Abstract
INTRODUCTION
Exisulind is an apoptotic agent with preclinical activity in non-small cell lung cancer (NSCLC). Vinorelbine is safe and effective in older patients with advanced NSCLC. We assessed these agents together as palliative treatment for older patients with advanced NSCLC.
METHODS
Chemotherapy-naïve patients ≥ 70 years old with stage IIIB-IV NSCLC and a performance status (PS) ≤ 2 were eligible. Primary endpoints were the maximum tolerated dose (MTD, phase I) and time-to-progression (TTP, phase II) of oral exisulind with 25 mg/m2/week of intravenous (IV) vinorelbine on a 28-day cycle. Patients with clinical benefit after six cycles of this combination received exisulind alone.
RESULTS
Fourteen phase I patients (median PS 1; median age 78 years) were enrolled. Dose-limiting toxicities included grade 3 constipation (one patient), grade 3 febrile neutropenia (one patient) and grade 3 diarrhea (one patient). The MTD of oral exisulind with 25 mg/m2/week of IV vinorelbine was 125 mg twice daily. Thirty phase II patients (median PS 1; median age 78 years) were enrolled. Grade ≥ 3 neutropenia occurred in 14/30 patients. Two patients experienced neutropenic fever. There were no complete responses, one partial response and 12 patients with stable disease as their best response. The objective response rate was 4.0% (95% CI: 0.1–20.4%). Phase II median TTP was 4.7 months (95% CI: 3.1 – 9.3 months) and median OS was 9.6 months (95% CI: 6.6 – 19.1 months).
CONCLUSIONS
This combination is safe, appears to have activity in the elderly with advanced NSCLC and a PS ≤ 2, and warrants further investigation.
Keywords: Elderly, exisulind, sulindac sulfone, non-small cell lung cancer, vinorelbine, phase I, phase II
INTRODUCTION
Lung cancer is common after age 70. Between 2001 and 2003, it was diagnosed in 1 in 15 males and 1 in 22 females in this age cohort, compared with 1 in 38 males and 1 in 54 females aged 60 to 69 years.1 Clinical researchers are increasingly recognizing that the elderly with cancer should be studied as a distinct population.2
Vinorelbine has demonstrated efficacy in older patients with advanced NSCLC. In a phase II study, Gridelli treated patients at least 70 years old with stage IIIB-IV NSCLC with first-line vinorelbine (30 mg/m2/week).3 Median age was 73 years and median Eastern Cooperative Oncology Group (ECOG) performance status (PS)3 was 2 (22/78 patients). Median time-to-progression (TTP) was 11 weeks and median overall survival (OS) was 36 weeks. The phase III Elderly Lung Cancer Vinorelbine Study (ELVIS) randomized patients at least 70 years old with stage IIIB/IV NSCLC to best supportive care (BSC) or BSC with weekly vinorelbine.4 The median ECOG PS was 2 (19/43 patients). Patients receiving vinorelbine experienced a superior median OS (28 versus 21 weeks) and improved quality of life.
Exisulind (sulindac sulfone; OSI Pharmaceuticals, Melville, NY) is an orally available inducer of apoptosis in cancer cells via inhibition of cyclic guanosine monophosphate phosphodiesterase and activation of protein kinase G.5–7 It may also inhibit angiogenesis.9 Exisulind demonstrated preclinical activity in NSCLC.8–10 Phase I testing of exisulind in patients aged 18–45 years with familial adenomatous polyposis (FAP) and subtotal colectomy recommended a phase II dose of 300 mg orally twice daily continuously.11 Treatment was well tolerated. Predominant toxicities included reversible grade ≤ 3 hepatotoxicity, grade ≤ 2 headaches and grade ≤ 2 gastrointestinal disturbance.
Based on the preclinical activity of exisulind in NSCLC models, its favorable toxicity profile, and the non-overlapping mechanisms of action of vinorelbine and exisulind, we conducted a phase I/II trial of first-line exisulind/vinorelbine for patients with stage IIIB-IV NSCLC who were at least 70 years old and ECOG PS ≤ 2. The phase I primary objective was to find dose limiting toxicities (DLT), the maximum tolerated dose (MTD) and recommended phase II dose of this combination. The phase II primary objective was to evaluate the clinical efficacy in prolonging TTP. The secondary objectives were to evaluate toxicities, objective RR and OS.
PATIENTS AND METHODS
Patient Eligibility
Eligible patients were ≥ 70 years of age, chemotherapy-naive, had an ECOG PS ≤ 2, histologically-confirmed stage IIIB (pleural effusion) or IV NSCLC, measurable disease and adequate major organ function [white blood cell count ≥ 3,500/mm3, absolute neutrophil count (ANC) ≥ 1,500/mm3, platelet count ≥ 100,000/mm3, creatinine ≤ 2.0 mg/dl or creatinine clearance ≥ 60 mg/dl, total bilirubin (TB) < institutional upper limit of normal (ULN), aspartate transaminase (AST) ≤ 1.5 ULN and alkaline phosphatase (ALP) ≤ 2.5 ULN]. Prior radiotherapy was allowed if there was measurable disease outside of or progressive disease within the radiotherapy port. Exclusion criteria included clinically unstable brain metastasis, use of non-steroidal anti-inflammatory medications within two weeks (except ibuprofen, naproxen and low dose aspirin) and subtotal colectomy. The Institutional Review Board of the University of Wisconsin and participating institutions approved the study protocol. All enrolled patients provided written informed consent.
Study Parameters and Tumor Assessment
Baseline laboratory and imaging studies were performed within two and four weeks prior, respectively, of enrollment. Subsequent lab studies were performed on day 1 of each cycle. A complete blood count was drawn weekly. Measurable disease was reimaged and graded using Response Evaluation Criteria in Solid Tumors14 every two cycles.
