Abstract
It has been shown that mTOR inhibitors activate Akt while inhibiting mTOR signaling. However, the underlying mechanisms and the impact of the Akt activation on mTOR-targeted cancer therapy are unclear. The present work focused on addressing the role of mTOR/rictor in mTOR inhibitor-induced Akt activation and the impact of sustained Akt activation on mTOR-targeted cancer therapy. Thus, we have demonstrated that mTOR inhibitors increase Akt phosphorylation through a mechanism independent of mTOR/rictor because the assembly of mTOR/rictor was inhibited by mTOR inhibitors and the silencing of rictor did not abrogate mTOR inhibitor-induced Akt activation. Moreover, Akt activation during mTOR inhibition is tightly associated with development of cell resistance to mTOR inhibitors. Accordingly, co-targeting mTOR and PI3K/Akt signaling prevents mTOR inhibition-initiated Akt activation and enhances antitumor effects both in cell cultures and in animal xenograft models, suggesting an effective cancer therapeutic strategy. Collectively, we conclude that inhibition of the mTOR/raptor complex initiates Akt activation independent of mTOR/rictor. As a result, the sustained Akt activation during mTOR inhibition will counteract mTOR inhibitors’ anticancer efficacy.
Keywords: mTOR inhibitors, rictor, Akt, cancer therapy
INTRODUCTION
The mammalian target of rapamycin (mTOR), a phosphatidylinositol 3 kinase (PI3K)-related serine/theronine kinase, plays a central role in regulating cell growth, proliferation and survival, in part by regulation of translation initiation, through interactions with other proteins such as raptor (forming mTOR complex 1, mTORC1) and rictor (forming mTOR complex 2, mTORC2) (1–3). The best characterized downstream effectors of mTORC1 are the 70 kDa ribosomal S6 kinase (p70S6K) and the eukaryotic translation initiation factor 4E (eIF4E) binding protein 1 (4E-BP1) (1). In response to mitogenic stimuli or nutrient availability, mTORC1 is activated (4), leading to phosphorylation of p70S6K and 4E-BP1, and the subsequent enhanced translation of mRNAs that are critical for cell cycle progression and proliferation (1).
PI3K/Akt signaling represents a major cell survival pathway. Its activation has long been associated with malignant transformation and apoptotic resistance (5, 6). It is generally thought that mTOR (i.e., mTORC1) functions downstream of the PI3K/Akt pathway and is phosphorylated (or activated) in response to stimuli that activate the PI3K/Akt pathway (1, 7). However, the recent discovery of mTORC2 as an Akt Ser473 kinase also places mTOR upstream of Akt. Although mTORC2 is thought to be insensitive to rapamycin, it has been shown that prolonged rapamycin exposure inhibits mTORC2 assembly and Akt activity in certain types of cancer cells (8). We and others have shown that mTOR inhibitors activate Akt while suppressing mTORC1 signaling in different types of cancer cell lines and clinical human tumor samples (9–11). Currently, it is unclear how mTOR inhibitors activate Akt survival signaling.
mTOR signaling has recently emerged as an attractive therapeutic target for cancer therapy (1, 12). The potential applications of mTOR inhibitors for treating various types of cancer have been actively studied both pre-clinically and clinically. In the United States, several phase II or III trials are ongoing that test the effects of mTOR inhibitors on various cancers (1, 13, 14). A recent study has shown encouraging results that the mTOR inhibitor CCI-779 improved overall survival among patients with metastatic renal-cell carcinoma (15).
In addition to the intrinsic resistance of cancer cells to mTOR inhibition by rapamycin, cancer cells can acquire resistance to rapamycin (16). Therefore, understanding the mechanisms by which cells become resistant to mTOR inhibitors such as rapamycin has long been an interesting subject and may eventually guide the development of successful mTOR-targeted cancer therapy by avoiding or overcoming cell resistance to mTOR inhibition.
The current study aimed at demonstrating the relationship between mTORC2 and mTORC1 inhibition-induced Akt activation, and particularly the biological significance of Akt activation in mTOR-targeted cancer therapy.
MATERIALS AND METHODS
For detailed information on reagents, cell lines, Western blot analysis, growth inhibition assay, colony formation assay, cell cycle analysis, immunohistochemistry (IHC) and statistic analysis, please see supplemental text.
Establishment of a Rapamycin-resistant Cell Line
The rapamycin-resistant A549 cell line (A549-RR) was established by exposing the rapamycin-sensitive A549 parental cells (A549-P) to gradually increased concentrations of rapamycin from the initial 1 nM to the final 20 µM over a 6-month period. A549-RR cells were routinely cultured in complete medium containing 1 µM rapamycin.
Immunoprecipitation (IP)
mTOR complexes were immunoprecipitated with goat polyclonal mTOR (FRAP; N-19) antibody according to the same procedure described previously (17, 18).
Gene Knockdown by Small Interfering RNA (siRNA)
Raptor and rictor siRNAs and lentiviral raptor, rictor and scramble shRNAs were described previously (18) and synthesized from Qiagen (Valencia, CA). For detailed sequences and transfection, please see Supplemental Text.
Lung Cancer Xenografts and Treatments
Animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Emory University. Four-to 6-week old (about 20 g of body weight) female athymic (nu/nu) mice were ordered from Taconic (Hudson, NY) and housed under pathogen-free conditions in microisolator cages with laboratory chow and water ad libitum. A549 cells at 5 × 106 in serum-free medium were injected s.c. into the flank region of nude mice. When tumors reached certain size ranges (100 to 500 mm3), the mice were randomized into four groups (n = 6/group) according to tumor volumes and body weights for the following treatments: vehicle control, formulated RAD001 (4 mg/kg/day, og), LY294002 in DMSO (25 mg/kg/day; ip), and the combination of RAD001 and LY294002. Tumor volumes were measured using caliper measurements once every two days and calculated with the formula V = π(length × width2)/6. After a 14-day treatment, the mice were sacrificed with CO2. The tumors were then removed, weighed and frozen in liquid nitrogen or fixed with formalin. Certain portions of tumor tissues from each tumor were homogenized in protein lysis buffer for preparation of whole-cell protein lysates as described previously (19). Western blotting results were quantitated using Kodak Image Station 2000R (Eastman Kodak Company; Rochester, NY).
