Abstract
Hippocampal epileptogenesis is hypothesized to involve secondary mechanisms triggered by initial brain injury. Chemoconvulsant-induced status epilepticus has been used to identify secondary epileptogenic mechanisms under the assumption that a seizure-free, pre-epileptic “latent period” exists that is long enough to accommodate delayed mechanisms. The latent period is difficult to assess experimentally because early spontaneous seizures may be caused or influenced by residual chemoconvulsant that masks the true duration of the epileptogenic process. To avoid the use of chemoconvulsants and determine the latency to hippocampal epileptogenesis and clinical epilepsy, we developed an electrical stimulation-based method to evoke hippocampal discharges in awake rats, and produce hippocampal injury and hippocampal-onset epilepsy reliably. Continuous video-monitoring and granule cell layer recording determined whether hippocampal epileptogenesis develops immediately or long after injury. Bilateral perforant pathway stimulation for 3 hr evoked granule cell epileptiform discharges and convulsive status epilepticus with minimal lethality. Spontaneous Stage 3−5 behavioral seizures reliably developed within 3 days post-stimulation, and all 72 spontaneous behavioral seizures recorded in 10 animals were preceded by spontaneous granule cell epileptiform discharges. Histological analysis confirmed a reproducible pattern of limited hippocampal and extra-hippocampal injury, including an extensive bilateral loss of hilar neurons throughout the hippocampal longitudinal axis. These results indicate that hippocampal epileptogenesis after convulsive status epilepticus is an immediate network defect coincident with neuron loss or other early changes. We hypothesize that the latent period is directly related and inversely proportional to the extent of neuron loss in brain regions involved in seizure initiation, spread, and clinical expression.
Keywords: epilepsy, hippocampus, dentate gyrus
INTRODUCTION
Brain injuries are suspected causes of acquired temporal lobe epilepsy (TLE; French et al., 1993; Engel, 1996; Chang and Lowenstein, 2003). The seizure-free “latent” period that often follows brain injury is of unknown mechanistic significance, but is commonly regarded as the duration of “epileptogenesis.” The temporal mismatch between a presumed initial injury and the onset of clinically detectable epilepsy was attributed early-on to the concept of the “ripening of the scar” (Earle et al., 1953), which refers to a presumably gradual epileptogenic process. The idea that epileptogenesis is a gradual secondary process, rather than an immediate network imbalance caused by neuron loss or other early effects, has had significant conceptual impact historically and experimentally despite the fact that epilepsy can begin without delay after brain injury, or in the absence of any known antecedent injury (French et al., 1993; Wieser, 2004; Mikaeloff et al., 2006).
Convulsive status epilepticus (SE)-induced brain damage in rats is the most commonly used method to investigate epileptogenic mechanisms experimentally, primarily because prolonged SE induced by chemoconvulsants (Pisa et al., 1980; Cavalheiro et al., 1982; Turski et al., 1987; Mello et al., 1993), or electrical stimulation (Lothman et al., 1990; Bertram and Cornett, 1993; Nissinen et al., 2000; Mazarati et al., 2002) produces a permanent epileptic state. Initial chance observations of spontaneous seizures long after SE was induced (Pisa et al., 1980), and the results of non-continuous behavioral monitoring after convulsive SE (Cavalheiro et al., 1982; Lothman et al., 1990; Priel et al., 1996; Arida et al., 1999; Nissinen et al. 2000; Glien et al., 2001; Brandt et al., 2003), established and reinforced the notion that a prolonged seizure-free latent period reliably follows convulsive SE in rats (Leite et al., 2002; Stables et al., 2003). The idea that rats are reliably “pre-epileptic” for several weeks post-SE is crucial for hypotheses that invoke delayed secondary processes as likely epileptogenic mechanisms (Tauck and Nadler, 1985; Parent et al., 1997; Chang and Lowenstein, 2003), and is also important because a “therapeutic window” must be open long enough after injury to permit practical anti-epileptogenic intervention (Stables et al., 2003; Stafstrom and Sutula, 2005).
Two recent studies that used continuous video-monitoring of pilocarpine-treated rats after prolonged behavioral SE reported that spontaneous behavioral seizures began within days after convulsive SE (Raol et al., 2006; Goffin et al., 2007). A third recent study in pilocarpine-treated rats, which involved video monitoring only on days 3 and 6 post-SE, also reported that pilocarpine-treated rats were spontaneously epileptic during the first week after convulsive SE that lasted only one hour (Jung et al., 2007). Thus, even a relatively brief duration of behavioral SE was associated with a minimal latency to clinical epilepsy. However, the presence of circulating chemoconvulsant in these studies confounds interpretation of the results because early seizures could be caused or influenced by residual chemoconvulsant in the days following chemoconvulsant injection. Thus, it remains unclear whether a “pre-epileptic” latent period long enough to accommodate relatively slowly developing secondary mechanisms exists following convulsive SE-induced injury.
A second assumption about rodents subjected to prolonged convulsive SE is that the spontaneous behavioral seizures that develop in these animals reflect completion of a process of hippocampal epileptogenesis, despite a lack of evidence in vivo that the hippocampus in general, or the dentate granule cells, in particular, are the source of the spontaneous seizures that define post-SE animals as “epileptic.” To the contrary, we observed during more than 200 spontaneous seizures in pilocarpine-treated rats that the hippocampus did not appear to be a source of the seizures (Harvey and Sloviter, 2005), suggesting that the predominantly extra-hippocampal pathology caused by convulsive SE (Schwob et al., 1980; Turski et al., 1983; Chen and Buckmaster, 2005; Harvey and Sloviter, 2005; Niessen et al., 2005) may not reliably involve hippocampal epileptogenesis. Given these issues inherent in the use of chemoconvulsant-induced SE as an epileptogenic insult, we developed an electrical stimulation model in awake rats that was designed to: 1) activate and injure the hippocampus reliably and reproducibly by forcing it to discharge throughout the duration of SE; 2) avoid the use of chemoconvulsants, the residual presence of which might mask the true duration of the latent period, and; 3) produce a model that reliably exhibits spontaneous dentate granule cell epileptiform discharges prior to the onset of each behavioral seizure, and do so without producing significant lethality or severe brain damage. We then used continuous (24/7) video monitoring and bilateral depth recording directly from the dentate granule cell layers to determine the latency to both hippocampal epileptogenesis and clinical epilepsy following prolonged convulsive SE.
METHODS
Animal treatment
Fifty-five Male Sprague-Dawley rats (350−450g; Harlan Sprague Dawley; Indianapolis, IN) were treated in accordance with the guidelines of the National Institutes of Health for the humane treatment of animals, and the methods used were approved by the University of Arizona Institutional Animal Care and Use Committee. The 55 rats included 25 rats given pilocarpine, 3 rats that received vehicle injections (controls for pilocarpine-treated rats), 19 implanted and stimulated rats, 4 implanted sham controls, and 4 implanted, stimulated rats used only to assess paired-pulse responses before and after stimulation.
Pilocarpine-induced status epilepticus
Rats were given atropine methylbromide (1mg/kg sc in saline vehicle; Sigma, St. Louis, MO), followed 30 min later by pilocarpine hydrochloride (350−380 mg/kg sc in saline; Sigma). Different doses were used in different groups when it was determined that few animals entered SE after the lower dose. Controls received two saline injections. After 3 hr of continuous behavioral SE (usuall ∼3.5 hr after pilocarpine injection), behavioral seizures were terminated abruptly by halothane inhalation, and their reoccurrence was suppressed by a sub-anesthetic dose of urethane (0.8 g/kg sc). This drug combination was used after it was determined in pilot studies using hippocampal depth recording and behavioral observation that this treatment fully suppressed both the behavioral seizures and the hippocampal electrographic discharges. Pilocarpine-treated rats appeared lethargic and did not eat for several days after SE, which necessitated subcutaneous injections of saline and provision of apple slices.
Perforant pathway stimulation-induced status epilepticus
Rats were implanted bilaterally with two stimulating and two recording electrodes in the angular bundles of the perforant pathways and dentate granule cell layers, respectively, under chloral hydrate anesthesia, as previously described (Harvey and Sloviter, 2005). At least one week after surgery, awake animals were stimulated bilaterally for 3 hr using a previously described stimulus train paradigm designed to evoke hippocampal granule and pyramidal cell discharges (Sloviter et al., 1996) throughout the duration of stimulation. Stimulation consisted of 15 − 20 V paired-pulse stimuli delivered at 2 Hz, with a 40 msec interpulse interval, plus 10 sec long, 20 Hz stimulus trains of single, 15 − 20 V stimuli delivered once per minute. After 3 hr of continuous behavioral SE, behavioral seizures were terminated abruptly by halothane inhalation, and their reoccurrence was suppressed by a sub-anesthetic dose of urethane (0.8 g/kg sc). Stimulated rats appeared to be fully recovered behaviorally approximately one day after urethane injection. Stimulated rats appeared healthier one day post-SE than pilocarpine-treated rats, and were usually eating and drinking ad libitum by two days post-SE.
Electrophysiological and video monitoring methods
Electrical stimulation in awake animals utilized stimuli (0.1 msec duration) generated by a Grass S88 stimulator and stimulus isolation unit (Grass Instruments, West Warwick, RI). Evoked and spontaneous granule cell layer activity during and after stimulation-induced SE was amplified and recorded digitally at a 10 kHz sampling rate (AD Instruments, Mountain View, CA). After the end of 3 hr of perforant pathway stimulation-induced SE, the granule cell layer recording electrodes recorded spontaneous activity continuously (24/7) and stored digitally and automatically in 6 hr epochs. Each day, the preceding 24 hr of recordings were assessed visually, and all events with amplitudes obviously larger than baseline were expanded and analyzed qualitatively and quantitatively. Each confirmed epileptiform event was related to behavior on the time-stamped video recordings of behavior, as described below.
Continuous (24/7) video-monitoring began immediately after the end of SE utilizing Panasonic model 9622 color CCD day/night infrared cameras. Video files were captured at 30 frames/sec and time-stamped for integration with the electrophysiological data using surveillance software (Ben Software, London, UK), and stored digitally. Video files were played back and observed at high speed (6−12X) to detect behavioral seizures, without knowledge of the electrophysiological data. Spontaneous behavioral seizures were scored according to the Racine scale (Racine, 1972). Only behavioral seizures clearly identifiable by video analysis on review at normal playback speed as Stage 3−5 motor seizures were counted during analysis. Due to the urethane anesthesia used to prevent reoccurrence of SE after its termination by halothane inhalation, analysis of the video data began 24 hr after the end of SE, when rats appeared to be fully recovered. Given these methodological considerations, the earliest seizure latency possible was on day 2 after SE (the 24 hr period starting 24 hr post-SE). Thus, the estimated seizure latencies and frequencies are conservative underestimates.
Perfusion-fixation and tissue treatment
Rats were anesthetized with urethane and perfused through the aorta by gravity-feed with saline for 2 min followed by 4% paraformaldehyde in 0.1M phosphate buffer, pH 7.4, for 10 min. After storage of the intact rats overnight at 4°C, brains were removed from the skull and 40 μm-thick sections were cut in 0.1M Tris (hydroxymethylaminomethane) buffer, pH 7.6, using a Vibratome. Brains were first cut in the horizontal plane from the base of the brain, and then the remaining block was cut coronally. All histological and immunocytochemical procedures were as previously described (Sloviter et al, 2006).
Immunocytochemistry protocol
Sections were mounted on Superfrost Plus slides, air dried, and placed in 0.1M TRIS buffer, pH 7.6 (TRIS). Slides were then immersed in 85−87°C TRIS for 1 min, washed in TRIS, and placed in TRIS containing 0.25% bovine serum albumin (BSA; fraction V; Sigma) and 0.1% Triton X-100. Slides were incubated overnight in mouse anti-NeuN (diluted 1:10,000; MAB377; Chemicon). Anti-NeuN was raised against purified cell nuclei from mouse brain, and recognizes 2−3 bands in Western blots in the 46−48 kDa range, and possibly another band at approximately 66 kDa, which constitutes unknown nuclear proteins (Mullen et al., 1992). After primary antibody incubation, slides were washed in TRIS-BSA-Triton X buffer, pH 7.6 (2×5 min minimum). Slides were incubated in biotinylated secondary antibody solution (1:2000 dilution of goat anti-mouse; Vector Labs, Burlingame, CA) in TRIS-BSA-Triton X buffer for 2 hr, washed in the same buffer, and then incubated for 2 hr in avidin-biotin-HRP complex (Vector Labs Elite kit diluted 1:1000 in TRIS-BSA-Triton X buffer). Slides were then washed in TRIS (3×5 min minimum) and incubated in a hydrogen peroxide-generating DAB solution (100ml TRIS containing 50 mg DAB, 40 mg ammonium chloride, 0.3 mg glucose oxidase, and 200 mg β-D-glucose). After incubation in DAB solution (20−30 min), slides were rinsed in TRIS, dehydrated in graded ethanols and xylene, and coverslipped with Permount. Other sections were Nissl-stained (1% cresyl violet) or stained with Fluoro-Jade B (Schmued and Hopkins, 2000) to visualize degenerating neurons.