Phase I and II Treatment
OSI Pharmaceuticals (Melville, NY) provided exisulind as 125 mg capsules. We used pill counts to monitor exisulind compliance.
During the phase I study, exisulind (dose level 1, 125 mg twice a day; dose level 2, 125 mg in the morning and 250 mg in the evening; and dose level 3, 250 mg in the morning and 250 mg in the evening) was combined with 25 mg/m2/week (without interruption) of intravenous vinorelbine on a 4-week cycle. The MTD was used as the phase II regimen.
Progressive disease (PD), treatment intolerance, treatment delay of either drug > 14 days and withdrawn consent were reasons for ceasing treatment. Otherwise, patients received up to six cycles of vinorelbine with exisulind followed by exisulind alone (initially at the cycle 6 dose).
Granulocyte colony simulating factor (G-CSF) support was not used in the phase I study. G-CSF was used during phase II for grade 4 neutropenia > 5 days and recurrent neutropenia, followed by prophylactic G-CSF in subsequent cycles.
Phase I Dose Limiting Toxicity, Dose Escalation and Maximum Tolerated Dose
Toxicities were graded according to the National Cancer Institute Common Toxicity Criteria, version 2.0. DLT was defined as grade 4 neutropenia ≥ 7 days, grade 4 neutropenia and fever (any oral temperature > 38.5°C, or three elevations > 38°C/24 hours) requiring parenteral antibiotics, grade 4 thrombocytopenia, delay in treatment > 14 days and other non-hematological grade ≥ 3 toxicity.
The MTD was determined during cycle 1 using a traditional 3-by-3 cohort design as previously described.12
Statistical Considerations
The primary endpoint of the phase I study was toxicity. The primary endpoint of the phase II study was TTP. Secondary endpoints included safety, tolerability, objective RR and OS.
The sample size estimate for phase II enrollment did not include phase I patients. Utilizing standard approximations based upon an exponential distribution for time to event assuming that patient accrual is uniform over time, we estimated that 27 disease progressions would need to be observed. This sample size would provide 90% power to detect a 50% increase in median TTP from 11 weeks for historical control13 to 16.5 weeks on the protocol therapy according to a one-sided logrank test at the 0.2 signifiance level. Our rationale was that we were not so much concerned about falsely claiming efficacy of this therapy, but rather we did not want to overlook a promising therapy because of limited power. We planned to accrue 30 patients with an additional follow up after the last patient was enrolled until there were 27 disease progressions.
According to this design, this combination would be considered for further testing if the observed median TTP was > 13 weeks, corresponding to the 80% lower confidence limit for the true median TTP with the protocol therapy when 27 disease progressions were observed in the study. TTP and OS were defined as the time elapsed between study enrollment and progressive disease (PD) and death, respectively. Patients completing two cycles of treatment were evaluable for objective response. All enrolled patients were considered evaluable for OS and TTP (intent-to-treat). TTP and OS were analyzed using the Kaplan-Meier method.14 Quantitative and qualitative toxicities, as well as objective RR were summarized with descriptive statistics.
RESULTS
Baseline Patient Characteristics
Fourteen patients enrolled in the phase I study between April 2001 and June 2002 (Table 1). Thirty patients enrolled in the phase II study between November 2002 and May 2004 (Table 1).
Table 1.
Baseline characteristics of all patients.
| Characteristic | Phase I (n=14) |
Phase II (n=30) |
Total (n=44) |
|---|---|---|---|
| Age, years | |||
| Median | 78 | 78 | 78 |
| Range | 72–84 | 71–91 | 71–91 |
| Male | 8 (57.1%) | 17 (56.7%) | 25 (56.8%) |
| Female | 6 (42.9%) | 13 (43.3%) | 19 (43.3%) |
| ECOG performance status | |||
| 0 | 5 (35.7%) | 12 (40%) | 17 (38.6%) |
| 1 | 9 (64.3%) | 16 (53.3%) | 25 (56.8%) |
| 2 | 0 | 2 (6.7%) | 2 (4.5%) |
| Ethnicity | |||
| African American | 0 | 1 (3.3%) | 1 (2.3%) |
| Caucasian | 14 (100%) | 29 (97.7%) | 43 (97.7%) |
| Histology | |||
| Adenocarcinoma | 10 (71.4%) | 16 (53.3%) | 26 (59.1%) |
| Squamous cell | 2 (14.3%) | 5 (16.7%) | 7 (15.9%) |
| Bronchoalveolar | 0 | 4 (13.3%) | 4 (9.1%) |
| Adenocarcinoma/bronchoalveolar | 0 | 1 (3.3%) | 1 (2.3%) |
| Unspecified | 2 (14.3%) | 4 (13.3%) | 6 (13.6%) |
| Stage IIIB | 1 (7.1%) | 0 | 1 (2.3%) |
| Stage IV | 13 (92.9%) | 30 (100%) | 43 (97.7%) |
| Metastatic sites | |||
| Liver | 2 (14.3%) | 5 (16.7%) | 7 (15.9%) |
| Bone | 4 (28.6%) | 7 (23.3%) | 11 (25.0%) |
| Brain | 0 | 3 (10.0%) | 3 (16.8%) |
| Adrenal gland | 4 (28.6%) | 3 (10.0%) | 7 (15.9%) |
| Pleural | 0 | 2 (6.7%) | 2 (4.5%) |
| Skin | 0 | 3 (10.0%) | 3 (6.8%) |
| Prior cancer-related therapy | |||
| Chemotherapy | 0 | 0 | 0 |
| Surgery | |||
| Early stage disease | 4 (28.6%) | 9 (30.0%) | 13 (29.5%) |
| Advanced stage disease | 0 | 0 | 0 |
| Radiotherapy | |||
| Early stage disease | 1 (7.1%) | 0 | 1 (2.3%) |
| Advanced stage disease | 2 (14.3%) | 11 (36.7%) | 13 (29.5%) |
| Surgery and radiotherapy | 2 (14.3%) | 4 (13.3%) | 6 (13.6%) |
| Gefitinib | 1 (7.1%) | 0 | 1 (2.3%) |
| Treatment site | |||
| UWCCC | 4 (28.6%) | 13 (43.3%) | 17 (38.6%) |
| WON | 10 (71.4%) | 17 (56.7%) | 27 (61.4%) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; UWCCC, University of Wisconsin Paul P. Carbone Comprehensive Cancer Center; WON, Wisconsin Oncology Network.
