Abstract
In cooperatively breeding species, parents often use helper contributions to offspring care to cut their own costs of investment (i.e. load-lightening). Understanding the process of load-lightening is essential to understanding both the rules governing parental investment and the adaptive value of helping behaviour, but little experimental work has been conducted. Here we report the results of field experiments to determine maternal provisioning rules in cooperatively breeding superb fairy-wrens (Malurus cyaneus). By manipulating carer : offspring ratios, we demonstrate that helpers allow females to reduce the rate at which they provision their brood. Female reductions, however, were less than that provided by helpers, so that chicks still received food at a faster rate in the presence of helpers. Despite this, chicks fed by parents and helpers were not heavier than those provisioned by parents alone. This is because maternal load-lightening not only occurs during the chick provisioning stage, but also at the egg investment stage. Theoretically, complete load-lightening is predicted when parents value themselves more highly than their offspring. We tested this idea by ‘presenting’ mothers with a ‘choice’ between reducing their own levels of care and increasing investment in their offspring. We found that mothers preferred to cut their contributions to brood care, just as predicted. Our experiments help to explain why helper effects on offspring success have been difficult to detect in superb fairy-wrens, and suggest that the accuracy with which theoretical predictions of parental provisioning rules are matched in cooperative birds depends on measuring maternal responses to helper presence at both the egg and chick stages.
Keywords: bi-parental care, helper effects, load-lightening, provisioning rules, cooperative breeding
1. Introduction
Parents provisioning young are selected to balance the benefits of providing care against the costs they will sustain as a result (Williams 1966; Trivers 1972; Clutton-Brock 1991). In cooperatively breeding species, the presence of helpers could change the nature of this trade-off, provided that helpers are responsive to the demands of the offspring (Härdling et al. 2003). When breeding in the presence of helpers, each breeder faces a choice: maintain levels of investment and so use helper contributions to increase the fitness of their current young; or reduce levels of investment and so use helper contributions to offset costs and save resources for their future young. Despite the theoretical interest in this choice, few experimental tests have been conducted (see Hatchwell & Davies 1990; Hatchwell & Russell 1996; Wright & Dingemanse 1999).
Predicting the choice that parents will make in the presence of helpers is essential to understanding the adaptive value of helping behaviour. In general, theoretical models of cooperative breeding systems predict that breeder responses to helper presence will depend on the relative costs and benefits of investment in young (Brown 1978; Emlen 1991; Crick 1992; Hatchwell & Russell 1996; Hatchwell 1999; Härdling et al. 2003; Heinsohn 2004). In particular, when the marginal benefits of parental care exceed the marginal costs (l.h.s. of fig. 3 in Hatchwell (1999)), then breeders should use helper contributions to enhance the fitness of their current young. In these circumstances, breeders are predicted to maintain (or reduce only partially) their unassisted level of investment, so that the brood receives greater overall investment in the presence of helpers. By contrast, when the marginal benefits of providing care are lower than the marginal costs sustained (r.h.s. of fig. 3 in Hatchwell (1999)), then breeders are predicted to use helper contributions to reduce their own investment in the brood, so that net brood investment is no greater when helpers are present than when they are absent.
In many cooperatively breeding species, observations suggest that parents feed offspring less when helpers are present than when they are absent (Brown 1978; Crick 1992; Hatchwell 1999; Heinsohn 2004). However, only three studies have attempted to test experimentally whether helpers cause these reductions in parental care and each is open to alternative interpretation. Wright & Dingemanse (1999) increased helper provisioning effort in Arabian babblers (Turdoides squamiceps) by supplementally feeding helpers and measuring parental responses. Supplementally fed helpers increased their provisioning effort dramatically, and parents reduced their levels by the same amount, suggesting that helpers cause complete reductions in parental investment. However, an alternative interpretation is that supplementally fed helpers provisioned chicks to satiation, leaving little opportunity for parents to feed offspring themselves (Wright & Dingemanse 1999). Hatchwell & Davies (1990) and Hatchwell & Russell (1996) used temporary helper removal experiments in dunnocks (Prunella modularis) and long-tailed tits (Aegithalos caudatus), respectively, in order to test parental provisioning rules. Both experiments suggested that helpers allow breeders to reduce their investment; partially in the case of dunnocks and fully in the case of long-tailed tits. However, here the problem is that helper removal experiments inherently cause reductions in group size. If such reductions reduce foraging competition or increase foraging efficiency within groups, then this, rather than removal of help per se, might cause the observed increases in chick feeding rates by parents (Jennions & Macdonald 1994; Cockburn 1998).
