Abstract
Background
Syphilis and human immunodeficiency virus (HIV) frequently coexist in patients, but the effects of immunosuppression on the course of syphilis are unknown. Our goal was to determine whether the degree of HIV-mediated immunosuppression and the use of highly active antiretroviral therapy impact syphilis serologic responses.
Methods
We assessed all cases of syphilis with positive serologic test results from 1990 through 2006 in a prospective, observational clinical cohort of HIV-infected patients. We defined seroreversion as the loss of reactivity in a patient who previously had a serologic test result positive for syphilis. We defined serologic failure as the lack of a 4-fold decrease in rapid plasma reagin titers 270–365 days after therapy or a 4-fold increase in titers ⩾30 days after therapy. We used Cox proportional hazards models with statistical adjustments for multiple failure instances.
Results
One hundred eighty subjects experienced 231 cases of syphilis. The median follow-up time was 5.3 years. A total of 71 episodes of serologic failure were documented. A CD4 cell count of <200 cells/mL at the time of syphilis diagnosis was associated with an increased risk of serologic failure (adjusted hazard ratio, 2.48; 95% confidence interval, 1.26–4.88). The receipt of highly active antiretroviral therapy was associated with a 60% reduction in the rate of serologic failure (adjusted hazard ratio, 0.40; 95% confidence interval, 0.21–0.75), independent of concomitant CD4 cell response. Rapid plasma reagin seroreversion was infrequent (16.1%) and inconsistent, and it was more likely to occur among patients who received macrolides.
Conclusion
The use of highly active antiretroviral therapy to reverse immunosuppression and the routine use of macrolides for the prevention of opportunistic infections may reduce syphilis serologic failure rates among HIV-infected patients who have syphilis.
Patients who have syphilis are frequently coinfected with HIV [1]. Syphilis affects HIV infection by increasing serum HIV levels and decreasing CD4 cell counts [2, 3], but the treatment of syphilis leads to the reversal of these abnormalities. Although coinfected patients may be more likely to experience serologic failure than are patients with monoinfection [4–7], this may not reflect microbiologic failure [8].
In animal models, humoral and T cell responses are important in mediating immunity to syphilis [9–11]. In humans, the impact of HIV-related immunosuppression on syphilis serologic responses following therapy is unclear. In 2 small studies, the degree of immunosuppression was not associated with serologic response to therapy [6, 12]. Our goal was to determine whether the degree of immunosuppression, as measured by CD4 cell count, HIV RNA level, and use of HAART, affected syphilis serologic responses.
METHODS
Population and data abstraction
This is a retrospective cohort study involving patients enrolled in the Johns Hopkins HIV Clinical Cohort who have been described elsewhere [13]. After informed consent is obtained for patients participating in the cohort, a baseline evaluation of medical and social histories, physical examination, and laboratory studies is recorded and is prospectively updated from the clinic-based medical record every 6 months with any new medical diagnoses, hospitalizations and procedures, filled prescriptions, and laboratory and radiographic results. At each visit, information is recorded regarding any prescribed therapy by treatment name, dosage, and number of refills; this information is also updated when prescriptions are filled over the phone or mailed to patients. Prescriptions obtained from outside providers are actively sought and documented. Use of this database for the analysis of patient outcomes is approved by the Institutional Review Board of the Johns Hopkins University School of Medicine (Baltimore, MD). Data used for this analysis included the results of serologic tests for syphilis, CD4 cell counts, HIV RNA measurements, and data on antiretroviral therapy use (including HAART). HIV RNA levels and CD4 cell counts (except for baseline CD4 cell count) were both analyzed as time-varying covariates. CD4 cell count at the time of initiation of syphilis therapy (baseline) and counts obtained at 6-month intervals during follow-up were used in the models. CD4 cell count was evaluated as a dichotomous variable (CD4 cell count, ≤200 cells/mL or >200 cells/mL). We defined a detectable HIV RNA level as >400 copies/mL. HIV RNA level was evaluated as a dichotomous variable (detectable or undetectable), as RNA quartiles, and as a log-transformed continuous variable. HAART was defined as concomitant use of 3 antiretroviral drugs, either the use of 3 drugs from 2 different classes (e.g., nucleoside reverse-transcriptase inhibitors, non-nucleoside reverse-transcriptase inhibitors, protease inhibitors, or fusion inhibitors) or the use of 3 nucleoside reverse-transcriptase inhibitors for a duration of ⩾6 months. We defined an immunologic response to HAART as a ⩾50% increase in CD4 cell count from baseline after the initiation of HAART. Data concerning antibiotic use other than that used for syphilis therapy were available for azithromycin, clarithromycin, doxycycline, and oral penicillins. Data were not available for intravenous penicillins or any cephalosporin (other than that used for syphilis therapy). We calculated median weekly dosages in milligrams for clarithromycin and azithromycin, because both antibiotics were typically used for extended periods for the prevention and treatment of Mycobacterium avium-intracellulare. We also calculated the total duration of azithromycin and clarithromycin use during follow-up. Use of doxycycline and oral penicillin (used mostly for respiratory infections) was generally limited to a short period of time (duration, <2 weeks) and was analyzed as “any” or “none”.
