Abstract
Clinical availability of genetic testing for cancer predisposition genes is generating a major challenge for U.S. health care systems to provide relevant genetic services to underserved populations. Here we present rates of study enrollment and utilization of genetic testing in a research study on BRCA1 testing acceptance in one large kindred. We also present data on baseline access to genetic information as well as enabling and obstructing factors to study enrollment. The study population included female and male members of an African-American kindred based in the rural southern United States with an identified BRCA1 mutation. A combination of quantitative and qualitative data were collected and analyzed. Of the 160 living, eligible and locatable kindred members, 105 (66%) enrolled in the study. Family, personal, and educational motivations were the most commonly endorsed reasons for study participation. The most commonly cited reasons for refusal to participate in the study were: lack of interest, time constraints, and negative experiences with prior participation in genetic research. Eighty three percent of the participants underwent BRCA1 testing. In multiple logistic regression analysis, age 40-49 (odds ratio (OR) = 6.9; 95% confidence interval (CI) = 1.2-39.5), increased perceived cancer risk (OR = 4.1; 95% CI = 1.1-14.6), and high cancer genetics knowledge levels (OR = 1.5; 95% CI = 1.1-2.3) were associated with BRCA1 testing acceptance. The results of this study indicate that cognitive and demographic factors may influence genetic research participation and genetic testing decisions among African Americans who are at increased risk of carrying a deleterious BRCA1 mutation.
Keywords: African American, genetic testing, BRCA1 gene mutation, blacks, cultural sensitivity, research enrollment
INTRODUCTION
Use of clinical genetic testing for individuals at high risk for developing breast and ovarian cancer is increasing. Identification of at-risk individuals is important in the development of cancer prevention and control strategies. When used appropriately, translation of genetic discoveries related to hereditary cancers into clinical practice has potential health benefits. Available U.S. data indicate that many generalists and non-cancer genetic specialists are either unfamiliar or uncomfortable with genetic risk assessment, providing genetic education and counseling to their patients, and recommending or interpreting genetic tests [Kutner, 1999; Mouchawar et al., 2001; Velicer and Taplin, 2001]. Further, clinical availability of genetic testing for cancer predisposition genes is generating a major challenge for the health care systems of the U.S. to provide relevant genetic services to an increasingly diverse population. Little is known about factors associated with use of genetic testing as well as behavioral outcomes of the testing process in African Americans.
While studies about the decision to undergo genetic testing and the clinical impact of receipt of test results have been major topics of research, relatively few have evaluated these issues in African Americans [Culver et al., 2001; Hughes et al., 2003; Thompson et al., 2002]. Much more work is needed to understand barriers to and facilitators of enrollment in genetic research and acceptance of clinical genetic testing in African Americans and other minority groups. This is particularly important as clinical genetic testing becomes more widespread and the utility of genetic information becomes more evident. A better understanding of these factors may help in the provision of culturally-sensitive family cancer clinic services and identification of factors contributing to racial disparities.
Although little is known about reasons why African Americans do not use cancer genetic services or refuse to participate in cancer genetic research studies, more is known about factors influencing their participation in health related-research. Careful consideration of these factors may help researchers address low enrollment rates in genetic research and resources such as cancer registries. Barriers to research participation among African-Americans may include: medical mistrust; inconvenience; anticipated time commitment; lack of knowledge about the health issue under study; lack of cultural sensitivity of researchers; and religious/spiritual beliefs [Advani et al., 2003; Gamble, 1993; Herring et al., 2004; Hoyo et al., 2003]. In a recent study, enrollment rates in a familial cancer registry were substantially lower for African American (15%) than for White women (36%); these differences were not explained by socioeconomic or cancer risk factors [Moorman et al., 2004].
There is an increasing body of work directed at understanding genetic risk factors for breast and ovarian cancer in African Americans [Gao et al., 2000; Gao et al., 1997; Olopade et al., 2003]. For all races and ethnicities, a strong family history of breast and/or ovarian cancer is an important risk factor for these diseases. Genetic epidemiologic research has led to the discovery of inherited breast/ovarian cancer susceptibility genes, BRCA1 and BRCA2. Approximately 5 to 10% of breast cancer cases and 10% of ovarian cancer cases are attributed to genetic predisposition [Claus et al., 1998; King et al., 2003; Narod and Boyd, 2002]. Differences in BRCA1 prevalence rates between African Americans and whites have not been observed [Frank et al., 2002; Gao et al., 1997; Gao et al., 2000; Olopade et al., 2003]. However, similarities between BRCA1 -related cancers and breast cancers in African-American women such as young age at diagnosis and specific pathologic characteristics (i.e., large tumor size and lymph node involvement) have been reported [Olopade et al., 2003].
Available data indicate high levels of interest in and high rates of favorable attitudes about the benefits of genetic testing relative to limitations and risks of genetic testing for deleterious BRCA mutations in African-American women [Hughes et al., 2003; Hughes et al., 2004; Kinney et al., 2001]. Endorsed advantages of genetic testing for hereditary breast-ovarian cancer susceptibility include enhancing knowledge about preventive options, assisting with decision-making regarding risk reduction, and reducing uncertainty [Halbert et al., 2005]. Perceived disadvantages and concerns about BRCA1/2 testing cited by African-American women include potential adverse familial and emotional impact, and confidentiality issues [Kessler et al., 2005; Thompson et al., 2003]. Lower income, lower education levels, and low levels of knowledge about genetic testing have been associated with higher levels of concern about genetic testing [Thompson et al., 2003]. Although African Americans are more likely to express preferences for adult genetic testing than non-Latino Whites, recent data indicate relatively low levels of utilization of BRCA testing, suggesting that barriers to participation in genetic testing among African Americans may exist [Armstrong et al., 2005; Culver et al., 2001; Hughes et al., 2003; Singer et al., 2004; Thompson et al., 2003]. Several recent reviews of psychosocial issues in genetic counseling and testing for inherited breast-ovarian cancer concluded that more investigation is needed to understand enabling and obstructing factors for utilization and the behavioral impact of genetic testing in this population [Halbert et al., 2005; Hughes et al., 2004; Pasacreta, 2003].
Few studies have been published that examined both demographic and psychosocial factors related to acceptance of genetic counseling and testing for BRCA mutations in high-risk African-American women. Participation rates ranged from 40-61% [Culver et al., 2001; Hughes et al., 2003; Thompson et al., 2002]. In these studies, beliefs and attitudes such as fatalistic views toward cancer and familial independence were associated with non-participation. Available data indicate that African-Americans may be less knowledgeable about genetic testing than non-Hispanic whites and are less likely to have access to genetic services because of lower incomes and limited health insurance coverage [Halbert et al., 2005]. A recent study reported that substantially fewer African American women used genetic testing than white women in a single health care system in a large northeastern U.S. city [Armstrong et al., 2005].
Efforts to provide genetic counseling and testing programs to African Americans require an understanding of factors that predict utilization of such services. Most prior research in this area has focused on urban African-American women. This report describes a prospective, observational study examining predictors of BRCA1 testing decisions in male and female members of an African-American kindred with a BRCA1 mutation, most of whom resided in the rural southern United States. In this report we also describe barriers and facilitators to participation in the genetic testing study, baseline patient-provider communication about genetic risk status and clinical genetic services, and perceptions about access to these services outside of this study.
MATERIALS AND METHODS
Conceptual Framework
We used the PRECEDE-PROCEED model, a heuristic conceptual model, to guide this study [Green and Kreuter, 1991]. The underlying premise of the model asserts that health problems are multi-dimensional and, thus, cannot be explained by any single behavioral theory. The model further emphasizes that measuring determinants of health problems is an essential prerequisite to intervention development, deployment, and outcome evaluation. It also emphasizes that determinants will vary in importance across communities or population subgroups. Thus, specific needs of communities should be assessed and considered when planning, implementing, and evaluating interventions and research studies.