In addition, these three experimental studies pre-date the more recent theoretical models which predict that parental provisioning rules should be governed by the relative marginal costs and benefits of parental care (Hatchwell 1999; Heinsohn 2004). Here we adopt the alternative experimental approach of temporary brood size manipulations (Clutton-Brock et al. 2001) to test theoretical predictions of helper effects on maternal provisioning rules in superb fairy-wrens Malurus cyaneus. Brood size manipulation experiments are advantageous for these purposes because they allow one to manipulate carer : offspring ratios without changing group size. Our brood size manipulation experiments served three purposes. First, we tested whether helpers cause load-lightening (i.e. reductions in maternal investment; sensu Brown 1978) through active contributions to offspring provisioning as opposed to through passive effects of group size (see Jennions & Macdonald 1994). Second, we determined the effect of helpers on the extent to which females load-lighten (i.e. by the same amount provided by helpers or not). Third, we examined whether the extent of load-lightening is predicted by the relative marginal costs and benefits of maternal care (see §2). For each purpose, we focused exclusively on maternal brood provisioning behaviour because females always have full maternity of the brood that they rear (Mulder et al. 1994; Rowley & Russell 1997). This removes the potential complication of varying relatedness from our analyses, which is a correlate of male behaviour in this species (Mulder et al. 1994; Green et al. 1995; Dunn & Cockburn 1996).
Superb fairy-wrens are a 10 g, sexually dichromatic, facultative cooperatively breeding passerine bird endemic to southeastern Australia (Rowley & Russell 1997). Typically, breeding begins in spring, clutches range from three to four eggs and up to two breeding attempts are successful in a season. A single female lays the eggs, but offspring are usually fathered by extra-pair males (Mulder et al. 1994). Females alone incubate during the two-week incubation period, but all group members contribute to chick provisioning during the 11-day nestling period. The presence of helpers appears unrelated to female quality (Cockburn et al. 2003); less than half of all breeding pairs have helpers and most helped nests have just a single helper (Cockburn et al. in press). Helpers are always male, often a son (up to 60% of instances), and contribute as much or more than the male breeder to offspring provisioning (Dunn et al. 1995).
2. Material and methods
Our study was conducted from October to December in 2003 and 2004 in Campbell Park, a 128 ha eucalypt woodland in northeastern Canberra, ACT, Australia (149°9′ E, 35°16′ S; see Langmore & Kilner (in press) for further details). The site is approximately 10 km from the long-term Botanic Garden study population; compared with the Botanic Garden site, wrens in Campbell Park occur at lower density and have shorter reproductive seasons, but otherwise have similar life history and prevalences of helpers (Russell et al. 2007; Cockburn et al. in press). Within our site, no differences were apparent between pairs and groups in terms of mean first lay dates (pairs=23 October versus 22 October: F1,298=1.91, p=0.17, controlling for significant inter-year differences), clutch sizes (median=4 in each, Mann–Whitney U-test: W=25 778, N=187,86, p=0.76) or brood sizes mid-way through the nestling period (day 7; pairs: mean (±s.e.)=3.17±0.21; groups: 3.12±0.13, t-test; t16=0.17, p=0.86).
We conducted brood size manipulation experiments on 20 nests (12 pairs and 8 groups each with one helper (N=6), two helpers (N=1) and three helpers (N=1)). Natural brood sizes in these 20 nests were two chicks (N=2), three chicks (N=13) and four chicks (N=5). Each nest experienced three treatments: brood reduction (by the removal of chicks); brood enlargement (by the addition of chicks); and a control treatment (the natural brood size). The sequence of brood size treatments (reduced, control and enlarged) was assigned arbitrarily to each nest tested, from a predetermined list, so that each possible sequence was used at least twice. In all except one case, all three treatments were carried out on the same day. Chicks were moved between nests when 5–8 days old (hatch day=0; mean=6). Provisioning rates were determined using hour-long nest observations, with the observer concealed 10–50 m from the nest, and started at least 30 min after the brood size manipulation, to allow all feeders to adjust to the change in brood size. This was gauged as being sufficient time for all birds to become accustomed to the new brood sizes, because each bird provisions every 6 min on average. Although the mother was obvious in all nests, the putative father was only obvious in pairs. As a consequence, we measured the provisioning rate of the mother at all nests, of the putative father in pair-nests and of all males combined in group-nests. Only the provisioning frequency of the mother is considered in detail here (see §1 for rationale).
The number of chicks swapped between nests was constrained to some degree by the availability of potential donor broods with chicks of the same age (within a day). In no case were all chicks from a nest removed. One of the primary aims of this study was to compare maternal provisioning rates in pairs versus groups when carer : offspring ratios were experimentally ‘equalized’ (see §2a). Consequently, the number of chicks that we removed from a pair-nest was determined by the number which would result in a carer : offspring ratio that was comparable to natural ratios in groups (i.e. 1.13 : 1) and at the same time, when added to a group-nest, would give us a carer : offspring ratio comparable to natural ratios in pairs (i.e. 0.65 : 1). One to three chicks (mean=2) were moved between nests within 20 min walk from each other (but usually much less) in a woollen hat. All statistical analyses were two-tailed and conducted in Genstat v. 9 (Rothamsted Experimental Station, Harpenden, UK).