Phase I Dose Escalation, Dose Limiting Toxicity and Maximum Tolerated Dose
After no DLTs were observed in three patients at dose level 1 (125 mg of exisulind twice a day), four patients enrolled at dose level 2 (125 mg of exisulind in the morning and 250 mg in the evening). Two dose level 2 patients experienced a DLT (grade 3 constipation and grade 3 diarrhea). Dose level 2 was expanded by five patients. Two were replaced because of death during cycle 1 unrelated to treatment (one patient) and withdrawn consent (one patient). One patient in this expanded cohort experienced a DLT (grade 4 neutropenic fever). Therefore, the MTD was designated as 125 mg exisulind twice a day orally continuously with 25 mg/m2/week intravenous vinorelbine on a 4-week cycle.
Phase I Treatment
Fourteen phase I patients received a median 2 cycles of vinorelbine with exisulind (41 cycles total; range, 0.5 to 6 cycles) and a median zero cycles of exisulind alone (60.0 cycles total; range, 0 to 28 cycles). Three patients (21.4%) completed six cycles of combination therapy (i.e., one patient at dose level 1 and two at dose level 2). These three patients then received 10, 22 and 28 cycles of exisulind alone. Reasons for removal from treatment included PD (eight patients), DLT (three patients), withdrawn consent (one patient), death unrelated to treatment (one patient), and serious adverse event unrelated to treatment (cerebrovascular accident in one patient).
Phase II Treatment
Thirty phase II patients received a median 4 cycles of vinorelbine with exisulind (114 cycles total; range, 0.3 to 6 cycles) and a median zero cycles of exisulind alone (65 cycles total; range, 0 to 13 cycles). Seventeen percent (5/30) of patients withdrew from treatment before completing cycle 2 because of declining PS and/or treatment intolerability. Forty percent (12/30) of patients completed the initial six cycles of vinorelbine with exisulind. Eleven of these patients continued exisulind alone (median 4 cycles; range, 2 to 13 cycles). Reasons for stopping treatment included PD (25/30 patients), declining PS (two patients), withdrawn consent (one patient), death unrelated to treatment (one patient), and grade 3 peripheral neuropathy (one patient). Vinorelbine dose intensity was 75.5% of planned (median, 75%; range, 25 to 100%). Examination of available exisulind pill calendars for the patients treated at the University of Wisconsin suggested that compliance was good.
Phase I Safety and Tolerability
Table 2 summarizes phase I toxicities. Sixteen (10%) of 165 planned vinorelbine doses were held and 37 (22%) were reduced because of grade ≥ 2 neutropenia. One patient had neutropenic fever (grade 4). Toxicity attributable to exisulind was infrequent. Liver function test elevation was ≤ grade 2 except for one occurrence of grade 3 ALP elevation. One incidence of grade 3 diarrhea was reported. One episode of grade 3 constipation occurred, attributable to both drugs. Exisulind was held for 12 (5%) of 240 planned weeks of administration and dose reduced for nine (4%).
Table 2.
Maximal severity of toxicities per phase I patient and type regardless of attribution to study drugs occurring in ≥ 20% patients.
| Exisulind Dose Level | Total (n=14) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 125 mg BID (n=3) |
125 mg qAM 250 mg qPM (n=11) |
|||||||||||
| Grade | Grade | Grade | ||||||||||
| Event* | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 |
| Anemia | 1 | 2 | 9 | 2 | 1 | 10 | 4 | 1 | ||||
| Fatigue | 2 | 4 | 4 | 2 | 4 | 6 | 2 | |||||
| Constipation | 2 | 1 | 4 | 2 | 1 | 6 | 3 | 1 | ||||
| Neutropenia | 1 | 1 | 1 | 4 | 2 | 2 | 4 | 3 | ||||
| Dyspnea | 2 | 1 | 3 | 2 | 1 | 5 | 2 | |||||
| Pain | 2 | 4 | 1 | 1 | 6 | 1 | 1 | |||||
| Anorexia | 2 | 2 | 3 | 4 | 3 | |||||||
| Leukopenia | 1 | 1 | 1 | 3 | 1 | 4 | 1 | 2 | ||||
| Asparate aminotransferase | 1 | 4 | 5 | |||||||||
| Alkaline phosphatase | 1 | 2 | 1 | 1 | 2 | 1 | ||||||
| Diarrhea | 3 | 1 | 3 | 1 | ||||||||
| Nausea | 4 | 4 | ||||||||||
| Cough | 1 | 2 | 3 | |||||||||
| Hyperglycemia | 1 | 1 | 1 | 2 | 1 | |||||||
| Infection | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Rash | 2 | 1 | 2 | 1 | ||||||||
| Vomiting | 3 | 3 | ||||||||||
National Cancer Institute Common Toxicity Criteria version 2.0
Abbreviations: BID, twice daily; n, number; q, every; AM, morning; PM, evening.
