Abstract
Adults have little difficulty perceiving objects as complete despite occlusion, but newborn infants perceive moving, partly occluded objects solely in terms of visible surfaces. The developmental mechanisms leading to perceptual completion have never been adequately explained. Here, we examine the potential contributions of oculomotor behavior and motion sensitivity to perceptual completion performance in individual infants. Young infants were presented with a center-occluded rod, moving back and forth against a textured background, to assess perceptual completion. Infants also participated in tasks to assess oculomotor scanning patterns and motion direction discrimination. Individual differences in perceptual completion performance were strongly correlated with scanning patterns, but were unrelated to motion direction discrimination. We present a new model of development of perceptual completion that posits a critical role for targeted visual scanning, an early-developing oculomotor action system.
Do infants experience a world of coherent objects? Veridical perception of objects presupposes perception of occlusion (Gibson, 1979). Occlusion is ubiquitous in the visual environment, because from any single vantage point, nearer objects and surfaces often overlap those that are farther away. Human adults, nevertheless, experience a world composed of coherent objects with regular shapes, rather than a world of surface fragments, and by 4 months after birth, infants provide evidence of occlusion perception in displays that depict partly occluded (Johnson & Náñez, 1995) and fully occluded (Johnson, Bremner, Slater, Mason, Foster, & Cheshire, 2003) objects. Newborn infants, in contrast, have been shown consistently to perceive similar partial occlusion displays solely in terms of their visible surfaces, failing to make the perceptual “inference” necessary to achieve perceptual completion of object surfaces (Slater, Johnson, Brown, & Badenoch, 1996; Slater, Johnson, Kellman, & Spelke, 1994; Slater, Morison, Somers, Mattock, Brown, & Taylor, 1990).
Evidence comes from tasks in which infants are shown a center-occluded rod, its visible portions undergoing common motion against a textured background, which is viewed repeatedly until infant looking times decline according to a predetermined habituation criterion (Figure 1, left). Following habituation, infants view two test displays, either side-by-side or in alternation, each matching the habituation display in a different way. Posthabituation looking time patterns are thought to reflect a novelty preference, and can be used to assess whether infants perceived the visible surfaces as connected in the habituation display. Older infants (i.e., 4-month-olds) consistently prefer the “broken” rod test display (Figure 1, center), implying that the “complete” rod is familiar relative to the habituation display—hence consistent with perception of unity. When tested with similar displays, in contrast, neonates consistently prefer the complete rod test display (Figure 1, right), implying that the broken rod is familiar relative to the habituation display—hence consistent with perception of disjoint surfaces, rather than perceptual completion. Preferences at 2 months tend to fall between these patterns, depending on display characteristics (Johnson, 2004). Together, these findings point to 0-4 months as an important time of transition toward veridical occlusion perception when infants view partly occluded object displays. When viewing displays in which a moving object becomes fully hidden and then reemerges, however, perception of trajectory continuity emerges a few months later (Bremner et al., 2005, 2007; Johnson et al., 2003).
Figure 1.

Stimuli used in experiments that investigate young infants’ perception of partly occluded objects. Left: Partly occluded rod display, presented repeatedly until the infant’s looking times decline to a predetermined criterion. Center: “Broken” rod posthabituation test display. Right: “Complete” rod posthabituation test display.
The mechanisms of development that lead to perceptual completion in infants are poorly understood. One prominent hypothesis highlights the role of common motion. When visible rod surfaces in a rod-and-box display move in tandem, and the outer contours of the rod surfaces are aligned, young infants provide evidence of unity perception; in static versions of these displays, however, infants do not seem to perceive unity (Kellman & Spelke, 1983; Jusczyk, Johnson, Spelke, & Kennedy, 1999). These findings have led to a hypothesized link between motion discrimination and perceptual completion. Discrimination between different directions of motion is limited in very young infants (Banton & Bertenthal, 1997), and perceptual completion in static occlusion displays has not been observed until 6-8 months (Craton, 1996). Moreover, motion direction discrimination and perceptual completion in moving-rod occlusion displays are first observed at about 2 months (Johnson & Aslin, 1995; Wattam-Bell, 1996), leading to speculation that limits in perceptual completion are rooted in a failure to detect common motion: If the immature visual system is unable to detect common motion, rendering it unavailable, then perceptual completion may be precluded in young infants (Kellman & Arterberry, 1998). On this account, therefore, the young infant’s visual system uses motion as a primary cue to identify unity of objects, and motion processing mechanisms must develop before this cue is available.
Two recent sets of findings provide mixed support for this proposal. Johnson (2004) tested 2-month-olds for perceptual completion in displays in which edge alignment and proximity of visible rod surfaces across the occluded gap were manipulated. The infants provided evidence of unity perception in a stimulus in which the rod parts above and below the occluder were in close proximity (i.e., across a narrow occluder), but not in stimuli in which the rod parts were misaligned, or when aligned rod parts were presented with a relatively wide occluder. In all three types of display (aligned rod parts, narrow occluder; misaligned rod parts, narrow occluder; and aligned rod parts, wide occluder), the visible portions of the rod underwent common lateral translation. In a second experiment, Johnson asked whether the failure to perceive unity in the “misaligned, narrow” and “aligned, wide” displays stemmed from a failure to detect common motion of the rod parts. If the failure were rooted in insensitivity to motion, infants would be unable to distinguish the common motion available in these displays from a motion pattern in which the rod parts move in opposite directions. Two-month-olds were presented with one of the three displays used in Experiment 1 until habituation of looking had occurred, and then viewed the same display on alternating trials with a stimulus in which the rod parts moved in opposite directions. Other aspects of the two stimuli were identical (size of occluder, orientations of the visible rod parts). In all three of these conditions, infants looked longer toward the opposite motion displays, implying that the differences in motion across habituation and test (i.e., common and opposite motions) were detected. Three further groups of 2-month-olds were habituated with opposing motion stimuli, and looked longer at test at common motion displays, leading to the same conclusion. Johnson interpreted these results to suggest that the infants analyzed the positions, orientations, and motions of visible surfaces, yet did not consistently perceive completion despite the availability of motion information. These results, therefore, do not support the primacy of motion information in perceiving unity.
A second recent study complicates this interpretation. Valenza, Leo, Gava, and Simion (2006) examined newborn infants’ perceptual completion with computer-generated rod-and-box stimuli in which the visible rod parts underwent so-called “stroboscopic” motion (more commonly called apparent or phi motion): The rod parts appeared alternately every 500 ms in two lateral positions without moving through the space between them. The background was black and untextured. Infants were reported to show preferences for broken rod test displays under these conditions, leading to claims of unity perception, perhaps, according to the authors, because the newborn visual system is sensitive to stroboscopic, but not actual (smooth) motion. Thus the infants were claimed to detect the common changes in position of visible rod surfaces, specified by smooth motion in the Slater et al. (1990, 1994, 1996) studies, and by apparent motion in the Valenza et al. study, but only perceived unity from apparent motion. If this is correct, it implies that perceptual completion is available early, in the absence of visual experience, given the right conditions (viz., information to which the newborn visual system is sensitive).