(a) Helper presence and evidence of load-lightening
Analysis of general levels of investment by mothers in pairs versus groups was conducted using the data from the control (unmanipulated) observations. Overall, we observed 186 nestling feeds by mothers during control observations (N=20 mothers: 12 in pairs and 8 in groups). There was no difference between pairs and groups in potential confounds such as brood size or lay date (see §2), nor in chick age (Mann–Whitney U-test: W=47.0, N=12, 8, p=0.93). Consequently, the effect of helper absence versus presence on maternal contributions to nestling provisioning was analysed using a two-sample Student's t-test.
Reductions in maternal provisioning rates in the presence of helpers may be caused by the active effects of helpers on offspring provisioning or through passive effects on group size (Jennions & Macdonald 1994). In order to test which of these two alternatives accounts for any evidence of load-lightening, we conducted a Linear mixed effects model (LME) in which we first fitted maternal provisioning frequency (across the three treatments) as the response term to a normal error structure in which carer : offspring ratio and group size were fitted as fixed effects. Maternal identity was fitted as a random term to control for repeated measures in mothers (N=3 in each analysis, corresponding to the three treatments). Squared functions of the two explanatory terms tested were non-significant, indicating that all significant relationships within the range of carer : offspring ratios observed were linear. Our analysis was conducted on 578 maternal feeds (including reduced, control and enlarged treatments).
Second, to test experimentally whether group size influences maternal provisioning rates, we compared maternal provisioning rates in the following cases: (i) pairs in which the carer : offspring ratios were at control levels (0.65 : 1) versus groups in which carer : offspring ratios had been experimentally reduced (0.69 : 1), and (ii) groups in which carer : offspring ratios were at control levels (1.13 : 1) versus pairs in which carer : offspring ratios had been experimentally increased (1.33 : 1). Overall, 174 and 216 maternal feeds were observed in each comparison, respectively (n=20 mothers in each). Again, since there were no differences between pairs and groups in potential confounds (see above), differences between maternal provisioning rates in each of the two cases were tested using two separate two-sample t-tests.
Third, to test experimentally whether or not carer : offspring ratios influenced maternal provisioning rates, we investigated maternal provisioning rates : (i) within pairs in which carer : offspring ratios were manipulated (reduced=0.4 : 1, control=0.65 : 1, increased=1.33 : 1), and (ii) within groups in which carer : offspring ratios were manipulated (reduced=0.69 : 1, control=1.13 : 1, increased=3.37 : 1). Overall, 396 and 182 maternal feeds were observed in each comparison, respectively (pairs, n=12 mothers; groups, n=8 mothers). Differences between maternal provisioning rates were analysed in each case using two separate repeated measures ANOVAs (one for pairs and another for groups).
(b) Helper presence and degree of load-lightening
We investigated whether maternal reductions in contributions to offspring provisioning in the presence of helpers were complete or incomplete. Overall, we observed 1100 chick feeds from our 60 nest observations (20 nests, each with three observations corresponding to the three brood size treatments), 578 of which were by mothers. We conducted two separate repeated measures two-way ANOVAs. In the two analyses, helper presence and treatment were fitted as the two fixed effects, while nest identity was fitted as a blocking function to account for repeated measures of nests. Maternal feeds per chick per hour and total feeds per chick per hour were fitted as the response terms, respectively, after logarithm transformation. The rationale is that if mothers reduce their investment proportionally to that provided by the helpers, then chicks will receive food at the same rate in pairs as they do in groups, whereas if they maintain levels (or reduce levels only partially) chicks will receive food at a faster rate in groups than in pairs.
(c) Marginal costs and benefits of care
Finally, using the brood size manipulation experiments described previously, we assessed whether the extent of load-lightening is predicted by the relative marginal costs and benefits of maternal care (Hatchwell 1999; Heinsohn 2004). We could not measure the marginal costs and benefits of care directly with our experiments. Instead, we assumed that our brood size manipulations corresponded with the potential benefits of maternal care, while the presence or absence of helpers potentially affected the costs of care, and that each independently influenced the levels of care provided. Our experiments effectively gave mothers a choice between increasing investment in their current brood and cutting their own costs of care. We predicted that if the marginal costs of maternal care were greater than the marginal benefits, then mothers should favour a reduction in their own levels of care over increased provisioning of their brood.
We first investigated whether our assumption that brood size and helper presence had separate independent influences on maternal provisioning rates by examining whether maternal responses to changes in brood size were the same, irrespective of group size. Consequently, we conducted two separate general(ized) linear models to compare the (per capita) amount of food received (in pairs versus groups) when chicks were (i) in control versus reduced brood sizes and (ii) in control versus enlarged brood sizes. In each analysis, the change in per capita food received between control and manipulated brood size was entered as the response term, with Poisson (control–reduced) and normal (control–enlarged) error structures, respectively.