Syphilis definitions
Patients in the cohort who received a diagnosis of and treatment for syphilis from 1990 through 2006 were eligible. Inclusion required ⩾2 serologic tests with results positive for syphilis (an initial positive result titer at the time of treatment and at least 1 follow-up positive result titer) within 365 days after the treatment date. We only included cases with clear documentation of the patient’s syphilis stage, their pre-treatment titer, and the date and type of syphilis therapy received. We abstracted the results of additional serologic tests for syphilis from the Baltimore City Health Department database, which captures any positive test results generated within the state of Maryland. Syphilis diagnoses were made by clinicians on the basis of the Centers for Disease Control and Prevention criteria [14]. Patients were screened with the nontreponemal rapid plasma reagin (RPR) test; positive results were confirmed using the fluorescence treponemal antibody absorption test. Serologic tests for syphilis were not batched. Cases of primary syphilis with nonreactive serologic characteristics at the time of treatment were excluded, because this study focused on serologic responses. Criteria for the diagnosis of neurosyphilis included positive serologic results and ⩾1 of the following: (1) ⩾1 abnormality on CSF examination (WBC count,> 10 cells/μL; protein level, >50 mg/dL; and/or a positive Venereal Diseases Research Laboratory serologic test result) and/or (2) an otherwise unexplained neurological manifestation consistent with neurosyphilis.
Seroreversion was defined as the reversion of serologic test results for syphilis, either the RPR test or the fluorescence treponemal antibody absorption test, from a positive result to a negative result. Serologic failure was defined as any ⩾4-fold increase in RPR titers ⩾30 days after treatment, the lack of a ⩾4-fold decrease in RPR titers at ⩾270 days after treatment for early syphilis (primary, secondary, or early latent stages) or⩾365 days after treatment for late infection, or clinical manifestations compatible with syphilis. Because we lacked any behavioral exposure data that would have allowed for the separation of cases of reinfection from cases of serologic failure, we attempted to discern between treatment failures and reinfection by applying objective criteria of patterns observed over time. Some experts suggest that patients whose RPR titers do not decrease in response to therapy more likely have experienced treatment failure, whereas patients whose titers decrease then subsequently increase ⩾4-fold are more likely to have been reinfected [15]. Other experts suggest that late failures (occurring >365 days after treatment for early syphilis and >730 days after treatment for late syphilis) are more suggestive of reinfection [16]. Therefore, we performed analyses using both type of failure and time of failure to determine whether significant differences existed. Because late latent disease may present with low pretreatment titers, individuals with baseline titers ≤1:2 who did not experience seroreversion (and showed no clinical evidence of serologic failure) were not considered to have experienced serologic failure [7].
Data analysis
We used time-to-event statistical models. Entry time for all analyses was the date of syphilis therapy. Date of failure was defined as the first date when serologic failure was documented. Patients were censored at their final clinic visit (if before 2006) or at the end of follow-up in 2006. The serologic failure analysis consisted of multiple failure-instance data, because 1 person could contribute >1 case of syphilis. We used the Andersen-Gill method to adjust for repeated measures [17]. The proportional hazard assumption with robust variance estimation was tested using scaled Schoenfeld residuals against the log of time. Identical multivariable models were constructed for the analyses, which restricted the overall model to type of failure and time of failure. We applied similar techniques to the RPR seroreversion analysis, in which “failure” date was the first date on which a negative RPR test result was documented. We included variables in the final multivariable models if they were biologically relevant or if their univariable Wald’s test P value was<.2. Cox-Snell residuals were used to assess overall model fit. P values<.05 were considered to be statistically significant. We used STATA/SE for Windows, version 10.0 (Stata).
RESULTS
From 1990 through 2006, 180 patients experienced 251 cases of syphilis. Of these 251 cases, 231 had follow-up data available, including syphilis stage, treatment given, immunologic test results, and medication use unrelated to syphilis. Of the 20 excluded cases, 15 were missing baseline RPR titer information, and 12 were missing information regarding syphilis stage and treatment. The median per-patient follow-up time was 5.3 years (range, 270 days to 13.9 years), and the median number of follow-up RPR tests performed per syphilis case was 5.0 (range, 1–13 tests). Table 1 summarizes patient demographic characteristics, and table 2 summarizes clinical characteristics for each case of syphilis.
Table 1.
Demographic characteristics of 180 syphilis patients and of the overall clinical cohort.
| Characteristic | Syphilis patients (n = 180) | Overall clinical cohort (n = 4850) |
|---|---|---|
| Male sex | 121 (67.2) | 3226 (66.5) |
| Median age, years (range) | 37.9 (22–68) | 39 (19–88) |
| Race | ||
| Black | 157 (87.2) | 3699 (76.3) |
| White | 19 (10.6) | 1042 (21.5) |
| Other | 4 (2.2) | 109 (2.2) |
| HIV infection risk factor category | ||
| Heterosexual person | 81 (45.0) | 1425 (29.4) |
| Man who has sex with men | 55 (30.6) | 1062 (21.9) |
| Injection drug user | 69 (38.3) | 2033 (41.9) |
NOTE. Data are no. (%) of patients, unless otherwise indicated.