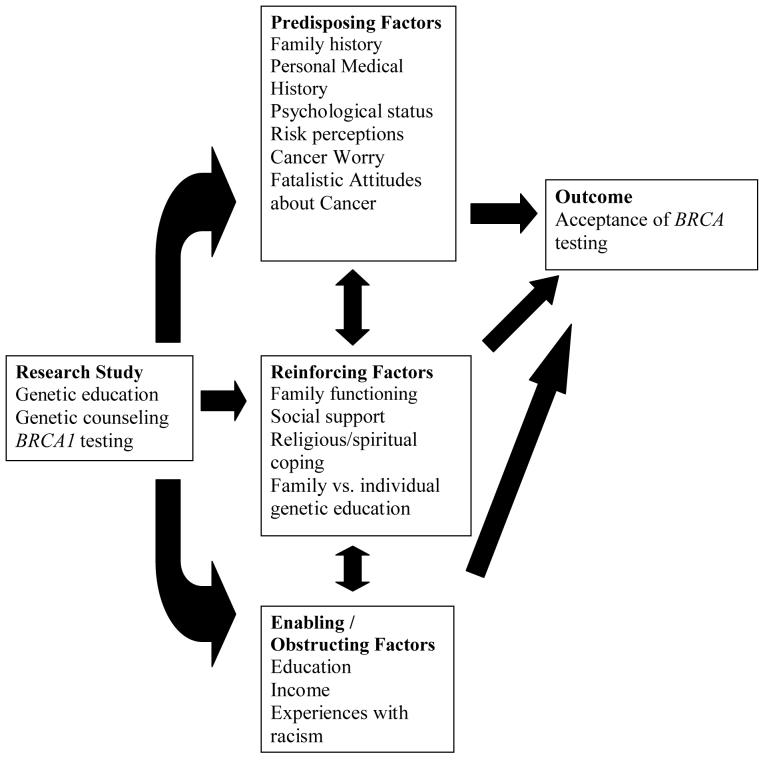
The PRECEDE-PROCEED model proposes broad constructs associated with health issues that are drawn from relevant theories and community needs: 1) Predisposing factors provide rationale or motivation for a behavior or health outcome to occur and include health beliefs and clinical factors, 2) Reinforcing factors provide incentive for a behavior or healthy state to persist and include social support and spirituality, 3) Enabling or obstructing factors are environmental conditions that facilitate or obstruct a behavior or healthy coping to occur or not to occur, 4) Behavioral factors are behaviors and lifestyles that contribute to the onset and severity of a health problem, and 5) Environmental factors are external social or physical factors that can be modified to promote healthy behaviors and coping. The health intervention or program that arises from consideration of these factors will typically modify the health problem by targeting predisposing, reinforcing, and enabling/obstructing factors in at-risk individuals to modify behavioral and environmental contributors to the health issue. Figure 1 presents the elements of the PRECEDE-PROCEED model and the associated factors for each that we selected as relevant to this study. This model is an ideal framework to link personal, familial, cultural, and social factors to health behaviors such as acceptance of genetic testing.
Figure I.
PRECEDE-PROCEED conceptual framework adapted for this study.
Study Design
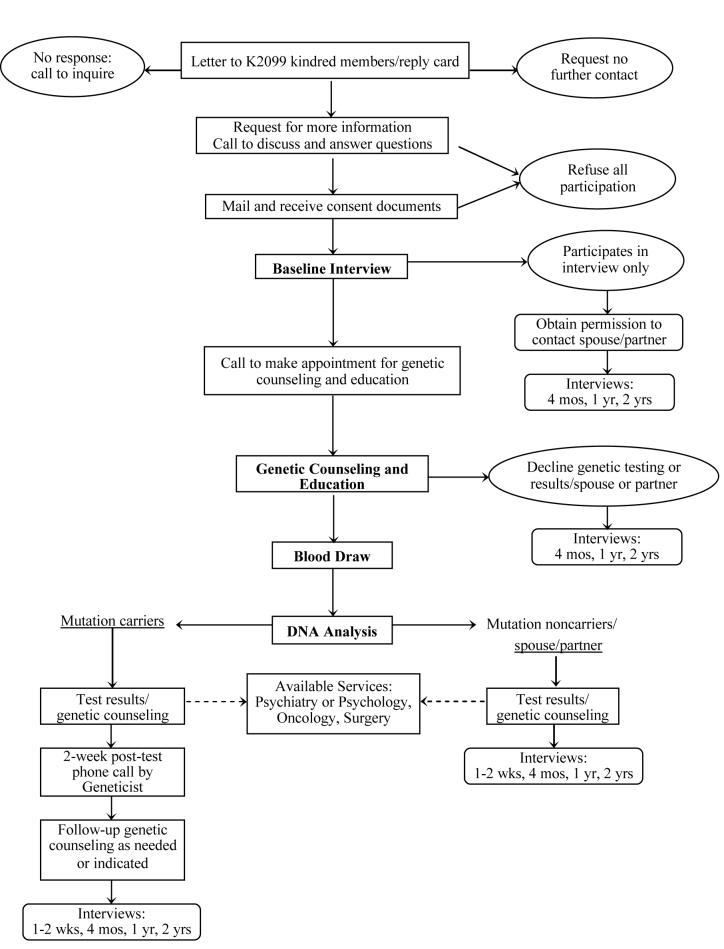
Figure II provides the study flow chart for this ongoing prospective observational study. Kindred members were offered genetic services, including BRCA1 testing and pre- and post-test education and counseling according to an established protocol. Follow-up interviews are conducted at four time points. The genetic education, counseling and testing phase of the study has been completed. Longitudinal follow-up information is currently being collected.
Figure II.
Family Health Study Flow Diagram
Study Population
The K2099 founder and many descendants are from the social and cultural milieu of a rural Louisiana bayou town on the Mississippi River. K2099 was originally ascertained for a genetic epidemiologic study conducted in 1993-94 to localize the BRCA1 gene [Miki et al., 1994]. Fifty-one of the adult kindred members enrolled in the current study participated in this prior research study, which identified a BRCA1 M1775R mutation [Miki et al., 1994]. Because the testing was done in a research lab, participants consented to the project after being informed that they would not receive their own results; this was consistent with IRB policies at the time the genetic epidemiologic study was conducted. In 1998-99, a K2099 needs assessment of psychosocial and health-related screening issues was conducted [Kinney et al., 2002; Kinney et al., 2001]. Results indicated high levels of interest in genetic testing, as well as a strong desire to increase knowledge about causes of cancer, familial cancer risk factors, and preventive measures. In 2001, after receiving funding for this prospective study, we initiated contact with kindred members.
Using the pedigree from the original linkage study [Miki et al., 1994], we initially contacted nuclear families, then expanded the pedigree to include those individuals who had not participated in prior research. For the current study, the five-generation K2099 pedigree includes 240 members with 56 known cases of breast (n=36), ovarian (n=7), prostate (n=6), and colorectal (n=7) cancer. Four kindred members have been diagnosed with two primary tumors (two breast/ovarian, one breast/colorectal, one ovarian/colorectal). Information on non-participants was obtained from our prior needs assessment, kindred key informants, and other kindred members. At enrollment, study participants were at least 18 years of age, provided written informed consent, had not undergone genetic education and counseling or clinical BRCA1 testing, and did not know their BRCA1 mutation status.