Second, to determine whether or not females were more responsive to helper presence (i.e. reproductive costs) or brood sizes (i.e. reproductive benefits), we conducted an LME model in which female feeds per hour was fitted as the response term, brood size and helper presence were fitted as fixed effects and nest identity was fitted as a random term to account for repeated sampling of nests. Subsequently, we investigated whether the magnitude of the effect sizes of the two fixed terms differed significantly using the formula: effect term A−effect term B/square root (s.e. term A+s.e. term B). We then compared the resulting value against a t-distribution with denominator d.f. equal to the mean denominator d.f. of the two fixed terms using Satterthwaite's correction in the statistical package SAS (SAS Institute, Inc., Release v. 8.02, 1999–2001).
3. Results
(a) Helper presence and load-lightening
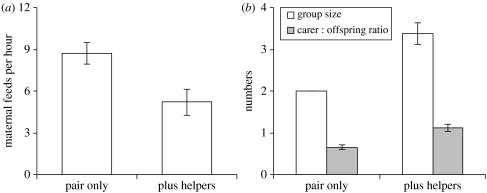
Comparisons of provisioning rates collected during control observations (i.e. natural brood sizes) revealed that mothers fed their brood 36% less frequently in the presence of helpers (figure 1a). This result could be caused by higher levels of foraging competition and reduced foraging success among those females breeding in the presence of helpers because group sizes were 40% larger when helpers were present versus absent (figure 1b; see §1). Alternatively, because carer : offspring ratios were 42% greater in groups than in pairs (figure 1b), reductions in maternal feeding rates might be a response to helper provisioning, as predicted by the load-lightening hypothesis (sensu Brown 1978).
Figure 1.
Differences in maternal provisioning rate in the presence of helpers and potential mechanisms. (a) Mothers provisioned their brood at a significantly reduced rate in the presence of helpers (two-sample t-test, t15=3.28, p=0.005). (b) Two potential mechanisms are larger group sizes (two individuals in pairs versus more than two in groups) and greater carer : offspring ratios in the presence of helpers (Mann–Whitney U-test, W=84, N=12, 8, p=0.0008). Figures show means±s.e. (except for group size in pairs, which is always two individuals).
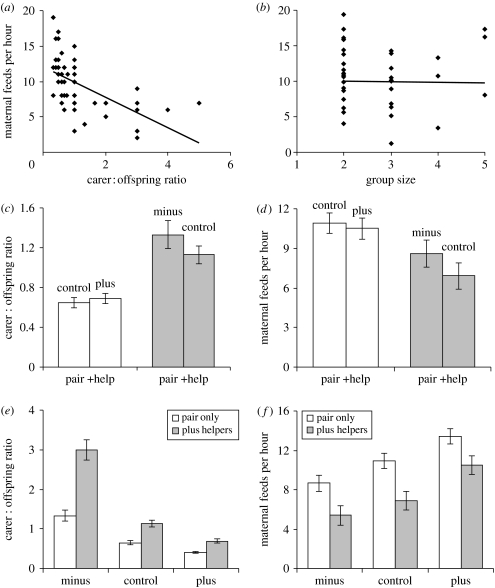
An LME model showed that carer : offspring ratio had a strong negative effect on maternal provisioning rate (R2=34%; figure 2a), while group size had no effect (R2=0.04%; figure 2b). Moreover, experimental equalization of carer : offspring ratios between pairs and groups (figure 2c) led to the disappearance of the difference between pairs and groups in maternal provisioning rates (figure 2d). This result was true irrespective of whether we compared levels in control pairs with experimental groups (figure 2d, l.h.s.) or levels in experimental pairs with control groups (figure 2d, r.h.s.). Conversely, experimental increases and decreases in carer : offspring ratios within pairs and groups (figure 2e) gave rise to significant decreases and increases, respectively, in maternal provisioning rates within both pairs and within groups (figure 2f). These results strongly suggest that it is helper effects on contributions to care that cause changes in maternal provisioning rates rather than helper effects on group size.
Figure 2.