RESULTS
Effects of Prolonged Treatment with mTOR Inhibitors on Akt Phosphorylation are Dose-Dependent
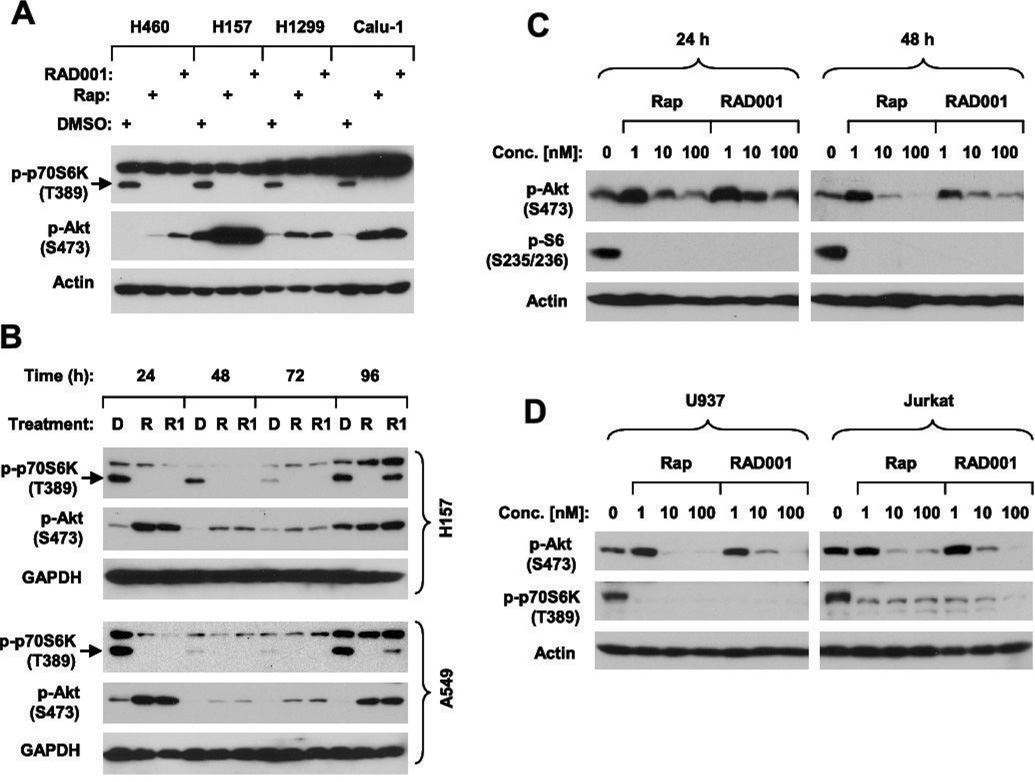
We and others previously showed that rapamycin induces a rapid and sustained increase in Akt phosphorylation in several types of cancer cells including lung, breast and prostate cancer cells (9, 10). However, two recent studies have shown that prolonged treatment with mTOR inhibitors decrease Akt phosphorylation in certain cancer cell lines (e.g., PC-3 and U937) (8, 20). In this study, we further examined the effects of RAD001 in comparison to rapamycin on Akt phosphorylation in a group of lung cancer cell lines after a prolonged treatment. Both RAD001 and rapamycin at 10 nM increased p-Akt levels while inhibiting p70S6K phosphorylation in all of the cell lines after a 24 h treatment (Fig. 1A). We also treated H157 and A549 lung cancer cells with 1 nM RAD001 or rapamycin for a prolonged period of time from 24 to 96 h and then harvested the cells for analysis of Akt phosphorylation. As shown in Fig. 1B, p-Akt levels remained elevated at all the tested times over the prolonged period of time, even when decreased p-p70S6K levels returned at 96 h (i.e., RAD001 at 96 h). This result clearly shows that mTOR inhibitors induce a sustained Akt activation in the tested cell lines. We noted that p-p70S6K levels recovered at 96 h post treatment with RAD001, but not with rapamycin (Fig. 1B). Since we treated cells only once, it is likely that rapamycin may have a longer half-life in cell culture than RAD001, resulting in better efficacy than RAD001 in inhibiting mTOR signaling.
Fig. 1. Effects of prolonged treatment with mTOR inhibitors on Akt phosphorylation.
A, The indicated cell lines were treated with DMSO, 10 nM rapamycin (Rap) or RAD001 for 24 h. B, The indicated cells lines were treated with DMSO (D), 1 nM rapamycin (R) or RAD001 (R1) for the given times. C, PC-3 cells were treated with the given concentrations of rapamycin (Rap) or RAD001 for the indicated times. D, U937 or Jurkat cells were treated with the given concentrations of rapamycin (Rap) or RAD001 for 24 h. The cells were then harvested from the aforementioned treatments for preparation of whole-cell protein lysates and subsequent Western blot analysis.