Procedures
Recruitment and Interviews
After receiving an introductory letter and completing a screening survey, all eligible kindred members were provided with a description of the study, the time commitment, the duration and timing of interviews, and options for genetic education, counseling, and testing. After providing written informed consent, a baseline in-person interview in a participant's home or at another mutually convenient location was conducted for those residing in Southeastern Louisiana. Telephone interviews were conducted for those who lived elsewhere. Follow-up interviews were conducted at 1 month, 4 months, 1 year and 2 years following receipt of genetic test results for those tested and following completion of the genetic education session for those who did not complete genetic testing. All in-person interviews were conducted by African-American staff. Participants received an incentive payment of $25 for completing each interview and a $35 incentive for completing all four follow-up interviews.
Genetic counseling and testing protocol
Study participants attended educational and counseling sessions conducted by one of four genetic counselors. Participants selected either a family education session followed by a private genetic counseling session, or a private combined education/counseling session; sessions used culturally-targeted and family tailored education materials [Baty et al., 2003]. We also offered a choice of locations for the counseling sessions. Those living around the ancestral hometown could meet with the counselor at LSUHSC, at a community center, or at their home. Those at distant locations could schedule visits in their home or another convenient site. The education session provided basic information about the incidence of sporadic and hereditary breast and ovarian cancer, risk factors for these cancers, inheritance of breast/ovarian cancer, review of risk reduction strategies for breast and ovarian cancer, and the benefits, limitations and risks associated with BRCA1 testing [Kinney et al., 2005a]. Approximately one month after the sample collection, a second counseling session was provided. Post-genetic test counseling included notifying participants of their carrier status, discussion of probability and age-specific cancer risk, implications for themselves and their relatives, and medical management recommendations. The post-test counseling also included written materials, which summarized the results of their genetic test result and the relevant risk reduction recommendations. These 1 to 2 hour sessions were conducted at convenient locations for the participants; the vast majority of sessions were conducted in a private area in the participants' homes. Genetic counseling and testing were provided at no cost to the participants.
Measures
Measures that were used in this report are described and internal consistency reliability (Cronbach's α) coefficients for scales are reported for the present study's sample. Unless otherwise indicated, measures were assessed at baseline.
Outcome Variables
Study Enrollment
Participants who signed an informed consent document were classified as enrollees. Open ended questions were used to evaluate facilitators and barriers to research participation. We were interested in understanding the motivations for study participation. For participants, questions asked at the baseline interview included: 1) “What would you say is the primary reason you decided to participate in this study?” and 2) “Are there any other reasons that led you to participate in this study?” During the recruitment telephone call, those who actively refused to participate in the study were asked to cite their reasons for refusal.
Acceptance of Genetic Test Results
Participants were classified as acceptors of BRCA1 testing if they received their test results during a post-test counseling session.
PRECEDE-PROCEED Model Constructs
Predisposing Factors
Clinical Factors
Personal and family cancer history were evaluated by standard single item measures.
Family composition
Number of living biologic children was assessed with a single item.
Perceived Risk of Carrying a BRCA Mutation
Perceived likelihood of carrying a mutation was assessed with one item, “In your opinion, how likely is it that you have an altered gene for breast cancer? (very likely, likely, neither likely nor unlikely, unlikely, very unlikely).
Cancer Worry
This cancer-specific, three-item scale was adapted from a measure developed by Lerman and colleagues [Stefanek et al., 1999; Tercyak et al., 2001]. Responses to each item were summed to obtain a scale score (α = 0.62).
Psychological Status
The 20-item Center for Epidemiologic Studies Depression (CES-D) scale evaluates depressive symptomatology (α=0.87) [Radloff, 1977]. The 20-item State Anxiety Scale of the State-Trait Anxiety Inventory (STAI-Form Y) measures apprehension (α=0.90) [Spielberger et al., 1971].
Cancer Genetics Knowledge
A 10-item knowledge questionnaire was used for this study. Questions were drawn from the Cancer Genetics Consortium genetics knowledge survey [Hughes et al., 1997].
Fatalistic Attitudes about Cancer
Cancer fatalism was measured with the 15-item Powe Fatalism Inventory. It is comprised of four subscales that measure fear about cancer, cancer pessimism, predetermination, and inevitability of death using a yes/no format (α = 0.73) [Powe, 1995].
Reinforcing Factors
Family Functioning
The 30-item Family Adaptability and Cohesiveness Survey (FACES II) was used to evaluate family functioning. The scale consists of two subscales, cohesion and adaptability. The cohesion subscale measures the strength of family members' attachment to each other while the adaptability subscale determines how flexible they are in their relationships with each other. Internal consistency reliability was high for both the cohesion (α=0.92) and adaptability (α=0.91) subscales [Olson et al., 1982].
Religious Coping
Problem-solving styles utilizing religiosity were assessed via Religious Problem-Solving Subscales [Pargament et al., 1988]. The collaborative subscale assesses the extent to which one solves problems through active personal exchanges with God. The self-directing style subscale assesses the extent to which an individual perceives freedom God gives individuals to direct their lives and solve problems. The deferring subscale assesses the extent to which individuals wait for God's solutions rather than solving the problems without awareness of God's intervention (α=0.92, 0.86 and 0.90, respectively).
Enabling/Obstructing Factors
Demographics
Age, education level, marital status, annual household income, health insurance status, and presence of living children were measured using standard single item measures.
Perceived Racism
Questions were adapted from the Perceptions of Prejudice scale [Facione, 1999] to assess perceptions of racism. Eight four-point Likert-style items were used to measure perceptions of experiences of racism in general and particularly in health care delivery settings (α = .60).
Social Support
The Medical Outcomes Study Social Support Survey scales measure tangible support (α=0.78), affection (α=0.76), interaction (α=0.92), and emotional support (α=0.95) [Sherbourne and Stewart, 1991].
Additional Measures
Patient-Communication
Several questions assessed patient-provider communication about hereditary breast and ovarian cancer (HBOC) risk and BRCA testing at baseline:“Has a physician or other health care provider ever told you that you have a higher than average risk of getting BREAST cancer? (yes, no); “Has a physician or other health care provider ever told you that you a have higher than average risk of getting OVARIAN cancer? (yes, no); and “Do you think that your regular doctor or health care provider has adequate knowledge to provide genetic counseling and testing for BRCA1? (yes, no) If no, please tell me why not” (asked at one-month interview).
Perceptions about Access to Genetic Information and Clinical Genetic Services
To assess perceived access to clinical cancer genetic counseling and testing outside of this study, close- and open-ended questions about these topics were asked at the one-month interview: “If genetic counseling and testing for BRCA1 were not available to you through this study, do you think you would have gone somewhere to get these services? “(yes, no) and if “no”, participants were asked why not; and “If the out-of-pocket expenses for the genetic tests involved in this study were $15 ($50, $150, $500, $1,000), what would you say is your level of interest in being tested?” (very interested, somewhat interested, and not at all interested).
Analyses
We first examined available demographics of participants and non-participants (i.e., age, sex, and place of residence) in relation to study enrollment using descriptive statistics and chi-square (standard and exact) tests. We next focused on select PRECEED-PROCEDE model constructs' associations with acceptance of genetic testing using descriptive statistics, chi-square tests and t-tests. Variables that were significantly associated with acceptance of genetic testing at the p < 0.20 level were entered into the logistic model. Using a backward elimination procedure, variables associated with acceptance of genetic testing at the p < 0.10 level were retained in the final adjusted model. Conventional logistic regression analyses assume that data from different participants are independent; however, related individuals such as siblings may report correlated observations. Therefore, using generalized estimating equations, we fit a random effect to account for potential correlation between observations among siblings and used likelihood based approaches to test whether associations between kindreds were statistically significant [Liang and Zeger, 1986]. The difference of the residual log likelihood statistics for models with and without the kindred factor was compared to the chi-square distribution for df=1 and was non-significant for all factors studied. Therefore, the final logistic model did not include the kindred random effect. Results were summarized using odds ratios (OR) and 95% confidence intervals (CI). All p values are two-sided and statistical significance was considered for p <0.05 values.
