeoxynucleotides (ODN) containing immunostimulatory “CpG motifs” to AVA (13–15). CpG ODN interact with Toll-like receptor 9 expressed by B cells and plasmacytoid dendritic cells (16–19), improving antigen presentation and triggering the production of Th1 and pro-inflammatory chemokines and cytokines (including IFNγ, IL-6, IL-12, IL-18 and TNFα) (16,17,20,21). Studies in mice, monkeys and humans verify that CpG ODN both accelerate and magnify the immune response elicited by AVA (13–15). However, neither the duration nor mechanism(s) underlying CpG-mediated improvements in protection are fully characterized.
In the current work, large numbers of mice vaccinated with AVA plus CpG ODN were followed long term, and their response to anthrax challenge monitored. Results show that CpG-adjuvanted AVA induced the production of significantly higher anti-PA titers that persisted in the protective range for significantly longer (>1 year) than AVA alone. Unexpectedly, many mice immunized with CpG-adjuvanted AVA (but not AVA alone) remained resistant to infectious challenge even after their anti-PA titers fell into the sub-protective range. A modification of the splenic fragment technique (SFT) was used to identify the mechanism underlying this persistence of protection. Results indicate that vaccination with CpG-adjuvanted AVA preferentially generated a large pool of high affinity memory B cells. This finding establishes a novel benefit of CpG ODN adjuvants, and has profound implications for the criteria used to assess the efficacy of future anthrax vaccines.
MATERIALS AND METHODS
Reagents
Phosphorothioate CpG ODN 1555 (GCTAGACGTTAGCGT) and 1466 (TCAACGTTGA) as well as control ODN 1612 (GCTAGAGCTTAGCGT) and 1471 (TCAAGCTTGA) were synthesized at the FDA Center for Biologics core facility. All ODN were free of endotoxin and protein contamination. AVA was obtained from BioPort Corporation (East Lansing, MI). Recombinant PA (rPA) was provided by USAMRIID (Fort Detrick, MD) and prepared as described (22). The toxinogenic (pXO1+), nonencapsulated (pXO2−) Sterne vaccine strain spores of B. anthracis were obtained from the culture collection of USARMIID. Spores were prepared and stored as previously described (23).
Animals
Specific pathogen free female A/J mice were obtained from the NCI (Frederick, MD). They were housed in micro-isolator cages in a barrier environment, and initially vaccinated at 8–12 wk of age. All animal experiments were conducted using ACUC approved protocols, and challenge studies were performed in a BSL-2 facility.
Immunization and challenge studies
Mice were immunized intraperitoneally (i.p.) with 2 – 10 ul of AVA ± 20 ug of CpG ODN in a final volume of 50 ul. These vaccine doses were selected on the basis of preliminary studies demonstrating that 2 ul of AVA induced a detectable but suboptimal IgG anti-PA response while 10 ul of AVA induced a response that protected most mice from low dose anthrax challenge (13).
Mice were bled monthly by tail nicking and serum stored at −20° C until use. To evaluate the effect of serum Ab titers on protection, animals were challenged as early as 10 wk (at the height of their Ab response) or as late as 20 mos post vaccination. Immunization times were staggered so that animals with different initial Ab titers could be challenged and studied simultaneously. Challenge consisted of Sterne strain anthrax spores suspended in 0.1 ml of sterile PBS administered i.p. at dose of 5 – 100 LD50 (1 LD50 = 1.1 × 103 Sterne strain spores). Survival was monitored for 21 days.
Splenic Fragment Technique
A/J mice were immunized with 2 – 10 ul of AVA ± 20 ug of CpG ODN. Spleens were aseptically removed from animals whose Ab titers fell into the sub-protective range. Each organ was diced into approximately 50 1 mm3 fragments which were cultured in individual wells of a 96-well microtiter plate (Costar, Corning, NY). Fragments were maintained in RPMI 1640 media supplemented with 10% FCS, 50 U/ml penicillin, 50 ug/ml streptomycin, 0.3 ug/ml glutamine, 1 uM non-essential amino acids, 1 uM sodium pyruvate, 10 mM HEPES, and 10−5 M 2-ME in a 5% CO2 incubator. For the first two days of culture, medium was supplemented with 10−8 – 10−12 M rPA. Culture supernatants were replaced every 3 days, and spent supernatants assayed for IgG anti-PA Ab content by ELISA.
IgG anti-PA and avidity assays
IgG anti-PA Ab titers were monitored as described (15). Briefly, 96-well microtiter plates (Immulon 2HB, Thermo Labsystems, Franklin, MA) were coated with 1 μg/ml of rPA in PBS at 4° C overnight. The plates then were blocked with 5% non-fat dry milk in PBS containing 0.1% Tween-20. Plates were washed, and overlaid with serially diluted serum or undiluted splenic fragment culture supernatants for 2 h at room temperature. The plates were washed, and for avidity assays overlaid for 15 min with 200 ul of 6 M urea. Bound Abs were detected after washing by adding HRP-labeled goat anti-mouse IgG (Southern Biotechnology, Birmingham, AL) followed by ABTS substrate (Kirkegaard & Perry, Gaithersburg, MD). Ab titers were determined by comparison to a standard curve generated using pooled sera from hyper-immunized mice, and were expressed as the reciprocal of the end point dilution. For avidity comparisons, titers were established by comparison to a standard curve generated using high-titered anti-PA serum. All serum samples were analyzed in duplicate.