Moreover, we examined the effects of prolonged treatment with rapamycin or RAD001 on Akt phosphorylation in two cell lines (i.e., PC-3 and U937), in which Akt phosphorylation was decreased by prolonged treatment with rapamycin (8), in a more detailed way. Previous studies used 100 nM rapamycin (8) or > 1000 nM CCI-779 (20), which decreased p-Akt levels after a 24 h treatment. In our study, we could repeat this result after both 24 and 48 h treatments with 100 nM rapamycin in PC-3 cells. However, when the concentration of rapamycin was reduced to 1 nM, we consistently observed an increase in Akt phosphorylation at both 24 h and 48 h treatments. Similar results were also obtained from cells treated with RAD001 (Fig. 1C). In U937 cells, prolonged treatment with either 1 nM rapamycin or RAD001 clearly increased the levels of p-Akt although at 10 nM or 100 nM they decreased p-Akt levels (Fig. 1D). Similar results with RAD001 were also observed in Jurkat cells (Fig. 1D). We noted that both rapamycin and RAD001 at 1 nM sufficiently inhibited mTORC1 signaling evidenced by reduction of p-S6 or p-p70S6K levels (Figs. 1C and 1D). Thus, the effects of prolonged treatment with mTOR inhibitors on Akt phosphorylation are clearly dose-dependent in these cell lines. We also noted that both rapamycin and RAD001 at 1–100 nM increased Akt phosphorylation at Thr308 in a dose-dependent manner in PC-3 cells (see supplemental Fig. S1), suggesting that mTOR inhibitors also activate PDK1 kinase. We noted that our data here on Akt phosphorylation at Thr308 by rapamycin or RAD001 in PC-3 cells are different from previous report that rapamycin at 100 nM slightly decreased Akt phosphorylation at Thr308 after a 24 h treatment (8). The reason for this inconsistency is not clear, but may be due to the different ways the cells were treated by us and other investigators.
Rapamycin Increases Akt Phosphorylation Accompanied with Inhibition of the Assembly of mTORC2
We were interested in the effects of rapamycin on the assembly of mTORC2 under the conditions that Akt phosphorylation is increased. To this end, we immunoprecipiated mTOR complexes from rapamycin-treated cell lysates using an mTOR-specific antibody and then detected raptor and rictor, respectively, in these immunoprecipitates by Western blotting. In the tested cell lines exposed to 10 nM rapamycin for 24 h, the amounts of raptor and particularly rictor in mTOR complexes were substantially reduced, indicating that both mTORC1 and mTORC2 were inhibited in cells exposed to rapamycin, although the levels of p-Akt remained elevated in these cell lines (Fig. 2A). Moreover, we detected mTORC2 in PC-3 cells after a prolonged treatment with rapamycin at either 1 nM (which increases p-Akt levels) or 100 nM (which decreases p-Akt levels) as we presented in Fig. 1C. Rapamycin at both 1 nM and 100 nM effectively decreased the levels of rictor in mTOR complexes precipitated by an mTOR antibody (i.e., inhibition of mTORC2 assembly) albeit with differential effects on alteration of Akt phosphorylation. These results clearly indicate that rapamycin inhibits mTORC2 assembly regardless of its differential effects on regulation of Akt phosphorylation.
Fig. 2. Rapamycin increases Akt phosphorylation independent of mTORC2.
A and B, Detection of assembly of mTORC1 and mTORC2 in cells exposed to rapamycin for a prolonged time. The whole-cell protein lysates were prepared from the indicated lung cancer cell lines exposed to 10 nM rapamycin (Rap) for 24 h (A) or from PC-3 cells treated with 1 nM or 100 nM rapamycin for 24 h (B). These lysates were then subjected to IP using an mTOR antibody. Cell lysates and mTOR immunoprecitates were analyzed by Western blotting. C and D, Knockdown of rictor dose not impair rapamycin’s ability to increase Akt phosphorylation. C, The indicated lung cancer cell lines were transfected with control (Ctrl), raptor and rictor (Ric) siRNAs, respectively, for 48 h. The cells were then treated with 10 nM rapamycin (Rap) for 1 h before harvesting them for preparation of whole-cell protein lysates. D, H157 cells were infected once with lentivirus carrying control, raptor or rictor shRNA and then subjected to selection with 1 µg/ml puromycin for 10 days. After the selection, the cells that survived were further cultured in puromycin-free medium for another 10 days. They were then seeded, treated with 10 nM rapamycin for 1 h and then harvested for preparation of whole-cell protein lysates. The indicated proteins were detected by Western blot analysis.
mTOR Inhibitor-induced Akt Activation is Secondary to mTORC1 Inhibition and cannot be Abrogated by Inhibition of mTORC2
To dissect the roles of mTORC1 and mTORC2 in mTOR inhibitor-induced Akt phosphorylation, we knocked down raptor and rictor expression, which would result in disruption of mTORC1 and mTORC2, respectively. In both Calu-1 and H157 cells, raptor knockdown alone increased p-Akt levels as did rapamycin without altering the levels of p-p70S6K (Fig. 2C, lanes 3 and 11), indicating that disruption of mTORC1 activates Akt. Upon treatment with rapamycin, p-Akt levels were even further increased (lanes 4 vs. 3 and 12 vs. 11), likely due to additional inhibition of the activity of the residual mTORC1. Silencing of rictor using two different siRNAs slightly decreased basal levels of p-Akt (lanes 5, 7, 13 and 15). However, rapamycin still increased p-Akt levels in these cells (lanes 5–8 and 13–16). Similar results were also generated from H157 cells exposed to rapamycin for 24 h, in which raptor and rictor were stably silenced using lentiviral raptor and rictor shRNAs, respectively. Under such conditions, stable silencing of raptor did reduce basal levels of p-p70S6K (Fig. 2D). Collectively, these results indicate that rapamycin-mediated increase in Akt phosphorylation is secondary to mTORC1 inhibition independent of mTORC2. Since transient knockdown of raptor in our system did not apparently decrease p-p70S6K but substantially increased p-Akt levels, these results also suggest that p-Akt is more susceptible than p-p70S6K to modulation by mTOR inhibition, suggesting that mTOR inhibition-induced Akt phosphorylation is unlikely a secondary event to p70S6K inhibition.