Table 2.
Clinical characteristics of 231 cases of syphilis.
| Characteristic | Syphilis cases (n = 231) |
|---|---|
| Previous history of syphilis | 101 (43.7) |
| Syphilis stage | |
| Earlya | 62 (26.8) |
| Late latent or unknown duration | 128 (55.4) |
| Neurosyphilis | 41 (17.8) |
| Median RPR titerb | |
| <1:16 | 28 (12.1) |
| 1:16 to 1:128 | 101 (43.7) |
| >1:128 | 102 (44.2) |
| Syphilis treatment | |
| Penicillin | 218 (94.4) |
| Ceftriaxone | 6 (2.6) |
| Doxycycline | 7 (3.0) |
| CD4 cell countb | |
| Median CD4 cell count, cells/mL (IQR) | 280 (118–454) |
| >200 cells/mL | 144 (62.3) |
| 50–200 cells/mL | 49 (21.2) |
| <50 cells/mL | 27 (11.7) |
| Unknown | 11 (4.8) |
| Median HIV RNA level, copies/mL (IQR)b | 8742 (322–79,860) |
| Antiretroviral therapy duration >6 monthsc | |
| Any antiretroviral therapy | 169 (73.2) |
| HAART | 142 (61.5) |
| Macrolide therapyc | |
| Any macrolide therapy | 80 (37.2) |
| Treatment duration >3 months | 52 (22.5) |
| Penicillin or doxycycline therapyc | 27 (11.7) |
NOTE. Data are no. (%) of cases, unless otherwise indicated. IQR, interquartile range; RPR, rapid plasma reagin.
Includes primary syphilis, secondary syphilis, and early latent syphilis.
At the time of syphilis diagnosis.
Any time during follow-up.
Serologic failure
Among the 180 patients, 71 (39.4%) experienced serologic failure during follow-up. One hundred twenty-four patients experienced no failures after their initial syphilis episode, 43 experienced 1 failure each, 11 experienced 2 failures each, and 2 patients experienced 3 failures each. Of the 71 instances of failure, 44 (62%) were attributable to a 4-fold increase in RPR titers, and 26 (37%) were attributable to the lack of a 4-fold decrease in RPR titers. One patient (1%) manifested clinical symptoms and CSF test results consistent with neurosyphilis but did not meet the definition of serologic failure.
The multivariable predictors of serologic failure (table 3) included a low baseline CD4 cell count (≤200 cells/mL). The receipt of HAART for >6 months during follow-up was associated with a 60% reduction in the risk of serologic failure overall; an 82% reduction in the risk of experiencing serologic failure occurred among patients who had a >50% increase in CD4 cell count from baseline value, compared with a 52% reduction among those who did receive HAART but did not experience an immunologic response (P> .05). HIV RNA level was not associated with serologic failure.
Table 3.
Multivariable predictors of serologic failure overall and by type of failure (4-fold increase in titers vs. lack of 4-fold decrease and early failure vs. late failure) among 180 HIV-infected patients.
| Serologic failure, hazard ratio (95% CI)
|
|||||
|---|---|---|---|---|---|
| Variable | Overall (n = 71) | ⩾4-fold titer increase (n = 44) | Lack of 4-fold decrease (n = 26) | Early failurea (n = 39) | Late failureb (n = 32) |
| Age, years | |||||
| ≤38 | Ref | Ref | Ref | Ref | Ref |
| >38 | 0.57 (0.34–0.97) | 0.67 (0.35–1.27) | 0.41 (0.17–0.98) | 0.45 (0.22–0.95) | 0.53 (0.25–1.12) |
| Baseline CD4 cell count, cells/mL | |||||
| >200 | Ref | Ref | Ref | Ref | Ref |
| ≤200 | 2.48 (1.26–4.88) | 2.26 (1.04–4.95) | 3.64 (1.33–9.94) | 3.14 (1.34–7.34) | 2.93 (1.10–7.84) |
| HIV RNA | |||||
| Undetectablec | Ref | Ref | Ref | Ref | Ref |
| Detectable | 1.01 (0.48–2.11) | 0.97 (0.11–2.29) | 1.86 (0.45–7.65) | 0.87 (0.31–2.50) | 1.29 (0.54–3.10) |
| HAART duration, months | |||||
| ≤6 | Ref | Ref | Ref | Ref | Ref |
| >6 | 0.40 (0.21–0.75) | 0.41 (0.19–0.89) | 0.32 (0.09–1.12) | 0.27 (0.11–0.67) | 0.36 (0.16–0.78) |
| HAART | |||||
| Duration ≤6 months | Ref | Ref | Ref | Ref | Ref |
| Immune responsed | 0.18 (0.05–0.62) | 0.06 (0.01–0.48) | 0.52 (0.10–2.83) | 0.18 (0.03–0.89) | 0.12 (0.03– 0.51) |
| No immune response | 0.48 (0.23–0.99) | 0.41 (0.16–1.00) | 0.45 (0.11–1.72) | 0.30 (0.10–0.89) | 0.49 (0.19–1.25) |
| Macrolidee therapy duration, months | |||||
| <3 | Ref | Ref | Ref | Ref | Ref |
| ⩾3 | 0.59 (0.43–0.82) | 0.62 (0.40–0.93) | 0.44 (0.26–0.75) | 0.36 (0.22–0.58) | 0.95 (0.92–0.99) |
| Any penicillin or doxycycline therapy | 0.56 (0.23–1.36) | 0.29 (0.07–1.24) | 1.68 (0.48–5.88) | 0.58 (0.18–1.85) | 0.43 (0.09–2.16) |
| RPR reversion | 0.52 (0.20–1.18) | 0.94 (0.35–2.56) | NAf | 0.21 (0.03–1.67) | 0.86 (0.24–3.10) |
NOTE. The multivariable model adjusted for sex, race, previous history of syphilis, baseline RPR titer, and type of syphilis therapy received. The Andersen-Gill method was used to adjust for the multiple failure-instance data (180 subjects had 231 cases of syphilis and 71 instances of serologic failure overall). NA, not applicable; Ref, reference; RPR, rapid plasma reagin test.