Yet such a conclusion may not be warranted, for three reasons. First, it is not clear why, on this account, neonates do not appear to detect common position changes from smooth motion. Even if the newborn visual system is better equipped to utilize apparent motion to perceive unity, smooth motion is detectable by neonates (Slater, 1995). Second, there is evidence that apparent motion can drive optokinetic nystagmus in newborn humans (Tauber & Koffler, 1966), but there is no evidence to our knowledge that infants perceive apparent motion as specifying a unitary object moving through space, although apparent motion can yield such a percept to adults (Yantis, 1995). Third, older infants’ unity perception in two-dimensional displays has been shown to depend on the presence of background texture (Johnson & Aslin, 1996), a finding that calls into question the likelihood that newborns can perceive unity under conditions in which background texture is absent. The Johnson (2004) study, too, leaves open questions, and cannot be considered to provide definitive evidence that 2-month-olds’ performance was rooted in sensitivity to differences in patterns of smooth motion. Instead, discrimination of common from opposite motions might have arisen simply from detection of position changes: “snapshots” of common and opposite motion displays that could be distinguished on the basis of static information—differences in position of visible rod parts only. Alternatively, it might be that motion sensitivity per se is unrelated to perceptual completion, a point to which we return in the Discussion section. Taken together, these questions imply that the relation between motion direction discrimination and perceptual completion is not yet clear.
Recently, an alternative possibility has emerged. Amso and Johnson (2006) and Johnson, Slemmer, and Amso (2004) observed 3-month-old infants in a perceptual completion task using the habituation paradigm described previously. The infants’ eye movements were recorded with a corneal reflection eye tracker during the habituation phase of the experiment. The authors reported differences in scanning patterns between infants whose posthabituation test display preferences indicated unity perception and those infants who provided evidence of perception of disjoint surfaces: “Perceivers” tended to scan more in the vicinity of the two visible rod segments and to scan back and forth between them. Johnson and Johnson (2000) found as well that there is a shift across 2-4 months in the extent to which young infants overcome a top bias, when visual attention is directed preferentially or exclusively toward the upper portions of a rod-and-box stimulus. Ontogeny of perceptual completion, therefore, seems to be accompanied by an inclination to direct visual attention to relevant aspects of a stimulus, in this case, the upper and lower moving rod parts.
Our goal in the present paper was to examine directly the possibility that emergence of motion direction discrimination in individual infants predicts performance on a perceptual completion task. Because there is a suggestion in the literature that visual attention plays a crucial role in unity perception, we also examined oculomotor behavior (scanning patterns) as infants viewed a rod-and-box display. We observed infant performance in four tasks on the same day, asking whether visual attention and motion perception are related directly to perceptual completion in individual infants. If so, we predicted a correlation between performance on motion direction discrimination and perceptual completion tasks, as well as a task assessing visual attention to rod-and-box displays. If not, we predicted that performance on the motion direction discrimination task would be independent of both perceptual completion and attention toward rod parts. As a manipulation check to ensure that our measure of motion perception was a valid index of true motion direction discrimination, and to provide a second, independent index of oculomotor performance, we observed infants’ responses to an oculomotor smooth pursuit task. It has been speculated that motion direction discrimination and smooth pursuit are subserved by the same cortical mechanisms (Johnson, 1990; Kellman & Arterberry, 1998) but to our knowledge this has never been tested directly.
In the present study, therefore, we observed young infants’ performance in four tasks:
Scanning: We recorded oculomotor behavior as infants viewed a rod-and-box occlusion display (Figure 1, left).
Smooth Pursuit: We recorded oculomotor behavior as infants viewed a small moving target moving against a black background (Figure 2).
Perceptual Completion: We recorded looking times as infants were habituated to a rod-and-box display, followed by broken and complete rod test displays (Figure 1).
Motion Direction Discrimination: We recorded oculomotor behavior as infants viewed pairs of random-dot kinematograms, presented side-by-side. In half of each display, the dots moved in the same direction; the other half was divided into three regions in which dots moved in opposing directions (Figure 3).
Figure 2.
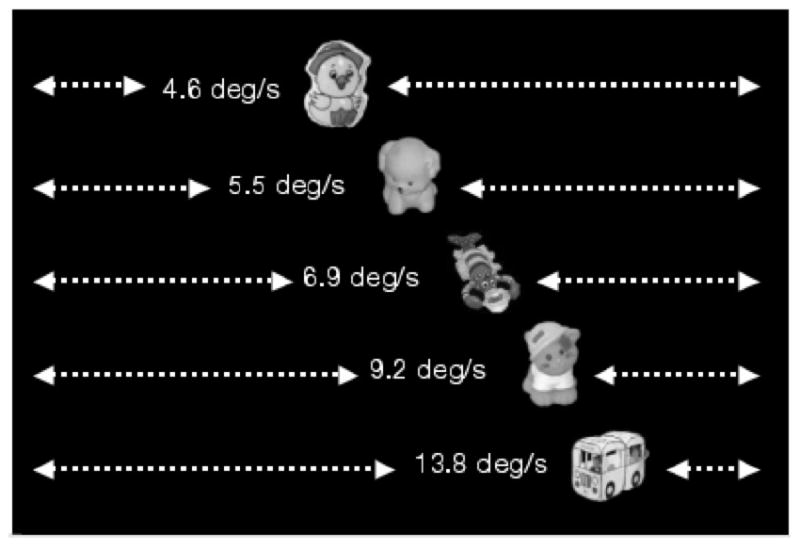
Schematic depiction of stimuli used to assess smooth pursuit. On each trial, a target was presented in one of five positions on the screen, moving on a single left-right cycle of motion at one of five speeds. The five vertical positions are shown here for illustrative purposes; only a single target was seen on each trial.
Figure 3.
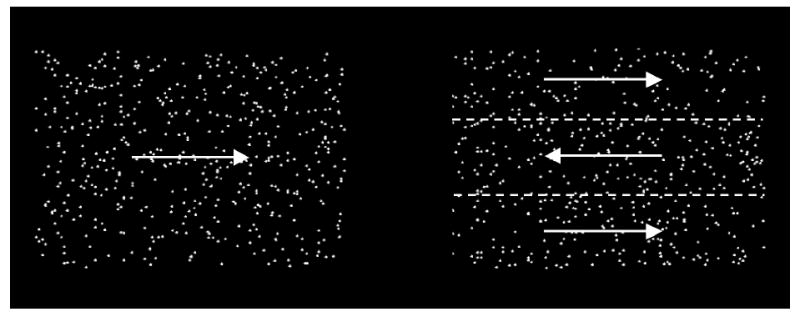
Schematic depiction of random-dot kinematograms used to assess motion direction discrimination. Left: Simple motion. Right: Complex motion. Arrows and dotted lines are shown here for illustrative purposes and were not visible in the stimuli shown to infants.
Methods
Participants
We collected a complete data set in each of the four tasks from 20 full-term infants (8 girls, 12 boys), ranging in age from 58 to 97 days (M = 74.9). An additional 22 infants were observed but not included in the final sample due to poor calibration of the point of gaze, or POG (5 infants), failure to calibrate or complete one of the four tasks due to fussiness (12 infants) or sleepiness (2 infants), or insufficient attention (<10%) toward the displays in either the Scanning, Smooth Pursuit, or Motion Direction Discrimination task (3 infants). The majority of infants were from middle-class families; detailed information about education and race/ethnicity was not available at the time of testing.
Stimuli
Scanning
The stimulus for the Scanning task consisted of a center-occluded green rod measuring 1.5 × 15.5 cm (1.4 × 14.7° visual angle at the infant’s 60-cm viewing distance), undergoing lateral translation at 4.3 cm/s (4.1°/s), reversing direction every 2.5 s. The occluder consisted of a 3.8 × 21.6 cm (3.6 × 20.4°) blue box. Rod parts and box were presented against a 12 × 20 grid of small white dots serving as background texture.
Smooth Pursuit
The stimuli for the Smooth Pursuit task consisted of five small color photos of children’s toys, presented individually, translating horizontally across a black background in one of five vertical positions on the screen and at one of five speeds, 4.8, 5.5, 6.9, 9.8, or 13.8°/s (see Figure 2). Each target was approximately 3° across. The target for each trial, location of its path, and its speed were presented pseudorandomly within each block of five trials to hold the infant’s interest. Also to hold the infant’s interest, between the first and second 5-trial blocks, a photo of two young girls in princess costumes was shown for 5 s, and between the second and third 5-trial blocks, a toy was shown bouncing across the screen on an irregular trajectory in tandem with a periodic “boing” sound for 5 s. Also to sustain interest, we played snappy music during the entire Smooth Pursuit task (Fun Fun Fun by the Beach Boys), which pilot testing revealed to be highly effective in maintaining alertness.