The Rapamycin-resistant Cell Line Exhibits Increased Levels of p-Akt with Disrupted mTORC2
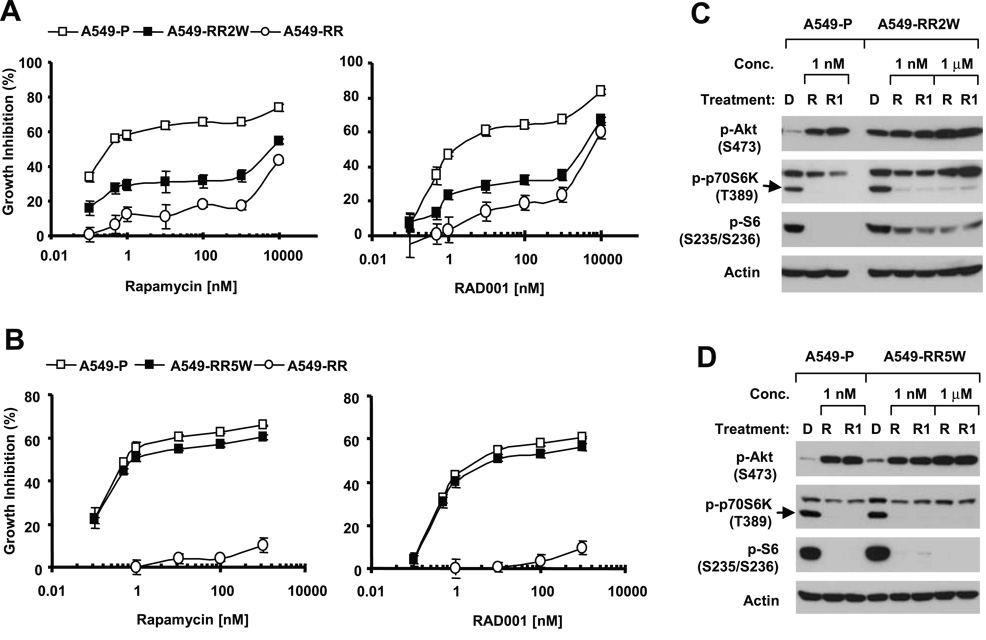
To further demonstrate the impact of long-term mTOR inhibitor exposure on Akt activity, we established a rapamycin-resistant cell line named A549-RR by exposing rapamycin-sensitive A549 cells (A549-P) to gradually increased concentrations of rapamycin from the initial 1 nM to the final 20 µM over a 6-month period. A549-RR cells were resistant not only to rapamycin but also to RAD001 (Fig. 3A) and were at least 10,000-fold more resistant to either rapamycin or RAD001 than A549-P cells by comparing their IC50s. The A549-RR cell line had a comparable growth rate to that of A549-P (Fig. 3B). To maintain the acquired resistance to rapamycin, we routinely cultured A549-RR cells in complete medium containing 1 µM of rapamycin. Twenty-four hours before each experiment, rapamycin was withdrawn from the medium. We observed that A549-RR cells had much higher basal levels of p-Akt than A549-P cells; these high levels of p-Akt were not increased further by either rapamycin or RAD001 (Fig. 3C). In A549-P cells, rapamycin at either 1 nM or 1 µM increased p-Akt levels. The total levels of Akt in both A549-P and A549-RR cell lines were not altered (Fig. 3C, bottom panel). Both GSK3β and FOXO3a are well-known substrates of Akt. The basal levels of p-GSK3β but not p-FOXO3a were accordingly elevated in A549-RR cells compared with those in A549-P cells (Fig. 3C). We noted that p-p70S6K levels were not decreased by rapamycin or RAD001 in A549-RR cells although the phospho-S6 (p-S6) levels were slightly decreased by high concentration (i.e., 1 µM) of rapamycin or RAD001 (Fig. 3C). There results indicate that A549-RR cells lose responses to mTOR inhibitor-mediated inhibition of mTORC1-p70S6K signaling while exhibiting increased levels of p-Akt.
Fig. 3. Comparison of cell sensitivities to mTOR inhibitors (A), growth rates (B), phosphorylation of p-Akt, p-p70S6K and p-S6 (C), and the levels of mTORC1 and mTORC2 (D) between A549 parental (A549-P) and rapamycin-resistant A549 cells (A549-RR).
A, The indicated cell lines were plated in 96-well plates and then treated with different concentrations of rapamycin or RAD001 as indicated on the second day. After 3 days, the cell numbers were estimated using the SRB assay. Each point represents a mean ± SD of four replicate determinations. B, The similar number of A549-P and A549-RR cells were seeded in 96-well plates. At the indicated times, one plate was fixed for estimation of cell number using the SRB assay. Each point represents a mean ± SD of four replicate determinations. C, The indicated cell lines were plated in 10-cm diameter cell culture dishes and treated on the second day with DMSO (D), the given concentrations of rapamycin (R) or RAD001 (R1) for 1 h. The cells were then harvested for preparation of whole-cell protein lysates and subsequent Western blot analysis. Rapamycin was removed from the culture medium for at least 24 h before A549-RR cells were used in the experiments. D, The whole-cell protein lysates were prepared from A549-P and A549-RR cells and then subjected to IP using mTOR antibody. Cell lysates and mTOR immunoprecitates were analyzed by Western blotting.
It has been suggested that downregulation of 4E-BP1 is associated with rapamycin resistance (21). Therefore, we compared the levels of 4E-BP1 and its phosphorylation between A549-P and A549-RR cell lines. As presented in Fig. 3C, we did not find an obvious difference in basal levels of 4E-BP1 between A549-P and A549-RR cell lines. The expression levels of 4E-BP1 were not altered by mTOR inhibitors in both cell lines. We found that both cell lines had comparable levels of phospho-4E-BP1 (p-4E-BP1). p-4E-BP1 levels were reduced by both low (1 nM) and high (1 µM ) concentrations of rapamycin or RAD001 in A549-P cells, but not in A549-RR cells except for the high dose (1 µM) of rapamycin. These results suggest that 4E-BP1 levels cannot account for cell resistance to mTOR inhibitors in our system.
Following these studies, we determined whether the assembly of mTOR complexes was altered in A549-RR cells. Therefore, we compared the levels of mTORC1 and mTORC2 between A549-P and A549-RR cells. The total levels of mTOR, raptor and rictor in cell lysates were not altered in A549-RR cells, however, the amounts of raptor and rictor in mTOR complexes precipitated by an mTOR antibody were strikingly decreased (Fig. 3D), indicating that both mTORC1 and mTORC2 were inhibited in A549-RR cells. Under such circumstances, the levels of p-Akt (S473), p-Akt (T308) and p-GSK3β (S9) were elevated in cell lysates from A549-RR cells compared with those from A549-P cells (Fig. 3D), indicating that A549-RR cells have increased Akt activity albeit with disrupted mTORC2.