Next, we examined provider communication and perceived access to genetic services outside of this study by using descriptive statistics (i.e., frequencies and proportions) and qualitative analytic techniques described below.
Analyses of qualitative data (audiotaped and transcribed verbatim open-ended responses (e.g., reasons for enrollment and non-enrollment, patient-provider communication and perceived access items) were categorized using procedures consistent with content analysis [Krippendorff, 1980]. Two of the authors (AK& SS) developed a coding system from a literature review and independently reviewed responses of all participants. Subsequently, the same two coders each independently rated all participant responses and then resolved discrepancies by consensus. Thus all analyses are based on a dataset with all discrepancies resolved. Reliability was determined by assessing the two raters agreement level with kappa statistics [Landis and Koch, 1977]. Inter-rater reliability for the coding system was very good to excellent (Kappas: 0.81-1.00).
RESULTS
Study Population Characteristics
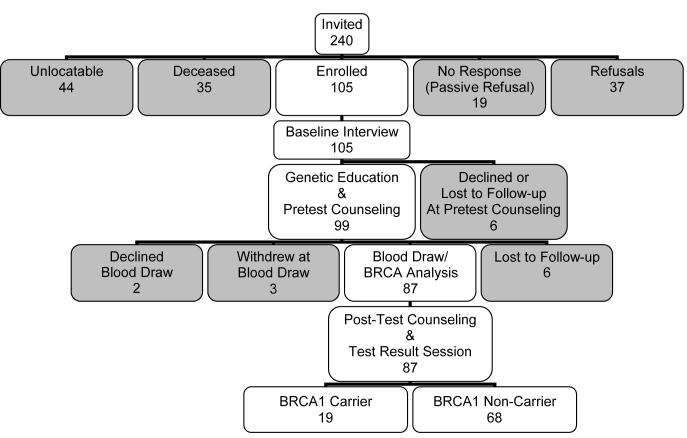
Of the 240 kindred members aged 18 years and older whom we identified as potential participants, 6 were ineligible (3 were disabled or mentally incompetent, 2 were incarcerated, 1 was overseas in the military), and 29 were deceased. Figure III depicts the study recruitment and genetic testing schema. We were able to contact 161 living and eligible kindred members. Of the living, eligible, and contactable kindred members, 105 enrolled. Response and cooperation rates as defined by the American Association for Public Opinion Research [The American Association for Public Opinion Research, 2004] were 51% and 65%, respectively and are reported in Table I. Neither sex (p = 0.31), prior genetic epidemiologic research participation (p = 0.90) nor residence in Louisiana influenced participation (p = 0.80).
Figure III.
Recruitment and Genetic Testing Schema
Table I.
Responses of individuals selected as potential participants for the Family Health Study by kindred status, gender and age.
| Variable | N % |
|---|---|
| K2099 pedigree | 240 (100%) |
| Ineligible (%)a | 6 (3%) |
| Deceased | 29 (12%) |
| Uncontactable (%) | 44 (18%) |
| Family member refuses study staff to contact (%) | 37 (15%) |
| Participant refusal (%) | 6 (6%) |
| Interviewed | 105 |
| Contact rate (%)b | 161/240 (67%) |
| Cooperation rate (%)c | 105/161 (65%) |
| Overall response rate (%)d | 105/205 (51%) |
Eligibility criteria include age 18 years and older, biological relationship in kindred, or married or living with a spouse partner, able to complete an interview in English.
Contact rate = number of individuals contacted/# of individuals identified as potential kindred members or spouse/partners
Cooperation rate = # of completed interviews/over those contacted and eligible
Overall response rate = # of completed interviews/# of individuals selected for study not including deceased or ineligible persons
The demographic, clinical and psychosocial characteristics of the 105 participants who completed a baseline interview and were offered genetic education, counseling, and testing are shown in Table II. All of the 105 participants in the current study self identified as African American and 90% also identified themselves as Black Creole [Dormon, 1992; Dubois and Melancon, 2000].
Table II.
Baseline sociodemographic and clinical characteristics among kindred members.
| Variable | Kindred Respondents | |
|---|---|---|
| N = 105* | % | |
| Gender | ||
| Female | 71 | 68 |
| Male | 34 | 32 |
| Age, years | ||
| < 40 | 46 | 44 |
| 40-49 | 36 | 34 |
| 50-64 | 15 | 14 |
| ≥ 65 | 8 | 8 |
| Residence | ||
| Southeastern Louisiana | 82 | 78 |
| Other | 23 | 22 |
| Educational attainment | ||
| Some High school or less | 13 | 12 |
| High school graduate or GED | 28 | 27 |
| Some college/vocational school | 43 | 41 |
| College/vocational school graduate | 19 | 18 |
| Marital status | ||
| Married/living as married | 83 | 79 |
| Unmarried or separated | 22 | 21 |
| Household Income | ||
| < $20,000 | 21 | 21 |
| $20,000-$40,000 | 32 | 31 |
| $41,000-$59,000 | 17 | 17 |
| >= $60,000 | 32 | 31 |
Cited Reasons for Study Enrollment and Non-enrollment
Reasons for enrollment and non-enrollment were elicited from participants and non-participants, respectively. Four major themes emerged for enrollment. Family and personal motivations constituted 62% of the responses. 28% of the responses revealed educational or informational motivations, and 9% noted environmental/societal concerns; respondents expressed their understanding that participation could have a positive and broad community impact. Six reasons were identified for declining to participate. Lack of interest was the primary reason for not enrolling in the study (54%). Other factors included time constraints (12%), personal problems (6%), and study logistics (8%). Rarely, negative BRCA1 test results in other relatives led to refusal to enroll (4%). Ten percent of K2099 members decided not to enroll because of negative attitudes about prior experiences involving their or their family member's participation in prior genetic research (e.g., research test results were not disclosed to them).
Predictors of Acceptance of BRCA Testing
Table III presents comparisons of acceptors and decliners of BRCA testing. Perceived increased risk of being a BRCA1 mutation carrier, age over 39 years, higher levels of cancer genetics knowledge, and collaborative religious coping style were significantly associated with acceptance of testing. As shown in Table IV, in logistic regression analysis, three factors were independently associated with BRCA1 testing acceptance: age, increased perceived cancer risk and high cancer genetics knowledge levels.
Table III.