Phase II Safety and Tolerability
Table 3 summarizes phase II toxicities. Fourteen percent (62/444) of vinorelbine doses were held and 19% (84/444) were reduced because of neutropenia. Two patients (7%) had grade 3 neutropenic fever. Toxicities attributable to exisulind were infrequent. One episode, each, of grade 3 ALP and grade 3 AST elevation occurred. Four patients experienced grade 3 constipation. Out of 704 planned weeks of administration, exisulind was held for 16 weeks (3%) and dose reduced for 75 weeks (11%). Toxicities observed during 65 cycles of exisulind maintenance were grade ≤ 2 except for one incidence of grade 3 anemia and one episode of grade 3 neutropenia.
Table 3.
Maximal severity of toxicities per phase II patient and type regardless of attribution to study drug occurring in ≥ 20% of patients (n=30).
| Grade | Frequency (n=30) | |||||||
|---|---|---|---|---|---|---|---|---|
| Event* | 1 | 2 | 3 | 4 | Grade 1–2 (n) |
Grade 1–2 (%) |
Grade 3–4 (n) |
Grade 3–4 (%) |
| Anemia | 18 | 8 | 1 | 26 | 80.0 | 1 | 3.3 | |
| Fatigue | 8 | 15 | 4 | 23 | 76.7 | 4 | 13.3 | |
| Neutropenia | 4 | 4 | 6 | 8 | 8 | 26.7 | 14 | 46.7 |
| Constipation | 9 | 8 | 4 | 17 | 56.7 | 4 | 13.3 | |
| Dyspnea | 3 | 9 | 8 | 1 | 12 | 40.0 | 9 | 30.0 |
| Cough | 16 | 2 | 2 | 18 | 60.0 | 2 | 6.7 | |
| Leukopenia | 5 | 5 | 8 | 2 | 10 | 33.3 | 10 | 33.3 |
| Nausea | 9 | 8 | 1 | 17 | 56.7 | 1 | 3.3 | |
| Anorexia | 9 | 8 | 17 | 56.7 | ||||
| Pain | 7 | 7 | 1 | 14 | 46.7 | 1 | 3.3 | |
| Hyperglycemia | 9 | 3 | 2 | 12 | 40.0 | 2 | 6.7 | |
| Diarrhea | 11 | 2 | 13 | 43.3 | ||||
| Peripheral neuropathy | 10 | 1 | 1 | 11 | 36.7 | 1 | 3.3 | |
| Infection | 3 | 8 | 11 | 36.7 | ||||
| Insomnia | 9 | 1 | 10 | 33.3 | ||||
| Lightheadedness | 7 | 1 | 1 | 8 | 26.7 | 1 | 3.3 | |
| Limb Edema | 6 | 3 | 9 | 30.0 | ||||
| Alkaline phosphatase | 6 | 1 | 1 | 7 | 23.3 | 1 | 3.3 | |
| Asparate aminotransferase | 6 | 1 | 1 | 7 | 23.3 | 1 | 3.3 | |
| Visual disturbance | 5^ | 2& | 7 | 23.3 | ||||
| Alopecia | 5 | 1 | 6 | 20.0 | ||||
| Chest pain | 4 | 1 | 1 | 5 | 16.7 | 1 | 3.3 | |
National Cancer Institute Common Toxicity Criteria, version 2.0.
Two patients had cataracts
One patient was status/post cerebrovascular accident and another patient had cataracts.
Abbreviations: n, number.
Phase I Efficacy
By January 2008, with a median follow up of 11.6 months (range, 0.4 to 54.5 months), all patients had died. Table 4 summarizes patient outcomes.
Table 4.
Efficacy.
| Variable | Phase I | Phase II |
|---|---|---|
| Best response | ||
| Evaluable | 8 | 25 |
| Complete response | 0 | 0 |
| Partial response | 1 | 1 |
| Stable disease | 3 | 12 |
| Progressive disease | 4 | 12 |
| TTP and Survival | ||
| Evaluable | 30 | 30 |
| Median TTP, months | 4.2 (95% CI: 1.9 – ∞) | 4.7 (95% CI: 3.1 – 9.3) |
| Median OS, months | 11.6 (95% CI: 6.0 – 52) | 9.6 (95% CI: 6.6 – 19.1) |
| 1-year survival, percent | 50 (30% standard error) | 47 (9% standard error) |
Abbreviations: TTP, time-to-progression; OS, overall survival; CI, confidence interval.