Light microscopic imaging methods
Brightfield images were acquired digitally on a Nikon E800M microscope with a Hamamatsu C5180 camera. Adobe Photoshop 7.0 was used to acquire images and optimize contrast and brightness, but not to enhance or change the image content. For comparison photographs, all tissue sections were photographed under identical conditions of exposure, and any changes made in brightness or contrast were applied uniformly to all photographs.
Hilar neuron counting
To estimate the extent of hilar neuron loss following perforant pathway stimulation-induced SE, we analyzed three non-consecutive NeuN-immunostained sections from the dorsal hippocampus and three sections from the ventral hippocampus (at the levels shown in Figure 7) of control and experimental rats. The hilar cell counts included all neuronal somata within the hilus that were not in contact with the granule cell layers, and were outside the somal and dendritic regions of area CA3c. Hilar neurons were not counted in Nissl-stained sections because neurons could not be reliably differentiated from many of the glial cells that proliferate after seizure-induced damage. Two groups of stimulated rats, which survived for 7−10 days or for 42−120 days, addressed the inaccuracy inherent in counting hilar neurons after SE. We hypothesized that rats killed shortly after stimulation, before neurogenesis had added a maximal number of new cells to the hilus (Parent et al., 1997), might provide a more accurate estimate of the number of hilar neurons that had survived the convulsive SE. In addition, although newly born granule cells are generally smaller than normal hilar neurons, a tangentially cut and stained soma of a large cell can be difficult to distinguish from a centrally-cut ectopic granule cell. Therefore, we counted the total number of NeuN-immunopositive hilar neurons in 6 sections from sham controls (n=3), from rats that survived for 7−10 days (n=4), and from rats that survived for 42−120 days (n=4). Group means were compared using Student's t test.
Figure 7.
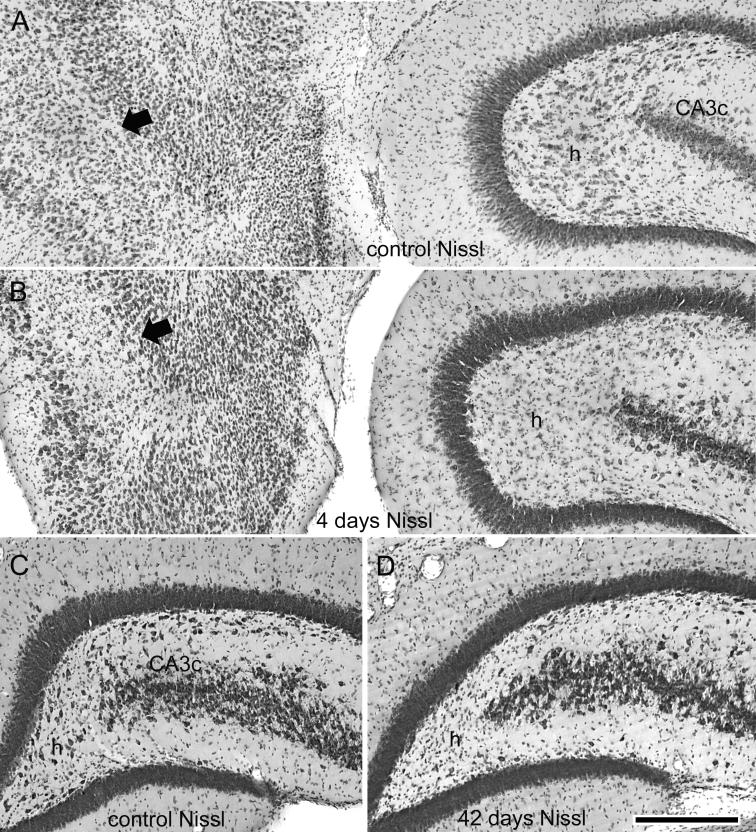
Neuronal injury and loss in the dentate hilus and entorhinal cortex after 3 hr of perforant pathway stimulation-induced convulsive SE. Nissl-stained sections adjacent to those shown in the previous figure, in which NeuN immunostaining illustrated the apparent loss of hilar neurons. (A) Nissl-stained horizontal section from a sham control animal. (B) 4 days post-SE. Note the extensive loss of large Nissl-stained hilar neurons (h) and neurons in the entorhinal cortex (arrow). (C) and (D) Nissl-stained coronal sections from a sham control animal (C) and a stimulated animal 42 days post-SE (D). Note the extensive loss of hilar neurons (h). Calibration bar: 400μm in A and B; 285 μm in C and D.
RESULTS
Experiment 1: Spontaneous seizure latency after 3 hr of pilocarpine-induced SE
We analyzed two weeks of continuous video recordings of 7 pilocarpine-treated rats that survived 3 hr of continuous convulsive SE. This was done primarily to confirm the results of three previous studies, all of which reported that pilocarpine-treated rats developed spontaneous behavioral seizures during the first week post-SE (Raol et al., 2006; Goffin et al., 2007; Jung et al., 2007). Seventeen of 25 pilocarpine-treated rats entered convulsive SE, of which ten died during or after 3 hr of convulsive SE. This high mortality rate is typical of pilocarpine use (Glien et al., 2001; Goffin et al., 2007). All 7 surviving pilocarpine-treated animals exhibited spontaneous Stage 3−5 behavioral seizures (Racine, 1972) during the first week post-SE. The first spontaneous seizures were observed an average of 4.9 ± 0.9 days post-SE (range: 2−9 days; median: 4 days; n=7). The average frequency of Stage 3−5 behavioral seizures per day over the 2 week observation period was 1.4 ± 0.5 behavioral seizures per rat per day (range: 0−19 seizures/ day; median: 0.6 seizures; n=7). The average total number of seizures per rat over the entire observation period was 19.1 ± 6.6 seizures (range: 5−50 seizures; median: 9 seizures; n=7). Three pilocarpine-treated rats that did not develop convulsive SE, and were assigned to a control group for that reason, exhibited no spontaneous behavioral seizures during the 2 week post-SE observation period. Similarly, 3 saline-treated rats exhibited no spontaneous seizures during the same observation period. Thus, all pilocarpine-treated rats that survived 3 hr of behavioral SE exhibited spontaneous seizures during the first week post-SE.
Pilocarpine-treated rats were not implanted with electrodes in this study because the purpose of this experiment was only to confirm, by continuous behavioral observation, the recent findings that pilocarpine-treated rats reliably exhibit a minimal latent period (Raol et al., 2006; Goffin et al., 2007; Jung et al., 2007). Spontaneous hippocampal activity during epileptic seizures in pilocarpine-treated rats was the subject of a previous study (Harvey and Sloviter, 2005), in which we reported that during more than 200 spontaneous seizures in pilocarpine-treated rats, none appeared to be hippocampal-onset seizures, a finding diametrically opposite to the observations in the perforant pathway-stimulated rats described below.
Experiment 2: Spontaneous seizure latency after 3 hr of perforant pathway stimulation-induced SE
We used perforant pathway stimulation to evoke hippocampal granule cell epileptiform discharges and behavioral SE throughout the 3 hr SE period, with the purposes of both avoiding the involvement of a chemoconvulsant drug, and consistently producing both hippocampal injury and hippocampal epileptogenesis.
Initiation of convulsive SE
After paired test stimuli were delivered at 0.1 Hz and a 40 msec interpulse interval to assess normal paired-pulse suppression (Fig. 1A), 3 hr of bilateral perforant pathway stimulation was initiated. Bilateral stimulation was used to replicate the bilateral seizure activity and brain damage produced by chemoconvulsant-induced SE. The afferent stimulation paradigm used to evoke dentate granule cell epileptiform discharges and behavioral SE consisted of continuous stimulation at 2 Hz with paired pulses 40 msec apart plus 10 sec-long trains of single pulses delivered at 20 Hz once per minute, as shown in Figure 1, B1. During the 50 sec periods between the 10 sec-long 20 Hz trains, 2 Hz paired stimuli evoked granule cell epileptiform discharges, as shown in the expanded trace in Figure 1, B1a. After 3 hr of continuous granule cell epileptiform activity and convulsive behavioral SE (3 hr after the start of stimulation), both were abruptly terminated by halothane inhalation, and maintained by a sub-anesthetic dose of urethane (0.8 g/kg sc).
Figure 1.
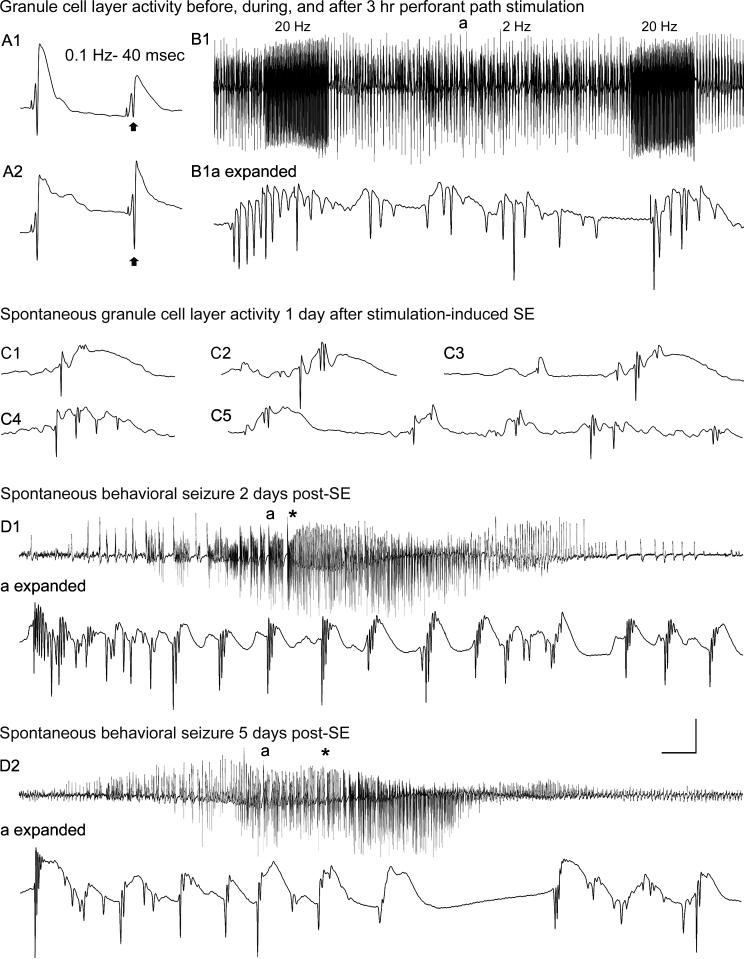
Dentate granule cell excitability and spontaneous activity before, and 1−5 days after, 3 hr of perforant pathway stimulation-induced convulsive status epilepticus (SE). (A1) Before SE, paired-pulse perforant pathway stimulation at 0.1 Hz and an inter-stimulus interval of 40 msec evokes granule cell responses that exhibit partial suppression of the amplitude of the second population spike (arrow). (A2) Three days after 3 hr of SE, the identical afferent stimulation failed to suppress the second population spike (arrow). (B1) Granule cell layer activity during 3 hr of perforant pathway stimulation in the same awake rat. The stimulation paradigm involved continuous stimulation at 2 Hz with paired pulses delivered at a 40 msec interpulse interval, plus 10 sec-long 20 Hz trains delivered once per minute. Note the morphology of the granule cell epileptiform discharges during the 2 Hz inter-train interval (a) in the expanded trace (B1a expanded). (C) On the first day after 3 hr of stimulation-induced SE, a granule cell layer electrode recorded spontaneous granule cell field “EPSPs” and population spikes that closely resemble the evoked responses in (A). (D) Granule cell layer activity during spontaneous behavioral seizures during the first week post-SE. (D1) On day 2 post-SE, granule cell layer activity amplitude increased before the behavioral onset of the second behavioral seizure on that day (marked by asterisk). (D1a expanded) expanded trace of the region above marked “a,” showing that the high-amplitude activity in (D1) consisted of granule cell epileptiform discharges that preceded the behavioral seizure-onset (asterisk). (D2) Three days later, the fourth spontaneous behavioral seizure exhibited nearly identical features including high-frequency granule cell epileptiform discharges (D2a expanded) that preceded the behavioral seizure-onset (asterisk). Calibration bars: (A) 14 msec and 9 mV; (B1) 7 sec and 9 mV; (B1 expanded) 46 msec and 9 mV; (C) 40 msec and 9 mV; (D1) 3.4 sec and 9 mV; (D1 expanded) 53 msec and 9 mV; (D2) 3.4 sec and 9 mV; (D2 expanded) 60 msec and 9 mV.