Carer: offspring ratio versus group size effects on maternal provisioning rates. (a) Increases in carer : offspring ratios were associated with linear reductions in maternal provisioning rates (LME model, Χ12=30.72, p<0.0001), but (b) increases in group size were not (LME, Χ12=0.07, p=0.73). (c) By adding chicks to groups, we made carer : offspring ratios in groups similar to those in natural pairs (l.h.s.: Mann–Whitney U-test, W=118, N=8, 12, p=0.55), while by removing chicks from pairs we made carer : offspring ratios in pairs similar to those in natural groups (r.h.s.: W=134, N=12, 8, p=0.47). (d) Correspondingly, maternal provisioning rates were similar in groups that had their carer : offspring ratios reduced to levels comparable to pairs (l.h.s.: t-test, t14=0.32, p=0.75), and similar in pairs that had their carer : offspring ratios increased to levels comparable to those in natural groups (r.h.s.: t15=1.43, p=0.18). (e) Manipulating brood sizes in pairs and groups had significant influences on carer : offspring ratios (pairs: Friedman test, S=23.13, d.f.=2, p<0.0001; groups: S=16.0, d.f.=2, p<0.0001). (f) Maternal provisioning rates changed in response to manipulations in carer : offspring ratios (pairs: repeated measures ANOVA, F2,22=12.37, p<0.001; groups: F2,14=10.17, p=0.002). In (a,b), adjusted values from the LME model are shown, while in (c–f) predicted means±s.e. are shown.
(b) Helper presence and degree of load-lightening
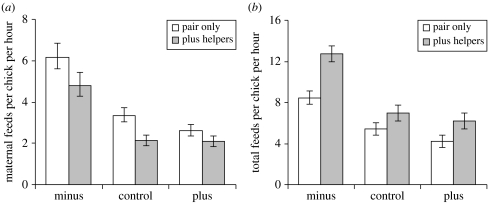
Helpers more than compensated for maternal reductions in offspring provisioning, irrespective of brood size treatment. Despite mothers reducing the rate at which they fed each chick in the presence of helpers by an average of 26% (across the three brood size treatments; figure 3a), total provisioning rates to chicks at nests with helpers were on average 29% higher than those without helpers (figure 3b). Breeding males decrease their provisioning rate in the presence of helpers (Dunn et al. 1995), so their behaviour cannot confound our interpretation of the results. All results stand even when the two groups with greater than one helper were removed from the analysis, confirming that the results were not driven by outlying group sizes (results not shown).
Figure 3.
Helper and brood size manipulation effects on maternal and overall levels of provisioning. (a) Maternal feeds to each chick were highest in reduced broods (brood size effect: F2,36=49.89, p<0.001) and lower in the presence of helpers (helper effect: F1,18=6.80, p=0.018). (b) Individual chicks received food at a faster rate when in experimentally reduced broods (brood size effect: F2,36=44.1, p<0.001) and when in the presence of helpers (helper effect: F1,18=17.22, p<0.001). In (a,b), back-transformed predicted means±s.e are shown. All interactions between helper presence and treatment were non-significant (range in p-values: p>0.35–0.75).
(c) Marginal costs and benefits of care
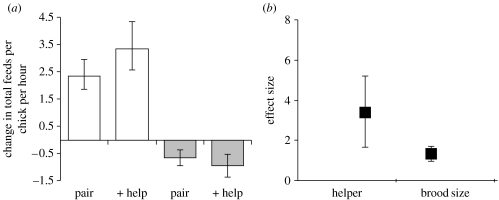
Maternal responses to changes in brood size were independent of helper presence. In experimentally reduced and enlarged broods, each chick received food at the same increased and decreased rate from their mother, respectively, irrespective of whether their mother was in a pair or in a group (figure 4a). These results uphold our assumption that brood size and helper presence influence maternal provisioning rates independently of each other.
Figure 4.
Maternal responsiveness to helpers versus chicks. (a) Each chick received similar changes in food delivery frequency from their mother irrespective of whether they were reared in pairs or groups (change in reduced broods, pairs versus groups (white bars): F1,18=1.70, p=0.21; change in enlarged broods, pairs versus groups (grey bars): F1,18=0.54, p=0.47). (b) The magnitude of helper effect on maternal provisioning rates was greater than the magnitude of the brood size effect (t30=2.08, p<0.05). (a) Means±s.e and (b) effect sizes±95% CI.
We know that maternal provisioning rates increase with increasing brood size (figure 2f) and decrease in the presence of helpers (figure 1a). Using an LME model to generate effect sizes for each of these two relationships, and comparing the magnitude in the effect size of each, we found that a mother's decrease in provisioning rate in the presence of helpers was greater than her increase for increasing brood size (figure 4b). This suggests that mothers value future offspring over current offspring and hence that the marginal costs of current care are greater than the marginal benefits.
4. Discussion
Our results show that helpers allow female superb fairy-wrens to reduce their own contributions to nestling care. Females worked less hard to provision offspring when assisted by helpers than when breeding in a pair. Despite this, offspring still received food at a faster rate in the presence of helpers, indicating that female reductions were more than compensated by helper contributions to care. Finally, we found that females changed their provisioning more in response to the presence or absence of helpers than they did in response to changes in brood size. This suggests that for female superb fairy-wrens, the marginal costs of providing care are greater than the marginal benefits.