Perceptual Completion
The habituation stimuli for the Perceptual Completion task consisted of the same center-occluded green rod, blue occluder, and textured background as in the Scanning task, except its size was scaled up by 167% to accommodate a greater viewing distance (100 cm). The broken rod test stimulus consisted of rod parts moving in the same manner as in the habituation stimulus, but there was no occluder and background dots were visible between the rod parts. In the complete rod test stimulus, the center of the rod was filled in (see Figure 1).
Motion Direction Discrimination
The stimuli for the Motion Direction Discrimination task consisted of 24 pairs of random-dot kinematograms. White dots (70 cd/m2), each 5 × 5 arc min, were randomly dispersed and covered approximately 1.2% of an otherwise black surface (.3 cd/m2). In one half of each display, the dots all moved in the same direction, which we termed simple motion. The other half was divided into thirds; the center portion of this half contained dots that moved in opposition to the others, such that there were three distinct regions of motion (Figure 3), which we termed complex motion. Direction of simple motion (up, down, left, or right), left-right placement, and direction of motion in the center of complex motion were randomized across trials. Motion in any single trial was either horizontal or vertical. Each region of dot motion (simple and complex) measured 10.2 × 14.0 cm (9.7 × 13.3°) and was separated by 4.76 cm (4.52°). Dots moved at 3.2 cm/s (3.0°/s).
Pilot testing revealed that infants were largely uninterested in viewing random-dot kinematograms. Therefore, we embedded the displays within a digitized 4-min segment of A Charlie Brown Christmas. The sound from the video (e.g., the children’s voices) was maintained throughout the task. Prior to presentation of each 4-s kinematogram pair, we presented a small audiovisual stimulus for 2 s in the center of the screen to re-center the infant’s POG. Each of these stimuli was a small toy, either looming/contracting, rotating, shaking, or bouncing within a 5° radius, and each paired in a unique sound. Each kinematogram pair was followed immediately by 4 s of Charlie Brown video.
Apparatus
The Scanning, Smooth Pursuit, and Motion Direction Discrimination tasks were conducted with a Tobii model ET-17 corneal-reflection eye tracker. Stimuli were viewed on a 43-cm flat panel (thin-film transistor) monitor. Eye movement data were recorded at 30 Hz. Experiments were controlled with ClearView software provided by Tobii.
The Perceptual Completion task was conducted with a G4 Macintosh and a 76-cm CRT monitor. An observer viewed the infant on a monitor attached to a closed-circuit camera. The experiment was controlled with Habit software (Cohen, Atkinson, & Chaput, 2004).
Procedure
Each infant was tested individually and seated in a parent’s lap for all tasks. The four tasks were presented in the same order for all infants. If needed, infants were given a short break between tasks. After the parent was briefed and provided informed consent, the infant’s POG was calibrated with the eye tracker. The POG was calibrated by comparing it to known coordinates on the screen as the infant viewed a target-patterned “attention-getter,” looming/contracting in synchrony with a rhythmic sound. The attention-getter was presented at five locations on the monitor, and the infant looked at each in turn.
Following calibration, each infant was observed in the Scanning task. Infants were shown four 15-s presentations of the rod-and-box display described previously as their eye movements were recorded. Between trials the attention-getter was shown for 6 s to center the POG and maintain the infant’s interest in the task. Total length of the task was 1 min 18 s.
Second, each infant was observed in the Smooth Pursuit task. The infant viewed fifteen trials, each consisting of a moving toy target translating back and forth once across the screen. Measures taken to maintain interest in the task were described previously. Total length of the task was 2 min 15 s.
Third, each infant was observed in the Perceptual Completion task. An observer (the first author), blind to the stimulus on the screen at any given time, recorded looking times by pressing a key as the infant looked, and released it when the infant looked away. The computer presented stimuli, stored the observer’s data, calculated the habituation criterion for each infant, and changed displays after the criterion had been met. Prior to the first trial, and between each trial, the attention-getter was shown to recruit the infant’s attention after she had looked away. The rod-and-box display was presented until the infant reached a predetermined habituation criterion, computed as a decline in looking times across four consecutive trials, beginning with the second trial, adding up to less than half the total looking times across the first four trials. When looking times declined to the habituation criterion, the computer switched automatically to test displays. Broken and complete rod displays were presented three times each in alternation. Order of initial presentation was counterbalanced.
Finally, each infant was observed in the Motion Direction Discrimination task, consisting of 4-s segments of the Charlie Brown video, a 2 s audiovisual stimulus, and 4-s moving dot displays. Segments of the Charlie Brown video were presented in sequence. Order of dot displays was randomized. Total task length was 4 min 26 s.
Data
For the Scanning, Smooth Pursuit, and Motion Direction Discrimination tasks, data consisted of x-y coordinates of the POG on the stimulus monitor, recorded at 30 Hz, saved on the computer’s hard drive. Gaze patterns were analyzed with Matlab. For the Perceptual Completion task, data consisted of looking times to broken and complete rod test displays.
Results
Data Scoring
Scanning
We examined a number of characteristics of individual infants’ scanning behavior that we thought might be related to perceptual completion, including mean number of fixations/s and mean saccade distance (to assess overall scanning activity), mean vertical position of each infant’s fixations (to assess a top bias), and mean dispersion of attention across the stimulus (to assess a tendency toward “local” vs. “global” scanning patterns: scanning limited portions of the stimulus vs. scanning more broadly, respectively). In addition, we reasoned that the most informative regions with respect to perceptual completion comprised the visible rod parts (Amso & Johnson, Johnson et al., 2004). Data from the Scanning task, therefore, also included the proportion of saccadic eye movements directed toward the moving rod parts, which we termed targeted scans.
Infants contributed a mean of 44.32 s (SD = 11.67) of total scanning data, out of 60 s possible, the cumulative exposure time of the rod-and-box display during the Scanning task. We recorded a mean of 69.85 fixations (SD = 16.58) per infant (M = 1.78 fixations/s, SD = .81). Mean saccade distance was 3.10° visual angle (SD = 1.04). The mean vertical position of fixations was 490.83 pixels from the top of the screen (SD = 75.79), not reliably different from the screen’s center, 512 pixels, t(19) = 1.25, ns (thus there was no top bias across the sample). Mean dispersion of attention, operationalized as the mean distance of saccades for each infant from his or her average x-y fixation position on the screen, was 2.22° visual angle (SD = .64). A mean of 27.61% (SD = 20.57) of saccades were targeted scans, directed toward the moving rod.
Smooth Pursuit
The principal index of Smooth Pursuit performance consisted of the ratio of oculomotor pursuit speed to target speed, or gain. (A gain of 1.0 would indicate that the speed of the POG matched perfectly target speed.) We used the following algorithm to identify infants’ smooth pursuit eye movements. We first identified all eye movements with instantaneous velocity greater than 25°/sec and labeled these as saccades. We then isolated segments of eye gaze that contained no saccades, were less than 10° away from the target to be tracked, that exceeded 200 ms in duration, and that occurred in trials during which there was more than 400 ms total of smooth pursuit. Gain for each of these segments was calculated by dividing the average velocity of the POG during the segment by the average velocity of the target during the same period of time; mean gain for a trial consisted of the average gain over segments weighted by the length of the segments.