Latency to spontaneous behavioral seizures after 3 hr of stimulation-induced SE
Of 19 chronically-implanted rats that began 3 hr of perforant pathway stimulation, one died during SE after a severe tonic-clonic seizure. Of the 18 remaining rats, 17 exhibited numerous spontaneous Stage 3−5 behavioral seizures throughout the 2 week post-SE observation period. Stage 3−5 behavioral seizures were not observed in the one remaining rat, which did exhibit two electrographic seizure discharges associated with Stage 2 behavioral seizures, on days 3 and 9 post-SE. Of the 17 rats that exhibited spontaneous Stage 3−5 behavioral seizures, 10 had their first detected Stage 3−5 behavioral seizure by day 2 post-SE (the period 24−48 hr post-SE), and 16 of the 17 rats exhibited their first Stage 3−5 behavioral seizures by day 4 post-SE. On average, the first spontaneous seizure occurred 2.6 ± 0.4 days post-SE (range: 1−8 days; median: 2 days; n=17). This is a conservative estimate because most rats were not fully ambulatory on the first day after SE (0−24 hr post-SE) and, therefore, behaviors were not assessed. In addition, we only counted obvious Stage 3−5 motor seizures (Racine, 1972) as “seizures” during the two week observation period. Less obvious Stage 1 and 2 behaviors were not counted because rats often did not face the camera, and subtle movements could not be accurately evaluated during video playback. The average frequency of Stage 3−5 seizures over the 2 week observation period was 0.6 ± 0.1 seizures per rat per day (range: 0−7 seizures per day; median: 0.6 seizures; n=18 rats). The average total number of Stage 3−5 behavioral seizures during the 2 wk observation period was 7.6 ± 1.1 behavioral seizures per rat (range: 0−17 seizures; median: 7.5 seizures; n=18). No spontaneous behavioral seizures were observed in 4 implanted control rats not subjected to stimulation-induced SE, but which were stimulated for 3 hr at 0.1 Hz and then given halothane and the sub-anesthetic dose of urethane.
Experiment 3: Evoked and spontaneous electrographic hippocampal events after 3 hr of perforant pathway stimulation-induced status epilepticus
Decreased paired-pulse suppression after convulsive SE
We intentionally avoided stimulating rats after the end of convulsive SE because afferent stimulation might affect the latency to spontaneous seizures. However, in one rat, which exhibited two spontaneous seizures on day 2 post-SE, and was therefore judged to be “epileptic,” we delivered 4 paired pulses to the perforant pathway at 0.1 Hz (two stimuli with a 20 msec interpulse interval and two stimuli with a 40 msec interpulse interval) on the 3rd day post-SE (Fig. 1A). This was done to determine whether the dentate granule cells were hyperexcitable coincident with acute neuron injury, as we have found in previous studies involving the chemoconvulsants kainic acid and pilocarpine (Sloviter and Damiano, 1981a; Sloviter, 1992; Harvey and Sloviter, 2005; Sloviter et al., 2006) or perforant pathway stimulation (Sloviter and Damiano, 1981b; Sloviter, 1987; 1991b). Granule cells exhibited decreased paired-pulse suppression after 3 hr of SE (Fig. 1A). Identical stimulation was performed in four additional rats not used to determine the latency to spontaneous behavioral seizures. Paired pulses delivered before and 3 days post-SE confirmed that all 5 rats tested exhibited decreased paired-pulse suppression at 0.1 Hz and a 40 msec interpulse interval compared to the response before stimulation, as illustrated in Figure 1A.
Spontaneous activity in the dentate granule cell layer post-SE
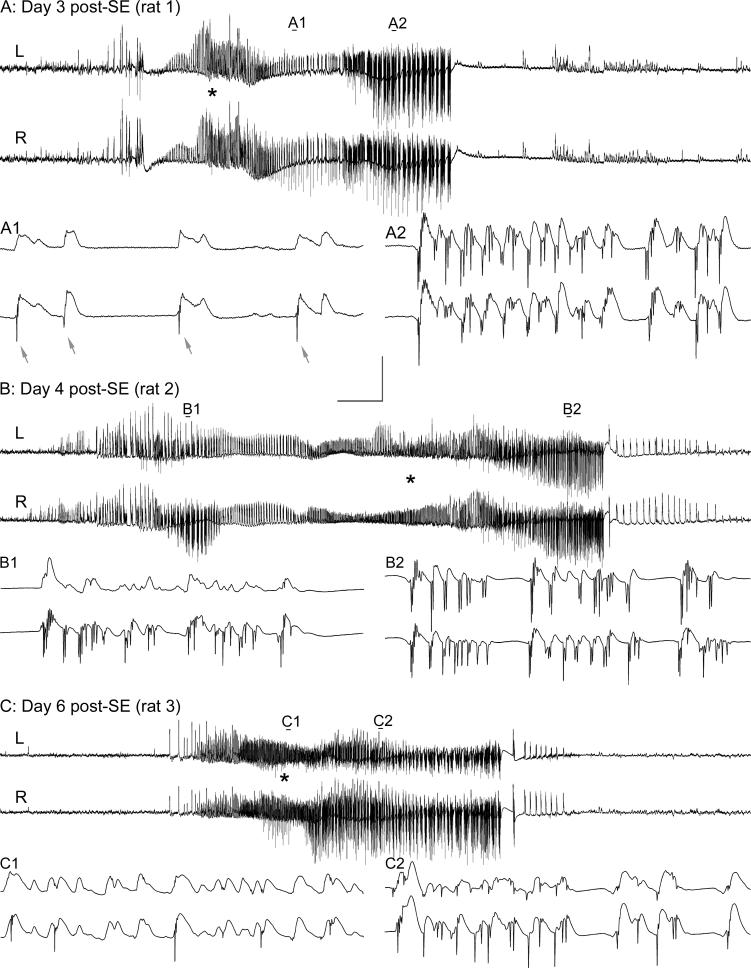
Of the 18 stimulated rats described above, 11 were chosen for continuous awake recording because their recording electrodes were judged to be optimally located within the granule cell layer on the basis of their characteristic responses to afferent stimulation (Andersen et al., 1966; Sloviter, 1991a). Continuous granule cell layer recording and synchronized video monitoring revealed that within one day after SE, dentate granule cells generated frequent spontaneous potentials (Fig. 1C) virtually identical to the potentials evoked by perforant pathway stimulation (Fig. 1A). Sham control animals (n=4), which also had stimulating electrodes implanted in the angular bundles of the perforant pathways, did not generate similar spontaneous potentials. By the second day post-SE, all 11 rats exhibited spontaneous granule cell potentials containing population spikes. Initially, these spontaneous population discharges consisted of single (Fig. 1, C1) and multiple population spikes (Fig. 1C, 2−5) that were not accompanied by obvious behavioral manifestations. More prolonged epileptiform granule cell discharges (Fig. 1, D1) were recorded in 7 of 11 rats by day 2 post-SE (24−48 hr post-SE; the first day of video analysis), and in 10 of 11 rats by day 3 post-SE. Whenever spontaneous granule cell layer discharges contained high-frequency, negative-going population spikes (Fig. 1, D1a expanded), the onset of Stage 3−5 behavioral seizures (asterisk in Fig. 1, D1) occurred within ∼20−30 seconds after the start of the initial high-amplitude granule cell layer activity. The granule cell layer activity that preceded the first signs of each behavioral seizure was a characteristic feature of the spontaneous behavioral seizures initiated by stimulation-induced SE (Figs. 1 and 2). Thus, all 72 Stage 3−5 behavioral seizures observed in the 10 continuously recorded rats that exhibited Stage 3−5 behavioral seizures appeared to be “hippocampal-onset” in nature. However, we emphasize that this term refers to hippocampal seizure discharges that precede or accompany behavioral seizure manifestations, but does not imply that the hippocampus must be the primary seizure source (Spencer and Spencer, 1994; Spencer, 1998).
Figure 2.
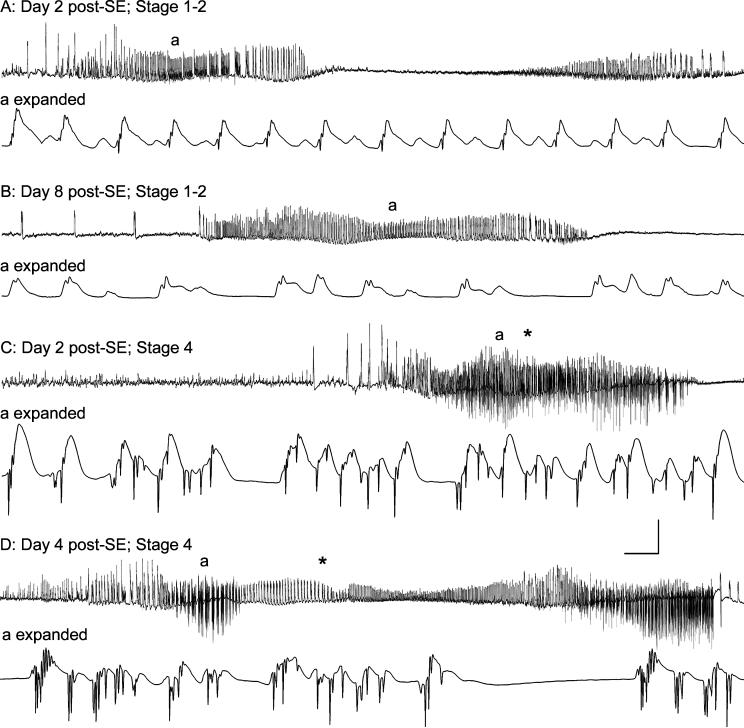
Correlation between spontaneous granule cell layer activity and behavioral seizure expression in an epileptic rat 2−8 days after 3 hr of perforant pathway stimulation-induced convulsive status epilepticus (SE). Two focal (subclinical) seizures (A) and (B) on days 2 and 8 post-SE, and two Stage 4 behavioral seizures (C) and (D) on days 2 and 4 post-SE. (A) On Day 2 post-SE, spontaneous high-amplitude activity, consisting of field “EPSPs” and small amplitude population spikes, was recorded from the granule cell layer electrode. During this spontaneous event, the animal exhibited only a frozen stare, followed by stereotyped chewing movements. (B) A similar spontaneous granule cell layer event was recorded on Day 8 post-SE, which was also associated with staring and stereotyped head movements only. (C) and (D) on Days 2 and 4 post-SE, spontaneous granule cell layer events included larger amplitude, downwardly deflected population spikes, and these events were invariably followed by Stage 3−5 behavioral seizures. Note that high-frequency spiking began prior to the first behavioral manifestation (asterisks in C and D) of each behavioral seizure. These events from a single rat (different from the rat shown in Fig. 1) are representative of all 72 Stage 3−5 behavioral seizures recorded in 10 chronically-implanted, continuously monitored rats. Calibration bars: (A) 1.4 sec and 9 mV; (A expanded) 56 msec and 9 mV; (B) 3.2 sec and 9 mV; (B expanded) 56 msec and 9 mV (C) 3.2 sec and 9 mV; (C expanded) 56 msec and 9 mV; (D) 4.5 sec and 9 mV; (D expanded) 56 msec and 9 mV.
There was a clear and consistent correlation between the presence or absence of high-frequency granule cell discharges, and the occurrence or absence of Stage 3−5 behavioral seizures (Fig. 2). High-amplitude granule cell layer activity that consisted of positive-going field “EPSPs” (Andersen at el., 1966), but few large-amplitude granule cell population spikes (Fig. 2, A and B), was never followed by Stage 3−5 behavioral seizures (n >300 individual granule cell layer population events lacking or containing few high-frequency population spikes). Granule cell population activity that lacked or contained few high-frequency population spikes occurred without any obvious behavioral correlate, or were followed solely by staring or head and chewing movements. Conversely, spontaneous granule cell layer events that started with field depolarizations, and then progressed to include high-frequency granule cell epileptiform discharges (Fig. 2, C and D), were invariably followed by Stage 3−5 behavioral seizures (n=72 Stage 3−5 behavioral seizures recorded in 10 rats).