Early failure is defined as serologic failure occurring ≤365 days after therapy for early stage syphilis and ≤730 days after therapy for the late stages.
Late failure is defined as serologic failure occurring >365 days after therapy for early stage syphilis and >730 days after therapy for the late stages.
Defined as <400 copies/mL.
Defined as >50% increase in CD4 cell count from baseline measured as a continuous change during follow-up.
Ninety-seven percent of macrolide therapy involved the administration of azithromycin (mean dosage, 1100 mg/week); 3% involved clarithromycin (mean dosage, 7000 mg/week).
NA denotes a lack of 4-fold decrease, which, by definition, precludes seroreversion.
There was a 2% decrease (95% CI, 1%–5%) in the incidence of serologic failure for every week of macrolide use (97% of macrolide use involved azithromycin) during follow-up. The median weekly dose of azithromycin was 1100 mg (most patients had received azithromycin as opportunistic infection pro-phylaxis at a dosage of 1200 mg/week; the remainder had received azithromycin for the treatment of an opportunistic infection or for the treatment of a respiratory tract infection at dosages of 850–1500 mg/week). Three percent of macrolide use consisted of clarithromycin at a median dosage of 7000 mg/week. The mean total number of weeks of macrolide use during follow-up intervals when the CD4 cell count was <50, 50–200, 201–500, and >500 cells/mL were 49.5, 27.1, 10.6, and 4.4 weeks, respectively. In a separate multivariable model that only included azithromycin and excluded the small number of patients treated with clarithromycin, the hazard ratio (HR) was similar to that found with a model that included both macrolides (HR, 0.98; 95% CI, 0.95–1.00; P = .035).
In univariate analyses looking at the total duration of macrolide use, both a >1-month (vs. ≤1-month) and >3-month (vs. ≤3-month) total duration of macrolide use was associated with a decreased risk for serologic failure. In a multivariate model, >1 month of macrolide use was only marginally statistically significantly associated with a decreased risk of serologic failure (HR, 0.76; 95% CI, 0.54–1.07, P = .07), but a duration of macrolide use >3 months retained statistical significance. Finally, macrolide use was stratified by CD4 cell counts. Patients with CD4 cell counts ≤200 cells/mL who used macrolides for>3 months during follow-up had a significant reduction in the rate of serologic failure (HR, 0.53; 95% CI, 0.36–0.79), whereas the reduction in the rate of serologic failure among those whose CD4 cell counts were >200 cells/mL and who received >3 months of macrolide therapy (HR, 0.73; 95% CI, 0.39–1.38) did not reach statistical significance. Among the 12% of patients who received additional doses of doxycycline and/or oral penicillin during follow-up, there was a non–statistically significant 44% reduction in the rate of serologic failure. Finally, RPR seroreversion was associated with a marginally decreased risk of serologic failure (P = .1); this was mostly driven by patients who experienced early serologic failures.