Statistics
Differences in the kinetic development of anti-PA immune responses were determined by two-way ANOVA. Differences in the IgG anti-PA response induced by various vaccine-adjuvant combinations were assessed by one-way ANOVA. Differences in survival were evaluated using Chi-square analysis of Kaplan-Meier curves. Correlation coefficients were determined by linear regression analysis. The frequency of positive wells in the splenic fragment assay was calculated based on the assumption of a binomial distribution, and evaluated using the z-test.
RESULTS
Magnitude and duration of the IgG anti-PA response induced by CpG-adjuvanted AVA
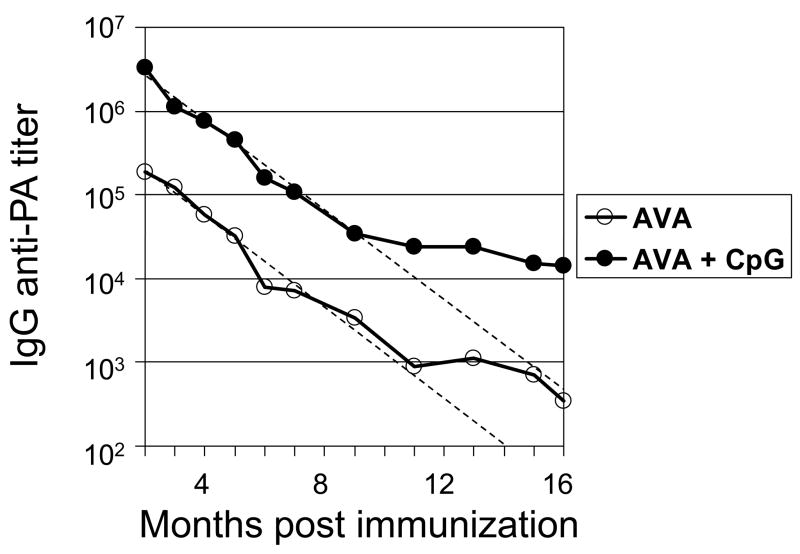
To evaluate the duration of the immune response induced by CpG-adjuvanted AVA, A/J mice were vaccinated and their serum IgG anti-PA titers monitored for >1 yr. A/J mice were selected for evaluation because these animals i) mount strong Ab responses to AVA + CpG ODN, ii) are highly susceptible to challenge by attenuated STI anthrax spores due to a defect in their complement cascade and iii) generate an anti-PA response to AVA + CpG ODN similar to that of other species, including Man (13,15,24–26). Consistent with previous reports, adding CpG ODN increased the mean Ab response when compared to AVA alone by >10-fold (from a GMT of 185,000 to 3,250,000, p <.01, Fig 1)(14,25). Over time, anti-PA titers declined in both groups of vaccinated mice. The half-life of the serum IgG anti-PA response was virtually identical between groups (AVA alone: 38.2 days vs AVA plus CpG ODN: 37.6 days, see dashed lines in Fig 1). The rate of decline in Ab titers slowed after one year (perhaps reflecting intermittent activation of memory B cells). Of note, IgG anti-PA titers remained significantly higher in the group vaccinated with CpG-adjuvanted AVA vs AVA alone for the duration of the study (p. <.01, Fig 1).
Fig 1. Magnitude and persistence of IgG anti-PA Abs elicited by vaccination with AVA + CpG ODN.
A/J mice were immunized i.p. with 10 ul of AVA alone (F) or adjuvanted with 20 ug of CpG ODN (M). Data represent the geometric mean serum IgG anti-PA titers from >30 independently studied mice/group combining results from 3 similar but independent experiments. The dashed lines represent the best fit first-order regression analysis of data from 2 – 10 months post vaccination, and were used to calculate the half-life of the serum Ab response.
Correlation between IgG anti-PA titers and protection from Sterne strain anthrax spore challenge
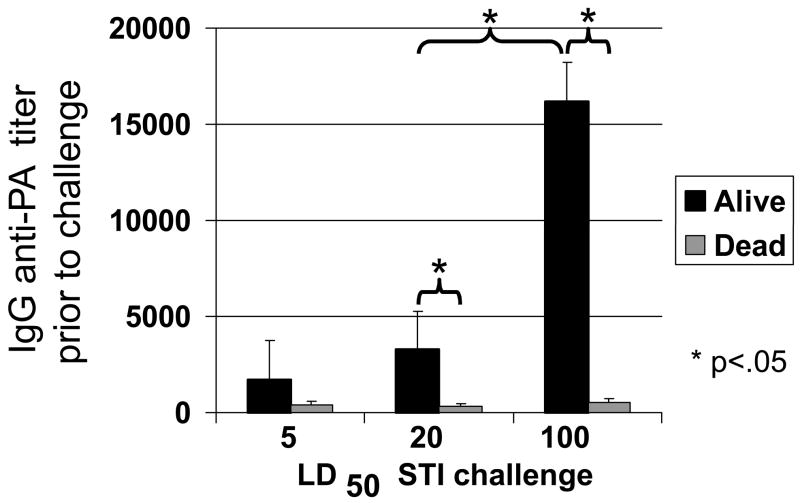
A/J mice immunized with 2–10 ul of AVA were challenged i.p. up to 6 months later with 5 – 100 LD50 of B. anthracis Sterne spores. Consistent with previous studies, survival correlated with serum IgG anti-PA titers at the time of challenge. More animals succumbed to infection as the challenge dose was increased, and a higher serum anti-PA titer was required to survive (Fig 2, 80% survival following 5 LD50 vs 26% survival following 100 LD50, p <.001)(15). Results from this experiment showed that serum IgG anti-PA titers in excess of 1:16,000 generally protected against the highest spore challenge examined (100 LD50). This Ab titer was taken as a “protective baseline”, and used to evaluate the duration of protective immunity in individual mice over time.