Granule cell layer activity during one 6 minute-long event in an awake epileptic rat well illustrated the apparently causal relationship between granule cell layer activity and clinical seizure onset (Fig. 3). At the beginning of this single 6 minute-long epoch, the granule cells began to generate positive-going “field EPSPs” with occasional population spikes at a frequency of ∼ 0.4 Hz (Fig. 3, top trace, expanded in trace 1; 57 events in 160 sec). This was followed by two distinct higher-frequency events beginning 3.0 and 3.7 min after the onset of granule cell layer activity (Fig. 1, top trace). Both of these events lacked maximal amplitude, negative-going epileptiform discharges (Fig. 3, traces 2−5), and were not accompanied by a behavioral seizure. Approximately 80 sec after the second electrographic event, however, larger amplitude epileptiform discharges occurred for the first time in this 6 minute-long event (Fig. 3, traces 6−8), and were rapidly accompanied by a Stage 4 behavioral seizure (asterisk in top trace marks behavioral seizure onset).
Figure 3.
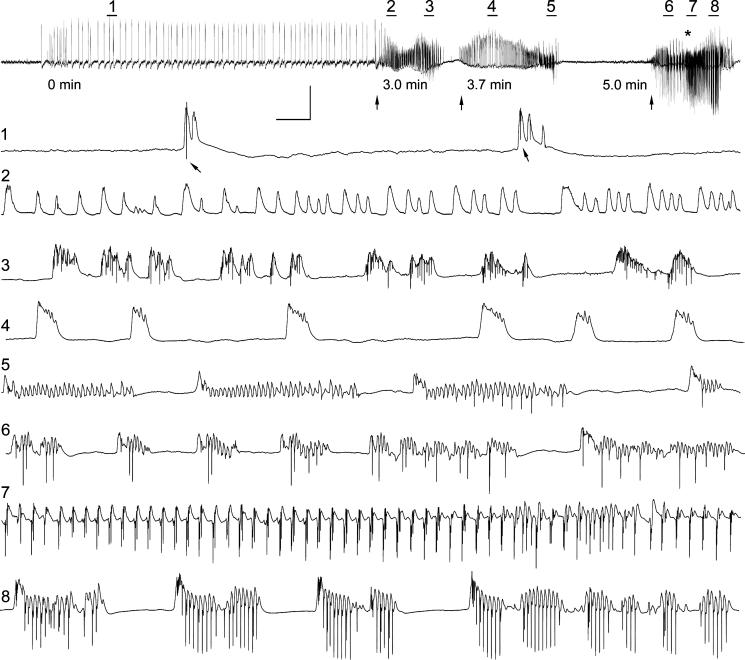
Granule cell layer activity and clinical seizure expression during one 6-minute period in an awake epileptic rat 9 days after 3 hr of perforant pathway stimulation-induced convulsive status epilepticus (SE). Top trace: 6 min of granule cell layer activity showing 3 distinct events (arrows), the last of which culminated in a Stage 4 spontaneous epileptic seizure (forepaw clonus and rearing) that began (asterisk) ∼19 sec after the onset of high amplitude granule cell population spikes. Prior to the first onset of high frequency granule cell layer activity at the 3 min marker (first arrow), high amplitude, low frequency activity consisted of positive-going waves with occasional superimposed granule cell population spikes (arrows in expanded trace 1). During the first of 3 distinct, high-frequency granule cell layer events, positive-going “field EPSPs” and population spikes were recorded (traces 2 and 3, respectively). These granule cell layer discharges lacked large amplitude granule cell population spikes that extended below baseline, and these potentials were not associated with a behavioral seizure. A second similar event began 40 sec later, and contained mostly positive-going events (traces 4 and 5) that were not associated with a behavioral seizure. The third event consisted of large amplitude, high-frequency granule cell population spikes that extended far below baseline (traces 6−8), and these features were uniquely associated with the onset of a spontaneous Stage 4 epileptic seizure. Note that forepaw clonus (asterisk in top trace) began ∼15 sec after the onset of high amplitude granule cell population spikes shown in trace 6. The top trace is 6.0 min in duration, and expanded traces 1−8 are 4.4 sec in duration. Calibration bars: top trace, 16.8 sec and 12.5 mV; traces 1−8: 205 msec and 12.5 mV.
Spontaneous granule cell layer discharges always preceded spontaneous behavioral seizures
At the start of some behavioral seizures, animals were not facing the camera, and the precise start of each behavioral seizure was difficult to determine. In other cases, animals were actively exploring the cage before the start of a behavioral seizure, and head or chewing movements could not be definitively judged to be the start of the seizure. Therefore, without knowledge of the electrophysiological data, we identified 20 spontaneous Stage 3−5 behavioral seizures from the video recordings (from a total of 72 seizures that occurred in 10 rats during the 2-week observation period) that began while the animals were asleep or still, and facing the camera. In these 20 instances, analysis of the electrographic granule cell layer activity revealed that high-amplitude, positive-going field depolarizations (Fig. 1D and Fig. 2, C and D) began an average of 24 seconds (range: 4−61 seconds; n=20 spontaneous behavioral seizures) before the first obvious behavioral seizure manifestations (sudden awakening, repetitive chewing movements, forepaw clonus, or rearing). The average duration of these 20 spontaneous granule cell seizure discharges was 44.0 ± 5.2 sec (range: 25−103 sec; median: 34.5 sec; n=20), and the average duration of the behavioral seizures was 26.1 ± 2.8 seconds (range: 11−55 sec, median: 21.5 sec; n=20). The average duration of all 72 spontaneous granule cell seizure discharges was 37.2 ± 1.8 sec (range: 17−103 sec; median: 34.0 sec; n=72), indicating that the epileptiform discharge durations of the 20 selected behavioral seizures were representative of the population from which they were selected.
Bilateral synchrony of granule cell layer spontaneous discharges
An unexpected and surprising finding that was reliably evident in all epileptic animals was the bilateral synchrony of all spontaneous granule cell layer events (Figure 4). Virtually all events, including dendritic field depolarizations, single and multiple population spikes, and epileptiform discharges, whether they occurred immediately, or weeks after the end of 3 hr of convulsive SE, were remarkably synchronous in both granule cell layers (Fig. 4). Despite the extraordinary degree of synchrony, the granule cell layer events were not identical, and there was no evidence that one recording electrode could detect activity in the contralateral dentate gyrus, because high amplitude spikes often occurred on one side without any spike being recorded by the contralateral electrode. The fact that recording electrodes were randomly implanted in different parts of the dorsal dentate gyrus in different animals, and the observation that all animals exhibited highly synchronous discharges, indicate that large expanses of the granule cell layers were generating highly synchronous, but not identical, spontaneous population discharges.
Figure 4.
Bilateral synchrony of spontaneous granule cell layer epileptiform discharges in three different epileptic rats. Bilateral recording electrodes with their tips in the dentate granule cell layers recorded highly synchronous spontaneous activity in all animals subjected to 3 hr of perforant pathway stimulation-induced convulsive SE. (A): Prior to a Stage 3−5 behavioral seizure onset, bilateral recording electrodes recorded superficially identical granule cell layer activity (top traces; 2.0 min in duration). Expanded views (all are 0.9 sec in duration) of two segments of these spontaneous discharges reveal highly synchronized, but clearly not identical, discharges. Note population spikes on the lower traces (arrows in A1), but only field depolarizations in the simultaneously recorded top trace in (A1). During the high frequency granule cell layer discharges (A2), spiking was highly synchronous but not identical. (B) and (C): Similar events recorded in two other epileptic rats 4 and 6 days post-SE, respectively. Note that these highly synchronized discharges are representative of all 72 spontaneous seizures recorded. Calibration bars: unexpanded traces top trace, 7.3 sec and 12.5 mV; expanded traces: 114 msec and 12.5 mV.
Experiment 4: Histological analysis after stimulation-induced convulsive SE
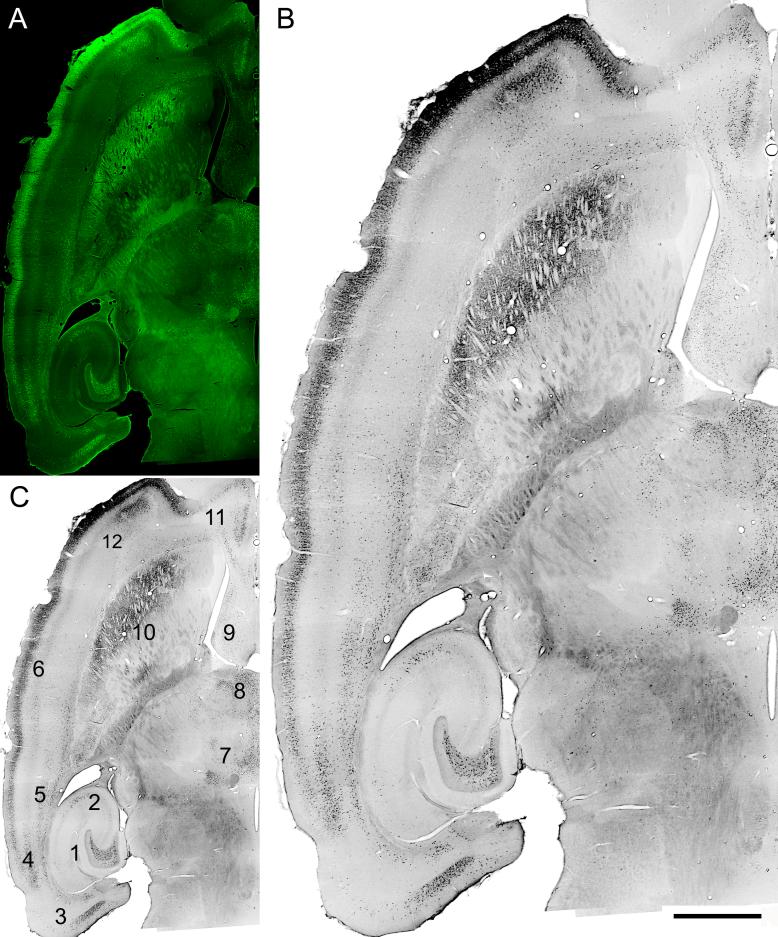
Stimulation-induced convulsive SE for 3 hr resulted in a pattern of bilateral hippocampal and extra-hippocampal brain damage (Fig. 5) that varied minimally among identically stimulated rats. Fluoro-Jade B (FJB) staining 4 days post-SE, by which time 16 of 17 rats had already exhibited their first spontaneous epileptic seizures, revealed selective and extensive acute neuronal injury in the hilus of the hippocampal dentate gyrus (Fig. 5, location 1), minor injury in area CA3a (Fig. 4, location 2), and selective damage to the entorhinal cortex, including Layer III (Fig. 5, location 3). In addition, acute injury was reliably produced in multiple layers of the perirhinal cortex (Fig. 5, locations 4 and 5), Layer II of the entire neocortex at the level of the section shown (Fig. 5, location 6), as well as in several thalamic nuclei, the lateral septum, caudate/putamen, and other cortical structures (Fig. 5, locations 7−12). A similar pattern of acute injury was evident in all 9 rats perfusion-fixed 4−14 days post-SE. We saw no evidence in stimulated animals of the variable pathology and vascular/ischemic abnormalities observed in pilocarpine-treated rats (Sloviter, 2005).
Figure 5.
Acute Fluoro-Jade B (FJB) staining showing neurodegeneration 4 days after 3 hr of perforant pathway stimulation-induced SE. (A) FJB fluorescence; (B and C) grayscale, inverted image of the same horizontal brain section. Note selective degeneration of neurons in the dentate hilar region (C-1), hippocampal area CA3a (C-2), entorhinal cortex layer III (C-3), perirhinal cortex (C-4 and 5), layer II throughout the neocortex (C-6), the parafascicular thalamic nucleus (C-7), the intermediodorsal-, mediodorsal-, paratenial-, paraventricular-, and centralmedial thalamic nuclei (C-8), lateral septum (C-9), lateral caudate/putamen (C-10), infralimbic cortex (C-11), and deep pyramidal cells of the agranular insular cortex (C-12). Calibration bar: 2mm in A and C; 1mm in B.