One of the most commonly documented helper effects in cooperatively breeding vertebrates is load-lightening, where helper presence is associated with reduced rates of offspring provisioning by parent(s) (Brown 1978; Crick 1992; Hatchwell 1999). However, experimental confirmation of the causality of these observations has been few and problematic (Jennions & Macdonald 1994; Cockburn 1998; see §1). In our study, we can be certain that females changed their behaviour directly in response to the care provided by helpers rather than as a consequence of helper effects on group size.
First, by equalizing carer : offspring ratios in pairs and groups using brood size manipulation experiments, we eliminated the difference between pairs and groups in maternal provisioning rates. This result shows that differences in maternal provisioning rates in pairs and groups cannot be caused by differences in group size. Furthermore, we showed by manipulation of carer : offspring ratios within units of the same size (i.e. within pairs and within groups) that changes in carer : offspring ratios have dramatic effects on maternal provisioning rates. Hence, it was helper effects on carer : offspring ratios that caused changes in maternal provisioning rates not helper effects on group size. Second, our results are unlikely to be confounded by any subtle changes in the size or type of food brought to the chicks in the presence and absence of helpers. We have shown experimentally in our population of superb fairy-wrens that chick growth is directly related to offspring provisioning rates (Russell et al. 2007). In addition, a previous study from a neighbouring population failed to note a difference in either prey type or size between groups and pairs (MacGregor & Cockburn 2002). Taken together, our results provide one of the few demonstrations that helper presence causes load-lightening in a cooperative vertebrate.
The reduction in the female's contribution to care was more than fully compensated by the extra work done by the helpers, so that overall chick provisioning rates were greater in the presence of helpers (see also Dunn & Cockburn 1996). According to the theory, the degree to which parents reduce their provisioning effort when assisted by helpers depends on the relative magnitude of the marginal costs and benefits of care (Hatchwell 1999). Circumstantial evidence suggests that the marginal benefits of extra care are likely to be relatively low in superb fairy-wrens because starvation is rare in this species (Rowley & Russell 1997) and so additional care is unlikely to provide much of a boost to offspring fitness (Dunn et al. 1995). Our experiments are consistent with this view because they show that female superb fairy-wrens were more responsive to the presence of helpers than to changes in brood size, when determining their provisioning rates at the nest. In other words, females value reductions in the costs of care more highly than any benefits they stand to gain from extra provisioning of their current young.
However, the theory also predicts that where this is the case, parents should lighten their load of care so much that overall levels of investment are unchanged by the presence of helpers (Hatchwell 1999). Yet our experiments, and previous observations (Dunn & Cockburn 1996), show that helper care increases overall chick provisioning rates, which seems to contrast with theoretical expectations. This apparent problem can be resolved if we extend our analysis of maternal investment to include nourishment of the eggs as well as provisioning of nestlings. We have shown previously in our population of superb fairy-wrens that mothers load-lighten during egg investment (Russell et al. 2007). If we consider levels of investment before and after hatching, then our results match theoretical predictions fully. The combined effects of maternal load-lightening before and after hatching are so great that helper contributions to chick care are completely obscured, and no effect of helper presence on nestling mass or fledging success can be detected (Dunn et al. 1995; Rowley & Russell 1997; Russell et al. 2007).
In superb fairy-wrens, mothers thus spread their load-lightening over two phases of the breeding attempt, each in isolation giving an incomplete picture of the true extent to which females reduce their contributions to parental investment in the presence of helpers. Perhaps load-lightening is spread out in this way because there are constraints on the lowest levels of investment that are possible at each stage of reproduction. For example, greater reductions in egg nourishment might compromise the viability of the embryo developing within (e.g. Nager et al. 2000). Alternatively, if females were to cease feeding offspring after hatching, they might trigger desertion of the brood by other group members, as has been observed in our study population in the context of cuckoo-chick desertion (Langmore et al. 2003). Determining the behavioural rules that regulate the degree of load-lightening after hatching in this species would be of considerable interest.
Although mothers use helper care to cut their own investment in superb fairy-wrens (Russell et al. 2007), it is probable that the precise way in which reductions in investment are split between egg nourishment and chick provisioning varies among years and among populations. Our observational data show that females worked less hard when assisted by helpers than when provisioning in a pair, but equivalent data collected at another study site show no such effect (Dunn & Cockburn 1996). A potential explanation is that maternal load-lightening happens primarily at the egg stage in most superb fairy-wren populations and is supplemented by further load-lightening at the chick stage only in harsh conditions. In contrast with previous studies on this species, our study was conducted in an area which is unmanaged and also coincided with a period of severe drought (Langmore & Kilner in press), which might explain why our observations are somewhat different from earlier work (see also Luck 2002).