Mean gain was .303 (SD = .082) across the sample. A one-way ANOVA on target speed (4.8, 5.5, 6.9, 9.8, 13.8°/s) yielded a reliable effect, F(4, 76) = 3.39, p < .05. There were no reliable differences in gain across the four slowest target speeds (Newman-Keuls tests; all ps > .45). Gain at the fastest target speed (13.8°/s) was significantly lower than gain at target speeds of 4.6°/s, 5.5°/s, and 9.8°/s (ps < .05), and lower as well than gain at the 6.9°/s target speed (p < .06). The fastest target speed, therefore, appears to have presented the greatest challenge to the infants’ smooth pursuit skills.
Perceptual Completion
Data from the Perceptual Completion task consisted of posthabituation preferences for the broken rod stimulus, computed as the percentage of looking toward the broken rod as a function of total looking at both test stimuli as judged by the observer. A second coder, blind to the displays and to the hypotheses under investigation, coded infants’ looking times during habituation and test, and these judgments were compared to the first observer. The correlation was high and significant, r(275) = .98, p < .01.
The mean preference for the broken rod was 47.99% (SD = 20.09). Nine infants looked longer overall at the broken rod at test, and 11 looked longer at the complete rod. Mean looking time to the broken test stimulus was 15.21 s (SD = 11.34); mean looking time to the complete test stimulus was 15.85 s (SD = 16.90). A 2 (broken vs. complete test stimulus) × 3 (trial block) ANOVA yielded no reliable effects. In this sample of infants and with this particular stimulus, therefore, there is no evidence for perceptual completion for the group as a whole. This result is consistent with previous literature demonstrating emergence of unity perception in rod-and-box displays by 2 months of age under limited circumstances (e.g., in narrow-occluder displays), and becoming more robust by 4 months (Johnson, 2004; Johnson & Aslin, 1995, 1996; Johnson & Náñez, 1995).
Motion Direction Discrimination
The index of Motion Direction Discrimination was the proportion of visual attention directed at complex motion, computed as the percentage of looking in the complex region as a function of total looking in both regions (simple + complex). Overall, a mean of 69.31% (SD = 14.90) of attention was directed at the complex region, which is greater than expected by chance, t(19) = 5.80, p < .0001, providing evidence for direction discrimination across the sample of infants as a whole.
Relations Between Measures of Task Performance
Our principal goal was to test relations among individual infants’ performance in the Scanning, Smooth Pursuit, Perceptual Completion, and Motion Direction Discrimination tasks. In our initial analysis of this question, we computed a series of correlations across measures in performance in these tasks.
There was a reliable correlation between Motion Direction Discrimination and Smooth Pursuit gain when target speed was 13.8°/s, which we reasoned was best diagnostic of developing smooth pursuit given that it was the most difficult to track (Figure 5). There were reliable correlations between age (in days) and performance on both measures (Figure 6). These findings provide convergent evidence for the validity of the Motion Direction Discrimination measure as an index of motion sensitivity, on an account that posits a common neural foundation for motion perception and smooth pursuit performance (e.g., Johnson, 1990). These results also corroborate the possibility that motion sensitivity and smooth pursuit rely on a common cortical mechanism, one that undergoes important developments at 2-3 months after birth (Johnson, 1990; Kellman & Arterberry, 1998).
Figure 5.
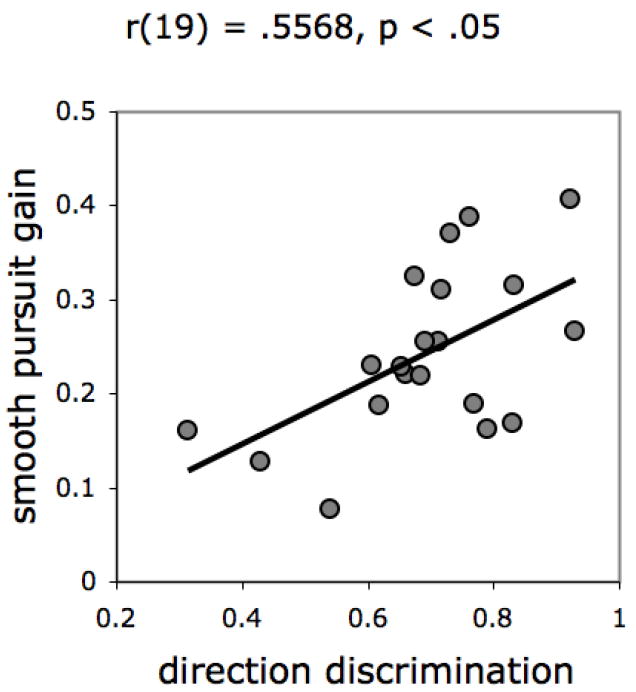
Correlation between performance on the Motion Direction Discrimination and Smooth Pursuit tasks.
Figure 6.
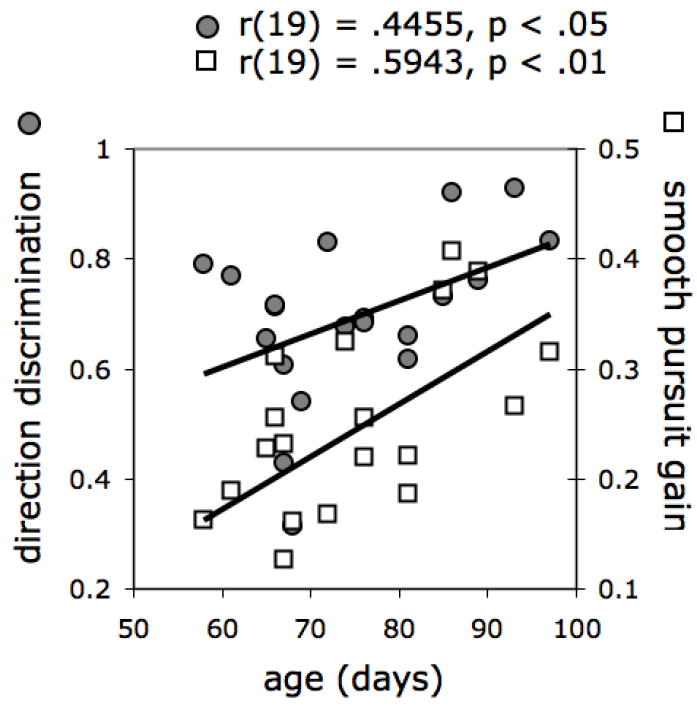
Correlations between age (in days) and performance on the Motion Direction Discrimination and Smooth Pursuit (target speed 13.8°/s) tasks.
There was no reliable correlation between Motion Direction Discrimination and posthabituation preference in the Perceptual Completion task (Figure 7), implying that perception of unity relies on mechanisms that are independent of those tapped by the motion sensitivity task. Nor was Performance on the Perceptual Completion task correlated with age, Pearson r(19) = -.26, ns.
Figure 7.
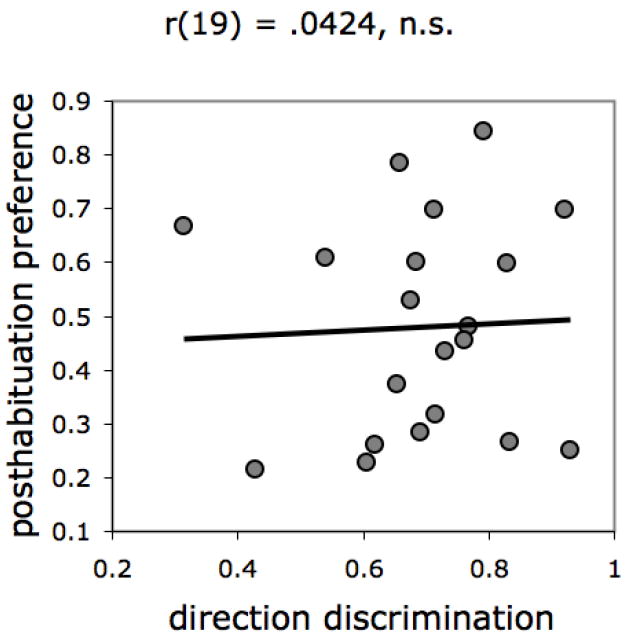
Correlation between performance on the Motion Direction Discrimination and Perceptual Completion tasks.