Bivariate Analysis of Predictors Associated with Acceptance of Genetic Testing (n = 105)
| Variable | Test Accepters n (%) 87 (83) | Test Decliners n (%) 18 (17) | P value |
| Gender | |||
| Female | 59 (68) | 11 (61) | |
| Male | 28 (32) | 7 (39) | 0.58 |
| Age | |||
| < 40 | 34 (39) | 14 (78) | |
| 40-49 | 34 (39) | 2 (11) | |
| 50+ | 19 (22) | 2 (11) | 0.02 |
| Educational attainment | |||
| HS graduate or less | 32 (37) | 7 (39) | |
| Some college or more | 55 (63) | 11 (61) | 0.87 |
| Household income | |||
| <$20,000 | 19 (22) | 6 (35) | |
| $20,000-$40,999 | 25 (30) | 5 (29) | |
| $41,000-$59,999 | 13 (15) | 3 (18) | |
| $60,000+ | 28 (33) | 3 (18) | 0.21 |
| Marital status | |||
| Married/Living as married | 51 (59) | 9 (50) | |
| Not married | 36 (41) | 9 (50) | 0.50 |
| Health insurance | |||
| None | 24 (28) | 5 (28) | |
| Public or Private Insurance | 63 (72) | 13 (72) | 1.00 |
| FDR with breast or ovarian cancer | |||
| None | 49 (57) | 7 (41) | |
| One | 21 (24) | 7 (41) | |
| Two or more | 16 (19) | 3 (18) | 0.50 |
| History of breast or ovarian cancer | 82 (94) | 18 (100) | |
| No | 5 (6) | 0 (0) | 0.59 |
| Yes | |||
| Living children | |||
| None | 23 (26) | 5 (28) | |
| Living sons or daughters | 64 (74) | 13 (72) | 1.00 |
| Likelihood of being BRCA1 carrier | |||
| Unlikely/Moderate Chance | 17 (20) | 8 (44) | 0.04 |
| Likely/Very Likely | 68 (80) | 10 (56) | |
| Participation in prior genetic linkage study | |||
| Yes | 39 (45) | 7 (39) | |
| No | 48 (55) | 11 (61) | 0.64 |
| Type of counseling session | |||
| Individual | 47 (54) | 6 (50) | |
| Group | 40 (45) | 6 (50) | 0.79 |
| Variable | Test Accepters mean (SD) | Test Decliners mean (SD) | p value |
| Depression | 11.64 (9.75) | 9.44 (9.75) | 0.39 |
| Anxiety | 32.47 (8.77) | 33.33 (11.07) | 0.71 |
| Social Support | 19.25 (3.33) | 20.61 (2.98) | 0.13 |
| Religious Coping Total | 18.46 (3.06) | 19.88 (2.52) | 0.09 |
| Collaborative Religious Coping | 22.70 (5.82) | 25.50 (4.69) | 0.07 |
| Self Directing Religious Coping | 12.61 (5.03) | 12.50 (5.59) | 0.94 |
| Deferring Religious Coping | 19.93 (6.33) | 21.63 (5.45) | 0.32 |
| Cancer Worry Total | 8.14 (2.38) | 8.24 (2.17) | 0.88 |
| Experiences of Racism | 17.67 (3.02) | 16.89 (2.47) | 0.31 |
| Cancer Genetics Knowledge | 7.14 (1.62) | 6.11 (2.08) | 0.02 |
| Cancer Fatalism | 3.45 (1.86) | 3.75 (1.98) | 0.57 |
| FACES II | |||
| Cohesion | 59.04 (6.91) | 60.06 (8.54) | 0.60 |
| Adaptability | 51.71 (7.53) | 55.92 (8.00) | 0.05 |
Table IV.
Logistic Regression Analysis of Predictors of BRCA1 Testing Uptake.
| Model Variables | Odds Ratio | 95% Confidence Interval |
|---|---|---|
| Age | ||
| < 40 | 1.0 | Referent |
| 40-49 | 6.91 | 1.2-39.9 |
| ≥ 50 | 4.97 | 0.7-34.3 |
| Likelihood of being BRCA1 carrier | ||
| Unlikely/Moderate Chance | 1.00 | Referent |
| Likely/Very Likely | 0.24 | 0.1-0.9 |
| Cancer Genetics Knowledge | 1.54 | 1.05-2.28 |
| Perceived Family Adaptability | 0.94 | 0.86-1.01 |
Odds ratios are adjusted for all variables in the model.
Patient-Provider Communication and Perceptions about Access to Clinical Cancer Genetic Services
Among women without a personal history of breast and/or ovarian cancer, 44% reported that a health care provider discussed their higher than average risk of developing breast cancer and only 9% indicated that a health care provider discussed their higher than average risk of developing ovarian cancer. None of the participants had undergone clinical genetic testing prior to study enrollment. Only 18% of participants indicated that they would have been tested for BRCA1 had it not been available through this study. The most common reasons for not getting clinical genetic testing prior to participation in this study were related to access to information, lack of knowledge about the test, and where to go for genetic counseling and testing. Further, only 35% of participants reported that they thought their regular doctor or health care provider had adequate knowledge to provide BRCA -related services, whereas 53% did not; 13% said that they did not know. Among those who responded “no”, the most common reasons they gave were: their provider lacked education and training in genetics, was unaware of the gene, or would recommend or refer to an experienced provider. The vast majority of participants indicated that they would be very interested in BRCA testing if the out-of-pocket expenses were $15 (81%) but only 49% and 15% would be very interested BRCA1 testing cost them $150, and $1,000, respectively.
DISCUSSION
This report included the largest number of high-risk African Americans involved in a prospective study of acceptance of BRCA1 testing in the literature. A primary aim of this study was to examine predictors of acceptance of BRCA1 testing. Of the 161 living, eligible and contactable kindred members, 54% chose to participate in the education and counseling study and to have BRCA1 testing. The genetic testing uptake rate is similar to uptake rates observed in other studies of high-risk kindreds [Biesecker et al., 2000; Botkin et al., 2003; Lerman et al., 1996] and in studies consisting of African-American community- and clinic-based samples [Culver et al., 2001; Hughes et al., 2003; Thompson et al., 2002]. Of the participants in our study who completed a baseline survey and participated in genetic education and counseling, 88% accepted genetic testing. This uptake rate is consistent with prior studies [Biesecker et al., 2000; Botkin et al., 2003].
In multiple logistic regression analysis, three factors were significantly associated with utilization of genetic testing. These were age over 39 years, higher levels of cancer genetics knowledge, and perceived increased personal risk of having a BRCA1 mutation. Consistent with other work in both non-Latino white and African-American high-risk study populations, [Armstrong et al., 2005; Codori et al., 1999; Culver et al., 2001], our study showed that increased risk perceptions and higher levels of cancer genetics knowledge [Thompson et al., 2002] were associated with acceptance of cancer susceptibility testing. Others have also observed significant positive associations with age and participation in genetic testing studies consisting predominately of high-risk non-Latino white women [Biesecker et al., 2000; Lee et al., 2002]. One explanation for this finding could be that older women have a heightened concern about their breast cancer risk. Another potential explanation could be that older age may be in part a proxy for concern about having older children who will be or are of childbearing age, thereby resulting in worry about passing on the risk to their offspring. Our finding, however, does not support other studies focusing on high-risk African-American women in which younger age was associated with testing uptake [Hughes et al., 2003; Thompson et al., 2002]. In contrast to other studies, we did not observe associations between the cancer genetic test uptake and baseline psychological distress, fatalistic beliefs about cancer, participation in prior genetic epidemiologic research, and social support [Armstrong et al., 2005; Hughes et al., 2003; Lerman et al., 1996; Thompson et al., 2002].
In our study, religious coping style was not associated with utilization of genetic testing. However, others have observed associations between religious/spiritual factors and decision-making about genetic testing for familial cancer and other cancer prevention activities in both African Americans and non-Latino Whites [Kinney et al., 2002; Kinney et al., 2005b; Schwartz et al., 2000]. Religious coping styles may be particularly important to evaluate among larger samples of African Americans, because African Americans are more likely to report church group membership and attendance [Strawbridge et al., 1997; Strawbridge et al., 2001], report higher levels of religiosity, and turn to religion as a coping resource when faced with health challenges [Ferraro and Koch, 1994] than non-Latino whites. Both collaborative and deferring religious coping styles have been associated with religious involvement (e.g, frequency of church attendance and prayer) [Pargament et al., 1988]. However, we did not evaluate religious/spiritual involvement and specific practices, which may be more important determinants of genetic testing behavior. Further, our study was not designed to examine how individuals make use of religious coping to understand and deal with HBOC risk and make preventive health-related decisions. We encourage future research in this area as well as examining the role of other factors (medical mistrust and temporal orientation) in acceptance of genetic testing [Hughes et al., 2003; Thompson et al., 2003].