Phase II Efficacy
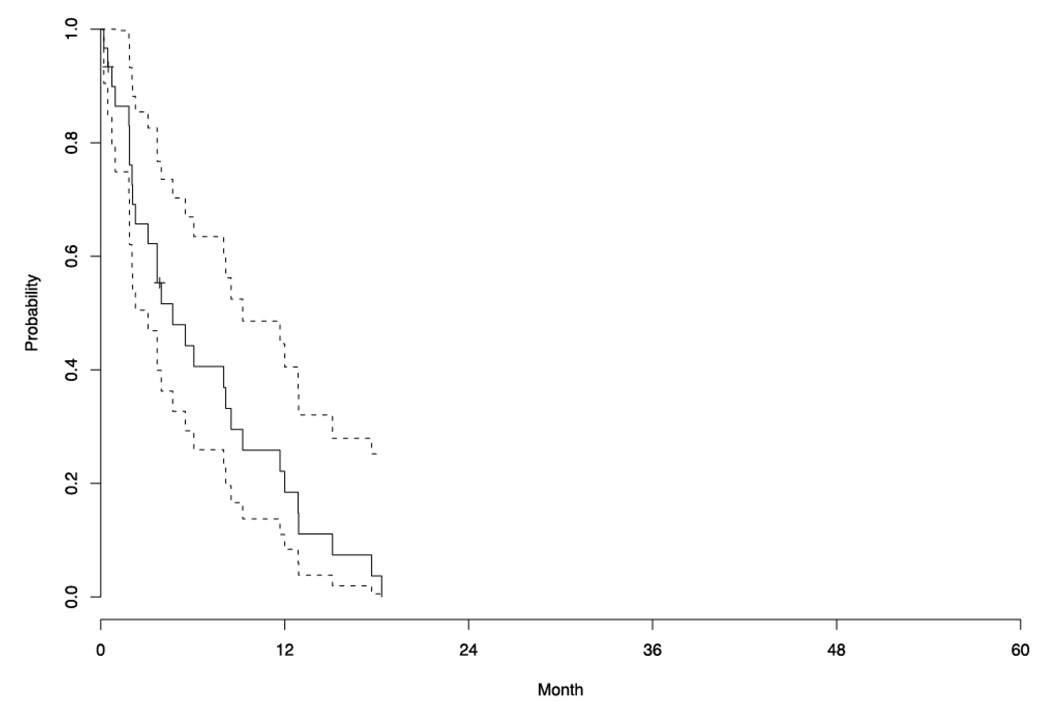
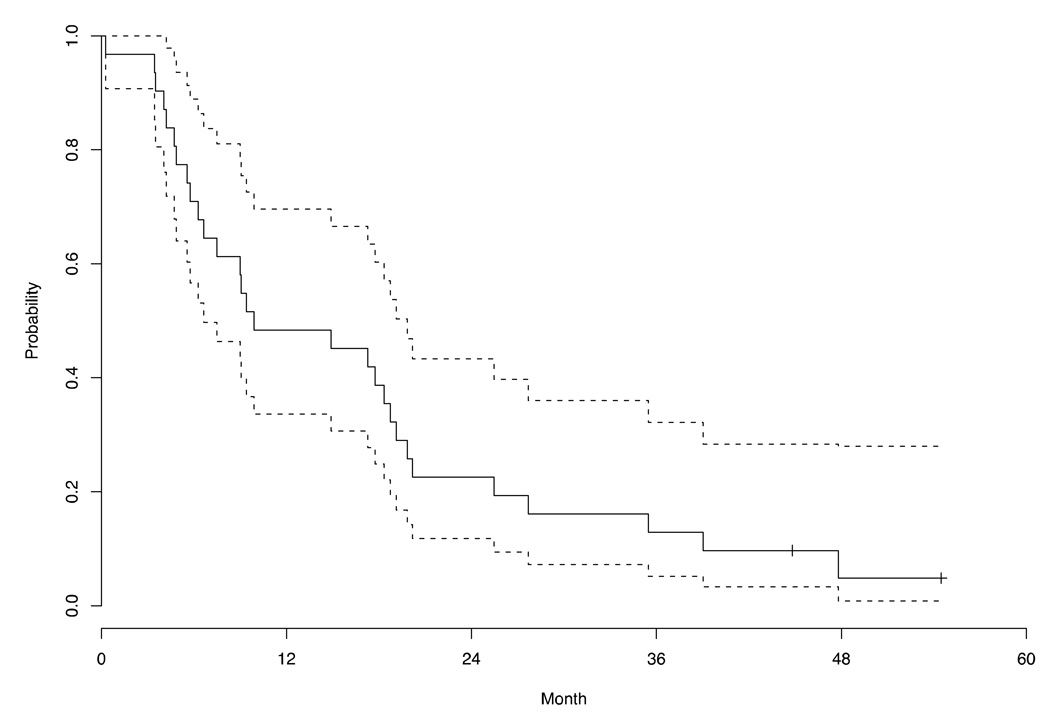
By January 2008, with a median follow up of 9.6 months (range, 0.3 to 61.6 months), 93.3% of patients had died. Table 4 summarizes patient outcomes. Five patients withdrew consent before completing two cycles due to decline in PS (three patients), grade 3 constipation (one patient) and progressive dyspnea (one patient). Reasons for stopping treatment in the other 25 patients included PD (24 patients) and grade 3 neuropathy (one patient). No CRs were seen. One patient experienced a PR, yielding an objective RR of 4.0% (95% CI: 0.1–20.4). The median duration of SD was 6.9 months. The median TTP was 4.7 months (95% CI: 3.1–9.3) and the median OS was 9.6 months (95% CI: 6.6–19.1). Figure 1 and Figure 2 are the Kaplan-Meier curves for TTP and OS, respectively.
Figure 1.
Kaplan-Meier estimate of the survival function for time to progression in the phase II patients (n=30).
Figure 2.
Kaplan-Meier estimate of the survival function for overall survival in the phase II patients (n=30).
DISCUSSION
Exisulind is a well-tolerated11 orally available pro-apoptotic agent6 with preclinical activity in NSCLC.8–10 Vinorelbine is the only drug shown to improve quality of life and survival as a single-agent in the first-line treatment of elderly patients with advanced NSCLC.4 Therefore, it was reasonable to assess these agents together.
Treatment in our phase I population yielded an MTD of 25 mg/m2/week of intravenous vinorelbine with 125 mg of oral exisulind twice daily continuously on a 28-day cycle. DLTs included constipation, diarrhea and febrile neutropenia. The phase II median TTP was 4.7 months (20.4 weeks) and the phase II median OS was 9.6 months (41.7 weeks). Our phase II primary endpoint was met, in that the median TTP exceeded 13 weeks. Neutropenia was the predominant toxicity, although neutropenic fever was rare. The median age of our patients was 78 years.