Fig 2. Effect of IgG anti-PA titer of susceptibility to anthrax infection.
To evaluate the effect of circulating IgG anti-PA levels on protection, A/J mice were immunized with 2 –10 ul of AVA (to induce a broad range of IgG anti-PA titers) and challenged when titers were maximal (at wk 10) or after they had declined >20-fold (at 6 mos) with 5, 20 or 100 LD50 of Sterne strain anthrax spores (N = 20 – 23 per challenge group). Data represent the mean serum IgG anti-PA Ab titer of mice that survived or succumbed to challenge. Note that low anti-PA titers protected against low dose challenge, while titers >1:16,000 protected against even the highest challenge dose.
*; p. <.05.
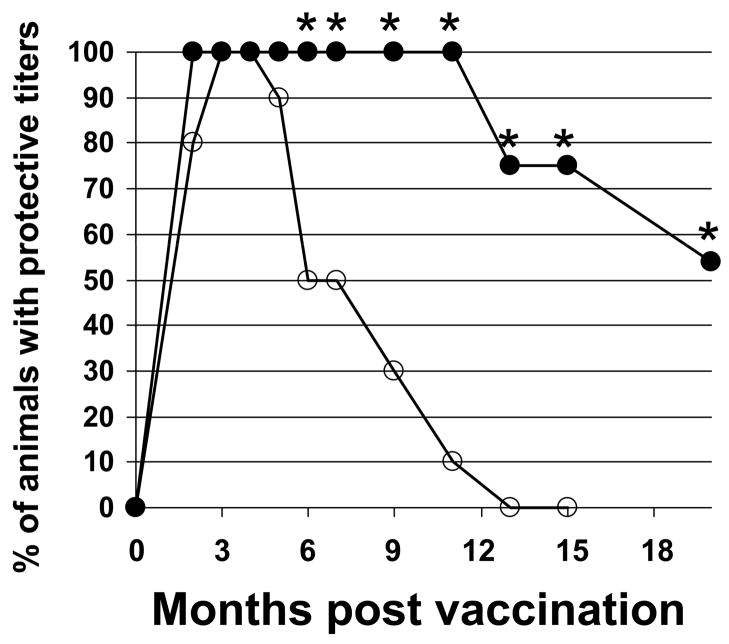
Additional animals were vaccinated with 10 ul of AVA ± CpG ODN and their Ab titers monitored for up to 20 months. As seen in Fig 3, every mouse immunized with CpG-adjuvanted vaccine maintained Ab titers >1:16,000 for at least one year, whereas titers fell below this level in half of the mice immunized with AVA alone within 6 months (p. <.001).
Fig 3. Persistence of protective Ab titers in mice immunized with AVA + CpG ODN.
A/J mice were immunized i.p. with 10 ul of AVA alone (F) or adjuvanted with 20 ug of CpG ODN (M). Serum IgG anti-PA titers were monitored individually for each of >30 mice/group over time. Data reflect the percent of animals in each group with anti-PA titers in the “protective range” (ie, in excess of 1:16,000) and represent the combined results of two similar but independent experiments
*; p. <.05 vs AVA alone.
Memory B cells contribute to vaccine-induced protective immunity
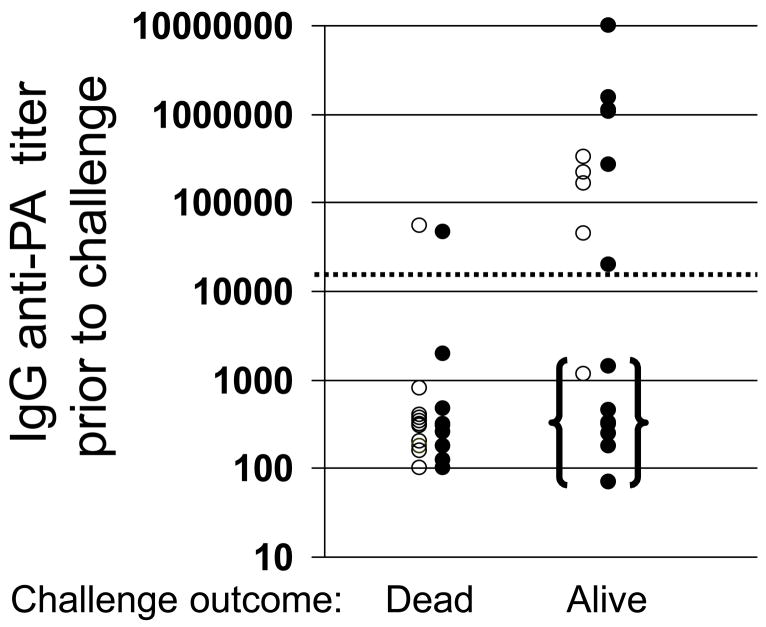
To determine whether IgG anti-PA titers accurately predicted susceptibility to infection over time, mice were immunized with 2 – 10 ul of AVA + CpG ODN. To compensate for differences in the magnitude of the initial IgG anti-PA response induced by specific vaccine/adjuvant combinations (see Fig 1) multiple independent groups of mice were vaccinated over a 10 mos period. Ab titers were monitored monthly in every animal. Mice with serum Ab levels in a broad but overlapping range were selected from among those vaccinated with AVA alone vs AVA + CpG ODN, and were challenged simultaneously. As expected, animals with circulating anti-PA titers >1:16,000 were largely resistant to infection, while those with lower titers were more susceptible (Fig 4, p <.02).