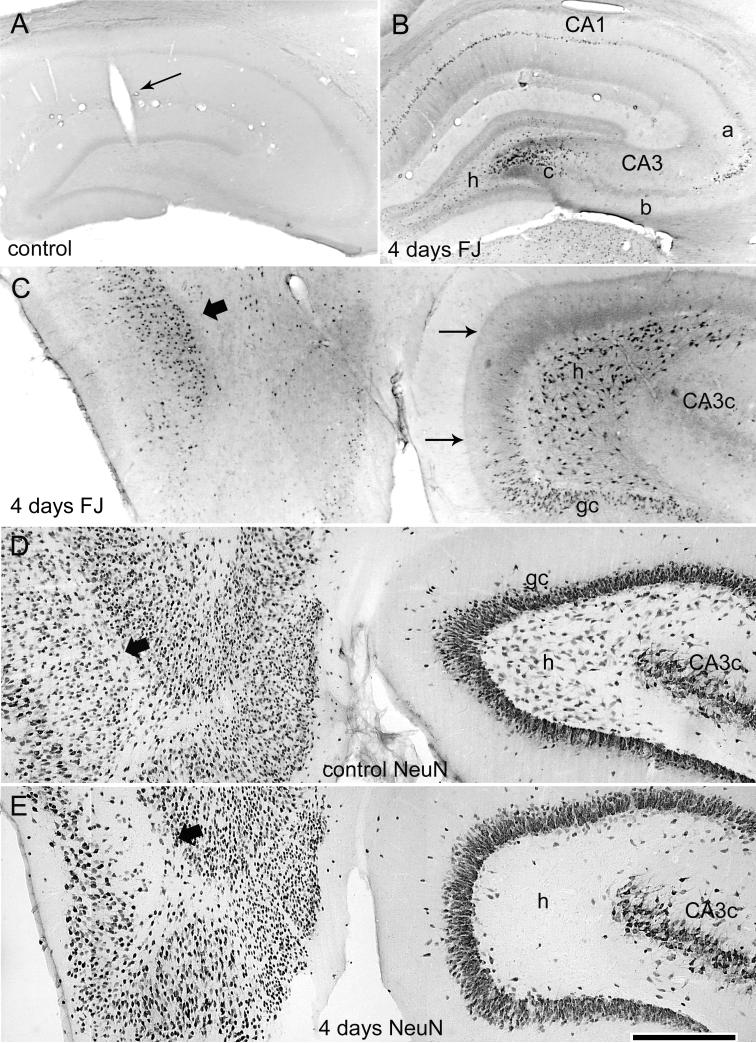
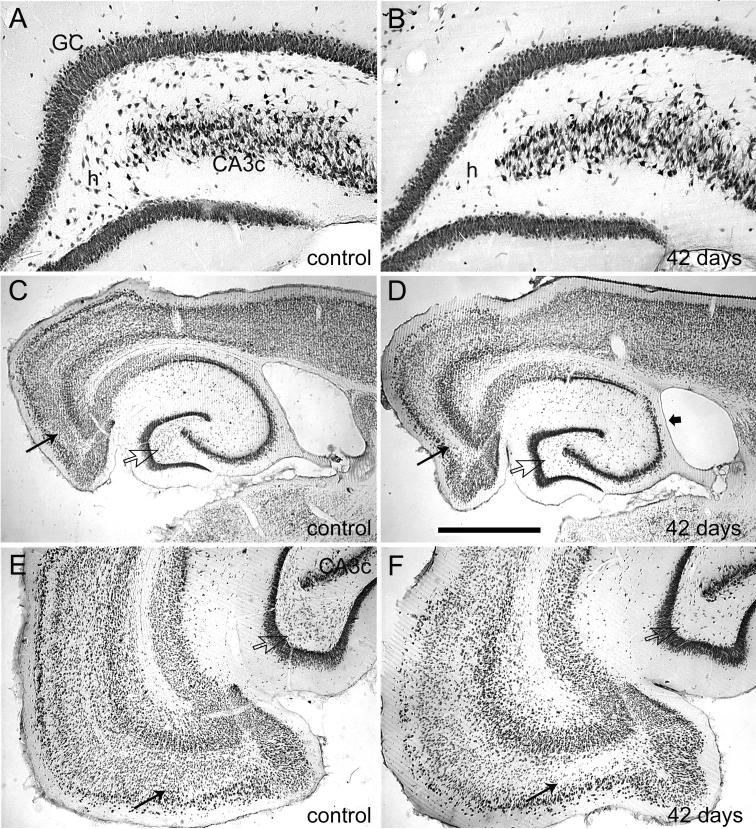
Within the hippocampus, coronal sections from 4 control animals showed that the presence of the recording electrodes and all sham treatments produced no detectable FJB staining (Fig. 6A). Conversely, 3 hr of perforant pathway stimulation-induced convulsive SE in awake rats produced degenerating neurons in the dentate hilus, in areas CA3a and CA3c of the CA3 pyramidal cell layer, and in area CA1 (Fig. 6B). In horizontal sections of the same epileptic animal, acutely degenerating neurons included hilar neurons, dentate granule cells of the inner blade, and neurons of the entorhinal cortex (Fig. 6C). This acute neuronal injury is highlighted by immunostaining for NeuN, a neuronal marker protein (Mullen et al., 1992), which revealed a bilateral loss of NeuN staining in hilar neurons in adjacent sections of the same animal (Fig. 6E). Adjacent Nissl-stained sections showed that loss of NeuN reflected degeneration of hilar neurons and cells of the entorhinal cortex (Fig. 7, A and B). Thus, in the dentate gyrus, which was hyperexcitable and generated spontaneous epileptiform discharges coincident with the acute neuronal injury, 3 hr of stimulation-induced SE produced primarily hilar neuron loss throughout the longitudinal axis of both hippocampi, with most animals exhibiting minor or moderate pyramidal layer injury (Fig. 8D). Despite 3 hr of continuous, verified hippocampal seizure activity, no animals exhibited the extensive pyramidal cell injury that defines classic hippocampal sclerosis (Sloviter et al., 2007). Consistent with the acute hippocampal pathology, all epileptic animals perfusion-fixed more than 4 weeks post-SE exhibited extensive bilateral neuron loss in the dentate hilus (Fig. 8, B and D) and in Layer III of the entorhinal cortex (Fig. 8, D and F), as previously described (Du et al., 1995).
Figure 6.
Acute neurodegeneration in hippocampus and entorhinal cortex 4 days after 3 hr of perforant pathway stimulation-induced SE. (A) Coronal section of a sham control rat (implanted, no SE) showing that the presence of the hippocampal recording electrode and the control treatment (electrode implantation, stimulation for 3 hr at 0.1 Hz, halothane anesthesia, and subanesthetic urethane treatment) produced no detectable hippocampal Fluoro-Jade B (FJB) staining (grayscale, inverted image as shown in preceding figure). (B) Coronal section of a stimulated rat (same rat as in the preceding figure) showing acute injury in the dorsal hippocampus 4 days after 3 hr of perforant pathway stimulation-induced SE. Note FJB-positive cells in the hilus, area CA3a and CA3c, and area CA1. (C) Horizontal brain section from the same rat stained with FJB, showing selective degeneration of neurons in the entorhinal cortex (wide arrow), the dentate hilus (h), and the dentate granule cell layer (gc). Note also the degenerating terminals in the inner dentate molecular layer (thin arrows) that originate from the degenerating hilar mossy cells. (D) Horizontal section from a sham control animal after immunostaining for the neuronal marker NeuN. (E) A horizontal section adjacent to the section shown in (C) after NeuN immunostaining showing the loss of NeuN immunoreactivity of neurons in the hilus (h) and entorhinal cortex. Calibration bar: 1mm in A and B; 400 μm in C-E.
Figure 8.
Neuron loss 42 days after 3 hr of perforant pathway stimulation-induced SE. (A) Dorsal hippocampal dentate gyrus in a sham control section; NeuN immunostaining. (B) Dentate gyrus in a coronal section 42 days after 3 hr of perforant pathway stimulation-induced SE. Note extensive loss of NeuN-positive hilar neurons, but minimal apparent loss of CA3c pyramidal cells. (C) and (E) Low and higher magnification views of a horizontal section from the control brain. (D) and (F) Low and higher magnification views of a horizontal section 42 days post-SE, showing hilar neuron loss in the ventral dentate gyrus (open arrows) and the loss of entorhinal cortex neurons (thin arrows). Also note in (D) relatively subtle CA3 pyramidal neuron loss (wide arrow). Calibration bar: 400 μm in A and B; 2mm in C and D; 1mm in E and F.
Hilar neuron loss
We counted NeuN-immunopositive hilar neurons in sections from sham controls, from rats that survived for 7−10 days after SE, and from rats that survived for 42−120 days post-SE. As stated in the Methods section, the post-SE hilus contains both surviving hilar neurons and newly-generated ectopic granule cells (Parent et al., 1997). Therefore, counting the total number of NeuN-immunostained hilar neurons after SE provides an inherently inaccurate estimate of the number of hilar neurons that were present prior to stimulation and that survived the insult. The reason for the comparison of shorter and longer survival groups was the hypothesis that the group that survived for 7−10 days, before neurogenesis had added a maximal number of new cells to the hilus, might provide a more accurate estimate of the number of hilar neurons that had survived the convulsive SE. Given these considerations, we present these quantitative results as inherently inaccurate underestimates of the extent of hilar neuron loss.
The numbers of NeuN-immunopositive hilar neurons were counted in 6 sections from each animal (3 from the dorsal hippocampus in coronal sections and 3 from the ventral hippocampus in horizontal sections from the same brain). In 3 sham controls, there was an average of 78.3 ± 12.1 hilar neurons per hippocampus per dorsal section and 151.1 ± 2.3 hilar neurons per hippocampus per ventral section. In 4 rats that survived for 7−10 days, there was an average of 17.6 ± 2.8 hilar neurons per dorsal section (78% fewer hilar neurons than control) and 50.8 ± 5.4 hilar neurons per ventral section (66% fewer hilar neurons than control). In the 4 rats that survived 42−120 days post-SE, there was an average of 29.7 ± 5.8 hilar neurons per dorsal section (62% fewer hilar neurons than control) and 56.1 ± 4.4 hilar neurons per ventral section (63% fewer hilar neurons than control). Both stimulated groups were significantly different from control (P<0.05), but not from each other (P>0.05). Given the inherent inaccuracy of counting cells in imperfectly matched sections, these quantitative data demonstrate only that there was a significant reduction (>60%) in the number of hilar neurons in both the dorsal and ventral dentate gyrus following 3 hr of convulsive SE.
DISCUSSION
The main original findings of this study are as follows. First, continuous perforant pathway stimulation that forced the hippocampus to discharge throughout the duration of convulsive SE, produced a highly reproducible pattern of bilateral hippocampal and extra-hippocampal injury, and nearly all animals exhibited spontaneous Stage 3−5 behavioral seizures within 3 days post-SE. Second, all spontaneous Stage 3−5 behavioral seizures were preceded by bilaterally synchronous dentate granule cell layer discharges, which represents the first unequivocal demonstration of hippocampal-onset epileptic seizures associated with SE-induced hippocampal injury. Third, hippocampal granule cell epileptogenesis was coincident with the initial pathology, not delayed secondary processes. Fourth, despite 3 hr of continuous, confirmed hippocampal seizure activity, no epileptic animals exhibited the extensive pyramidal cell loss that defines classic hippocampal sclerosis (Margerison and Corsellis, 1966; Bruton, 1988; Jackson et al., 2004).
Minimal latency to hippocampal epileptogenesis following convulsive SE
Our data suggest that the notion that animals are in a “pre-epileptic” or “non-epileptic” state for an extended period after prolonged convulsive SE, and that they then become “epileptic” because a delayed secondary epileptogenic process has gradually matured, is no longer tenable. The results of three recent studies in pilocarpine-treated rats (Raol et al., 2006; Goffin et al., 2007; Jung et al., 2007), and our results in pilocarpine-treated rats reported here, indicate that the pilocarpine model of epileptogenesis exhibits a latent period too short to accommodate delayed secondary epileptogenic mechanisms. Our finding that electrically stimulated rats, like pilocarpine-treated rats, exhibited spontaneous clinical epilepsy without delay after initial injury demonstrates that the minimal latency to clinical epilepsy after convulsive SE is not model-specific or related to the presence of residual chemoconvulsant, but is an immediate consequence of prolonged convulsive SE per se.