This is the first clinical study of exisulind with vinorelbine. Four other phase II studies combining exisulind with cytotoxic agents for the treatment of advanced NSCLC have been reported. Masters17 and Jones15 evaluated first-line exisulind combined with carboplatin/gemcitabine and carboplatin/docetaxel, respectively. Hoang12 and Weiss19 assessed second-line exisulind combined with gemcitabine and docetaxel, respectively. The results of these four studies were similar to what we would expect with their respective cytotoxic agents alone. In each, exisulind did not worsen outcomes or exacerbate toxicity. These studies’ findings are in line with others in which a targeted agent, such as erlotinib,16 or a matrix metalloproteinase inhibitor,17 was combined with cytotoxic agents for the treatment of advanced NSCLC. The notable exception to this record is the prolongation of survival resulting from the addition of bevacizumab to cytotoxic agents.22, 23
Our efficacy results compare favorably with those of prior single-agent vinorelbine studies treating advanced NSCLC in the elderly. In a phase II study by Gridelli, patients at least 70 years of age with stage IIIB-IV NSCLC and an ECOG PS ≤ 2 were treated with first-line vinorelbine (30 mg/m2/week).13 The median age was 73 years and median ECOG PS was 2 (22/43 patients). Median TTP was 11 weeks and median OS was 36 weeks. A fair assertion when comparing this study with ours is that our patients were “fit elderly” with a median ECOG PS of 1. Nonetheless, the finding in our elderly phase II population of a median TTP of 4.7 months and a median OS of 9.6 months is provocative when compared to single-agent historical control.13 A randomized trial of vinorelbine with and without exisulind would be necessary to identify any clinical benefit in adding exisulind.
It is unclear why the results of our combination study incorporating exisulind yielded more favorable efficacy results than seen with prior exisulind combination studies in advanced NSCLC.12, 15, 18, 19. Although small in number, our patients were selected from multiple sites in our academic-community network. In addition, there do not appear to be significant discrepancies between our eligibility criteria and those from the other studies.16–19 Our findings are consistent with the prior exisulind studies, in that the addition of exisulind to a cytotoxic regimen did not appear to exacerbate toxicity. Although preclinical evidence to date suggests a lack of potentiation between exisulind and cytotoxics,20 our favorable experience with this combination prompts us to consider the use of pro-apoptotic agents in NSCLC worthy of further study.
The predominant toxicity in our patients was neutropenia attributable to vinorelbine. This was expected.4, 21 Nearly half of phase II patients experienced grade ≥ 3 neutropenia. However, neutropenic fever was uncommon. Prophylactic G-CSF support may have been useful in this setting to avoid treatment delay and dose reduction. Prophylactic pegfilgrastim starting with the first cycle of chemotherapy reduced the rate of neutropenic fever and hospitalizations in a randomized trial of 852 patients at least 65 years old.22 Since the initiation of our study, several organizations23–25 have recommended consideration of prophylactic G-CSF in elderly patients receiving standard-dose myelotoxic chemotherapy for whom neutropenia is expected.
The addition of exisulind to vinorelbine did not worsen toxicity in our patients. This is evidenced by the fact that 40% of our phase II patients completed six cycles of combination treatment. Additionally, in 175 phase II cycles of exisulind as part of combination therapy or as maintenance, exisulind-related toxicities were ≤ grade 2 in all but two cases (grade 3 ALP and grade 3 AST elevation). This profile is similar to that seen in a phase I study by von Stolk of single-agent exisulind in patients aged 18–45 years with FAP and subtotal colectomy.11 Here, there was only one grade ≥ 3 event (elevated alanine aminotransaminase) over approximately 108 cycles. We did not perform pharmacokinetic analyses. Therefore, one may theorize that the von Stolk study found a higher MTD because of an undetected interaction between exisulind and vinorelbine. However, it is also possible that our patients, who had intact colons, had greater resorption of exisulind leading to a prolonged residence time.26, 27
It is noteworthy that among our 30 phase II patients, eight developed grade 3, and one developed grade 4, dyspnea. Dyspnea is a nearly ubiquitous symptom in this population, in whom pleural effusions, pulmonary infections, pulmonary emboli, atelectasis, emphysema, anemia, cardiovascular disease, and prior treatments with radiotherapy are frequent.28 For example, Gridelli et al. reported a baseline incidence of dyspnea of 67% in their elderly cohort.3 Dyspnea is not a known adverse effect of exisulind, and prior evaluation of vinorelbine in the elderly do not report the incidence of dyspnea during treatment.4, 11, 13 It does not appear that exisulind contributed to the worsening of dyspnea in our patients.
Strengths of this study include its novelty as the first clinical study of vinorelbine with exisulind and its focus on older patients. Additionally, the conduct of this trial in both the academic and community settings may provide less bias in patient selection and a more realistic profile of toxicities and patient outcomes.
There are several limitations to interpreting this study. First, we did not use a uniform method of classifying comorbidities and functional impairment.29–33 We relied on ECOG PS3 in determining eligibility. As has been documented, the use of ECOG PS alone may not be the best way to assess the functionality of the elderly.34
Second, pharmacokinetic analyses were not conducted. Pharmacokinetic studies in elderly cancer patients have provided conflicting results, including reports analyzing vinorelbine.35–37 To our knowledge, no data exist concerning the clearance of oral exisulind in the elderly. However, Sitar found no differences in the clearance of sulindac sulfoxide, a prodrug of exisulind, when comparing elimination data from young, healthy individuals with those older than 65 years taking 150 mg twice a day.38 The type, frequency and severity of our phase II patients’ toxicities were in line with our expectations. This observation implies there is low likelihood of significant pharmacologic interaction.