Sustained Akt Activation is Associated with Development of Cell Resistance to mTOR Inhibitors
We were interested in the biological significance of sustained Akt activation in mTOR-targeted cancer therapy. To this end, we took advantage of the rapamycin-resistant cell line (i.e., A549-RR) that has elevated levels of p-Akt as described above. We first determined whether the acquired rapamycin resistance in A549-RR cells was reversible. To do so, we cultured A549-RR cells in rapamycin-free complete medium for up to five months and monitored cell responses to mTOR inhibitors and p-Akt levels at one-month intervals. At two months after rapamycin withdrawal, the cell line, which was named A549-RR2W, was slightly more sensitive than A549-RR cells to either rapamycin or RAD001 (Fig. 4A). Even at 3 or 4 months after rapamycin withdrawal, the cells (i.e., A549-RR3W and A549-RR4W) were still partially resistant to mTOR inhibitors although their sensitivities to rapamycin or RAD001 were increased as compared to A549-RR2W cells (data not shown). After a 5-month withdrawal of rapamycin, the cell line, which was named A549-RR5W, was as sensitive as A549-P cells to both rapamycin and RAD001 (Fig. 4B), indicating a complete restoration of rapamycin sensitivity. Collectively, these results indicate that the acquired rapamycin resistance in A549 cells is reversible although it sustains for over 5 months.
Fig. 4. Impact of removal of the selective pressure rapamycin on cell resistance to mTOR inhibitors (A and B) and phosphorylation of Akt, p70S6K and S6 (C and D).
A549-RR cells were cultured in complete medium without rapamycin for two months and 5 months to generate A549-RR2W and A549-RR5W cell lines, respectively. A549-RR2W and A549-RR5W cell lines were then used for the experiments. A and B, The indicated cell lines were plated in 96-well plates and then treated with different concentrations of rapamycin or RAD001 as indicated on the second day. After 3 days, the cell numbers were estimated using the SRB assay. Each point represents a mean ± SD of four replicate determinations. C and D, The indicated cell lines were plated in 10-cm diameter cell culture dishes and treated on the second day with DMSO (D), the given concentrations of rapamycin (R) or RAD001 (R1) for 1 h. The cells were then harvested for preparation of whole-cell protein lysates and subsequent Western blot analysis.
Accordingly, we examined basal p-Akt levels and their modulation by mTOR inhibitors in rapamycin-resistant cell lines during rapamycin withdrawal. After a two-month withdrawal of rapamycin, we found that the basal levels of p-Akt in A549-RR2W cells were still much higher than that in A549-P cells and were only increased by high concentrations of rapamycin or RAD001 (i.e., 1 µM) (Fig. 4C). The basal levels of p-p70S6K in A549-RR2W and A549-P cells were comparable and could be effectively inhibited by both rapamycin and RAD001. Similarly, the p-S6 levels in A549-RR2W and A549-P cells were also comparable and inhibited by mTOR inhibitors (Fig. 4C). After five-month withdrawal of rapamycin when cell sensitivity to rapamycin is fully restored, we noted that p-Akt levels in A549-RR5W cells were as low as those in A549-P cells (Fig. 4D). Upon treatment with rapamycin or RAD001, p-Akt levels were substantially increased in A549-RR5W cells as was observed in A549-P cells (Fig. 4D). As we already demonstrated in A549-RR2W cells, p-p70S6K levels in A549-RR5W cells were comparable to those in A549-P cells and could be effectively decreased by rapamycin or RAD001 (Fig. 4D). Collectively, our results clearly indicate that sustained Akt activation (i.e., increase in p-Akt levels) during mTOR-targeted cancer therapy is associated with cell resistance to mTOR inhibitors.
To further demonstrate this association, we examined whether enforced reduction of p-Akt levels by Akt siRNA alter cell sensitivity to rapamycin. To this end, we decreased p-Akt levels by knocking down the levels of total Akt using Akt siRNA and then examined its impact on cell sensitivity to rapamycin. As presented in supplemental Fig. S2, silencing of Akt by Akt siRNA substantially reduced the levels of p-Akt (Fig. S2A). Accordingly, these cells were much more sensitive than control siRNA-transfected cells to rapamycin (Fig. S2B), indicating that enforced reduction of p-Akt levels restore cell sensitivity to rapamycin. Thus, these results further support the notion that sustained increase in p-Akt levels is associated with the development of cell resistance to mTOR inhibitors.
The Rapamycin-resistant Cell Line Retains Sensitivity to PI3K Inhibitors
Because of the increased levels of p-Akt in A549-RR cells, we determined whether A549-RR cells were cross-resistant to PI3K inhibitors. A549-RR cells responded as well as A549-P cells to either LY294002 or wortmannin in terms of a 3-day monolayer culture assay (supplemental Fig. S3A). By a long-term (10-day) colony formation assay, we found that LY29400 effectively inhibited the growth of both A549-P and A549-RR cells (supplemental Fig. S3B). At the tested concentrations of up to 15 µM, LY294002 failed to induce apoptosis in either A549-P or A549-RR cells by examining cell morphological changes and analysis of sub-G1 populations (data not shown). However, LY294002 induced G1 arrest in both A549-P and A549-RR cells with comparable potencies (supplemental Fig. S3C). Moreover, we compared the effects of LY294002 on p-p70S6K and p-Akt in A549-P and A549-RR cells and found that LY294002 effectively decreased the levels of not only p-p70S6K and p-S6, but also p-Akt in both cell lines although A549-RR cells had very high basal levels of p-Akt (supplemental Fig. S3D). Collectively, these results indicate that A549-RR cells do not exhibit cross-resistance to PI3K inhibitors.