Continuous monitoring via bilateral granule cell layer recording electrodes in awake animals revealed that the onset of spontaneous granule cell epileptiform discharges, i.e. confirmed hippocampal epileptogenesis after hippocampal injury, was coincident with the initial insult and the acute neurodegenerative phase, rather than a delayed secondary process. Dentate granule cells in awake rats generated spontaneous population spikes (“fast ripples;” Bragin et al., 2002) and full epileptiform granule cell discharges shortly after stimulation-induced SE, and these epileptiform discharges preceded each and every spontaneous behavioral seizure in all rats that exhibited Stage 3−5 epileptic seizures. Thus, many of the abnormal granule cell population events observed in hippocampal slices from chronically epileptic chemoconvulsant-treated rats, and attributed to the reactive synaptic reorganization (Tauck and Nadler, 1985; Wuarin and Dudek, 1996) triggered by hilar mossy cell degeneration (Jiao and Nadler, 2007), are unlikely to be causally related to any delayed secondary process. A similar conclusion was reached by Strafstrom and colleagues in kainate-treated rats when they noted that spontaneous seizures began before synaptic reorganization (Stafstrom et al., 1992). The close temporal association between initial hippocampal neuron loss, granule cell hyperexcitability, and directly recorded hippocampal granule cell seizure discharges in awake epileptic animals is consistent with the hypothesis that hippocampal principal cell hyperexcitability is an immediate network defect caused by the initial neuron loss (Sloviter, 1987; 1991b, 1994), or other rapid changes (Brooks-Kayal et al., 1998; Goodkin et al., 2005).
Although spontaneous granule cell epileptiform discharges always occurred before, or coincident with, the spontaneous behavioral seizure-onsets, these hippocampal events could have been triggered by discharges originating from within the damaged entorhinal cortex (Schwob et al., 1980; Du et al., 1993; 1995; de Guzman et al., 2008), or other brain nuclei (Spencer and Spencer, 1994; Cohen et al., 2002), and may not have arisen de novo from within the dentate gyrus. The early appearance of spontaneous field potentials and population spikes virtually identical to the potentials evoked by perforant pathway stimulation is consistent with a seizure origin in the entorhinal cortex, although the ultimate seizure source is unknown. Regardless of the site of seizure origin, immediate granule cell hyperexcitability and spontaneous granule cell seizure discharges were associated with acute neuronal injury that occurred throughout the longitudinal extent of the dentate hilus, and in the entorhinal cortex (as well as other regions). The extraordinarily synchronous discharges that arose spontaneously from the granule cell layers in both hippocampi suggests an origin in one entorhinal cortex, via ipsilateral and contralateral excitatory projections (Sloviter, 1983), or in both cortices simultaneously. The network mechanism that synchronizes both hippocampi in vivo cannot be inferred from our data, but the bilateral synchronicity of each and every spontaneous granule cell discharge is evidence that such a mechanism exists.
With regard to the properties of this new animal model of controlled hippocampal and convulsive SE, several advantages are apparent over existing models that utilize chemoconvulsants or self-sustained SE initiated by electrical stimulation. First, we found previously that convulsive SE induced by kainate rarely involved continuous granule cell discharges during convulsive SE (Sloviter et al., 2003), which presumably explains the highly variable and often less than extensive hilar neuron loss after kainate-induced SE (Schwob et al., 1980; Buckmaster and Dudek, 1997; Zappone and Sloviter, 2004). By forcing the dentate granule cells to discharge throughout the period of convulsive SE, the perforant pathway stimulation paradigm we used produced a highly uniform pattern of damage, including extensive dorsal and ventral hilar neuron loss bilaterally, and virtually all animals exhibited the same minimal latency to the first spontaneous granule cell layer epileptiform discharge and behavioral epileptic seizure. Second, we have previously reported that both kainate- and pilocarpine-induced SE involve a vascular abnormality arising from blood vessels of the hippocampal fissure that causes ischemic injury in addition to excitotoxic injury (Sloviter, 2005). We saw no evidence of this phenomenon in animals subjected to stimulation-induced SE. Third, presumably as a result of initiating convulsive SE in the dentate gyrus (via perforant pathway stimulation), hippocampal injury was reliably produced, and all spontaneous behavioral seizures involved hippocampal epileptiform discharges that preceded each behavioral seizure. This contrasts sharply with our previous finding that pilocarpine-treated rats, monitored under identical conditions, never exhibited hippocampal-onset seizures (Harvey and Sloviter, 2005). Thus, this new SE model, based on continuous afferent stimulation throughout the duration of convulsive SE, reliably involves hippocampal endfolium sclerosis and hippocampal epileptogenesis, with minimal mortality and minimal variability among animals.
Limitations of interpretation
Several problems of interpretation were addressed in the experimental design. The main issues from previous studies on the latency to hippocampal epileptogenesis and clinical epilepsy include the following. First, non-continuous video monitoring produces an inherently inaccurate estimate of the latent period. Second, the use of chemoconvulsants makes any estimation of the latent period uncertain because of the possible effects of the circulating excitatory substances used to initiate convulsive SE (i.e. kainate or pilocarpine). Third, the use of EEG recording, which cannot identify the structures generating the spontaneous discharges, and low sampling rates typical of EEG recording, which cannot differentiate between low and higher frequency events, makes it difficult to determine whether hippocampal neurons are involved, or whether high amplitude events are true, high-frequency “epileptiform” discharges (Fig. 3 in this paper; Harvey and Sloviter, 2005). Our use of electrical stimulation to induce SE, continuous (24/7) video recording starting immediately after SE, and depth recording from the dentate granule cell layers at a sampling rate of 10 kHz avoided these three issues entirely.
However, other factors that can confound interpretation were not eliminated in this study. First, the use of bilateral electrical stimulation to replicate the bilateral status epilepticus produced by chemoconvulsants eliminated chemoconvulsants, but introduced the issue of the possible role of permanently implanted electrodes. Although epileptic seizures were not observed in chronically-implanted sham controls, the presence of electrodes could have had some additive or synergistic effect in the stimulated, epileptic animals. This issue is addressed to some degree by our observation that animals that lost their electrode assembly after SE still became epileptic in the post-SE period (Bumanglag and Sloviter, unpublished), but this does not eliminate the issue of electrodes being present before and during convulsive SE, or the direct damage being present following loss of the electrode assembly. Second, we noted that the minimal latency to granule cell-onset epilepsy was associated with extensive hilar neuron loss throughout the longitudinal axis. Although this is consistent with our earlier hypothesis that mossy cell loss is the cause of the granule cell hyperexcitability (Sloviter, 1987; 1991b; 1994), this is only a correlation, and the granule cell epileptogenesis we observed was also correlated with all other pathology, and any other unmeasured early changes, such as altered receptors, gliosis, and other cellular changes.
What explains the widespread notion of an extended latent period after convulsive SE in rats?
We attribute the enduring notion of a seizure-free, “pre-epileptic” period of several weeks duration after convulsive SE in rats to the way the discovery of spontaneous seizures in kainate-treated rats was first reported, and how it has been reinforced by sporadic behavioral monitoring methods that unavoidably overestimate the duration of the latent period. In the first description of spontaneous seizures after convulsive SE of which we are aware, Pisa and colleagues (1980) reported seeing epileptic seizures 35−77 days post-SE. However, these authors only started their behavioral observations on day 35, presumably because they were addressing the issue of whether, not when, spontaneous seizures develop. The notion of the post-SE latent period as an extended silent period of at least several weeks duration became the primary rationale for proposing that delayed secondary events are likely epileptogenic mechanisms (Tauck and Nadler, 1985; Parent et al., 1997; Chang and Lowenstein, 2003). Although this view has been repeatedly reinforced by studies that used sporadic monitoring methods (Cavalheiro et al., 1982; Nissinen et al., 2000; Glien et al., 2001; Brandt et al., 2003; Kobayashi and Buckmaster, 2003), continuous monitoring of pilocarpine-treated rats has consistently detected latent periods of less than one week (Raol et al., 2006; Goffin et al., 2007). In addition, a recent study reported that pilocarpine-induced SE that lasted for only one hour also resulted in spontaneous seizures during the first week post-SE (Jung et al., 2007). Our data from pilocarpine-treated rats are identical to those of these three recent studies, and our data from stimulated animals demonstrate that early seizures after SE are not model-dependent or related to the presence of residual chemoconvulsant.
Although 3 hr of confirmed hippocampal epileptiform discharging and convulsive SE was reliably associated with a minimal latency to the onset of spontaneous epileptic seizures, we do not deny that injured animals can exhibit an extended period before behavioral seizures begin, as some studies that have used continuous video observation have reported (Bertram and Cornett, 1993; Hamani and Mello, 2002; Gorter et al., 2001; Nissinen et al., 2001; Mazarati et al., 2002; Riban et al., 2002; van Vliet et al., 2004; D'Ambrosio et al., 2005; El-Hassar et al., 2007), and as we found in this study in one stimulated rat that was continuously monitored. Several factors undoubtedly explain why some rats do not exhibit spontaneous behavioral seizures in the first days after convulsive SE-induced injury. First, damage to the perirhinal cortex and related structures can be so severe after chemoconvulsant-induced SE (Schwob et al., 1980; Nissinen et al., 2001; Hamani and Mello, 2002; Harvey and Sloviter, 2005; Sloviter, 2005; Niessen et al., 2005) that clinical seizure expression may be blocked by the loss of this critical link in the serial seizure circuit, at least until connections can be re-established, or seizure spread re-routed (McIntyre and Gilby, 2007). Second, recent results with perforant pathway stimulation in anesthetized rats show that remarkably subtle damage to hilar neurons of the dorsal hippocampus, and to neurons of the entorhinal cortex, amygdala, and thalamus (Sloviter et al., 2007) resulted in a confirmed 5 week latent period before spontaneous behavioral seizures were observed (Bumanglag and Sloviter, unpublished observation). The uniformly minimal latencies we observed in this study may simply be due to the bilaterally extensive neuron loss we reliably observed. Thus, latent periods, when they occur, may simply reflect limited or unilateral brain damage (Gorter et al., 2001; Brandt et al., 2003) in more variable animal models, which keeps focal discharges subclinical for an extended period of time. Our observation that brief spontaneous granule cell discharges reliably failed to cause Stage 3−5 behavioral seizures suggests that subtle brain damage, or a lack of brain damage in particular brain nuclei, may result in an extended period of subclinical seizures (Mazarati et al., 2002) that has been mistaken for a seizure-free, “pre-epileptic” latent period.
The finding that hippocampal epileptogenesis and clinical epilepsy developed rapidly after stimulation-induced convulsive SE suggests that the epileptogenic process may have two main constituent mechanisms, depending on the severity or location of initial injury: 1) initial neuron loss (Sloviter, 1991b; 1994) and other acute changes (Brooks-Kayal et al., 1998; Goodkin et al., 2005) that are sufficient to cause clinical epilepsy without a delayed secondary mechanism, and; 2) a “kindling” process (Goddard et al., 1969; Bertram, 2007) that may occur after injury in less severely damaged animals. Early clinical seizure expression after extensive injury may be the result of damage to all brain regions that normally act as serial barriers to the clinical expression of focal seizure activity. According to this hypothesis, the presence of a prolonged seizure-free latent period in less severely damaged rats, and in many human patients, may reflect the time needed for initially subclinical epileptic discharges (Mazarati et al., 2002; Bragin et al., 2004) to “kindle” through undamaged seizure barriers, or to recruit nuclei not damaged by the initial insult (Cavalheiro et al., 1991; Bertram and Cornett, 1993; 1994). Therefore, we hypothesize that the latent period is directly related, and inversely proportional, to the extent of initial neuron loss in the brain regions involved in seizure initiation, spread, and clinical expression. Other delayed secondary mechanisms triggered by neuron loss or injury could certainly play pathophysiological roles in models that exhibit a verified latent period, but the hypothesized roles of synaptic reorganization, neurogenesis, and other delayed secondary processes need to be studied in models first shown to exhibit a “pre-epileptic” state after injury, as well as granule cell-onset seizures. Regardless, our data primarily indicate that hippocampal epileptogenesis occurs rapidly after prolonged convulsive SE, and is coincident with initial cell loss or other rapid effects.