The results of our study have at least three important implications. First, as predicted by theoretical models (Hatchwell 1999; Heinsohn 2004), our results suggest that estimates of the marginal costs and benefits of parental care can be used to predict when reductions in parental effort are probable in the presence of helpers and whether these reductions should be partial or complete. Second, however, our results (this study; Russell et al. 2007) also highlight that theoretical predictions can only be tested effectively by measuring maternal responses to helper presence at both the egg and chick stages. Finally, we suggest that in species where helper presence/number is unpredictable (e.g. long-tailed tits: Hatchwell et al. 2004), females will concentrate exclusively on reducing their contributions to nestling provisioning, if helpers eventually arrive. Load-lightening after hatching may also be favoured by species that increase their clutch size in anticipation of helper care (e.g. dunnocks: Davies & Hatchwell 1992; Arabian babblers: Wright 1998). By contrast, females may load-lighten primarily by reducing their investment in eggs in species where help is predictable, and where it has been shown that helper provisioning increases brood provisioning rates without apparently improving nestling fitness (e.g. green woodhoopoes Phoenicerus purpureus: DuPlessis (1993), and other examples in Emlen (1991) and Cockburn (1998)). A challenge for future work is to account for these contrasting tactics of load-lightening by females and to determine the implications they have for measuring selection on cooperative breeding in vertebrates.
Acknowledgments
All work was conducted with ethics approval (Australian National University Animal Experimentation Ethics Committee Protocol F.BTZ.61.03).
We are grateful to Environment ACT for permission to work at Campbell Park and to Andrew Cockburn for his support throughout our work. Ben Hatchwell, Jon Wright and two anonymous referees provided helpful comments on the manuscript. The work was funded by grants from the Royal Society UK (A.F.R. and R.M.K.) and the Australian Research Council (R.M.K. and N.E.L.). A.F.R. and R.M.K. contributed equally to this study.
References
- Brown J.L. Avian communal breeding systems. Annu. Rev. Ecol. Syst. 1978;9:123–155. doi:10.1146/annurev.es.09.110178.001011 [Google Scholar]
- Clutton-Brock T.H. Princeton University Press; Princeton, NJ: 1991. The evolution of parental care. [Google Scholar]
- Clutton-Brock T.H, Russell A.F, Sharpe L.L, Brotherton P.N.M, McIlrath G.M, White S, Cameron E.Z. Effects of helpers on juvenile development and survival in meerkats. Science. 2001;293:2446–2449. doi: 10.1126/science.1061274. doi:10.1126/science.1061274 [DOI] [PubMed] [Google Scholar]
- Cockburn A. Evolution of helping in cooperatively breeding birds. Annu. Rev. Ecol. Syst. 1998;29:141–177. doi:10.1146/annurev.ecolsys.29.1.141 [Google Scholar]
- Cockburn A, Osmond H.L, Mulder R.A, Green D.J, Double M.C. Divorce, dispersal and incest avoidance in the cooperatively breeding superb fairy-wren Malurus cyaneus. J. Anim. Ecol. 2003;79:189–202. doi:10.1046/j.1365-2656.2003.00694.x [Google Scholar]
- Cockburn, A., Simms, R. A., Osmond, H. L., Green, D. J., Double, M. C. & Mulder, R. A. In press. Can we measure the benefits of help in cooperatively breeding birds: the case of the superb fairy-wren Malurus cyaneus J. Anim. Ecol [DOI] [PubMed]
- Crick H.Q.P. Load-lightening in cooperatively breeding birds and the cost of reproduction. Ibis. 1992;134:56–61. [Google Scholar]
- Davies N.B, Hatchwell B.J. The value of male parental care and its influence on reproductive allocation by male and female dunnocks. J. Anim. Ecol. 1992;61:259–272. doi:10.2307/5319 [Google Scholar]
- Dunn P.O, Cockburn A. Evolution of male parental care in a bird with almost complete cuckoldry. Evolution. 1996;50:2542–2548. doi: 10.1111/j.1558-5646.1996.tb03643.x. doi:10.2307/2410724 [DOI] [PubMed] [Google Scholar]
- Dunn P.O, Cockburn A, Mulder R.A. Fairy-wren helpers often care for young to which they are unrelated. Proc. R. Soc. B. 1995;259:339–343. doi:10.1098/rspb.1995.0050 [Google Scholar]
- DuPlessis M.A. Helping behaviour in cooperatively-breeding green woodhoopoes: selected or unselected trait? Behaviour. 1993;127:49–65. [Google Scholar]