Third, 30% of our phase II patients presented for first-line chemotherapy after prior surgery, and 37% after prior radiation. This may indicate that our cohort carried a more favorable prognosis and may have contributed to our provocative efficacy outcomes.
Last, most of our patients had a PS of 1, and only two of 30 had a PS of 2. Therefore, our results are confined to the “fit elderly” and may not generalize to a broader geriatric cancer population.
We demonstrate that a first-line regimen of vinorelbine/exisulind was reasonably well tolerated by an older cohort of patients with advanced NSCLC. Median TTP and median OS were prolonged compared to historical control. We conclude that this combination is safe, appears to have activity in patients at least 70 years of age with advanced NSCLC and a PS ≤ 2, and that the use of pro-apoptotic agents in NSCLC warrants further investigation. At present, however, the study sponsor does not plan further clinical development of exisulind.
ACKNOWLEDGMENTS
We thank our patients and their families for their participation and support. We acknowledge funding from National Institutes of Health grant T32 CA009614 Physician Scientist Training in Cancer Medicine (S.A.), grant P30 CA14520 (K.M.K.) and OSI Pharmaceuticals. COI disclosure: K.M.K serves as consultant to OSI on an unrelated project, not involving exisulind.
FUNDING
We acknowledge funding from grant P30 CA14520 (K.M.K.) and National Institutes of Health grant T32 CA009614 Physician Scientist Training in Cancer Medicine (S.A.) and OSI Pharmaceuticals (Melville, NY).
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Gridelli C, Aapro M, Ardizzoni A, et al. Treatment of advanced non-small-cell lung cancer in the elderly: results of an international expert panel. J Clin Oncol. 2005;23:3125–3137. doi: 10.1200/JCO.2005.00.224. [DOI] [PubMed] [Google Scholar]
- 3.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 4.Gridelli C. The ELVIS trial: a phase III study of single-agent vinorelbine as first-line treatment in elderly patients with advanced non-small cell lung cancer. Elderly Lung Cancer Vinorelbine Italian Study. Oncologist. 2001;6 Suppl 1:4–7. doi: 10.1634/theoncologist.6-suppl_1-4. [DOI] [PubMed] [Google Scholar]
- 5.Piazza GA, Rahm AK, Finn TS, et al. Apoptosis primarily accounts for the growth-inhibitory properties of sulindac metabolites and involves a mechanism that is independent of cyclooxygenase inhibition, cell cycle arrest, and p53 induction. Cancer Res. 1997;57:2452–2459. [PubMed] [Google Scholar]
- 6.Thompson WJ, Piazza GA, Li H, et al. Exisulind induction of apoptosis involves guanosine 3',5'-cyclic monophosphate phosphodiesterase inhibition, protein kinase G activation, and attenuated beta-catenin. Cancer Res. 2000;60:3338–3342. [PubMed] [Google Scholar]
- 7.Soh JW, Mao Y, Kim MG, et al. Cyclic GMP mediates apoptosis induced by sulindac derivatives via activation of c-Jun NH2-terminal kinase 1. Clin Cancer Res. 2000;6:4136–4141. [PubMed] [Google Scholar]
- 8.Chan DC, Earle KA, Zhao TL, et al. Exisulind in combination with docetaxel inhibits growth and metastasis of human lung cancer and prolongs survival in athymic nude rats with orthotopic lung tumors. Clin Cancer Res. 2002;8:904–912. [PubMed] [Google Scholar]
- 9.Soriano AF, Helfrich B, Chan DC, et al. Synergistic effects of new chemopreventive agents and conventional cytotoxic agents against human lung cancer cell lines. Cancer Res. 1999;59:6178–6184. [PubMed] [Google Scholar]
- 10.Whitehead CM, Earle KA, Fetter J, et al. Exisulind-induced apoptosis in a non-small cell lung cancer orthotopic lung tumor model augments docetaxel treatment and contributes to increased survival. Mol Cancer Ther. 2003;2:479–488. [PubMed] [Google Scholar]
- 11.van Stolk R, Stoner G, Hayton WL, et al. Phase I trial of exisulind (sulindac sulfone, FGN-1) as a chemopreventive agent in patients with familial adenomatous polyposis. Clin Cancer Res. 2000;6:78–89. [PubMed] [Google Scholar]
- 12.Hoang T, Kim K, Merchant J, et al. Phase I/II study of gemcitabine and exisulind as second-line therapy in patients with advanced non-small cell lung cancer. J Thorac Oncol. 2006;1:218–225. doi: 10.1016/s1556-0864(15)31571-9. [DOI] [PubMed] [Google Scholar]
- 13.Gridelli C, Perrone F, Gallo C, et al. Vinorelbine is well tolerated and active in the treatment of elderly patients with advanced non-small cell lung cancer. A two-stage phase II study. Eur J Cancer. 1997;33:392–397. doi: 10.1016/s0959-8049(97)89011-9. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan E, Meier P. Nonparametric estimation for incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 15.Jones SF, Kuhn JG, Greco FA, et al. A phase I/II study of exisulind in combination with docetaxel/carboplatin in patients with metastatic non-small-cell lung cancer. Clin Lung Cancer. 2005;6:361–366. doi: 10.3816/clc.2005.n.016. [DOI] [PubMed] [Google Scholar]
- 16.Herbst RS, Prager D, Hermann R, et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:5892–5899. doi: 10.1200/JCO.2005.02.840. [DOI] [PubMed] [Google Scholar]