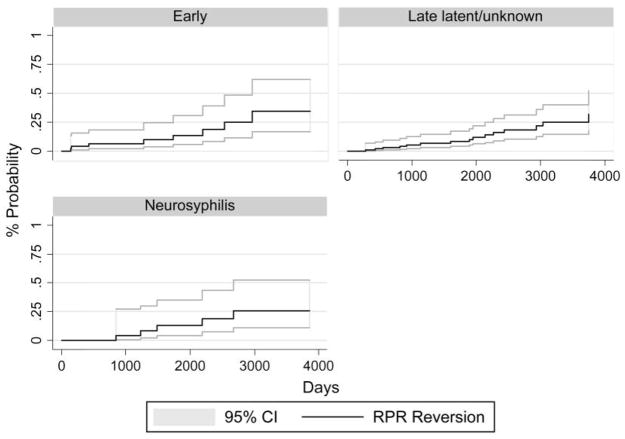
Seroreversion
Of the 180 patients, 29 (16.1%) had evidence of RPR seroreversion (figure 1). The median time to seroreversion was 4.8 years. The earliest seroreversion occurred at 136 days after therapy. In a multivariable Cox proportional hazards model (table 4), younger age, lower pretreatment RPR titer, early syphilis stage, and macrolide use for >3 months were statistically significantly associated with an increased incidence of seroreversion. HIV RNA level, HAART use >6 months, or penicillin or doxycycline use during follow-up were not statistically significantly associated with seroreversion.
Figure 1.
Probability of first rapid plasma reagin (RPR) seroreversion, by syphilis stage, among 180 patients. Follow-up time begins on the date of syphilis therapy and ends at the first documented time of RPR reversion; lines representing 95% CIs are provided.
Table 4.
Multivariable predictors of rapid plasma reagin (RPR) seroreversion in the HIV cohort.
| Variable | Hazard ratio (95% CI) (n = 180) |
|---|---|
| Age, years | |
| ≤38 | Ref |
| >38 | 0.40 (0.19–0.83) |
| Initial RPR titer | |
| ≤ 1:8 | Ref |
| 1:16 to 1:128 | 0.14 (0.05–0.37) |
| >1:128 | 0.08 (0.02–0.26) |
| Syphilis stage | |
| Late latent | Ref |
| Earlya | 4.33 (1.39–13.47) |
| Neurosyphilis | 3.40 (0.77–14.99) |
| Baseline CD4 cell count, cells/mL | |
| >200 | Ref |
| ≤200 | 1.03 (0.37–2.90) |
| HIV RNA | |
| Undetectableb | Ref |
| Detectable | 1.03 (0.50–2.15) |
| Macrolide therapy duration >3 monthsc | 1.79 (1.10–2.90) |
| HAART duration >6 months | 0.56 (0.22–1.38) |
| Any penicillin or doxycycline therapy | 0.84 (0.16–4.53) |
NOTE. The multivariable model adjusted for sex, race, previous history of syphilis, type of syphilis therapy received. Ref, reference.
Early syphilis includes primary, secondary, and early latent stages of syphilis.
Defined as <400 copies/mL.
Ninety-seven percent of macrolide therapy involved the administration of azithromycin (mean dosage, 1100 mg/week); 3% involved clarithromycin (mean dosage, 7000 mg/week).
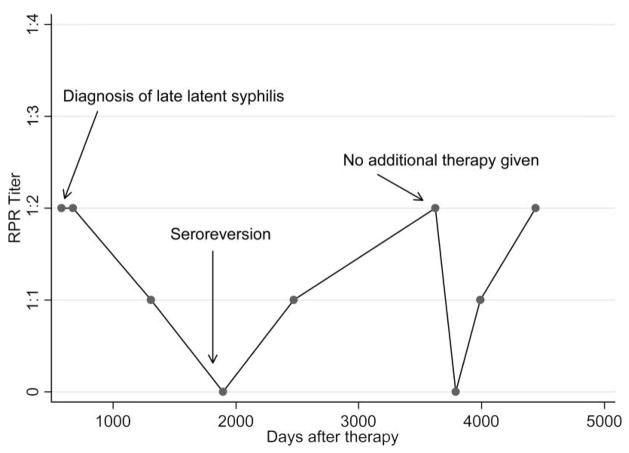
Among 26 of 29 subjects with postseroreversion serologic test results, 23 (88.5%) of 26 had subsequent positive RPR serologic test results after documented seroreversion with fluctuating titers ranging from 1:1 to 1:2. Figure 2 depicts RPR titer results for 1 such patient. None of these 23 patients experienced clinical failure, and none were treated again. Three (11.5%) of the 26 patients had subsequent >4-fold increases in RPR titers with no clinical symptoms and were treated again; none experienced another seroreversion.
Figure 2.
Example of a patient who experienced posttreatment rapid plasma reagin (RPR) seroreversion with evidence of fluctuating titers; therapy was given at the time of diagnosis. Twenty-three (88.5%) of 26 patients who had evidence of RPR seroreversion exhibited a similar course.
Only 1 patient (0.6%) had evidence of serologic reversion of their treponemal-specific fluorescence treponemal antibody absorption test; the test was repeated on 3 separate occasions in a 2-year period, and the fluorescence treponemal antibody absorption test result was found to be persistently negative. That patient did not experience RPR seroreversion.
DISCUSSION
Our data suggest that the treatment of HIV infection with HAART and the use of macrolides improve syphilis serologic responses in coinfected patients. These findings are notable, because 16% of all patients who have syphilis are also HIV infected [1], and coinfected patients may be at an increased risk for serologic failure [4–7].