Animal models and the problematic concept of the latent period in human epilepsy
The clear causal relationship between a known injury (e.g. traumatic brain injury in a previously normal person) in some patients, and the often delayed emergence of clinical epileptic seizures, led to the concept of the “latent” period as the time interval during which a slowly progressing epileptogenic process “ripens” (Earle et al., 1953). However, the notion that the latent period is a reliable indicator of the duration of the epileptogenic mechanism in humans is problematic, in part, because the latent period can be very short (French et al., 1993; Wieser, 2004; Mikaeloff et al., 2006) or extremely long (French et al., 1993; Lhatoo et al., 2001). It is difficult to conceive of a metabolic or structural process that takes 30 years to mature, and impractical to study it. Importantly, many early insults are remembered events that may or may not have played any causal epileptogenic role. For example, cases of extremely long latent periods (>30 years) have been calculated as the period between childhood febrile seizures and the subsequent appearance of afebrile epilepsy (French et al., 1993; Lhatoo et al., 2001). If the early febrile seizures were the cause of the later epileptic state, it might be reasonable to consider the interval between the two events to be a “latent” period, and perhaps to conclude that a slow process lasting for years or decades had finally matured. However, if both the childhood febrile seizures and the epilepsy were two separate results of a pre-existing abnormality, then the interval between the two events cannot be considered a “latent” period, because the first event did not cause the second (Grünewald, 2002). Importantly, some patients exhibit little or no latent period between a known injury and epilepsy (Wieser, 2004), indicating that epileptogenesis can occur rapidly, particularly after prolonged convulsive SE (Mikaeloff et al., 2006).
Although it is conceivable that latent periods of different durations could reflect different mechanisms that progress at different rates, the stereotypical clinical similarities among patients with acquired MTLE (French et al., 1993; Engel, 1996) suggest a common underlying network defect, at least as a logical starting point for generating testable hypotheses. We have previously hypothesized that the latent period is inversely proportional to the extent of dentate hilar mossy cell loss throughout the longitudinal axis of the hippocampal dentate gyrus (Sloviter, 1994) because mossy cells are hypothesized to constitute an inhibitory neuron-activating system that maintains granule cell inhibition throughout the dentate gyrus (Zappone and Sloviter, 2004). Based on our finding in this study that extensive bilateral hilar neuron loss throughout the dorsal and ventral dentate gyrus was associated with minimal latency to hippocampal epileptogenesis, and the recent report that bilateral hippocampal sclerosis in patients was associated with no apparent latent period in human patients that survived prolonged convulsive SE (Mikaeloff et al., 2006), we hypothesize that the presence of a latent period, when one occurs, may be related to the asymmetrical hippocampal injury exhibited by many MTLE patients with hippocampal sclerosis (Margerison and Corsellis, 1966; Bruton, 1988; Jackson et al., 2004). That is, disproportionate initial damage to one hippocampus may cause focal, initially subclinical seizures that do not spread to cause clinical seizures until the undamaged or less damaged contralateral hippocampus can be recruited by the unilateral hippocampal seizure focus. If the undamaged or less damaged hippocampus is initially a barrier to the spread of seizure activity, as indicated by the relative resistance of the hippocampus to kindling (McIntyre et al., 1999; McIntyre and Gilby, 2007), the latent period may simply be a time-consuming process in which a unilateral seizure focus gradually overcomes the resistance to seizure spread in structures that initially retain the capacity to resist the recruitment process. Although exactly how initially subclinical network defects become clinical disorders remains a subject of speculation, animal models that more closely resemble the limited pathology and pathophysiology of human temporal lobe epilepsy, and that exhibit a confirmed latent period, are needed if epileptogenic mechanisms other than initial neuron loss are to be identified, and if neuroprotective and anti-epileptogenic treatments are to be developed successfully.
ACKNOWLEDGEMENTS
This work was supported by grant NS18201 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health.
LITERATURE CITED
- Andersen P, Holmqvist B, Voorhoeve PE. Entorhinal activation of dentate granule cells. Acta Physiol Scand. 1966;66:448–460. doi: 10.1111/j.1748-1716.1966.tb03223.x. [DOI] [PubMed] [Google Scholar]
- Arida RM, Scorza FA, Peres CA, Cavalheiro EA. The course of untreated seizures in the pilocarpine model of epilepsy. Epilepsy Res. 1999;34:99–107. doi: 10.1016/s0920-1211(98)00092-8. [DOI] [PubMed] [Google Scholar]
- Bertram E. The relevance of kindling for human epilepsy. Epilepsia. 2007;48(Suppl 2):65–74. doi: 10.1111/j.1528-1167.2007.01068.x. [DOI] [PubMed] [Google Scholar]
- Bertram EH, Cornett J. The ontogeny of seizures in a rat model of limbic epilepsy: evidence for a kindling process in the development of chronic spontaneous seizures. Brain Res. 1993;625:295–300. doi: 10.1016/0006-8993(93)91071-y. [DOI] [PubMed] [Google Scholar]
- Bertram EH, Cornett JF. The evolution of a rat model of chronic spontaneous limbic seizures. Brain Res. 1994;661:157–162. doi: 10.1016/0006-8993(94)91192-4. [DOI] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Almajano J, Mody I, Engel J., Jr High-frequency oscillations after status epilepticus: epileptogenesis and seizure genesis. Epilepsia. 2004;45:1017–1023. doi: 10.1111/j.0013-9580.2004.17004.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Engel J., Jr Rate of interictal events and spontaneous seizures in epileptic rats after electrical stimulation of hippocampus and its afferents. Epilepsia. 2002;43(Suppl 5):81–85. doi: 10.1046/j.1528-1157.43.s.5.22.x. [DOI] [PubMed] [Google Scholar]
- Brandt C, Glien M, Potschka H, Volk H, Loscher W. Epileptogenesis and neuropathology after different types of status epilepticus induced by prolonged electrical stimulation of the basolateral amygdala in rats. Epilepsy Res. 2003;55:83–103. doi: 10.1016/s0920-1211(03)00114-1. [DOI] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective changes in single cell GABA(A) receptor subunit expression and function in temporal lobe epilepsy. Nat Med. 1998;4:1166–1172. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- Bruton CJ. The neuropathology of temporal lobe epilepsy. Oxford University Press; Oxford: 1988. [Google Scholar]
- Buckmaster PS, Dudek FE. Neuron loss, granule cell axon reorganization, and functional changes in the dentate gyrus of epileptic kainate-treated rats. J Comp Neurol. 1997;385:385–404. [PubMed] [Google Scholar]
- Cavalheiro EA, Riche DA, Le Gal La Salle G. Long-term effects of intrahippocampal kainic acid injection in rats: a method for inducing spontaneous recurrent seizures. Electroencephalogr Clin Neurophysiol. 1982;53:581–589. doi: 10.1016/0013-4694(82)90134-1. [DOI] [PubMed] [Google Scholar]
- Cavalheiro EA, Leite JP, Bortolotto ZA, Turski WA, Ikonomidou C, Turski L. Long-term effects of pilocarpine in rats: structural damage of the brain triggers kindling and spontaneous recurrent seizures. Epilepsia. 1991;32:778–782. doi: 10.1111/j.1528-1157.1991.tb05533.x. [DOI] [PubMed] [Google Scholar]
- Chang BS, Lowenstein DH. Epilepsy. N Engl J Med. 2003;349:1257–1266. doi: 10.1056/NEJMra022308. [DOI] [PubMed] [Google Scholar]
- Chen S, Buckmaster PS. Stereological analysis of forebrain regions in kainate-treated epileptic rats. Brain Res. 2005;1057:141–152. doi: 10.1016/j.brainres.2005.07.058. [DOI] [PubMed] [Google Scholar]
- Cohen I, Navarro V, Clemenceau S, Baulac M, Miles R. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science. 2002;298:1418–1421. doi: 10.1126/science.1076510. [DOI] [PubMed] [Google Scholar]
- D'Ambrosio R, Fender JS, Fairbanks JP, Simon EA, Born DE, Doyle DL, Miller JW. Progression from frontal-parietal to mesial-temporal epilepsy after fluid percussion injury in the rat. Brain. 2005;128:174–188. doi: 10.1093/brain/awh337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guzman P, Inaba Y, Baldelli E, de Curtis M, Biagini G, Avoli M. Network hyperexcitability within the deep layers of the pilocarpine-treated rat entorhinal cortex. J Physiol. 2008;586:1867–1883. doi: 10.1113/jphysiol.2007.146159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du F, Eid T, Lothman EW, Kohler C, Schwarcz R. Preferential neuronal loss in layer III of the medial entorhinal cortex in rat models of temporal lobe epilepsy. J Neurosci. 1995;15:6301–6313. doi: 10.1523/JNEUROSCI.15-10-06301.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du F, Whetsell WO, Jr, Abou-Khalil B, Blumenkopf B, Lothman EW, Schwarcz R. Preferential neuronal loss in layer III of the entorhinal cortex in patients with temporal lobe epilepsy. Epilepsy Res. 1993;16:223–233. doi: 10.1016/0920-1211(93)90083-j. [DOI] [PubMed] [Google Scholar]
- Earle KM, Baldwin M, Penfield W. Incisural sclerosis and temporal lobe seizures produced by hippocampal herniation at birth. AMA Arch Neurol Psychiatry. 1953;69:27–42. doi: 10.1001/archneurpsyc.1953.02320250033003. [DOI] [PubMed] [Google Scholar]
- El-Hassar L, Milh M, Wendling F, Ferrand N, Esclapez M, Bernard C. Cell domain-dependent changes in the glutamatergic and GABAergic drives during epileptogenesis in the rat CA1 region. J Physiol. 2007;578:193–211. doi: 10.1113/jphysiol.2006.119297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J., Jr Introduction to temporal lobe epilepsy. Epilepsy Res. 1996;26:141–150. doi: 10.1016/s0920-1211(96)00043-5. [DOI] [PubMed] [Google Scholar]
- French JA, Williamson PD, Thadani VM, Darcey TM, Mattson RH, Spencer SS, Spencer DD. Characteristics of medial temporal lobe epilepsy: I. Results of history and physical examination. Ann Neurol. 1993;34:774–780. doi: 10.1002/ana.410340604. [DOI] [PubMed] [Google Scholar]
- Glien M, Brandt C, Potschka H, Voigt H, Ebert U, Loscher W. Repeated low-dose treatment of rats with pilocarpine: low mortality but high proportion of rats developing epilepsy. Epilepsy Res. 2001;46:111–119. doi: 10.1016/s0920-1211(01)00272-8. [DOI] [PubMed] [Google Scholar]
- Goddard GV, McIntyre DC, Leech CK. A permanent change in brain function resulting from daily electrical stimulation. Exp Neurol. 1969;25:295–330. doi: 10.1016/0014-4886(69)90128-9. [DOI] [PubMed] [Google Scholar]
- Goffin K, Nissinen J, Van Laere K, Pitkanen A. Cyclicity of spontaneous recurrent seizures in pilocarpine model of temporal lobe epilepsy in rat. Exp Neurol. 2007;205:501–505. doi: 10.1016/j.expneurol.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Goodkin HP, Yeh JL, Kapur J. Status epilepticus increases the intracellular accumulation of GABA-A receptors. J Neurosci. 2005;25:5511–5520. doi: 10.1523/JNEUROSCI.0900-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorter JA, van Vliet EA, Aronica E, Lopes da Silva FH. Progression of spontaneous seizures after status epilepticus is associated with mossy fibre sprouting and extensive bilateral loss of hilar parvalbumin and somatostatin-immunoreactive neurons. Eur J Neurosci. 2001;13:657–669. doi: 10.1046/j.1460-9568.2001.01428.x. [DOI] [PubMed] [Google Scholar]
- Grünewald R. Childhood seizures and their consequences for the hippocampus. Brain. 2002;125:1935–1936. doi: 10.1093/brain/awf195. [DOI] [PubMed] [Google Scholar]
- Hamani C, Mello LE. Spontaneous recurrent seizures and neuropathology in the chronic phase of the pilocarpine and picrotoxin model epilepsy. Neurol Res. 2002;24:199–209. doi: 10.1179/016164102101199611. [DOI] [PubMed] [Google Scholar]
- Harvey BD, Sloviter RS. Hippocampal granule cell activity and c-Fos expression during spontaneous seizures in awake, chronically epileptic, pilocarpine-treated rats; implications for hippocampal epileptogenesis. J Comp Neurol. 2005;488:442–463. doi: 10.1002/cne.20594. [DOI] [PubMed] [Google Scholar]