- Emlen S.T. Evolution of cooperative breeding in birds and mammals. In: Krebs J.R, Davies N.B, editors. Behavioural ecology. Blackwell Scientific; Oxford, UK: 1991. pp. 301–337. [Google Scholar]
- Green D.J, Cockburn A, Hall M.L, Osmond H.L, Dunn P.O. Increased opportunities for cuckoldry may be why dominant male fairy-wrens tolerate helpers. Proc. R. Soc. B. 1995;262:297–303. doi:10.1098/rspb.1995.0209 [Google Scholar]
- Härdling R, Kokko H, Arnold K.E. Dynamics of the caring family. Am. Nat. 2003;161:395–412. doi: 10.1086/367587. doi:10.1086/367587 [DOI] [PubMed] [Google Scholar]
- Hatchwell B.J. Investment strategies of breeders in avian cooperative breeding systems. Am. Nat. 1999;154:205–219. doi: 10.1086/303227. doi:10.1086/303227 [DOI] [PubMed] [Google Scholar]
- Hatchwell B.J, Davies N.B. Provisioning of nestlings by dunnocks, Prunella modularis, in pairs and trios-compensation reactions by males and females. Behav. Ecol. Sociobiol. 1990;27:199–209. doi:10.1007/BF00180304 [Google Scholar]
- Hatchwell B.J, Russell A.F. Provisioning rules in cooperatively breeding long-tailed tits: an experimental study. Proc. R. Soc. B. 1996;263:83–88. doi:10.1098/rspb.1996.0014 [Google Scholar]
- Hatchwell B.J, Russell A.F, MacColl A.D.C, Ross D.J, Fowlie M.K, McGowan A. Helpers increase long-term but not short-term productivity in cooperatively breeding long-tailed tits. Behav. Ecol. 2004;15:1–10. doi:10.1093/beheco/arg091 [Google Scholar]
- Heinsohn R.G. Parental care, load-lightening and costs. In: Koenig W.D, Dickinson J.L, editors. Ecology and evolution of cooperative breeding in birds. Cambridge University Press; Cambridge, MA: 2004. pp. 67–80. [Google Scholar]
- Jennions M.D, Macdonald D.W. Cooperative breeding in mammals. Trends Ecol. Evol. 1994;9:89–93. doi: 10.1016/0169-5347(94)90202-X. doi:10.1016/0169-5347(94)90202-X [DOI] [PubMed] [Google Scholar]
- Langmore, N. E. & Kilner, R. M. In press. Breeding site and host selection in Horsfield's bronze-cuckoos Chalcites basalis [DOI] [PubMed]
- Langmore N.E, Hunt S, Kilner R.M. Escalation of a coevolutionary arms race through host rejection of brood parasitic young. Nature. 2003;422:157–160. doi: 10.1038/nature01460. doi:10.1038/nature01460 [DOI] [PubMed] [Google Scholar]
- Luck G.W. The parental investment strategy of an avian cooperative breeder differs between a fragmented and an unfragmented landscape. Am. Nat. 2002;160:809–814. doi: 10.1086/343881. doi:10.1086/343881 [DOI] [PubMed] [Google Scholar]
- MacGregor N.A, Cockburn A. Sex differences in parental response to begging nestlings in superb fairy-wrens. Anim. Behav. 2002;63:923–932. doi:10.1006/anbe.2001.1991 [Google Scholar]
- Mulder R.A, Dunn P.O, Cockburn A, Lazenby-Cohen K.A, Howell M.J. Helpers liberate female fairy-wrens from constraints on extra-pair mate choice. Proc. R. Soc. B. 1994;255:223–229. doi:10.1098/rspb.1994.0032 [Google Scholar]
- Nager R.G, Monaghan P, Houston D.C. Within-clutch trade-offs between the number and quality of eggs. Ecology. 2000;81:1339–1350. [Google Scholar]
- Rowley I, Russell E.M. Oxford University Press; Oxford, UK: 1997. Fairy-wrens and grasswrens: maluridae. [Google Scholar]
- Russell A.F, Langmore N.E, Cockburn A, Astheimer L.B, Kilner R.M. Reduced egg investment can conceal helper effects in cooperatively breeding birds. Science. 2007;317:941–944. doi: 10.1126/science.1146037. doi:10.1126/science.1146037 [DOI] [PubMed] [Google Scholar]
- Trivers R.L. Parental investment and sexual selection. In: Campbell B, editor. Sexual selection and the descent of man 1871–1971. Heinemann; London, UK: 1972. pp. 136–179. [Google Scholar]
- Williams G.C. Natural selection, the costs of reproduction, and a refinement of Lack's principle. Am. Nat. 1966;100:687–690. doi:10.1086/282461 [Google Scholar]
- Wright J. Helping-at-the-nest and group size in the Arabian Babbler Turdoides squamiceps. J. Avian Biol. 1998;29:105–112. doi:10.2307/3677187 [Google Scholar]
- Wright J, Dingemanse N.J. Parents and helpers compensate for experimental changes in the provisioning effort of others in the Arabian babbler. Anim. Behav. 1999;58:345–350. doi: 10.1006/anbe.1999.1152. doi:10.1006/anbe.1999.1152 [DOI] [PubMed] [Google Scholar]