The impact of HAART on the response of syphilis patients was consistently observed independent of the type or timing of serologic failure (table 3). Whether this is simply the result of better immunologic responses among those who received HAART is difficult to ascertain, because of the significant serologic benefits observed even in those who did not manifest an overt immunologic response to HAART. However, even when patients who receive HAART do not experience CD4 cell reconstitution, there are data to suggest that there are immunologic benefits [18]. An alternate behavioral hypothesis for the effect of HAART is that individuals who receive HAART define a more adherent and compliant behavior group that may be less likely to be reexposed to risky partners and may be more likely to practice safe sex. A meta-analysis by Crepaz et al. [19] suggested that the use of HAART in HIV-infected patients did not result in increased sexual risk-taking behaviors, even in cases in which the patient achieved an undetectable viral load.
Macrolides have well-documented treponemicidal activity [20, 21], but the recent development of resistant Treponema pallidum strains has limited their widespread use [22]. In our cohort, most macrolide use was for opportunistic infection prophylaxis. Thus, the observed serologic benefits of macrolides may have been attributable to the enhancement of the incomplete treponemicidal activity of penicillin, or possibly it was attributable to postexposure prophylaxis, because the effects of macrolide use appeared to extend across all groups (table 3). Previous approaches to treatment of HIV-infected patients who had syphilis have focused on evaluating the efficacy of more-intensive short-term treatment. A multicenter, randomized, controlled trial showed that enhanced therapy for early syphilis did not lead to improved serologic or clinical outcomes at 1 year [6]. Enhanced therapy in that study consisted of a 10-day course of oral amoxicillin with probenecid. Whether macrolide exposure might exert serologic benefit through enhanced CNS penetration, compared with oral β-lactams [23], or by extending the duration of therapy is unclear. The effects of macrolides in our study were observed in individuals with more-advanced immunosuppression. One explanation is that those patients with preserved cellular immunity are better able to control syphilis infection and are, thus, less dependent on enhanced macrolide therapy; alternatively, the number of patients with preserved immune responses who did receive macrolides was relatively small, thus limiting our ability to detect a beneficial effect in that group.
The clinical significance of RPR seroreversion in the penicillin era is unclear. In the prepenicillin era, patients who had early syphilis who did not manifest nontreponemal-specific test reversions at the end of therapy were at an increased risk for experiencing treatment failure [24]. In the pre-HIV penicillin era, documented treatment failures were exceedingly rare [25], but RPR seroreversions were relatively common after penicillin therapy. Studies in the pre-HIV penicillin era documented close to a 100% rate of RPR seroreversion 1–2 years (depending on the stage of syphilis and the duration of symptoms) after penicillin therapy (4.8 MU of benzathine penicillin G) among patients with primary and secondary syphilis [26, 27]. During the early years of HIV, Romanowski et al. [28] demonstrated that up to 72% of patients with an initial episode of primary syphilis and 56% of patients with secondary syphilis experienced RPR seroreversions at 36 months after therapy (usually, 2.4 MU of benzathine penicillin G). The influence of HIV on the rate of seroreversion could not be assessed. In our study, a smaller fraction of patients experienced RPR seroreversion (figure 1) despite a relatively long follow-up period. This raises several interesting questions. Is the decreasing incidence of seroreversion in the penicillin era the result of lower dosages of penicillin or of immunosuppression due to HIV infection? Does the lack of seroreversion (especially among patients who have early syphilis) increase the subsequent risk of treatment failure? In our study, only 11.5% of patients who experienced seroreversion experienced subsequent serologic failure. Overall, there was a non–statistically significant 52% reduction in the risk of serologic failure among patients who experienced RPR seroreversion, compared with those who did not. Subgroup analysis suggests that this was mostly driven by patients who experienced early serologic failure, suggesting that seroreversion may impact treatment failure but not reinfection. The corollary is whether enhanced syphilis therapy with the goal of RPR seroreversion (rather than a 4-fold decrease in RPR titers) in HIV-infected patients would result in improved serologic outcomes.
This study has several limitations. First, our endpoint of serologic failure included patients who experienced either treatment failure or reinfection. In any syphilis study that lacks behavioral or network data, the distinction between these 2 events is very difficult to make. We did not see an impact of HIV transmission risk group on the rate of serologic failure, but this behavioral information may be too crude to detect meaningful differences. We further analyzed the data using both the type of serologic failure and the timing of failure, because both of these approaches have been used to differentiate between treatment failure and reinfection. We did observe consistent findings of improved serologic outcomes with the use of HAART and macrolides across these groups, which suggests that these findings are likely to be clinically meaningful.
Second, serologic response after syphilis therapy in HIV-infected patients may be slower than serologic response in non–HIV-infected patients [7]. The Centers for Disease Control suggests a relatively wide window of time for determining serologic nonresponse: 6–12 months after treatment of early syphilis and 12–24 months after treatment of late latent infection [29]. In our observational study, the median time to retreatment for early syphilis was 483 days, and it was 528 days for late latent infection. There may have been fewer patients classified as nonresponders if clinicians had waited the full 730 days before treating the late latent nonresponders again.