- Jackson GD, Briellmann RS, Kuzniecky RI. In: Temporal Lobe Epilepsy. Kuzniecky RI, Jackson GD, editors. Magnetic Resonance in Epilepsy; Neuroimaging Techniques; Elsevier, Amsterdam: 2004. pp. 99–176. [Google Scholar]
- Jiao Y, Nadler JV. Stereological analysis of GluR2-immunoreactive hilar neurons in the pilocarpine model of temporal lobe epilepsy: correlation of cell loss with mossy fiber sprouting. Exp Neurol. 2007;205:569–582. doi: 10.1016/j.expneurol.2007.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Jones TD, Lugo JN, Sheerin JH, Miller JW, D'Ambrosio R, Anderson AE, Poolos NP. Progressive dendritic HCN channelopathy during epileptogenesis in the rat pilocarpine model of epilepsy. J Neurosci. 2007;27:13012–13021. doi: 10.1523/JNEUROSCI.3605-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Buckmaster PS. Reduced inhibition of dentate granule cells in a model of temporal lobe epilepsy. J Neurosci. 2003;23:2440–2452. doi: 10.1523/JNEUROSCI.23-06-02440.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite JP, Garcia-Cairasco N, Cavalheiro EA. New insights from the use of pilocarpine and kainate models. Epilepsy Res. 2002;50:93–103. doi: 10.1016/s0920-1211(02)00072-4. [DOI] [PubMed] [Google Scholar]
- Lhatoo SD, Sander JW, Fish D. Temporal lobe epilepsy following febrile seizures: unusually prolonged latent periods. Eur Neurol. 2001;46:165–166. doi: 10.1159/000050796. [DOI] [PubMed] [Google Scholar]
- Lothman EW, Bertram EH, Kapur J, Stringer JL. Recurrent spontaneous hippocampal seizures in the rat as a chronic sequela to limbic status epilepticus. Epilepsy Res. 1990;6:110–118. doi: 10.1016/0920-1211(90)90085-a. [DOI] [PubMed] [Google Scholar]
- Margerison JH, Corsellis JA. Epilepsy and the temporal lobes. A clinical, electroencephalographic and neuropathological study of the brain in epilepsy, with particular reference to the temporal lobes. Brain. 1966;89:499–530. doi: 10.1093/brain/89.3.499. [DOI] [PubMed] [Google Scholar]
- Mazarati A, Bragin A, Baldwin R, Shin D, Wilson C, Sankar R, Naylor D, Engel J, Wasterlain CG. Epileptogenesis after self-sustaining status epilepticus. Epilepsia. 2002;43(Suppl 5):74–80. doi: 10.1046/j.1528-1157.43.s.5.25.x. [DOI] [PubMed] [Google Scholar]
- McIntyre DC, Gilby KL. Mapping seizure pathways in the temporal lobe. Epilepsia. 2007;49(Suppl. 3):23–30. doi: 10.1111/j.1528-1167.2008.01507.x. [DOI] [PubMed] [Google Scholar]
- McIntyre DC, Kelly ME, Dufresne C. FAST and SLOW amygdala kindling rat strains: comparison of amygdala, hippocampal, piriform and perirhinal cortex kindling. Epilepsy Res. 1999;35:197–209. doi: 10.1016/s0920-1211(99)00012-1. [DOI] [PubMed] [Google Scholar]
- Mello LE, Cavalheiro EA, Tan AM, Kupfer WR, Pretorius JK, Babb TL, Finch DM. Circuit mechanisms of seizures in the pilocarpine model of chronic epilepsy: cell loss and mossy fiber sprouting. Epilepsia. 1993;34:985–995. doi: 10.1111/j.1528-1157.1993.tb02123.x. [DOI] [PubMed] [Google Scholar]
- Mikaeloff Y, Jambaque I, Hertz-Pannier L, Zamfirescu A, Adamsbaum C, Plouin P, Dulac O, Chiron C. Devastating epileptic encephalopathy in school-aged children (DESC): a pseudo encephalitis. Epilepsy Res. 2006;69:67–79. doi: 10.1016/j.eplepsyres.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Niessen HG, Angenstein F, Vielhaber S, Frisch C, Kudin A, Elger CE, Heinze HJ, Scheich H, Kunz WS. Volumetric magnetic resonance imaging of functionally relevant structural alterations in chronic epilepsy after pilocarpine-induced status epilepticus in rats. Epilepsia. 2005;46:1021–1026. doi: 10.1111/j.1528-1167.2005.60704.x. [DOI] [PubMed] [Google Scholar]
- Nissinen J, Halonen T, Koivisto E, Pitkanen A. A new model of chronic temporal lobe epilepsy induced by electrical stimulation of the amygdala in rat. Epilepsy Res. 2000;38:177–205. doi: 10.1016/s0920-1211(99)00088-1. [DOI] [PubMed] [Google Scholar]
- Nissinen J, Lukasiuk K, Pitkänen A. Is mossy fiber sprouting present at the time of the first spontaneous seizures in rat experimental temporal lobe epilepsy? Hippocampus. 2001;11:299–310. doi: 10.1002/hipo.1044. [DOI] [PubMed] [Google Scholar]
- Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisa M, Sandberg PR, Corcoran ME, Fibiger HC. Spontaneous recurrent seizures after intra-cerebral injections of kainic acid in rats: a possible model of human temporal lobe epilepsy. Brain Res. 1980;200:481–487. doi: 10.1016/0006-8993(80)90938-5. [DOI] [PubMed] [Google Scholar]
- Priel MR, dos Santos NF, Cavalheiro EA. Developmental aspects of the pilocarpine model of epilepsy. Epilepsy Res. 1996;26:115–121. doi: 10.1016/s0920-1211(96)00047-2. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Raol YH, Lund IV, Bandyopadhyay S, Zhang G, Roberts DS, Wolfe JH, Russek SJ, Brooks-Kayal AR. Enhancing GABA(A) receptor alpha 1 subunit levels in hippocampal dentate gyrus inhibits epilepsy development in an animal model of temporal lobe epilepsy. J Neurosci. 2006;26:11342–11346. doi: 10.1523/JNEUROSCI.3329-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riban V, Bouilleret V, Pham-Lê BT, Fritschy JM, Marescaux C, Depaulis A. Evolution of hippocampal epileptic activity during the development of hippocampal sclerosis in a mouse model of temporal lobe epilepsy. Neuroscience. 2002;112:101–111. doi: 10.1016/s0306-4522(02)00064-7. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874:123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- Schwob JE, Fuller T, Price JL, Olney JW. Widespread patterns of neuronal damage following systemic or intracerebral injections of kainic acid: a histological study. Neuroscience. 1980;5:991–1014. doi: 10.1016/0306-4522(80)90181-5. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. “Epileptic” brain damage in rats induced by sustained electrical stimulation of the perforant path. I. Acute electrophysiological and light microscopic studies. Brain Res Bull. 1983;10:675–697. doi: 10.1016/0361-9230(83)90037-0. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy. Science. 1987;235:73–76. doi: 10.1126/science.2879352. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. Feedforward and feedback inhibition of hippocampal principal cell activity evoked by perforant path stimulation: GABA-mediated mechanisms that regulate excitability in vivo. Hippocampus. 1991a;1:31–40. doi: 10.1002/hipo.450010105. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. Permanently altered hippocampal structure, excitability, and inhibition after experimental status epilepticus in the rat: the dormant basket cell hypothesis and its possible relevance to temporal lobe epilepsy. Hippocampus. 1991b;1:41–66. doi: 10.1002/hipo.450010106. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. Possible functional consequences of synaptic reorganization in the dentate gyrus of kainate-treated rats. Neurosci Lett. 1992;137:91–96. doi: 10.1016/0304-3940(92)90306-r. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. The functional organization of the hippocampal dentate gyrus and its relevance to the pathogenesis of temporal lobe epilepsy. Ann Neurol. 1994;35:640–654. doi: 10.1002/ana.410350604. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. The neurobiology of temporal lobe epilepsy: too much information, not enough knowledge. CR Biol. 2005;328:143–153. doi: 10.1016/j.crvi.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Damiano BP. On the relationship between kainic acid-induced epileptiform activity and hippocampal neuronal damage. Neuropharmacology. 1981a;20:1003–1011. doi: 10.1016/0028-3908(81)90088-5. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Damiano BP. Sustained electrical stimulation of the perforant path duplicates kainate-induced electrophysiological effects and hippocampal damage in rats. Neurosci Lett. 1981b;24:279–284. doi: 10.1016/0304-3940(81)90171-3. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Dichter MA, Rachinsky TL, Dean E, Goodman JH, Sollas AL, Martin DL. Basal expression and induction of glutamate decarboxylase and GABA in excitatory granule cells of the rat and monkey hippocampal dentate gyrus. J Comp Neurol. 1996;373:593–618. doi: 10.1002/(SICI)1096-9861(19960930)373:4<593::AID-CNE8>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Zappone CA, Bumanglag AV, Norwood B, Kudrimoti H. On the relevance of prolonged convulsive status epilepticus in animals to the etiology and neurobiology of human temporal lobe epilepsy. Epilepsia. 2007;48(Suppl 8):6–10. doi: 10.1111/j.1528-1167.2007.01335.x. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Zappone CA, Harvey BD, Bumanglag AV, Bender RA, Frotscher M. “Dormant basket cell” hypothesis revisited: relative vulnerabilities of dentate gyrus mossy cells and inhibitory interneurons after hippocampal status epilepticus in the rat. J Comp Neurol. 2003;459:44–76. doi: 10.1002/cne.10630. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Zappone CA, Harvey BD, Frotscher M. Kainic acid-induced recurrent mossy fiber innervation of dentate gyrus inhibitory interneurons: possible anatomical substrate of granule cell hyper-inhibition in chronically epileptic rats. J Comp Neurol. 2006;494:944–960. doi: 10.1002/cne.20850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SS. Substrates of localization-related epilepsies: biological implications of localizing findings in humans. Epilepsia. 1998;39:114–123. doi: 10.1111/j.1528-1157.1998.tb01349.x. [DOI] [PubMed] [Google Scholar]
- Spencer SS, Spencer DD. Entorhinal-hippocampal interactions in medial temporal lobe epilepsy. Epilepsia. 1994;35:721–727. doi: 10.1111/j.1528-1157.1994.tb02502.x. [DOI] [PubMed] [Google Scholar]
- Stables JP, Bertram E, Dudek FE, Holmes G, Mathern G, Pitkanen A, White HS. Therapy discovery for pharmacoresistant epilepsy and for disease-modifying therapeutics: summary of the NIH/NINDS/AES models II workshop. Epilepsia. 2003;44:1472–1478. doi: 10.1111/j.0013-9580.2003.32803.x. [DOI] [PubMed] [Google Scholar]
- Stafstrom CE, Sutula TP. Models of epilepsy in the developing and adult brain: implications for neuroprotection. Epilepsy Behav. 2005;7(Suppl 3):S18–24. doi: 10.1016/j.yebeh.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Stafstrom CE, Thompson JL, Holmes GL. Kainic acid seizures in the developing brain: status epilepticus and spontaneous recurrent seizures. Dev Brain Res. 1992;65:227–236. doi: 10.1016/0165-3806(92)90184-x. [DOI] [PubMed] [Google Scholar]
- Tauck DL, Nadler JV. Evidence of functional mossy fiber sprouting in hippocampal formation of kainic acid treated rats. J Neurosci. 1985;5:1016–1022. doi: 10.1523/JNEUROSCI.05-04-01016.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turski L, Cavalheiro EA, Czuczwar SJ, Turski WA, Kleinrok Z. The seizures induced by pilocarpine: behavioral, electroencephalographic and neuropathological studies in rodents. Pol J Pharmacol Pharm. 1987;39:545–555. [PubMed] [Google Scholar]
- Turski WA, Cavalheiro EA, Schwarz M, Czuczwar SJ, Kleinrok Z, Turski L. Limbic seizures produced by pilocarpine in rats: behavioural, electroencephalographic and neuropathological study. Behav Brain Res. 1983;9:315–335. doi: 10.1016/0166-4328(83)90136-5. [DOI] [PubMed] [Google Scholar]
- van Vliet EA, Aronica E, Tolner EA, Lopes da Silva FH, Gorter JA. Progression of temporal lobe epilepsy in the rat is associated with immunocytochemical changes in inhibitory interneurons in specific regions of the hippocampal formation. Exp Neurol. 2004;187:367–379. doi: 10.1016/j.expneurol.2004.01.016. [DOI] [PubMed] [Google Scholar]
- Wieser HG. Mesial temporal lobe epilepsy with hippocampal sclerosis. Epilepsia. 2004;45:695–714. doi: 10.1111/j.0013-9580.2004.09004.x. [DOI] [PubMed] [Google Scholar]
- Wuarin JP, Dudek FE. Electrographic seizures and new recurrent excitatory circuits in the dentate gyrus of hippocampal slices from kainate-treated epileptic rats. J Neurosci. 1996;16:4438–4448. doi: 10.1523/JNEUROSCI.16-14-04438.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappone CA, Sloviter RS. Translamellar disinhibition in the rat hippocampal dentate gyrus after seizure-induced degeneration of vulnerable hilar neurons. J Neurosci. 2004;24:853–864. doi: 10.1523/JNEUROSCI.1619-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]