Co-targeting mTOR and PI3K/Akt Signaling Augments Inhibition of Tumor Growth
Given that sustained Akt activation is associated with development of cell resistance to mTOR inhibitors, whereas mTOR inhibitor-induced Akt activation was suggested to be PI3K-dependent (9), it was plausible to speculate that blockage of mTOR inhibitor-induced Akt activation by a PI3K inhibitor would enhance mTOR inhibitors’ anticancer efficacy and prevent development of cell resistance to mTOR inhibitors. Thus, we examined the effects of RAD001 combined with LY294002 on the growth of lung cancer cells in cell culture. The RAD001 and LY294004 combination exhibited growth-inhibitory effects that are greater than that caused by each single agent in a 3-day monolayer culture (Fig. 5A). In the long-term colony formation assay, we obtained similar results. This combination worked better than either single agent in decreasing colony size and number (Fig. 5B).
Fig. 5. Combination of RAD001 and LY294002 augments growth inhibition of lung cancer cells in cells culture (A and B) and in nude mice (C and D).
A. The individual cell lines, as indicated, were seeded in 96-well plates. On the second day, they were treated with the indicated concentrations of RAD001 (RAD) alone, 2 µM LY294002 alone, and their respective combinations. After 3 days, plates were subjected to determination of cell number using a SRB assay. Each column represents a mean ± SD of four replicate determinations. B. H460 cells at a density of approximately 250 cells/well were seeded in 12-well plates. On the second day, cells were treated with the indicated concentrations of RAD001 alone, 2.5 µM LY294002 alone, and their respective combinations. The same treatments were repeated every 3 days. After 10 days, the plates were stained for the formation of cell colonies with crystal violet dye. The picture of the colonies was then taken using a digital camera. C and D, Four groups of mice with either A549 (C) or H460 (D) xenografts were treated with vehicle control, RAD001 (RAD) alone, LY294002 (LY) alone and RAD001 plus LY294002 on the same day after grouping. After 14 days, the mice were sacrificed and the tumors were removed. Tumor sizes were measured once every two days. Each measurement is a mean ± SD (n=6). * p < 0.05, ** p < 0.01, and *** p < 0.001 compared to vehicle control; # p < 0.05 compared to RAD001 treatment.
Furthermore, we tested the effects of the combination of RAD001 and LY294002 on the growth of lung cancer xenografts in nude mice. In agreement with the results in cell cultures, the combination of RAD001 and LY294002 exhibited a significantly greater effect than RAD001 or LY294002 alone in inhibiting the growth of A549 xenografts (p < 0.001) (Fig. 5C). During the two-week period of treatment, the tumor sizes in mice receiving both RAD001 and LY294002 were smaller in comparison with other groups receiving either vehicle or single agent treatment (Fig. 5C), indicating an effective anticancer efficacy for the combination treatment. In a H460 xenograft model, we began treatments with relatively larger tumors (in average 300–400 mM3). Both RAD001 and LY294002 alone failed to achieve significant effects on inhibiting the growth of tumors; however, the combination of RAD001 and LY294002 significantly inhibited the growth of H460 xenografts compared to control (p < 0.05 or 0.01) (Fig. 5D). Collectively, these results clearly demonstrate that co-targeting mTOR and PI3K/Akt signaling exhibits enhanced anticancer efficacy.
Co-targeting mTOR and PI3K/Akt Signaling Enhances Inhibition of mTORC1 Signaling while Preventing Akt Phosphorylation in vivo
We also determined whether continuous RAD001 treatment in cancer xenograft models led to an increase in Akt phosphorylation as we observed in cell cultures. By Western blot analysis, we detected p-Akt levels in tumors exposed to RAD001 for 14 days and found that p-Akt levels were significantly increased (p < 0.05) in the RAD001-treated group compared to the vehicle control group in both A549 and H460 xenografts (Fig. 6A). As expected, p-Akt levels in tumors exposed to the combination of RAD001 and LY294002 were not increased (Fig. 6A). Immunohistochemical analysis of p-Akt in H460 xenografts also showed that p-Akt levels was increased in RAD001-treated tumors, but not in tumors exposed to the combination treatment of RAD001 and LY294002 (Fig. 6C). Thus, these results clearly indicate that continuous treatment of lung tumors with an mTOR inhibitor in nude mice leads to an increase in Akt phosphorylation and this increase can be abrogated by inclusion of a PI3K inhibitor.
Fig. 6. Detection of p-Akt and p-S6 levels in tumor tissues.
A and B, Tissue from each tumor generated in the experiments described in Figs. 5C and 5D was homogenized for preparation of whole-cell protein lysates and subsequent analyses of p-Akt (A) and p-S6 (B) by Western blot analysis. The results were quantitated using Kodak Image Station 2000R and presented as a mean ± SD (n=6). C, p-Akt in H460 xenografts was detected with IHC.
Moreover, we determined whether the presence of LY294002 impacted the inhibitory effect of RAD001 on mTORC1 signaling in tumor tissues. As presented in Fig. 6B, RAD001 alone significantly decreased the levels of p-S6 (p < 0.001), indicating that RAD001 indeed inhibits mTORC1 signaling; however, the presence of LY294002 further reduced the levels of p-S6, which were significantly lower than those in tumors exposed to RAD001 alone (p < 0.05 or 0.01). Thus, these results indicate that co-treatment of tumors with an mTOR inhibitor (e.g., RAD001) and a PI3K inhibitor (e.g., LY294002) not only blocks RAD001-induced Akt phosphorylation, but also exhibits an enhanced effect on inhibiting mTORC1 signaling.