Third, we did not have data concerning penicillin and cephalosporin use during follow-up other than the use of oral penicillin, amoxicillin, and amoxicillin plus clavulanic acid. This may have biased our combined penicillin and doxycycline data towards a null finding. Furthermore, some of the data on medication use was obtained from patient self-reports. Serologic testing was not performed in batches; thus, variation in laboratory test performance may have led to misclassifications of serologic failure. However, we expect this misclassification to be nondifferential across groups. Changes in RPR titers may be a manifestation of abnormal B cell dysregulation associated with HIV infection rather than with serologic failure [30]. Our population represented a heterogeneous group with respect to HIV risk factors. Whether our findings would be generalizable to other populations is unknown. Finally, the number of RPR serologic tests was consistent among all patients, thus the potential for outcome identification bias (i.e., less frequent serologic testing among individuals with advanced immunosuppression may lead to fewer opportunities to detect serologic failure) was limited.
The interaction between syphilis and HIV infection is complex. In the future, a better understanding of the relationship between syphilis serologies and host immunity may help determine whether current treatment targets should be modified. Currently, the only difference in syphilis treatment recommendations between HIV-infected and non–HIV-infected patients is a more aggressive follow-up schedule for serologic testing in HIV-infected patients [29]. Unfortunately, this is not always easy to achieve in clinical practice, because follow-up is often lacking [7]. On the basis of our data, coupling the recommendation of more-frequent serologic follow-up of coinfected patients to aggressive HIV infection management—including the use of HAART and the use of macrolides for opportunistic infection prophylaxis, particularly among patients who are more severely immunosuppressed—may improve syphilis serologic responses.
Acknowledgments
This article is part of a Doctor of Philosophy thesis manuscript, Syphilis as an Opportunistic Infection in HIV (K.G.G.), at the Johns Hopkins Bloom-berg School of Public Health.
Financial support. National Institutes of Health (K23HD047395 to K.G.G.); the National Institute on Aging (R01-AG026250); and the National Institute on Drug Abuse (K23-DA00523, K24-DA00432, and R01-DA-11602 to K.A.G. and R.D.M.).
Footnotes
Presented in part: International Society for Sexually Transmitted Diseases Research Conference, Seattle, Washington, July 2007 (abstracts O-45 and P-269);
Potential conflicts of interest. All authors: no conflicts.
References
- 1.Blocker ME, Levine WC, St Louis ME. HIV prevalence in patients with syphilis, United States Sex. Transm Dis. 2000;27:53–9. doi: 10.1097/00007435-200001000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Buchacz K, Patel P, Taylor M, et al. Syphilis increases HIV viral load and decreases CD4 cell counts in HIV-infected patients with new syphilis infections. AIDS. 2004;18:2075–9. doi: 10.1097/00002030-200410210-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kofoed K, Gerstoft J, Mathiesen LR, Benfield T. Syphilis and human immunodeficiency virus (HIV)–1 coinfection: influence on CD4 T-cell count, HIV-1 viral load, and treatment response. Sex Transm Dis. 2006;33:143–8. doi: 10.1097/01.olq.0000187262.56820.c0. [DOI] [PubMed] [Google Scholar]
- 4.Malone JL, Wallace MR, Hendrick BB, et al. Syphilis and neurosyphilis in a human immunodeficiency virus type-1 seropositive population: evidence for frequent serologic relapse after therapy. Am J Med. 1995;99:55–63. doi: 10.1016/s0002-9343(99)80105-3. [DOI] [PubMed] [Google Scholar]
- 5.Yinnon AM, Coury-Doniger P, Polito R, Reichman RC. Serologic response to treatment of syphilis in patients with HIV infection. Arch Intern Med. 1996;156:321–5. [PubMed] [Google Scholar]
- 6.Rolfs RT, Joesoef MR, Hendershot EF, et al. A randomized trial of enhanced therapy for early syphilis in patients with and without human immunodeficiency virus infection. The Syphilis and HIV Study Group. N Engl J Med. 1997;337:307–14. doi: 10.1056/NEJM199707313370504. [DOI] [PubMed] [Google Scholar]