An aim of our study was to examine provider- and system-level influence on use of clinical genetic testing. We found that communication between providers and our study participants with regard to familial cancer risk was suboptimal. Prior to genetic counseling as part of this study, less than half of female participants without a personal history of breast/ovarian cancer reported communication with their providers about their potential genetic risk of breast cancer and fewer than 10% reported discussions about risk of ovarian cancer. Many participants in our study were not aware of the availability of clinical BRCA testing and settings in which cancer genetic services were provided. Others have also reported low levels of awareness about the availability of BRCA testing in moderate- to high-risk African Americans [Halbert et al., 2005]. Prior research comparing knowledge levels between racial groups observed lower levels among African-American women compared to non-Latino white women [Lerman et al., 1999]. Further, many of the participants in our study perceived that their health care providers lacked sufficient knowledge and training to offer cancer genetic services. It is well recognized that practice settings and individual care providers play a central role in coordinating, endorsing, and integrating virtually all aspects of patient care. The defining characteristics of health care such as accessibility (organizational) and clinical interaction (e.g., clinician-patient communication) have been strongly associated with favorable health outcomes such as patients' willingness to comply with health-related advice or treatment, patients' understanding of and efficacy managing chronic health conditions, and patients' self-reported health improvements [Safran et al., 1998]. There is a dearth of data on relationships between provider-level and system-level factors and use of clinical genetic services in diverse settings and among diverse populations.
Enrollment (response) rates in our study were comparable to prior BRCA testing kindred studies of predominantly non-Latino whites [Botkin et al., 1996; Lerman et al., 1996] and population-based samples of African Americans. Our response rate was also comparable to other BRCA testing studies of a community-based study population [Thompson et al., 2003] and a self-referred genetic education and testing research program [Hughes et al., 2003]; both prior studies focused on African-American women. Because of reluctance among many African Americans to participate in research and concomitant recruitment challenges [Corbie-Smith et al., 2002], we assessed factors related to study enrollment and refusal. Addressing this aim was particularly important because of the under-representation of racial and ethnic minorities in research studies in general and in cancer genetic studies in particular [Hughes et al., 2004]. Mistrust of the medical community and the research process are among the commonly cited barriers to research participation in African Americans. In our study, 10% of kindred members who decided not to enroll cited negative views of research.
Our data had several limitations. About half of the participants took part in prior genetic research and are related to a common ancestor, which could result in potential selection bias. We chose to limit this study population to kindred members because the kindred was known to have a BRCA1 mutation and specific mutation testing could be performed. In this study, confidential genetic counseling and testing were offered free of charge and were not recorded on a medical record. Our data suggest that participants may not be willing or able to pay out-of-pocket expenses that may be required for them to obtain these services. It is unknown what proportion of insured participants had adequate insurance coverage that would fully or partially reimburse for these services. Few clinical settings will offer free genetic counseling and testing services and none will provide the protection of confidentiality and privacy that are inherent in genetic testing research studies in non-clinical settings. These access factors may influence test acceptance. Additional limitations are that some of the scales had limited reliability in our study population and the time of administration (one month following genetic counseling) of some of the items assessing clinical services utilization (e.g., willingness to pay out of pocket expenses). This may have resulted in misclassification bias because participants may have answered these questions differently if they were asked before rather than after exposure to genetic education and counseling.
Selection bias, free genetic testing, provision of services in settings (e.g., participants' homes) and at times that were convenient for participants (e.g., nights and weekends), as well as the low response rate limit generalizability of our findings. The small sample size resulted in limited statistical power, particularly in relation to testing for PRECEDE-PROCEED model constructs as predictors of acceptance of genetic testing. Therefore our results on this important and high-risk African-American kindred of Creole origin should be interpreted with caution.
Despite these limitations, our results suggest that rates of acceptance of free BRCA testing for HBOC among high-risk African Americans in the southern United States in the context of a genetic counseling and testing research study may be similar to non-Latino whites and African Americans based in other geographic areas. Our findings also suggest the need to find ways to make genetic testing more accessible to those who might benefit. There is a need to develop effective approaches to promote education and train primary care providers to facilitate communication with patients about familial cancer risk and cancer genetics. Moreover, research on determinants of community-based primary care and specialist provider referral to cancer genetic services is needed. In this way, access to genetic services can be increased among persons from diverse sociodemographic backgrounds. As clinical genetic testing becomes more widespread and the utility of genetic information becomes more evident, we need to understand how to communicate genetic information effectively and provide genetic services for adult onset diseases to diverse populations. Further understanding of factors influencing testing uptake such as access issues and cultural factors such as the role of religion in medical decision-making will be important if we are to provide cancer genetic services that are responsive to ethnic minority subpopulations. Education about the availability of testing and cancer genetics as well as development of culturally sensitive approaches is of paramount importance for this to occur.
ACKNOWLEDGMENTS
This project was funded by the National Human Genome Research Institute, National Institute of Nursing Research and the National Cancer Institute (1 R01 HG02241 and 1 R01 HG02241-02S1). We thank Jeffrey Botkin and Jean Wylie for assistance with the design and implementation of the FHS, Chanita Hughes-Halbert, Claudia Branch, Lindsey Bloor, France Davis, Sharon Steib, and Susan Schulman for their comments on an earlier version of this manuscript. We would also like to thank Regan Challinor and Kelly Jackson for conducting the genetic education and counseling sessions. We also thank Jucynthia Taylor Ford, Ashely Holmes, Josalin Hunter, Kendra Rockwell, Berneice Parker, Andrea Wiley, and Carolyn Ross for their help in conducting this project.