DISCUSSION
In the current study, we further showed that prolonged treatment with either rapamycin or RAD001 increased p-Akt levels in several human lung cancer cell lines (Fig. 1). A549-RR cells, which were routinely cultured in the presence of 1 µM rapamcyin, still exhibited increased levels of p-Akt compared to the parental A549 cells (Fig. 3). Moreover, we detected significantly increased levels of p-Akt in lung cancer xenografts exposed to RAD001 for 14 days (Fig. 6). In current studies, we used 1 or 10 nM rapamycin or RAD001, which is lower than concentrations (100 or >1000 nM) used in other studies showing that prolonged treatment with an mTOR inhibitor decreases p-Akt levels (8, 20). At 100 nM (or even at 10 nM), both rapamycin and RAD001 indeed decreased p-Akt levels after a 24 h or 48 h treatment in PC-3, U937 and Jurkat cells as reported (8, 20). However, both rapamycin and RAD001 at 1 nM consistently increased p-Akt levels even after a 48 h exposure in these cell lines (Fig. 1). Thus, it appears that there are two types of cancer cells: one type exhibits increased levels of p-Akt after a prolonged treatment with an mTOR inhibitor regardless of concentrations (e.g., A549 and H157 cells), whereas another type shows dose-dependent alterations in p-Akt levels after prolonged treatment with an mTOR inhibitor (e.g., PC-3 and U937 cells). In the latter cell type, low doses (e.g., 1 nM) of mTOR inhibitors, which sufficiently blocks mTORC1 signaling (9), clearly increase p-Akt levels.
It has been suggested that mTORC2 is rapamycin-insensitive (17), although it can be inhibited by prolonged rapamycin treatment (8). It has been suggested that an equilibrium may exist between mTORC1 and mTORC2 complexes (7). Therefore, it is possible that inhibition of mTORC1 by an mTOR inhibitor somehow shifts the equilibrium to favor or facilitate formation and activation of mTORC2, leading to increase in Akt phosphorylation. In our study, we found that a prolonged treatment with rapamycin (i.e., 24 h) inhibited not only mTORC1 but also mTORC2 (e.g., H157 and A549) with increased Akt phosphorylation in all three lung cancer cell lines (Fig. 2A). In rapamycin-resistant A549-RR cells where p-Akt levels were increased, the assembly of both mTORC1 and mTORC2 were also clearly inhibited (Fig. 3D). Thus, our results clearly indicate that p-Akt levels can be increased under the condition that mTORC2 activity is inhibited.
Although mTORC2 has been recently demonstrated to be an Akt Ser473 kinase (18), our results indicate that mTOR inhibitor-induced Akt phosphorylation is unlikely to be mediated by mTORC2 because it is inhibited during mTOR inhibitor treatment. This notion is further supported by our findings that disruption of mTORC2 by knocking down rictor did not block rapamycin-induced Akt phosphorylation (Fig. 2). In agreement with previous findings that raptor knockdown increases Akt phosphorylation (18), we also observed that inhibition of mTORC1 by silencing raptor was sufficient to increase Akt levels in our cell lines tested. These results indicate that mTOR inhibitor-induced Akt activation is the consequence of mTORC1 inhibition. Collectively, we conclude that mTOR inhibitors induce Akt activation through an mTORC1-dependent mechanism independent of mTORC2.
It is well documented that PI3K/Akt represents a major survival pathway that is often associated with resistance to cancer therapy (22–24). The biological significance of mTOR-inhibitor-induced Akt activation in mTOR-targeted cancer therapy is unclear. In our study, we observed that p-Akt levels were drastically increased in the rapamycin-resistant cell line (A549-RR). Moreover, when the selective pressure (i.e., rapamycin) was removed, the acquired high levels of p-Akt remained for a long period of time and were tightly associated with cell resistance to mTOR inhibitors. When the sensitivity of rapamycin-resistant (A549-RR) cells to mTOR inhibitors was fully restored after a five-month removal of rapamycin, p-Akt levels dropped to normal levels comparable to those in rapamycin-sensitive parental cells (A549-P) (Fig. 4). Additionally, enforced reduced p-Akt levels by silencing total Akt levels with Akt siRNA increases cell sensitivity to rapamycin (see supplemental Fig. S2). Thus, our results suggest a critical role of Akt activation in the development of cell resistance to mTOR inhibitors. Although we suggest the association between sustained Akt activation and development of acquired resistance to mTOR inhibitors, the mechanistic insights into how sustained Akt activation negatively regulates mTOR inhibitors’ efficacies are still unclear and need further investigation.
PI3K/Akt works upstream of mTORC1 and regulates mTORC1 activity. Therefore, inhibition of PI3K/Akt signaling using PI3K inhibitors should affect mTORC1 activity as well. Moreover, mTOR is a PI3K-related serine/theronine kinase, and its activity can be directly inhibited by the PI3K inhibitors, LY294002 and wortmannin (25, 26). Thus, it has been proposed that PI3K inhibitors may share similar signaling pathways with rapamycin such as mTOR/p70S6K to exert their biological function (25). If PI3K inhibitors suppress cell growth solely through inhibition of mTOR signaling, cells resistant to rapamycin should be cross-resistant to PI3K inhibitors as was seen with RAD001. In our study, LY294002 or wortmannin was equally effective in inhibiting the growth of A549-P and A549-RR cells. Moreover, LY294002 induced G1 arrest in both A549-P and A549-RR cells with comparable potencies. We also found that LY294002 effectively decreased the levels of p-p70S6K, p-S6 and p-Akt in both A549-P and A549-RR cells (see supplemental Fig. S3). Together, these results indicate that rapamycin resistance does not interfere with the action of PI3K inhibitors, suggesting that mTOR inhibitors and PI3K inhibitors exert their biological functions through different mechanisms or PI3K inhibitors suppress cell growth through other mechanisms in addition to inhibition of mTOR signaling.
Rapamycin resistance is an important subject of mTOR-targeted cancer therapy in the clinic. Our finding that rapamycin-resistant cells retain sensitivity to PI3K inhibitors has important clinical implications. To overcome or avoid cell resistance to mTOR inhibitors during mTOR-targeted cancer therapy, combination of an mTOR inhibitor with a PI3K inhibitor or intermittent use of a PI3K inhibitor and an mTOR inhibitor may be good approaches. Indeed, our results clearly show that RAD001 combined with LY294002 exhibited enhanced inhibitory effects on the growth of human lung cancer cells in cell cultures (Fig. 5). Importantly, the RAD001 and LY294002 combination worked better than each single agent alone in inhibiting the growth of human lung cancer xenografts in nude mice (Fig. 5), indicating an enhanced anticancer activity in vivo. As expected, treatment of xenografts with RAD001 increased p-Akt levels, which could be abrogated by co-treatment with LY294002. Besides, we found that RAD001 plus LY294002 also exerted an enhanced effect on reduction of p-S6 levels, indicating that inhibition of PI3K/Akt enhances mTOR inhibitor’s effect on inhibition of mTORC1 signaling (Fig. 6). Collectively, our results validate the strategy for cancer therapy by co-targeting mTOR and PI3K/Akt signaling and warrant clinical evaluation of this strategy for cancer therapy.