- 7.Ghanem KG, Erbelding EJ, Wiener ZS, Rompalo AM. Serological response to syphilis treatment in HIV-positive and HIV-negative patients attending sexually transmitted diseases clinics. Sex Transm Infect. 2007;83:97–101. doi: 10.1136/sti.2006.021402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yobs AR, Rockwell DH, Clark JW., Jr Treponemal survival in humans after penicillin therapy: a preliminary report. Br J Vener Dis. 1964;40:248–53. doi: 10.1136/sti.40.4.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arroll TW, Centurion-Lara A, Lukehart SA, Van Voorhis WC. T-cell responses to Treponema pallidum subsp. pallidum antigens during the course of experimental syphilis infection. Infect Immun. 1999;67:4757–63. doi: 10.1128/iai.67.9.4757-4763.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morgan CA, Lukehart SA, van Voorhis WC. Protection against syphilis correlates with specificity of antibodies to the variable regions of Treponema pallidum repeat protein K. Infect Immun. 2003;71:5605–12. doi: 10.1128/IAI.71.10.5605-5612.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leader BT, Godornes C, VanVoorhis WC, Lukehart SA. CD4+ lymphocytes and gamma interferon predominate in local immune responses in early experimental syphilis. Infect Immun. 2007;75:3021–6. doi: 10.1128/IAI.01973-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gourevitch MN, Selwyn PA, Davenny K, et al. Effects of HIV infection on the serologic manifestations and response to treatment of syphilis in intravenous drug users. Ann Intern Med. 1993;118:350–5. doi: 10.7326/0003-4819-118-5-199303010-00005. [DOI] [PubMed] [Google Scholar]
- 13.Moore RD. Understanding the clinical and economic outcomes of HIV therapy: the Johns Hopkins HIV clinical practice cohort. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17(Suppl 1):S38–41. doi: 10.1097/00042560-199801001-00011. [DOI] [PubMed] [Google Scholar]
- 14.Wharton M, Chorba TL, Vogt RL, Morse DL, Buehler JW. Case definitions for public health surveillance. MMWR Recomm Rep. 1990;39(RR13):1–43. [PubMed] [Google Scholar]
- 15.Zetola NM, Engelman J, Jensen TP, Klausner JD. Syphilis in the United States: an update for clinicians with an emphasis on HIV coinfection. Mayo Clin Proc. 2007;82:1091–102. doi: 10.4065/82.9.1091. [DOI] [PubMed] [Google Scholar]
- 16.Idsoe O, Guthe T, Willcox RR. Penicillin in the treatment of syphilis: the experience of three decades. Bull World Health Organ. 1972;47:1–68. [PMC free article] [PubMed] [Google Scholar]
- 17.Andersen PK, Gill RD. Cox’s regression model for counting processes: a large sample study. Ann Stat. 1982;10:1100–20. [Google Scholar]
- 18.Nicastri E, Chiesi A, Angeletti C, et al. Clinical outcome after 4 years follow-up of HIV-seropositive subjects with incomplete virologic or immunologic response to HAART. J Med Virol. 2005;76:153–60. doi: 10.1002/jmv.20352. [DOI] [PubMed] [Google Scholar]
- 19.Crepaz N, Marks G. Towards an understanding of sexual risk behavior in people living with HIV: a review of social, psychological, and medical findings. AIDS. 2002;16:135–49. doi: 10.1097/00002030-200201250-00002. [DOI] [PubMed] [Google Scholar]
- 20.Hook EW, 3rd, Stephens J, Ennis DM. Azithromycin compared with penicillin G benzathine for treatment of incubating syphilis. Ann Intern Med. 1999;131:434–7. doi: 10.7326/0003-4819-131-6-199909210-00007. [DOI] [PubMed] [Google Scholar]
- 21.Riedner G, Rusizoka M, Todd J, et al. Single-dose azithromycin versus penicillin G benzathine for the treatment of early syphilis. N Engl J Med. 2005;353:1236–44. doi: 10.1056/NEJMoa044284. [DOI] [PubMed] [Google Scholar]
- 22.Lukehart SA, Godornes C, Molini BJ, et al. Macrolide resistance in Treponema pallidum in the United States and Ireland. N Engl J Med. 2004;351:154–8. doi: 10.1056/NEJMoa040216. [DOI] [PubMed] [Google Scholar]
- 23.Dunlop EM, Al-Egaily SS, Houang ET. Penicillin levels in blood and CSF achieved by treatment of syphilis. JAMA. 1979;241:2538–40. [PubMed] [Google Scholar]
- 24.Moore JE, Padget P. The problem of seroresistant syphilis. JAMA. 1938;110:96–100. [Google Scholar]
- 25.Perdrup A, Jorgensen BB, Pedersen NS. The profile of neurosyphilis in Denmark: a clinical and serological study of all patients in Denmark with neurosyphilis disclosed in the years 1971–1979 incl. by Wassermann reaction (CWRM) in the cerebrospinal fluid. Acta Derm Venereol Suppl (Stockh) 1981;96:1–14. [PubMed] [Google Scholar]
- 26.Fiumara NJ. Treatment of seropositive primary syphilis: an evaluation of 196 patients. Sex Transm Dis. 1977;4:92–5. doi: 10.1097/00007435-197707000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Fiumara NJ. Treatment of secondary syphilis: an evaluation of 204 patients. Sex Transm Dis. 1977;4:96–9. doi: 10.1097/00007435-197707000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Romanowski B, Sutherland R, Fick GH, Mooney D, Love EJ. Serologic response to treatment of infectious syphilis. Ann Intern Med. 1991;114:1005–9. doi: 10.7326/0003-4819-114-12-1005. [DOI] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006;55(RR11):1–94. [PubMed] [Google Scholar]
- 30.De Milito A. B lymphocyte dysfunctions in HIV infection. Curr HIV Res. 2004;2:11–21. doi: 10.2174/1570162043485068. [DOI] [PubMed] [Google Scholar]