Performance on Perceptual Completion was also uncorrelated with three of our measures of Scanning: fixations/s, saccade distance, and dispersion of attention, all rs < .31, ns, suggesting that these general differences in individual oculomotor behavior do not contribute to veridical occlusion perception in this age group. There was a marginally reliable negative correlation between top bias and Perceptual Completion, r(19) = -.38, p = .096, which may reflect a tendency of infants who exhibited a top bias to perceive disjoint objects rather than unity, consistent with the findings of Johnson and Johnson (2000). Most importantly, there was a strong correlation between the proportion of targeted scans produced by individual infants and Perceptual Completion (Figure 8). This result also is consistent with previous findings highlighting the importance of specific patterns of visual attention to unity perception (Amso & Johnson, 2006; Johnson et al., 2004).
Figure 8.
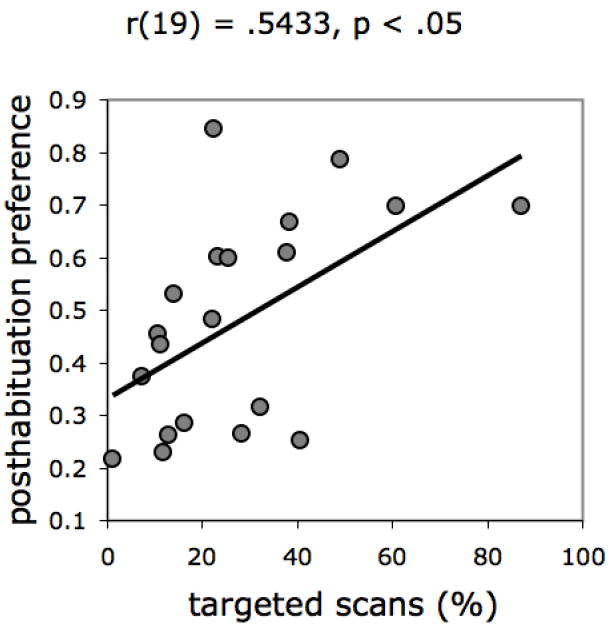
Correlation between performance on the Perceptual Completion and Scanning tasks.
In our next analysis, we used multiple regression to compare directly the contributions of Motion Direction Discrimination and Scanning to Perceptual Completion, by regressing broken rod test preference onto proportion of looking toward complex motion and proportion of targeted scans. Initially we included the two-way interaction in the model, but it failed to reach statistical significance and was dropped from the final analysis. Overall the regression was reliable, F(2, 17) = 3.68, p < .05. The effect of targeted scans was strong and significant, B = .5478 (SE = .2025), t(17) = 2.70, p < .05, Beta = .5628; the effect of motion preference was not, B = -.1149 (SE = .2794), t(17) = -.41, ns, Beta = -.0856. Finally, we compared the difference between these two regression coefficients (using formulas described by Cohen, Cohen, West, & Aiken, 2003, p. 641). The difference was significant, t(17) = 2.18, p < .05, providing evidence that Scanning uniquely contributed to Perceptual Completion, but Motion Direction Discrimination had little bearing on performance on that task.
Taken together, these results provide evidence that emergence of perceptual completion skills in infancy stems from mechanisms of visual attention, in particular targeted patterns of saccades, but is independent of developing motion discrimination abilities.
Discussion
We examined relations between motion sensitivity and perceptual completion in young infants who are at an age of transition toward perception of occlusion (Johnson, 2004). We found that our measure of motion sensitivity (detection of discrepant motion direction in random-dot kinematograms) was strongly correlated with a measure of oculomotor performance (smooth pursuit) that has been thought to rely on cortical motion-sensitive mechanisms. Performance on both tasks improved significantly with age in our sample. These results suggest that development of smooth pursuit does not rely solely on lower-level mechanisms that direct gaze, such as the oculomotor musculature or subcortical structures (e.g., brain stem and superior colliculus), but rather on mid-level cortical mechanisms, likely involving the human analogues of areas MT and MST in the primate visual system, that are implicated in motion processing (Atkinson, 2000; Johnson, 1990; Schiller, 1998). It is possible that motion sensitivity and smooth pursuit performance are supported by separate neural mechanisms that happen to mature at the same time; nevertheless, our findings are consistent with a view positing a role for cortical maturation in development of motion perception, in particular pathways to and from areas of the visual system that specialize in processing motion. Motion sensitivity performance was uncorrelated with perceptual completion. Perceptual completion also was unrelated to the age of the infants in our sample. Most importantly, perceptual completion was strongly related to the tendency to direct visual attention toward the visible portions of the partly occluded surface. This latter finding is consistent with a constructivist account of development of perceptual completion, according to which infants’ emerging attention to relevant parts of the visual scene plays a vital role in perceiving objects veridically.
Taken together, these results suggest that our tasks tapped into two separate visual functions, both undergoing development at 2-3 months. One function codes for direction of motion, and supports control of eye movements to track smooth motion of small targets in the environment. The second function identifies objects, and supports perception of the unity of the visible portions of a partly occluded surface. This account bears a superficial resemblance to the distinction between dorsal and ventral streams, two visual pathways that are largely anatomically and physiologically segregated through early areas of the visual system (Milner & Goodale, 1995; Ungerleider & Mishkin, 1982). In general, the dorsal stream is tuned to motion coherence and visually guided action, and the ventral stream is tuned to form and shape information. There is evidence that the two visual processing modes develop along different trajectories (Atkinson, 1992). Psychophysical experiments on coherence thresholds, for example, reveal that motion processing lags behind orientation processing by several weeks in very young infants, implying that the ventral stream is functional somewhat earlier than the dorsal stream (Braddick, Atkinson, & Wattam-Bell, 2003; Braddick, Birtles, Wattam-Bell, & Atkinson, 2005). (On the other hand, developments in ventral function extend into adolescence under some conditions; Kovács, Kozma, Fehér, & Benedek, 1999).
The Motion Direction Discrimination and Smooth Pursuit tasks we used in the present report clearly tap the “dorsal” portion of the dorsal-ventral distinction, but it is less clear that our Perceptual Completion task maps cleanly onto the “ventral” portion. Johnson (2004) found that 2-month-olds perceived unity in rod-and-box occlusion stimuli when the visible surfaces moved in tandem and were aligned across the occluder, but not when rod parts were misaligned, providing evidence for sensitivity to edge orientation, presumably a ventral stream operation. Thus some ventral function is necessarily in place at (or prior to) 2 months (cf. Braddick et al., 2005), and clearly plays a vital role in unity perception. (Adults, likewise, are more likely to assign aligned edges that lead behind an occluder to a single surface, relative to misaligned edges; Jusczk et al., 1999; Kellman, Garrigan, & Shipley, 2005.) We have demonstrated in the present experiment that motion sensitivity, a dorsal stream function, is unrelated to performance in a perceptual completion task, yet infants younger than 6 months perceive object unity only when the visible surfaces move together (Craton, 1996; Jusczyk et al., 1999; Kellman & Spelke, 1983). Thus motion, too, is vital to infants’ perceptual completion, but its precise role is not yet fully understood. The results of the present experiment also highlight the importance of visual attention (scanning patterns) to perceptual completion, a finding consistent with other recent reports (Amso & Johnson, 2006; Johnson et al., 2004; Johnson & Johnson, 2000).