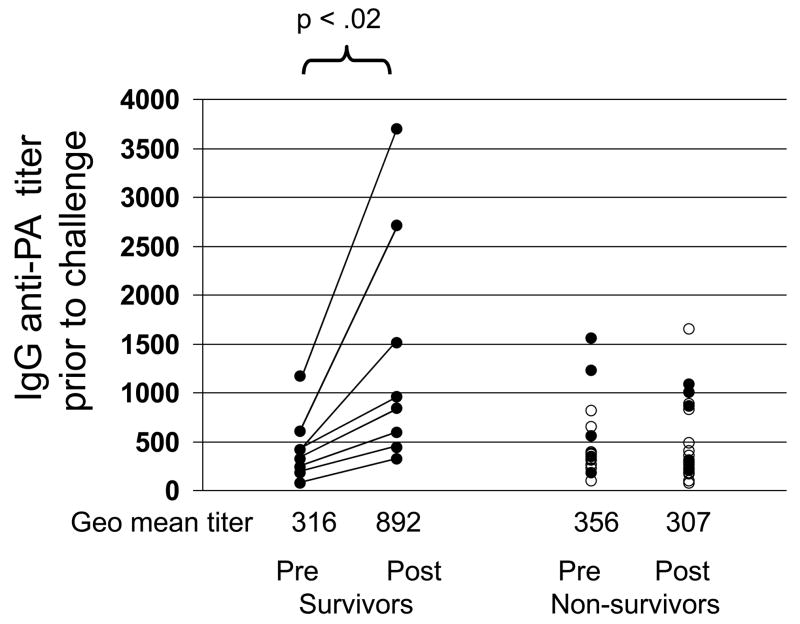
Fig 4. Effect of immunization with CpG-adjuvanted AVA on survival.
A/J mice were immunized i.p. with 2 –10 ul of AVA alone (F) or adjuvanted with 20 ug of CpG ODN (M). Multiple independent groups of mice were vaccinated over a 10 mos period. Ab titers were monitored in all animals, and mice that had been vaccinated with AVA alone vs AVA + CpG ODN whose titers were distributed through the same broad range were simultaneously challenged with 100 LD50 of Sterne strain anthrax spores. Those with titers in the “protective” range (marked by a dotted line at 1:16,000) were significantly better protected than those in the “sub-protective” range (p <.01). Of particular interest were those animals with sub-protective titers that survived infection (bracketed data).
Despite this general trend, a subset of mice was identified that survived infection despite having anti-PA titers 10-fold below the protective baseline (bracketed data, Fig 4). Almost all of these survivors had been immunized with CpG-adjuvanted AVA (8/16 vs 1/18 immunized with AVA alone, p<.01) The mechanism underlying the survival of animals with low serum IgG anti-PA titers was investigated. Mice immunized with 2 or 10 ul of AVA ± CpG ODN were challenged one year later, after their serum anti-PA titers had fallen into the sub-protective range. Serum was collected from all animals before and three days after challenge (as susceptible mice began to die by day 4). Consistent with the pattern noted above, 8/19 mice immunized with CpG-adjuvanted AVA survived infection vs 0/17 mice vaccinated with AVA alone (p<.01). Survival was not a function of circulating anti-PA Ab titers before challenge, since i) the geometric mean titer of protected vs non-protected mice was similar and ii) anti-PA titers in all mice were substantially below the protective baseline of 1:16,000. Of interest, IgG anti-PA titers rose in the cohort of mice that survived infection (average increase 3.2 fold by day 3) but not among those that succumbed to infection (p. <.02, Fig 5). These results suggest that the rapid production of IgG anti-PA Abs was protecting hosts with initially low circulating anti-PA titers.
Fig 5. Mice with low anti-PA titers that survive anthrax challenge mount a rapid anamnestic response.
A/J mice were immunized i.p. with 2 –10 ul of AVA alone (F) or adjuvanted with 20 ug of CpG ODN (M). As described in Fig 4, vaccinations were staggered over a 10 month period. Those mice with IgG anti-PA titers ranging from 1:100 – 1: 2,000 were selected from both the AVA alone and AVA + CpG ODN vaccination groups and challenged simultaneously with 100 LD50 of Sterne strain anthrax spores. Serum Ab titers are shown for each animal one week before (pre) and 3 days after challenge (post). Note that anti-PA titers rose ·3-fold in animals that survived (p. <.02), but did not change in animals that succumbed to infection.
Effect of CpG ODN on the generation of Ag-specific memory B cells
Two non-exclusive mechanisms might account for the rapid production of protective Abs by mice immunized with CpG-adjuvanted AVA and then challenged with anthrax. 1) These mice might harbor a larger pool of memory B cells than their more susceptible counterparts and/or 2) their memory B cells might be of higher affinity (and thus more responsive to the small amount of Ag present immediately after challenge). To examine these alternatives, a modification of the splenic fragment technique (SFT) (27,28) was developed to monitor the speed and affinity of the memory B cell response from AVA-immunized mice.
Splenic fragments were prepared from donor mice with IgG anti-PA titers in the sub-protective range. Fragments were cultured with ≥3×10−9 M rPA (to stimulate all memory B cells), or with ≤10−11 M rPA (to preferentially stimulate high affinity memory B cells) (29). After 2 days of Ag stimulation, the fragments were washed, cultured for 3–6 days, and supernants collected and monitored for Ab content.