REFERENCES
- Advani AS, Atkeson B, Brown CL, Peterson BL, Fish L, Johnson JL, Gockerman JP, Gautier M. Barriers to the participation of African-American patients with cancer in clinical trials: a pilot study. Cancer. 2003 March 15;97(6):1499–506. doi: 10.1002/cncr.11213. [DOI] [PubMed] [Google Scholar]
- Armstrong K, Micco E, Carney A, Stopfer J, Putt M. Racial differences in the use of BRCA1/2 testing among women with a family history of breast or ovarian cancer. JAMA. 2005 April 13;293(14):1729–36. doi: 10.1001/jama.293.14.1729. [DOI] [PubMed] [Google Scholar]
- Baty BJ, Kinney AY, Ellis SM. Developing culturally sensitive cancer genetics communication aids for African Americans. Am J Med Genet. 2003 April 15;118A(2):146–55. doi: 10.1002/ajmg.a.10150. [DOI] [PubMed] [Google Scholar]
- Biesecker BB, Ishibe N, Hadley DW, Giambarresi TR, Kase RG, Lerman C, Struewing JP. Psychosocial factors predicting BRCA1/BRCA2 testing decisions in members of hereditary breast and ovarian cancer families. Am J Med Genet. 2000 August 14;93(4):257–63. doi: 10.1002/1096-8628(20000814)93:4<257::aid-ajmg1>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Botkin JR, Croyle RT, Smith KR, Baty BJ, Lerman C, Goldgar DE, Ward JM, Flick BJ, Nash JE. A model protocol for evaluating the behavioral and psychosocial effects of BRCA1 testing. J Natl Cancer Inst. 1996 July 3;88(13):872–82. doi: 10.1093/jnci/88.13.872. [DOI] [PubMed] [Google Scholar]
- Botkin JR, Smith KR, Croyle RT, Baty BJ, Wylie JE, Dutson D, Chan A, Hamann HA, Lerman C, McDonald J, Venne V, Ward JH, Lyon E. Genetic testing for a BRCA1 mutation: Prophylactic surgery and screening behavior in women 2 years post testing. Am J Med Genet. 2003 April 30;118A(3):201–9. doi: 10.1002/ajmg.a.10102. [DOI] [PubMed] [Google Scholar]
- Claus EB, Schildkraut J, Iverson ES, Berry D, Parmigiani G. Effect of BRCA1 and BRCA2 on the association between breast cancer risk and family history. J Natl Cancer Inst. 1998;90:1824–9. doi: 10.1093/jnci/90.23.1824. [DOI] [PubMed] [Google Scholar]
- Codori AM, Petersen GM, Miglioretti DL, Larkin EK, Bushey MT, Young C, Brensinger JD, Johnson K, Bacon JA, Booker SV. Attitudes toward colon cancer gene testing: factors predicting test uptake. Cancer Epidemiol Biomarkers Prev. 1999 April 8;8(4 Pt 2):345–51. [PubMed] [Google Scholar]
- Corbie-Smith G, Thomas SB, St George DM. Distrust, race, and research. Arch Intern Med. 2002 November 25;162(21):2458–63. doi: 10.1001/archinte.162.21.2458. [DOI] [PubMed] [Google Scholar]
- Culver J, Burke W, Yasui Y, Durfy S, Press N. Participation in breast cancer genetic counseling: the influence of education level, ethnic background, and risk perception. Journal of Genetic Counseling. 2001;10(3):215–31. [Google Scholar]
- Dormon JH. Louisiana's ‘Creoles of color’: Ethnicity, marginality, and identity. Soc Sci Q. 1992 October;73(3):615–27. [Google Scholar]
- Dubois S, Melancon M. Creole is, Creole ain't: Diachronic and synchronic attitudes toward Creole identity in southern Louisiana. Language in Society. 2000;29:237–58. [Google Scholar]
- Facione NC. Breast cancer screening in relation to access to health services. Oncol Nurs Forum. 1999 May;26(4):689–96. [PubMed] [Google Scholar]
- Ferraro KF, Koch JR. Religion and health among black and white adults: Examining social support and consolation. Journal for the Scientific Study of Religion. 1994 December;33(4):362–75. [Google Scholar]
- Frank TS, Deffenbaugh AM, Reid JE, Hulick M, Ward BE, Lingenfelter B, Gumpper KL, Scholl T, Tavtigian SV, Pruss DR, Critchfield GC. Clinical characteristics of individuals with germline mutations in BRCA1 and BRCA2: analysis of 10,000 individuals. J Clin Oncol. 2002 March 15;20(6):1480–90. doi: 10.1200/JCO.2002.20.6.1480. [DOI] [PubMed] [Google Scholar]
- Gamble VN. A legacy of distrust: African Americans and medical research. Am J Prev Med. 1993 November;9(6 Suppl):35–8. [PubMed] [Google Scholar]
- Gao Q, Neuhausen S, Cummings S, Luce M, Olopade OI. Recurrent germ-line BRCA1 mutations in extended African American families with early-onset breast cancer. Am J Hum Genet. 1997 May;60(5):1233–6. [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Tomlinson G, Das S, Cummings S, Sveen L, Fackenthal J, Schumm P, Olopade OI. Prevalence of BRCA1 and BRCA2 mutations among clinic-based African American families with breast cancer. Hum Genet. 2000 August;107(2):186–91. doi: 10.1007/s004390000290. [DOI] [PubMed] [Google Scholar]
- Green LW, Kreuter MW. Health Promotion Planning: An Educational and Environmental Approach. 2nd ed Mayfield Pub. Co.; Mountain View, CA: 1991. [Google Scholar]
- Halbert CH, Kessler LJ, Mitchell E. Genetic testing for inherited breast cancer risk in African Americans. Cancer Invest. 2005;23(4):285–95. doi: 10.1081/cnv-58819. [DOI] [PubMed] [Google Scholar]
- Herring P, Montgomery S, Yancey AK, Williams D, Fraser G. Understanding the challenges in recruiting blacks to a longitudinal cohort study: the Adventist health study. Ethn Dis. 2004;14(3):423–30. [PubMed] [Google Scholar]
- Hoyo C, Reid ML, Godley PA, Parrish T, Smith L, Gammon M. Barriers and strategies for sustained participation of African-American men in cohort studies. Ethn Dis. 2003;13(4):470–6. [PubMed] [Google Scholar]
- Hughes C, Fasaye GA, LaSalle VH, Finch C. Sociocultural influences on participation in genetic risk assessment and testing among African American women. Patient Educ Couns. 2003 October;51(2):107–14. doi: 10.1016/s0738-3991(02)00179-9. [DOI] [PubMed] [Google Scholar]
- Hughes C, Gomez-Caminero A, Benkendorf J, Kerner J, Isaacs C, Barter J, Lerman C. Ethnic differences in knowledge and attitudes about BRCA1 testing in women at increased risk. Patient Educ Couns. 1997 September;32(1-2):51–62. doi: 10.1016/s0738-3991(97)00064-5. [DOI] [PubMed] [Google Scholar]
- Hughes C, Peterson SK, Ramirez A, Gallion KJ, McDonald PG, Skinner CS, Bowen D. Minority recruitment in hereditary breast cancer research. Cancer Epidemiol Biomarkers Prev. 2004 July;13(7):1146–55. [PubMed] [Google Scholar]
- Kessler L, Collier A, Brewster K, Smith C, Weathers B, Wileyto EP, Halbert CH. Attitudes about genetic testing and genetic testing intentions in African American women at increased risk for hereditary breast cancer. Genet Med. 2005 April;7(4):230–8. doi: 10.1097/01.gim.0000159901.98315.fe. [DOI] [PubMed] [Google Scholar]
- King MC, Marks JH, Mandell JB. New York Breast Cancer Study Group. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003 October 24;302(5645):643–6. doi: 10.1126/science.1088759. [DOI] [PubMed] [Google Scholar]
- Kinney AY, Bloor LE, Mandal D, Simonsen SE, Baty BJ, Holubkov R, Seggar K, Neuhausen S, Smith K. The impact of receiving genetic test results on general and cancer-specific psychologic distress among members of an African-American kindred with a BRCA1 mutation. Cancer. 2005 December 1a;104(11):2508–16. doi: 10.1002/cncr.21479. [DOI] [PubMed] [Google Scholar]
- Kinney AY, Bloor LE, Martin C, Sandler RS. Social ties and colorectal cancer screening among Blacks and Whites in North Carolina. Cancer Epidemiol Biomarkers Prev. 2005 Januaryb;14(1):182–9. [PubMed] [Google Scholar]