We propose a model of development of perceptual completion that accounts for these results and other related findings. We suggest that perceptual completion proceeds in several steps (Figure 9). Each of these steps likely involves different mechanisms and develops along a different trajectory; if any of these subprocesses are disrupted, veridical object perception may be precluded. First, the observer must parse the visual scene into its constituents (segmentation), proceeding from an initial analysis of individual feature elements and differences in surface appearance: edge orientations, intersections (e.g., T-, L-, and X-junctions), texture, contour, color, luminance and surface motions (Marr, 1982). Next, a viewer-centered description of relative distances of surfaces is constructed (depth placement). The observer must determine in which depth plane each surface resides, a process that relies on distance information such as disparity, accretion and deletion of background texture, assignment of contours to appropriate surfaces via interposition and other cues, and motion (Nakayama, He, & Shimojo, 1996; Nakayama & Shimojo, 1990). Finally, the observer must perceive the units in the visual scene across occlusion (unit formation), a process that may proceed via selective visual attention and its complement, inhibition (i.e., filtering out irrelevant information), exploiting some visual biases (such as attention to motion) and overcoming others (such as a top bias). Analysis of contour ownership continues from the earlier step of depth placement, and may rely on edge alignment; when edges are nonaligned, they may be perceived as belonging to separate objects, as if the contours of the rod ended at the occluder. Finally, an object-centered representation of the visual environment is realized (not shown in Figure 9), incorporating complete “object permanence” (Piaget, 1954/1937).
Figure 9.
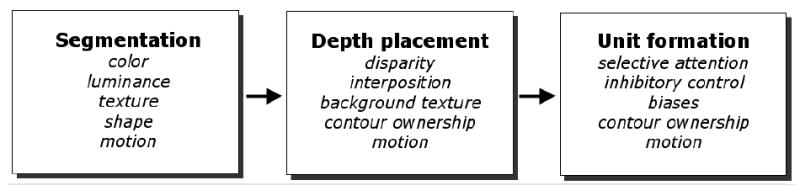
A three-part model of infants’ perceptual completion.
Research on infants’ perception of partly occluded objects suggests that segmentation is available from birth. Newborns have been found consistently to perceive visible rod parts as separate from the background, occluder, and each other (Slater et al., 1990, 1994, 1996). It is not clear precisely what visual information serves as the foundation for segmentation in newborns, however (e.g., differences in color, luminance, and so forth), because systematic investigations of this question have not been undertaken to our knowledge. (Later in the first year after birth, infants use a variety of visual cues, and top-down information, to segregate object surfaces; Needham, Baillargeon, & Kaufman, 1997; Needham & Ormsbee, 2003.) Depth placement is operational from at least 2 months, the youngest age at which perceptual completion in occlusion stimuli has been observed (Johnson, 2004; Johnson & Aslin, 1995). Here, many clues as to how the young infant’s visual system achieves depth placement have emerged. We know, for example, that background texture is critical for perceptual completion (Johnson & Aslin, 1996), that 3D depth information provides an important cue for unity (Smith, Johnson, & Spelke, 2003), that interposition is used as a depth cue (Shuwairi, Albert, & Johnson, 2007), and that young infants are sensitive to edge alignment (Johnson & Aslin, 1996) and use color, luminance, and motion information in assigning depth relations to surfaces (Johnson & Aslin, 1998, 2000; Johnson & Mason, 2002).
What, then, are the mechanisms of development of unit formation? Results from our experiments highlight the importance of targeted visual exploration in perceiving object unity. Targeted visual exploration emerges within the first several months after birth and stems from increasing cortical and endogeous control of oculomotor behavior (Amso and Johnson, 2005, 2006, in press; Johnson, 2001; Johnson et al., 2004). On this account, very young infants’ ability to perceive occlusion may be compromised because they do not obtain sufficient visual information for unity during the time the stimulus is available for viewing, information such as edge alignment, common motion, good form, and other Gestalt cues. On the other hand, insufficient information acquisition during habituation may lead to a “default” response to the visible disjoint surfaces, characteristic of younger infants, yielding a novelty preference for the complete rod at test. Either possibility is consistent with the idea that efficient visual exploration is an important agent of development in perceptual completion skills. An alternative possibility is that as infants come to perceive occlusion, their eye movement patterns change to support or confirm this percept. This possibility is unlikely, however, given recent experiments in which infants’ perceptual completion, and scanning patterns when viewing rod-and-box displays, were found to be strongly related to performance in a visual search task in which targets were selected amongst distracters (Amso & Johnson, 2006). This finding is inconsistent with the likelihood that scanning patterns were tailored specifically to perceptual completion, and instead suggests that a general facility with targeted visual behavior leads to improvements across multiple tasks. This is precisely what we found in the present experiments.
Targeted visual exploration supports the detection and subsequent processing of some parts of a visual scene while others are ignored or suppressed, contributing to information extraction. In the case of perceptual completion, our results imply that developments attentional engagement resulted in superior information acquisition as infants participated in the Scanning task, and perhaps as well during the habituation phase of the Perceptual Completion task (cf. Amso & Johnson, 2006; Johnson et al., 2004). The ability to attend to the rod parts and ignore irrelevant yet salient display elements increases the likelihood of gathering the relevant information for unity perception.
Targeted visual exploration incorporates complementary processes of selective attention and inhibitory control of eye movements, supporting the detection and subsequent processing of certain parts of a visual scene while others are ignored or suppressed. Selective attention, defined as increasing endogenous control of eye movements and a simultaneous decrease in exogenous control, emerges across the first several postnatal months with developments in cortical oculomotor control systems (Johnson, 1990, 2005; Richards & Hunter, 2002; Schiller, 1998). Control of saccades is initially exogenously driven and is thought to rely largely on subcortical circuitry. Reflexive eye movements involve a pathway involving connections from retinal ganglion cells, the lateral geniculate nucleus, and the superior colliculus, with only limited input from primary cortical visual areas. Maturation of other visual pathways supports more endogenous oculomotor control, in particular the frontal eye fields for overt shifts of attention, the parietal cortex for covert shifts of attention, and the prefrontal cortex for attentional control involving delays. Voluntary control over saccadic eye movements, such as those involved in visual inspection, involves connections between visual areas V1, V2, and V4, parietal cortex, and the frontal eye fields. Less is known about developments in inhibitory control of oculomotor behavior. Amso and Johnson (2005) recently reported evidence for functional inhibitory visual control mechanisms in 9-month-olds, whose oculomotor latencies to locations occupied by previously visible (but ignored) targets were delayed relative to control locations (thus the locations were processed covertly, and subsequently inhibited upon later selection). These mechanisms are at least partly functional in infants as young as 3 months (Amso & Johnson, 2006, in press). The cortical foundations of inhibitory oculomotor control, however, remain poorly understood.
A final question concerns the precise role of motion to infants’ perceptual completion. We found that motion sensitivity was unrelated to perceptual completion performance, yet previous experiments have found infants unable to perceive object unity without it (Kellman & Spelke, 1983; Jusczyk et al., 1999). These apparently conflicting findings can be reconciled under our model of the development of perceptual completion described previously (Figure 9). Note that each component of the model, segregation, depth placement, and unit formation, includes motion information. As noted previously, motion contributes both to infants’ surface segregation and to infants’ depth placement (Johnson & Aslin, 1998; Johnson & Mason, 2002). Its role in unit formation may be direct, serving to unify visible surfaces. Its role might also, or instead, be indirect, by increasing the salience of the moving parts, thus recruiting visual attention. Support for this possibility comes from experiments in which both moving and static versions of occlusion stimuli were shown to infants: Kellman and Spelke (1983) and Jusczyk et al. (1999) found that looking times were substantially higher during habituation to moving stimuli, relative to static displays.