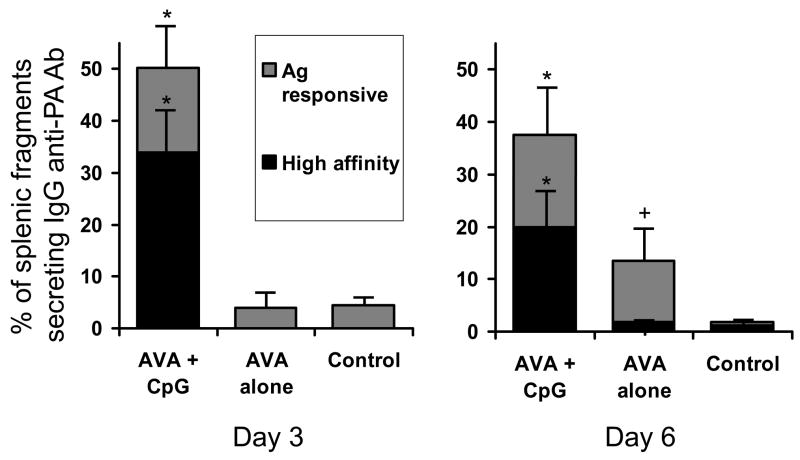
Analysis of supernatants collected on day 6 showed that the fraction of splenic fragments secreting IgG anti-PA Abs was significantly greater in mice vaccinated with AVA ± CpG ODN vs unimmunized controls (p. <.05, Fig 6A). Moreover, memory B cells from mice vaccinated with AVA alone responded to stimulation with high but not low concentrations of rPA, indicating that they were of relatively low affinity (Fig 6A). By comparison, the number of splenic fragments secreting IgG anti-PA Abs from donors vaccinated with CpG-adjuvanted AVA was nearly 3-fold higher than from donors vaccinated with AVA alone on day 6, and a majority of these responded to low concentrations of rPA (and thus were of high affinity, p <.05, Fig 6A).
Fig 6. Splenic fragments from mice immunized with CpG-adjuvanted AVA rapidly produce high affinity IgG anti-PA Abs when stimulated with Ag in vitro.
A/J mice were immunized with 10 ul of AVA (F) ± 20 ug of CpG ODN (M). 6 – 18 months later, fragment cultures were established from the spleens of mice with Ab titers in the sub-protective range. These fragments were stimulated ex vivo with high (≥3×10−9 M;○) or low (≤10−11 M;○) concentrations of rPA. A,B) Results represent the percent of fragments (avg + SD) per spleen secreting IgG anti-PA Abs 3 or 6 days post stimulation. Data reflect the combined results from 6 independent experiments involving a total of 22 mice immunized with CpG-adjuvanted AVA, 17 immunized with AVA alone, and 8 naive mice. C) The avidity of the IgG anti-PA Abs produced after 6 days of in vitro culture was examined. Supernatants known to contain IgG anti-PA Abs (N > 25 supernatants/group) were bound to rPA and then treated with 6 M urea. As previously described, urea induces the dissociation of low but not high affinity anti-PA Abs (25). Results show the percent of IgG anti-PA containing supernatants whose binding to rPA did not decline significantly after treatement with urea.
*; p. <.05 vs AVA alone, +; p. <.05 vs naive mice.
To confirm that mice immunized with CpG-adjuvanted AVA generated high affinity memory B cells, the continued binding of these Abs to their target Ag was examined after the addition of 6 M urea (30,31). As seen in Fig 6C, 27% of the splenic fragments from mice vaccinated with AVA plus CpG ODN produced Abs whose binding to rPA was not significantly reduced by 6 M urea compared to less than 5% the of IgG anti-PA secreting fragments from mice vaccinated with AVA alone (p. <.01).
Of even greater interest were results from culture supernatants collected after only 3 days of splenic fragment culture. The number of fragments secreting IgG anti-PA Abs from AVA vaccinated donors did not exceed background at this early time point. In contrast, the frequency of IgG anti-PA secreting fragments was increased 10-fold among donors that had been vaccinated with CpG-adjuvanted AVA (p. <.01, Fig 6B). The memory B cells from these CpG-adjuvanted vaccine donors were typically of high affinity (ie, responded to low Ag concentrations), whereas none of the splenic fragments from donors vaccinated with AVA alone were of high affinity at day 3 (p. <.01). These results suggest that vaccination with CpG-adjuvanted AVA generates a significantly larger and higher affinity population of memory B cells than AVA alone.
DISCUSSION
Producing effective vaccines against conventional and biothreat pathogens is an important goal of biomedical research. Towards that end, immunostimulatory adjuvants such as CpG ODN are being evaluated for their ability to improve the immunogenicity of vaccines targeting anthrax, smallpox, HIV and other infectious agents (reviewed in (32). Previous research established that CpG ODN accelerated and magnified AVA induced immunity in mice, macaques and humans (13–15,26). Current results confirm and extend these findings by demonstrating that CpG ODN extend the duration of protective immunity through 1 year. They further document that vaccination with CpG-adjuvanted AVA generates a large and long-lived population of high affinity memory B cells that respond so rapidly to challenge that they protect otherwise susceptible animals.
AVA requires six immunizations delivered over 18 months to induce and maintain protective Ab titers in humans, a regimen associated with deleterious side effects including joint pain, gastrointestinal disorders and pneumonia (9–11). Pre-clinical and phase I clinical studies show that adding CpG ODN to AVA increases serum IgG anti-PA titers by 6 – 20 fold (26). As seen in Fig 1, the serum half-life of anti-PA Abs was similar in mice vaccinated with AVA vs CpG-adjuvanted AVA. However, protection persisted significantly longer in recipients of CpG-adjuvanted vaccine due to their initially higher anti-PA titers (Fig 3).