- 17.Leighl NB, Paz-Ares L, Douillard JY, et al. Randomized phase III study of matrix metalloproteinase inhibitor BMS-275291 in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: National Cancer Institute of Canada-Clinical Trials Group Study BR.18. J Clin Oncol. 2005;23:2831–2839. doi: 10.1200/JCO.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 18.Masters GA, Li S, Dowlati A, et al. A phase II trial of carboplatin and gemcitabine with exisulind (IND #65,056) in patients with advanced non-small cell lung cancer: an Eastern Cooperative Oncology Group study (E1501) J Thorac Oncol. 2006;1:673–678. [PubMed] [Google Scholar]
- 19.Weiss GJ, Vokes EE, Bunn PA, Jr, et al. Docetaxel and exisulind in previously treated non-small cell lung cancer (NSCLC) patients: a multicenter, phase II clinical trial. J Thorac Oncol. 2007;2:933–938. doi: 10.1097/JTO.0b013e3181462051. [DOI] [PubMed] [Google Scholar]
- 20.Duffy CP, Elliott CJ, O'Connor RA, et al. Enhancement of chemotherapeutic drug toxicity to human tumour cells in vitro by a subset of non-steroidal anti-inflammatory drugs (NSAIDs) Eur J Cancer. 1998;34:1250–1259. doi: 10.1016/s0959-8049(98)00045-8. [DOI] [PubMed] [Google Scholar]
- 21.Buccheri G, Ferrigno D. Vinorelbine in elderly patients with inoperable nonsmall cell lung carcinoma: a phase II study. Cancer. 2000;88:2677–2685. doi: 10.1002/1097-0142(20000615)88:12<2677::aid-cncr5>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 22.Balducci L, Al-Halawani H, Charu V, et al. Elderly cancer patients receiving chemotherapy benefit from first-cycle pegfilgrastim. Oncologist. 2007;12:1416–1424. doi: 10.1634/theoncologist.12-12-1416. [DOI] [PubMed] [Google Scholar]
- 23.Smith TJ, Khatcheressian J, Lyman GH, et al. 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006;24:3187–3205. doi: 10.1200/JCO.2006.06.4451. [DOI] [PubMed] [Google Scholar]
- 24.Aapro MS, Cameron DA, Pettengell R, et al. EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphomas and solid tumours. Eur J Cancer. 2006;42:2433–2453. doi: 10.1016/j.ejca.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Repetto L, Biganzoli L, Koehne CH, et al. EORTC Cancer in the Elderly Task Force guidelines for the use of colony-stimulating factors in elderly patients with cancer. Eur J Cancer. 2003;39:2264–2272. doi: 10.1016/s0959-8049(03)00662-2. [DOI] [PubMed] [Google Scholar]
- 26.Ray GF, Lanman RC, Fu CJ, et al. Determination of FGN-1 (an active metabolite of sulindac) in human plasma, urine, and feces by HPLC. J Pharm Biomed Anal. 1995;14:213–220. doi: 10.1016/0731-7085(95)01631-7. [DOI] [PubMed] [Google Scholar]
- 27.Strong HA, Warner NJ, Renwick AG, et al. Sulindac metabolism: the importance of an intact colon. Clin Pharmacol Ther. 1985;38:387–393. doi: 10.1038/clpt.1985.192. [DOI] [PubMed] [Google Scholar]
- 28.Tishelman C, Petersson LM, Degner LF, et al. Symptom prevalence, intensity, and distress in patients with inoperable lung cancer in relation to time of death. J Clin Oncol. 2007;25:5381–5389. doi: 10.1200/JCO.2006.08.7874. [DOI] [PubMed] [Google Scholar]
- 29.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–386. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 30.Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol. 2007;25:1824–1831. doi: 10.1200/JCO.2007.10.6559. [DOI] [PubMed] [Google Scholar]
- 31.Hurria A, Gupta S, Zauderer M, et al. Developing a cancer-specific geriatric assessment: a feasibility study. Cancer. 2005;104:1998–2005. doi: 10.1002/cncr.21422. [DOI] [PubMed] [Google Scholar]
- 32.Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc. 1968;16:622–626. doi: 10.1111/j.1532-5415.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- 33.Miller MDTA. A Manual of Guidelines for Scoring the Cumulative Illness Rating Scale for Geriatrics (CIRS-G) Pittsburg, Pa: University of Pittsburgh; 1991. [Google Scholar]
- 34.Repetto L, Fratino L, Audisio RA, et al. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: an Italian Group for Geriatric Oncology Study. J Clin Oncol. 2002;20:494–502. doi: 10.1200/JCO.2002.20.2.494. [DOI] [PubMed] [Google Scholar]
- 35.Gauvin A, Pinguet F, Culine S, et al. Bayesian estimate of vinorelbine pharmacokinetic parameters in elderly patients with advanced metastatic cancer. Clin Cancer Res. 2000;6:2690–2695. [PubMed] [Google Scholar]
- 36.Hurria A, Lichtman SM. Pharmacokinetics of chemotherapy in the older patient. Cancer Control. 2007;14:32–43. doi: 10.1177/107327480701400105. [DOI] [PubMed] [Google Scholar]
- 37.Sorio R, Robieux I, Galligioni E, et al. Pharmacokinetics and tolerance of vinorelbine in elderly patients with metastatic breast cancer. Eur J Cancer. 1997;33:301–303. doi: 10.1016/s0959-8049(96)00426-1. [DOI] [PubMed] [Google Scholar]
- 38.Sitar DS, Owen JA, MacDougall B, et al. Effects of age and disease on the pharmacokinetics and pharmacodynamics of sulindac. Clin Pharmacol Ther. 1985;38:228–234. doi: 10.1038/clpt.1985.163. [DOI] [PubMed] [Google Scholar]