- Kinney AY, Croyle RT, Dudley WN, Bailey CA, Pelias MK, Neuhausen SL. Knowledge, attitudes, and interest in breast-ovarian cancer gene testing: a survey of a large African-American kindred with a BRCA1 mutation. Prev Med. 2001 December;33(6):543–51. doi: 10.1006/pmed.2001.0920. [DOI] [PubMed] [Google Scholar]
- Kinney AY, Emery G, Dudley WN, Croyle RT. Screening behaviors among African American women at high risk for breast cancer: do beliefs about god matter? Oncol Nurs Forum. 2002 June;29(5):835–43. doi: 10.1188/02.ONF.835-843. [DOI] [PubMed] [Google Scholar]
- Krippendorff K. Content analysis: an introduction to its methodology. Sage Publications; Beverly Hills, CA: 1980. [Google Scholar]
- Kutner SE. Breast cancer genetics and managed care. The Kaiser Permanente experience. Cancer. 1999 December 1;86(11 Suppl):2570–4. doi: 10.1002/(sici)1097-0142(19991201)86:11+<2570::aid-cncr14>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977 March;33(1):159–74. [PubMed] [Google Scholar]
- Lee SC, Bernhardt BA, Helzlsouer KJ. Utilization of BRCA1/2 genetic testing in the clinical setting: report from a single institution. Cancer. 2002 March 15;94(6):1876–85. doi: 10.1002/cncr.10420. [DOI] [PubMed] [Google Scholar]
- Lerman C, Hughes C, Benkendorf JL, Biesecker B, Kerner J, Willison J, Eads N, Hadley D, Lynch J. Racial differences in testing motivation and psychological distress following pretest education for BRCA1 gene testing. Cancer Epidemiol Biomarkers Prev. 1999;8(4 Pt 2):361–7. [PubMed] [Google Scholar]
- Lerman C, Narod S, Schulman K, Hughes C, Gomez-Caminero A, Bonney G, Gold K, Trock B, Main D, Lynch J, Fulmore C, Snyder C, Lemon SJ, Conway T, Tonin P, Lenoir G, Lynch H. BRCA1 testing in families with hereditary breast-ovarian cancer. A prospective study of patient decision making and outcomes. JAMA. 1996 June 26;275(24):1885–92. [PubMed] [Google Scholar]
- Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986 April;73(1):13–22. [Google Scholar]
- Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S, Liu Q, Cochran C, Bennett LM, Ding W, Bell R, Rosenthal J, Hussey C, Tran T, McClure M, Frye C, Hattier T, Phelps R, Haugen-Strano A, Katcher H, Yakumo K, Gholami Z, Shaffer D, stone S, Bayer S, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994 October 7;266(5182):66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- Moorman PG, Skinner CS, Evans JP, Newman B, Sorenson JR, Calingaert B, Susswein L, Crankshaw TS, Hoyo C, Schildkraut JM. Racial differences in enrolment in a cancer genetics registry. Cancer Epidemiol Biomarkers Prev. 2004 August;13(8):1349–54. [PubMed] [Google Scholar]
- Mouchawar J, Klein CE, Mullineaux L. Colorado family physicians' knowledge of hereditary breast cancer and related practice. J Cancer Educ. 2001;16(1):33–7. doi: 10.1080/08858190109528721. [DOI] [PubMed] [Google Scholar]
- Narod SA, Boyd J. Current understanding of the epidemiology and clinical implications of BRCA1 and BRCA2 mutations for ovarian cancer. Curr Opin Obstet Gynecol. 2002 February;14(1):19–26. doi: 10.1097/00001703-200202000-00004. [DOI] [PubMed] [Google Scholar]
- Olopade OI, Fackenthal JD, Dunston G, Tainsky MA, Collins F, Whitfield-Broome C. Breast cancer genetics in African Americans. Cancer. 2003 January 1;97(1 Suppl):236–45. doi: 10.1002/cncr.11019. [DOI] [PubMed] [Google Scholar]
- Olson DH, Portner J, Bell R. Family adaptability and cohesion evaluation scales (FACES II) University of Minnesota, Family Social Services; St. Paul: 1982. [Google Scholar]
- Pargament KI, Kennell J, Hathaway W, Grevengoed N, Newman J, Jones W. Religion and the Problem-Solving Process: Three Styles of Coping. Journal for the Scientific Study of Religion. 1988 March;27(1):90–104. [Google Scholar]
- Pasacreta JV. Psychosocial issues associated with genetic testing for breast and ovarian cancer risk: an integrative review. Cancer Invest. 2003;21(4):588–623. doi: 10.1081/cnv-120022380. [DOI] [PubMed] [Google Scholar]
- Powe BD. Cancer fatalism among elderly Caucasians and African Americans. Oncol Nurs Forum. 1995 October;22(9):1355–9. [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale:A self-report depression scale for research in the general population. Appl Psychol Measure. 1977;1(3):385–401. [Google Scholar]
- Safran DG, Kosinski M, Tarlov AR, Rogers WH, Taira DH, Lieberman N, Ware JE. The Primary Care Assessment Survey: tests of data quality and measurement performance. Med Care. 1998 May;36(5):728–39. doi: 10.1097/00005650-199805000-00012. [DOI] [PubMed] [Google Scholar]
- Schwartz MD, Hughes C, Roth J, Main D, Peshkin BN, Isaacs C, Kavanagh C, Lerman C. Spiritual faith and genetic testing decisions among high-risk breast cancer probands. Cancer Epidemiology Biomarkers and Prevention. 2000 April;9:381–5. [PubMed] [Google Scholar]
- Sherbourne C, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32(6):705–14. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- Singer E, Antonucci T, Van HJ. Racial and ethnic variations in knowledge and attitudes about genetic testing. Genet Test. 2004;8(1):31–43. doi: 10.1089/109065704323016012. [DOI] [PubMed] [Google Scholar]
- Spielberger C, Gorsuch F, Lushene R. STAI Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, CA: 1971. [Google Scholar]
- Stefanek M, Enger C, Benkendorf J, Flamm HS, Lerman C. Bilateral prophylactic mastectomy decision making: A vignette study. Prev Med. 1999 September;29(3):216–21. doi: 10.1006/pmed.1999.0524. [DOI] [PubMed] [Google Scholar]
- Strawbridge WJ, Cohen RD, Shema SJ, Kaplan GA. Frequent attendance at religious services and mortality over 28 years. Am J Public Health. 1997 June;87(6):957–61. doi: 10.2105/ajph.87.6.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawbridge WJ, Shema SJ, Cohen RD, Kaplan GA. Religious attendance increases survival by improving and maintaining good health behaviors, mental health, and social relationships. Ann Behav Med. 2001;23(1):68–74. doi: 10.1207/s15324796abm2301_10. [DOI] [PubMed] [Google Scholar]
- Tercyak KP, Lerman C, Peshkin BN, Hughes C, Main D, Isaacs C, Schwartz MD. Effects of coping style and BRCA1 and BRCA2 test results on anxiety among women participating in genetic counseling and testing for breast and ovarian cancer risk. Health Psychol. 2001 May;20(3):217–22. [PubMed] [Google Scholar]
- Standard Definitions: Final Dispositions of Case Codes and Outcome Rates for Surveys. 3rd AAPOR; Lenexa, Kansas: 2004. [Google Scholar]
- Thompson HS, Valdimarsdottir HB, Duteau-Buck C, Guevarra J, Bovbjerg DH, Richmond-Avellaneda C, Amarel D, Godfrey D, Brown K, Offit K. Psychosocial predictors of BRCA counseling and testing decisions among urban African-American women. Cancer Epidemiol Biomarkers Prev. 2002 December;11(12):1579–85. [PubMed] [Google Scholar]
- Thompson HS, Valdimarsdottir HB, Jandorf L, Redd W. Perceived disadvantages and concerns about abuses of genetic testing for cancer risk: differences across African American, Latina and Caucasian women. Patient Educ Couns. 2003 November;51(3):217–27. doi: 10.1016/s0738-3991(02)00219-7. [DOI] [PubMed] [Google Scholar]
- Velicer CM, Taplin S. Genetic testing for breast cancer: where are health care providers in the decision process? Genet Med. 2001 March;3(2):112–9. doi: 10.1097/00125817-200103000-00005. [DOI] [PubMed] [Google Scholar]