Multiple mechanisms have been proposed to explain the ability of CpG ODN to improve AVA immunogencity. Unmethylated CpG DNA directly trigger immune cells that express TLR 9, initiating an innate immune response characterized by the production of pro-inflammatory and Th1 cytokines/chemokines capable of promoting the development of adaptive humoral responses (17,20,21,33). CpG ODN also induce the functional maturation of professional APCs (34,35). The increased availability of such “help” may explain why CpG-adjuvanted AVA induces protection more rapidly than AVA alone, and generates a larger and more avid population of memory B cells. The presence of such help may also facilitate the development of a protective secondary response.
It is well established that serum anti-PA Abs protect against infection, and that resistance is maintained by repeated re-immunization (36–38). However, the literature provides examples of animals remaining resistant to infection after their serum Ab response has waned (39). We therefore examined the susceptibility of mice to challenge after their anti-PA titers fell into the sub-protective range. Surprisingly, half of the mice immunized with CpG-adjuvanted AVA with anti-PA titers 10-fold below the protective baseline survived a 100 LD50 Sterne strain spore challenge (Figs 4,5). This contrasted with only 1/35 mice with the same Ab titer that had been immunized with AVA alone (p. <.01). The survival of mice with sub-protective titers did not correlate with the maximal Ab titer achieved following vaccination, time post vaccination, or dose of AVA (p>.45 for each parameter). Rather, protection correlated with how rapidly the host mounted a humoral response following pathogen challenge (Fig 5). Specifically, IgG anti-PA titers rose rapidly in mice that survived, but were unchanged in animals that succumbed.
Among survivors, serum anti-PA titers did not reach protective levels (>1:16,000) until >10 days post challenge (by which time virtually all susceptible mice had died). This suggests that the cumulative Ab response over the course of infection, rather than solely at the time of challenge, determines host survival. This interpretation is consistent with results from an earlier study showing that mice challenged shortly after vaccination (when serum anti-PA titers were low) survived infection if their anti-PA response was rising towards protective levels (14).
We hypothesize that mice with low serum Ab levels can survive infection if their high-affinity memory B cells respond rapidly to the Ag released following challenge. This possibility could not be evaluated in vivo due to the rapid demise of AVA-vaccinated animals. Instead, the frequency and speed with which PA-specific memory B cells responded to Ag stimulation ex vivo was examined using the splenic fragment technique (SFT). The SFT maintains the splenic micro-environment, thereby facilitating the detection of Ag-specific memory B cells (27,28). Splenic fragments from all vaccinated mice secreted anti-PA Abs within 6 days of culture with rPA, unlike those from naive mice (Fig 6). However, three important differences were noted between the response of splenic fragments from mice immunized with AVA alone vs AVA plus CpG ODN. First, significantly more memory B cells were present in the spleens of mice vaccinated with the CpG-adjuvanted vaccine (p. <.05, Fig 6). Second, these cells responded more rapidly to Ag stimulation, producing anti-PA Abs by day 3 post stimulation vs day 6 in mice vaccinated only with AVA (Fig 6). Finally, these B cells responded to lower concentrations of rPA, and produced Ab of higher affinity, that those from mice vaccinated with AVA alone (Fig 6). These results are consistent with the in vivo observation that mice immunized with CpG-adjuvanted AVA responded rapidly to anthrax challenge by producing protective anti-PA Abs (Figs 4–6). While several mechanism(s) might contribute to the rapid activation of memory B cells from mice immunized with the CpG-adjuvanted vaccine, data suggest that a critical factor is their high affinity for PA. As seen in Fig 6, significantly more memory B cells from CpG-adjuvanted animals i) responded to low concentrations of rPA and ii) produced high affinity Abs, than those from mice immunized with AVA alone.
A detailed analysis of the SFT results showed marked intra-animal variability in the response of CpG-adjuvanted mice. Specifically, splenic fragments from approximately one-third of donor mice behaved like those from AVA vaccinated animals: they contained relatively few anti-PA secreting B cells and these were primarily of low affinity. By comparison, splenic fragments from the majority of mice vaccinated with CpG-adjuvanted AVA contained large numbers of memory B cells, many of which secreted Abs of such high affinity that they remained bound to their target Ag despite treatment with 6 M urea (which strips low affinity Abs from rPA (25)). We speculate that the latter group of mice are those destined to survive challenge. Thus, while other immune elements (such as Ag-responsive T cells) might also contribute to protection, current findings suggest that an important but previously unrecognized goal of anthrax vaccine development should be the generation long-lasting high-affinity memory B cells.
Conventional phase III clinical trials are designed to test whether a vaccine reduces the risk of human infection. Serious technical and ethical limitations prevent this conduct of such studies for vaccines targeting biothreat pathogens. Recognizing this problem, the FDA developed an “animal rule” that allows surrogate markers of protection derived from animal challenge studies to be substituted for evidence of clinical efficacy in human licensure decisions. Vaccine-induced anti-PA Abs correlate with survival from anthrax challenge in multiple animal models, and thus represent one such marker (13,36–38). However, current results indicate that high affinity memory B cells also reduce host susceptibility to infection. Thus, relying on serum anti-PA Ab levels alone for licensure decisions could underestimate the protection conferred by novel vaccines. This leads us to suggest that second and third generation anthrax vaccines should also be evaluated for their ability to generate a durable pool of high-affinity memory B cells.
Acknowledgments
The assertions herein are the private ones of the authors and are not to be construed as official or as reflecting the views of DTRA or the NCI at large. Support for this work was provided in part by the Joint Science and Technology Office for Chemical and Biological Defense of the Defense Threat Reduction Agency.