Taken together, these findings motivate a new view of the contributions of motion information to development of object perception in infancy. On this new account, motion provides support for segmentation and depth placement, two foundational perceptual achievements antecedent to unit formation, but it may play no direct part in unit formation as such. If this is correct, infants in the Valenza et al. (2006) experiment who were presented with apparent motion did not achieve perceptual completion, but instead may have perceived rod parts and occluder as part of the same object, a possibility made more likely by the black background in their displays; for young infants, an untextured background precludes perceptual completion by impeding depth placement in 2D stimuli (Johnson & Aslin, 1996). This led to a posthabituation preference for the broken rod not because it was novel relative to a connected object behind the occluder, but rather because it was novel relative to a perceived larger, unified object—rod-parts-plus-occluder. Thus in the absence of motion to force perceptual segregation of rod parts and occluder, these surfaces were never perceived as separate, barring the first step toward perceptual completion under our model. Notably, full motion sensitivity is not necessary to exploit motion-induced segmentation. That is, the very young infant’s visual system may be unresponsive to direction of motion, yet still can discriminate changes in positions of surfaces when provided sufficient horizontal translation (Johnson, 2004).
In conclusion, the results of the present report and other recent findings suggest that development of perceptual completion relies less on cortical maturation and on motion sensitivity than on the infant’s own oculomotor patterns. Infants “assemble” surfaces in the visual environment that are spatially segregated via active examination of visible parts, implying that developing object concepts are neither abstract nor innate, but instead are tied closely to the infant’s own experience and behavior, and his or her interactions with the environment. With the emergence of selective attention, infants become active participants in their own perceptual processes rather than passive recipients of information, a possibility that is compatible with other approaches emphasizing a strong role for the infant’s behavior in his or her development (e.g., Bremner, 1993; Campos, Anderson, Barbu-Roth, Hubbard, Hertenstein, & Witherington, 2000; Thelen, 2000).
Figure 4.
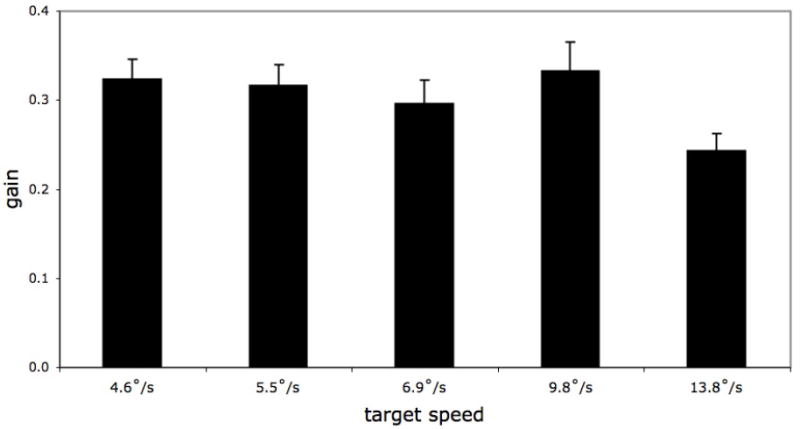
Smooth pursuit gain at each target speed.
Acknowledgments
This research was supported by NSF grant BCS-0418103 and NIH grants R01-HD40432 and R01-HD048733. We gratefully acknowledge the efforts of the infants and parents who participated in the studies. We thank also Lauren Clepper for assistance recruiting the infant participants, and Kerri L. Johnson and Masumi Ido for helpful advice.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at http://www.apa.org/journals/dev/
Contributor Information
Scott P. Johnson, University of California, Los Angeles
Juliet Davidow, Sackler Institute for Developmental Psychobiology.
Cynthia Hall-Haro, New York University.
Michael C. Frank, Massachusetts Institute of Technology
References
- Amso D, Johnson SP. Selection and inhibition in infancy: Evidence from the spatial negative priming paradigm. Cognition. 2005;95:B27–B36. doi: 10.1016/j.cognition.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Amso D, Johnson SP. Learning by selection: Visual search and object perception in young infants. Developmental Psychology. 2006;6:1236–1245. doi: 10.1037/0012-1649.42.6.1236. [DOI] [PubMed] [Google Scholar]
- Amso D, Johnson SP. Development of visual selection in 3- to 9-month-olds: Evidence from saccades to previously ignored locations. Infancy. doi: 10.1080/15250000802459060. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson J. Early visual development: Differential functioning of parvocellular and magnocellular pathways. Eye. 1992;6:129–135. doi: 10.1038/eye.1992.28. [DOI] [PubMed] [Google Scholar]
- Atkinson J. The developing visual brain. Oxford, UK: Oxford University Press; 2000. [Google Scholar]
- Banton T, Bertenthal BI. Multiple developmental pathways for motion processing. Optometry and Vision Science. 1997;74:751–760. doi: 10.1097/00006324-199709000-00023. [DOI] [PubMed] [Google Scholar]
- Braddick O, Atkinson J, Wattam-Bell J. Normal and anomalous development of visual motion processing: Motion coherence and “dorsal stream vulnerability.”. Neuropsychologia. 2003;41:1769–1784. doi: 10.1016/s0028-3932(03)00178-7. [DOI] [PubMed] [Google Scholar]
- Braddick O, Birtles D, Wattam-Bell J, Atkinson J. Motion- and orientation-specific cortical responses in infancy. Vision Research. 2005;45:3169–3179. doi: 10.1016/j.visres.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Bremner JG. Motor abilities as causal agents in infant cognitive development. In: Savelsbergh G, editor. The development of coordination in infancy. Amsterdam: Elsevier; 1993. pp. 47–77. [Google Scholar]
- Bremner JG, Johnson SP, Slater A, Mason U, Cheshire A, Spring J. Conditions for young infants’ failure to perceive trajectory continuity. Developmental Science. 2007;10:613–624. doi: 10.1111/j.1467-7687.2007.00616.x. [DOI] [PubMed] [Google Scholar]
- Bremner JG, Johnson SP, Slater AM, Mason U, Foster K, Cheshire A, Spring J. Conditions for young infants’ perception of object trajectories. Child Development. 2005;74:1029–1043. doi: 10.1111/j.1467-8624.2005.00895.x. [DOI] [PubMed] [Google Scholar]
- Campos JJ, Anderson DI, Barbu-Roth MA, Hubbard EM, Hertenstein MJ, Witherington D. Travel broadens the mind. Infancy. 2000;1:149–219. doi: 10.1207/S15327078IN0102_1. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. 3. Mahwah, NJ: Erlbaum; 2003. [Google Scholar]
- Cohen LB, Atkinson DJ, Chaput HH. Habit X: A new program for obtaining and organizing data in infant perception and cognition studies (version 1.0) Austin: University of Texas; 2004. [Google Scholar]
- Cohen LB, Chaput HH, Cashon CH. A constructivist model of infant cognition. Cognitive Development. 2003;17:1323–1343. [Google Scholar]
- Craton LE. The development of perceptual completion abilities: Infants’ perception of stationary, partly occluded objects. Child Development. 1996;67:890–904. [PubMed] [Google Scholar]
- Gibson JJ. The ecological approach to visual perception. Boston: Houghton Mifflin; 1979. [Google Scholar]
- Johnson MH. Cortical maturation and the development of visual attention in early infancy. Journal of Cognitive Neuroscience. 1990;2:81–95. doi: 10.1162/jocn.1990.2.2.81. [DOI] [PubMed] [Google Scholar]
- Johnson MH. Developmental cognitive neuroscience. 2. London: Blackwell; 2005. [Google Scholar]