Supplementary Material
ACKNOWLEDGEMENT
We are grateful to Drs. Y. Jing for kindly providing cell lines and Dr. H. Elrod in our laboratory for editing of the manuscript.
Grant Support: NIH RO1 CA118450-01 (S-Y. S.) and PO1 CA116676-01 (Project 1 to F. Khuri and S-Y. Sun), Georgia Cancer Coalition Distinguished Cancer Scholar award (S-Y. S.), DOD IMPACT award W81XWH-05-0027 (Project 5 to F.R. K. and S-Y. S.) and BATTLE award W81XWH-06-1-0303 (Project 4 to F.R. K. and S-Y. S.).
Note: S-Y. Sun, H. Fu, and F. R. Khuri are Georgia Cancer Coalition Distinguished Cancer Scholars.
REFERENCES
- 1.Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335–348. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 2.Guertin DA, Sabatini DM. An expanding role for mTOR in cancer. Trends Mol Med. 2005;11:353–361. doi: 10.1016/j.molmed.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 4.Manning BD, Cantley LC. United at last: the tuberous sclerosis complex gene products connect the phosphoinositide 3-kinase/Akt pathway to mammalian target of rapamycin (mTOR) signalling. Biochem Soc Trans. 2003;31:573–578. doi: 10.1042/bst0310573. [DOI] [PubMed] [Google Scholar]
- 5.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 6.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 7.Hay N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell. 2005;8:179–183. doi: 10.1016/j.ccr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Sarbassov dos D, Ali SM, Sengupta S, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 9.Sun SY, Rosenberg LM, Wang X, et al. Activation of Akt and eIF4E Survival Pathways by Rapamycin-Mediated Mammalian Target of Rapamycin Inhibition. Cancer Res. 2005;65:7052–7058. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 10.O'Reilly KE, Rojo F, She QB, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tabernero J, Rojo F, Calvo E, et al. Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol. 2008;26:1603–1610. doi: 10.1200/JCO.2007.14.5482. [DOI] [PubMed] [Google Scholar]
- 12.Sawyers CL. Will mTOR inhibitors make it as cancer drugs? Cancer Cell. 2003;4:343–348. doi: 10.1016/s1535-6108(03)00275-7. [DOI] [PubMed] [Google Scholar]
- 13.Houghton PJ, Huang S. mTOR as a target for cancer therapy. Curr Top Microbiol Immunol. 2004;279:339–359. doi: 10.1007/978-3-642-18930-2_20. [DOI] [PubMed] [Google Scholar]
- 14.Rowinsky EK. Targeting the molecular target of rapamycin (mTOR) Curr Opin Oncol. 2004;16:564–575. doi: 10.1097/01.cco.0000143964.74936.d1. [DOI] [PubMed] [Google Scholar]
- 15.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. The New England journal of medicine. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 16.Huang S, Bjornsti MA, Houghton PJ. Rapamycins: mechanism of action and cellular resistance. Cancer Biol Ther. 2003;2:222–232. doi: 10.4161/cbt.2.3.360. [DOI] [PubMed] [Google Scholar]
- 17.Sarbassov DD, Ali SM, Kim DH, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 18.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 19.Sun SY, Yue P, Dawson MI, et al. Differential effects of synthetic nuclear retinoid receptor-selective retinoids on the growth of human non-small cell lung carcinoma cells. Cancer Res. 1997;57:4931–4939. [PubMed] [Google Scholar]
- 20.Zeng Z, Sarbassov dos D, Samudio IJ, et al. Rapamycin derivatives reduce mTORC2 signaling and inhibit AKT activation in AML. Blood. 2007;109:3509–3512. doi: 10.1182/blood-2006-06-030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dilling MB, Germain GS, Dudkin L, et al. 4E-binding proteins, the suppressors of eukaryotic initiation factor 4E, are down-regulated in cells with acquired or intrinsic resistance to rapamycin. J Biol Chem. 2002;277:13907–13917. doi: 10.1074/jbc.M110782200. [DOI] [PubMed] [Google Scholar]
- 22.West KA, Castillo SS, Dennis PA. Activation of the PI3K/Akt pathway and chemotherapeutic resistance. Drug Resist Updat. 2002;5:234–248. doi: 10.1016/s1368-7646(02)00120-6. [DOI] [PubMed] [Google Scholar]
- 23.Kim D, Dan HC, Park S, et al. AKT/PKB signaling mechanisms in cancer and chemoresistance. Front Biosci. 2005;10:975–987. doi: 10.2741/1592. [DOI] [PubMed] [Google Scholar]
- 24.Martelli AM, Tabellini G, Bortul R, et al. Involvement of the phosphoinositide 3-kinase/Akt signaling pathway in the resistance to therapeutic treatments of human leukemias. Histol Histopathol. 2005;20:239–252. doi: 10.14670/HH-20.239. [DOI] [PubMed] [Google Scholar]
- 25.Brunn GJ, Williams J, Sabers C, Wiederrecht G, Lawrence JC, Jr, Abraham RT. Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin and LY294002. Embo J. 1996;15:5256–5267. [PMC free article] [PubMed] [Google Scholar]
- 26.McMahon LP, Yue W, Santen RJ, Lawrence JC., Jr Farnesylthiosalicylic acid inhibits mammalian target of rapamycin (mTOR) activity both in cells and in vitro by promoting dissociation of the mTOR-raptor complex. Mol Endocrinol. 2005;19:175–183. doi: 10.1210/me.2004-0305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.