Abbreviations
- AVA
Anthrax Vaccine Adsorbed
- Ab
antibody
- ODN
oligodeoxynucleotide
- Ag
antigen
- PA
protective antigen
- APC
antigen presenting cell
- LD50
50% lethal dose
Reference List
- 1.Hanna P. Anthrax pathogenesis and host response. Curr Top Microbiol Immunol. 1998;225:13–35. 13–35. doi: 10.1007/978-3-642-80451-9_2. [DOI] [PubMed] [Google Scholar]
- 2.Chowdhury K, Light SE, Garon CF, Ito Y, Israel MA. A cloned polyoma DNA fragment representing the 5′ half of the early gene region is oncogenic. J Virol. 1980;36:566–574. doi: 10.1128/jvi.36.2.566-574.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Little sF, Ivins BE. Molecular pathogenesis of Bacillus anthracis infection. Microbes Infect. 1999;1:131–139. doi: 10.1016/s1286-4579(99)80004-5. [DOI] [PubMed] [Google Scholar]
- 4.Welkos S, Little S, Friedlander A, Fritz D, Fellows P. The role of antibodies to Bacillus anthracis and anthrax toxin components in inhibiting the early stages of infection by anthrax spores. Microbiology. 2001;147:1677–1685. doi: 10.1099/00221287-147-6-1677. [DOI] [PubMed] [Google Scholar]
- 5.Welkos SL, Friedlander AM. Comparative safety and efficacy against Bacillus anthracis of protective antigen and live vaccines in mice. Microb Pathog. 1988;5:127–139. doi: 10.1016/0882-4010(88)90015-0. [DOI] [PubMed] [Google Scholar]
- 6.Friedlander AM, Brachman PS. Anthrax. In: Plotkin SA, Mortimer EA, editors. Vaccines. W.B. Saunders; Philadelphia, PA: 1998. p. 729. [Google Scholar]
- 7.Ivins BE, Welkos SL. Recent advances in the development of an improved, human anthrax vaccine. Eur J Epidemiol. 1988;4:12–19. doi: 10.1007/BF00152686. [DOI] [PubMed] [Google Scholar]
- 8.Pittman PR, Gibbs PH, Cannon TL, Friedlander AM. Anthrax vaccine: short-term safety experience in humans. Vaccine. 2001;20:972–978. doi: 10.1016/s0264-410x(01)00387-5. [DOI] [PubMed] [Google Scholar]
- 9.Brachman PS, Gold H, Plotkin SA, Fedety FR, Werrin NR, Ingraham M. Field evaluation of a human anthrax vaccine. Am J Publ Hlth. 1962;52:632–645. doi: 10.2105/ajph.52.4.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ready T. US soldiers refuse to fall in line with anthrax vaccination scheme. Nat Med. 2004;10:112. doi: 10.1038/nm0204-112b. [DOI] [PubMed] [Google Scholar]
- 11.Geier DA, Geier MR. Anthrax vaccination and joint related adverse reactions in light of biological warfare scenarios. Clin Exp Rheumatol. 2002;20:217–220. [PubMed] [Google Scholar]
- 12.Lane HC, Montagne JL, Fauci AS. Bioterrorism: a clear and present danger. Nat Med. 2001;7:1271–1273. doi: 10.1038/nm1201-1271. [DOI] [PubMed] [Google Scholar]
- 13.xie H, Gursel I, Ivins BE, Singh M, O’Hagan DT, Ulmer JB, Klinman DM. CpG oligodeoxynucleotides adsorbed onto polylactide-co-glycolide microparticles improve the immunogenicity and protective activity of the licensed anthrax vaccine. Infect Immun. 2005;73:828–833. doi: 10.1128/IAI.73.2.828-833.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klinman DM, Currie D, Lee G, Grippe V, Merkel T. Systemic but not mucosal immunity induced by AVA prevents inhalational anthrax. Microbes Infect. 2007;9:1478–1483. doi: 10.1016/j.micinf.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klinman DM, xie H, Ivins BE. CpG oligonucleotides improve the protective immune response induced by the licensed anthrax vaccine. Ann N Y Acad Sci. 2006;1082:137–150. doi: 10.1196/annals.1348.030. [DOI] [PubMed] [Google Scholar]
- 16.Krieg AM, Yi A, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, Koretzky GA, Klinman DM. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–548. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 17.Klinman DM, Yi A, Beaucage SL, Conover J, Krieg AM. CpG motifs expressed by bacterial DNA rapidly induce lymphocytes to secrete IL-6, IL-12 and IFNg. Proc Natl Acad Sci USA. 1996;93:2879–2883. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takeshita F, Leifer CA, Gursel I, Ishii K, Takeshita S, Gursel M, Klinman DM. Cutting Edge: role of toll-like receptor 9 in CpG DNA-induced activation of human cells. J Immunol. 2001;167:3555–3558. doi: 10.4049/jimmunol.167.7.3555. [DOI] [PubMed] [Google Scholar]
- 19.Hemmi H, Takeuchi O, Kawai T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 20.Ballas ZD, Rasmussen WL, Krieg AM. Induction of NK activity in murine and human cells by CpG motifs in oligodeoxynucleotides and bacterial DNA. J Immunol. 1996;157:1840–1847. [PubMed] [Google Scholar]
- 21.Halpern MD, Kurlander RJ, Pisetsky DS. Bacterial DNA induces murine interferon-gamma production by stimulation of IL-12 and tumor necrosis factor-alpha. Cell Immunol. 1996;167:72–78. doi: 10.1006/cimm.1996.0009. [DOI] [PubMed] [Google Scholar]