- Johnson SP. Neurophysiological and psychophysical approaches to visual development. In: Kalverboer AF, Gramsbergen A, Hopkins JB, editors. Handbook of brain and behaviour in human development: IV. Development of perception and cognition. Amsterdam: Elsevier; 2001. pp. 653–675. [Google Scholar]
- Johnson SP. Development of perceptual completion in infancy. Psychological Science. 2004;15:769–775. doi: 10.1111/j.0956-7976.2004.00754.x. [DOI] [PubMed] [Google Scholar]
- Johnson SP, Aslin RN. Perception of object unity in 2-month-old infants. Developmental Psychology. 1995;31:739–745. [Google Scholar]
- Johnson SP, Aslin RN. Perception of object unity in young infants: The roles of motion, depth, and orientation. Cognitive Development. 1996;11:161–180. [Google Scholar]
- Johnson SP, Aslin RN. Young infants’ perception of illusory contours in dynamic displays. Perception. 1998;27:341–353. doi: 10.1068/p270341. [DOI] [PubMed] [Google Scholar]
- Johnson SP, Aslin RN. Infants’ perception of transparency. Developmental Psychology. 2000;36:808–816. [PubMed] [Google Scholar]
- Johnson SP, Johnson KL. Early perception-action coupling: Eye movements and the development of object perception. Infant Behavior & Development. 2000;23:461–483. [Google Scholar]
- Johnson SP, Náñez JE. Young infants’ perception of object unity in two-dimensional displays. Infant Behavior & Development. 1995;18:133–143. [Google Scholar]
- Johnson SP, Bremner JG, Slater A, Mason U, Foster K, Cheshire A. Infants’ perception of object trajectories. Child Development. 2003;74:94–108. doi: 10.1111/1467-8624.00523. [DOI] [PubMed] [Google Scholar]
- Johnson SP, Mason U. Perception of kinetic illusory contours by 2-month-old infants. Child Development. 2002;73:22–34. doi: 10.1111/1467-8624.00389. [DOI] [PubMed] [Google Scholar]
- Johnson SP, Slemmer JA, Amso D. Where infants look determines how they see: Eye movements and object perception performance in 3-month-olds. Infancy. 2004;6:185–201. doi: 10.1207/s15327078in0602_3. [DOI] [PubMed] [Google Scholar]
- Jusczyk PW, Johnson SP, Spelke ES, Kennedy LJ. Synchronous change and perception of object unity: Evidence from adults and infants. Cognition. 1999;71:257–288. doi: 10.1016/s0010-0277(99)00026-8. [DOI] [PubMed] [Google Scholar]
- Kellman PJ, Arterberry ME. The cradle of knowledge: Development of perception in infancy. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- Kellman PJ, Garrigan P, Shipley TF. Object interpolation in three dimensions. Psychological Review. 2005;112:586–609. doi: 10.1037/0033-295X.112.3.586. [DOI] [PubMed] [Google Scholar]
- Kellman PJ, Spelke ES. Perception of partly occluded objects in infancy. Cognitive Psychology. 1983;15:483–524. doi: 10.1016/0010-0285(83)90017-8. [DOI] [PubMed] [Google Scholar]
- Kovács I, Kozma P, Fehér Á, Benedek G. Late maturation of visual spatial integration in humans. Proceedings of the National Academy of Sciences (USA) 1999;96:12204–12209. doi: 10.1073/pnas.96.21.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr D. Vision. San Francisco: Freeman; 1982. [Google Scholar]
- Milner AD, Goodale MA. The visual brain in action. Oxford, UK: Oxford University Press; 1995. [Google Scholar]
- Nakayama K, He ZJ, Shimojo S. Visual surface representation: A critical link between lower-level and higher-level vision. In: Kosslyn SM, Osherson DN, editors. Visual cognition: Vol. 2. An invitation to cognitive science. 2. Cambridge, MA: MIT Press; 1996. pp. 1–70. [Google Scholar]
- Nakayama K, Shimojo S. Toward a neural understanding of visual surface representation. Cold Spring Harbor Symposia on Quantitative Biology. 1990;40:911–924. doi: 10.1101/sqb.1990.055.01.085. [DOI] [PubMed] [Google Scholar]
- Needham A, Baillargeon R, Kaufman L. Object segregation in infancy. In: Rovee-Collier C, Lipsitt L, editors. Advances in infancy research. Vol. 11. Greenwich, CT: Ablex; 1997. pp. 1–44. [Google Scholar]
- Needham A, Ormsbee SM. The development of object segregation during the first year of life. In: Kimchi R, Behrmann M, Olson C, editors. Perceptual organization in vision: Behavioral and neural perspectives. Mahwah , NJ: Erlbaum; 2003. pp. 205–232. [Google Scholar]
- Piaget J. In: The construction of reality in the child. Cook M, translator. New York: Basic Books; 1954. Original work published 1937. [Google Scholar]
- Richards JE, Hunter SK. Testing neural models of the development of infant visual attention. Developmental Psychobiology. 2002;40:226–236. doi: 10.1002/dev.10029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller PH. The neural control of visually eye movements. In: Richards JE, editor. Cognitive neuroscience of attention: A developmental perspective. Mahwah, NJ: Erlbaum; 1998. pp. 3–50. [Google Scholar]
- Shuwairi SM, Albert MK, Johnson SP. Discrimination of possible and impossible objects in infancy. Psychological Science. 2007;18:303–307. doi: 10.1111/j.1467-9280.2007.01893.x. [DOI] [PubMed] [Google Scholar]
- Slater A. Visual perception and memory at birth. In: Rovee-Collier C, Lipsitt LP, editors. Advances in infancy research. Vol. 9. Norwood, NJ: Ablex; 1995. pp. 107–162. [Google Scholar]
- Slater A, Johnson SP, Brown E, Badenoch M. Newborn infants’ perception of partly occluded objects. Infant Behavior & Development. 1996;19:145–148. [Google Scholar]
- Slater A, Johnson SP, Kellman PJ, Spelke ES. The role of three-dimensional depth cues in infants’ perception of partly occluded objects. Early Development and Parenting. 1994;3:187–191. [Google Scholar]
- Slater A, Morison V, Somers M, Mattock A, Brown E, Taylor D. Newborn and older infants’ perception of partly occluded objects. Infant Behavior & Development. 1990;13:33–49. [Google Scholar]
- Smith WC, Johnson SP, Spelke ES. Motion and edge sensitivity in perception of object unity. Cognitive Psychology. 2003;46:31–64. doi: 10.1016/s0010-0285(02)00501-7. [DOI] [PubMed] [Google Scholar]
- Tauber ES, Koffler S. Optomotor response in human infants to apparent motion: Evidence of innateness. Science. 1966;152:382–383. doi: 10.1126/science.152.3720.382. [DOI] [PubMed] [Google Scholar]
- Thelen E. Grounded in the world: Developmental origins of the embodied mind. Infancy. 2000;1:3–28. doi: 10.1207/S15327078IN0101_02. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M. Two cortical visual systems. In: Ingle DJ, Goodale MA, Mansfield RJW, editors. Analysis of visual behavior. Cambridge, MA: MIT Press; 1982. pp. 549–586. [Google Scholar]
- Valenza E, Leo I, Gava L, Simion F. Perceptual completion in newborn human infants. Child Development. 2006;77:1810–1821. doi: 10.1111/j.1467-8624.2006.00975.x. [DOI] [PubMed] [Google Scholar]
- Wattam-Bell J. Visual motion processing in 1-month-old infants: Habituation experiments. Vision Research. 1996;36:1679–1685. doi: 10.1016/0042-6989(95)00237-5. [DOI] [PubMed] [Google Scholar]
- Yantis S. Perceived continuity of occluded visual objects. Psychological Science. 1995;6:182–186. [Google Scholar]