- 22.Ivins BE, Pitt ML, Fellows PF, Farchaus JW, Benner GE, Waag DM, Little sF, Anderson GW, Jr, Gibbs PH, Friedlander AM. Comparative efficacy of experimental anthrax vaccine candidates against inhalation anthrax in rhesus macaques. Vaccine. 1998;16:1141–1148. doi: 10.1016/s0264-410x(98)80112-6. [DOI] [PubMed] [Google Scholar]
- 23.Ivins BE, Welkos SL, Knudson GB, Little sF. Immunization against anthrax with aromatic compound-dependent (Aro-) mutants of Bacillus anthracis and with recombinant strains of Bacillus subtilis that produce anthrax protective antigen. Infect Immun. 1990;58:303–308. doi: 10.1128/iai.58.2.303-308.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harvill ET, Lee G, Grippe VK, Merkel TJ. Complement depletion renders C57BL/6 mice sensitive to the Bacillus anthracis Sterne strain. Infect Immun. 2005;73:4420–4422. doi: 10.1128/IAI.73.7.4420-4422.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klinman DM, xie H, Little sF, Currie D, Ivins BE. CpG oligonucleotides improve the protective immune response induced by the anthrax vaccination of rhesus macaques. Vaccine. 2004;22:2881–2886. doi: 10.1016/j.vaccine.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 26.Rynkiewicz D, Rathkopf M, Ransom J, Sim I, Giri L, Quinn J, Waytes T, Al-Adhami M, Johnson W, Nielsen C. Marked enhancement of antibody response to anthrax vaccine adsorbed with CpG 7909 in healthy volunteers. 2005 doi: 10.1016/j.vaccine.2011.05.047. [DOI] [PubMed] [Google Scholar]
- 27.Klinman NR. The mechanism of antigenic stimulation of primary and secondary clonal precursor cells. J Exp Med. 1972;136:241–260. doi: 10.1084/jem.136.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klinman NR, Aschinazi G. The stimulation of splenic foci in vitro. J Immunol. 1971;106:1338–1344. [PubMed] [Google Scholar]
- 29.Riley RL, Klinman NR. The affinity threshold for antigenic triggering differs for tolerance susceptible immature precursors vs mature primary B cells. J Immunol. 1986;136:3147–3154. [PubMed] [Google Scholar]
- 30.Eggers M, Bader U, Enders G. Combination of microneutralization and avidity assays: improved diagnosis of recent primary human cytomegalovirus infection in single serum sample of second trimester pregnancy. J Med Virol. 2000;60:324–330. doi: 10.1002/(sici)1096-9071(200003)60:3<324::aid-jmv11>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 31.Cozon GJ, Ferrandiz J, Nebhi H, Wallon M, Peyron F. Estimation of the avidity of immunoglobulin G for routine diagnosis of chronic Toxoplasma gondii infection in pregnant women. Eur J Clin Microbiol Infect Dis. 1998;17:32–36. doi: 10.1007/BF01584360. [DOI] [PubMed] [Google Scholar]
- 32.Klinman DM, Currie D, Gursel I, Verthelyi D. Use of CpG oligodeoxynucleotides as immune adjuvants. Immunol Rev. 2004;199:201–16. 201–216. doi: 10.1111/j.0105-2896.2004.00148.x. [DOI] [PubMed] [Google Scholar]
- 33.Klinman DM. Adjuvant activity of CpG oligodeoxynucleotides. Int Rev Immunol. 2006;25:135–154. doi: 10.1080/08830180600743057. [DOI] [PubMed] [Google Scholar]
- 34.Sparwasser T, Koch E, Vabulas RM, Heeg K, Lipford GB, Ellwart JW, Wager H. Bacterial DNA and immunostimulatory CpG oligonucleotides trigger maturation and activation of murine dendritic cells. Eur J Immunol. 1998;28:2045–2054. doi: 10.1002/(SICI)1521-4141(199806)28:06<2045::AID-IMMU2045>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 35.Gursel M, Verthelyi D, Klinman DM. CpG oligodeoxynucleotides induce human monocytes to mature into functional dendritic cells. Eur J Immunol. 2002;32:2617–2622. doi: 10.1002/1521-4141(200209)32:9<2617::AID-IMMU2617>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 36.Weiss S, Kobiler D, Levy H, Marcus H, Pass A, Rothschild N, Altboum Z. Immunological correlates for protection against intranasal challenge of Bacillus anthracis spores conferred by a protective antigen-based vaccine in rabbits. Infect Immun. 2006;74:394–398. doi: 10.1128/IAI.74.1.394-398.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reuveny S, White MD, Adar YY, Kafri Y, Altboum Z, Gozes Y, Kobiler D, Shafferman A, Velan B. Search for correlates of protective immunity conferred by anthrax vaccine. Infect Immun. 2001;69:2888–2893. doi: 10.1128/IAI.69.5.2888-2893.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Little sF, Ivins BE, Fellows PF, Pitt ML, Norris SL, Andrews GP. Defining a serological correlate of protection in rabbits for a recombinant anthrax vaccine. Vaccine. 2004;22:422–430. doi: 10.1016/j.vaccine.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 39.Ivins BE, Fellows PF, Pitt MLM, Estep JE, Welkos SL, Worsham PLFAM. Efficacy of a standard human anthrax vaccine against Bacilus anthracis aerosol spore challenge in rhesus monkeys. Salisbury Medical Bulletin Special supplement No. 87. 1985:125–126. [Google Scholar]