Abstract
Owing to the great morphological diversity of domestic dogs (Canis familiaris), the study of historical shape change in dog skulls provides an excellent opportunity for investigating the dynamics of morphological evolution. Breed standards make known which features were selected by breeders. Here we use the methods of geometric morphometrics to study change of skull shape in a series of purebred St Bernard dogs spanning nearly 120 years. A regression of shape on time was highly significant and revealed a consistent trend of shape change that corresponded to the features deemed desirable by the breed standard. Historical shape change in St Bernards involves a broadening of the skull and a tilting of the palate and upper jaw relative to the rest of the skull. This trend appears to be linear throughout the entire period and appears to be continuing. Allometry was ruled out as a contributing factor to this change because there was no consistent trend of historical change in skull size and because neither the patterns of static nor ontogenetic allometry corresponded to the historical shape change. The dramatic modification of the St Bernard skull demonstrates that selection can achieve sustained and substantial change and can completely overcome constraints such as allometry.
Keywords: allometry, Canis familiaris, evolutionary rate, geometric morphometrics, Procrustes analysis
1. Introduction
Quantification of the magnitude and types of morphological variation produced under strong selection can inform our understanding of phylogenetic patterns. In addition, the rate at which populations of organisms diversify can give us insight into speciation processes. Rapid or contemporary evolutionary change has been documented in a variety of organisms, demonstrating that diversification can occur on small time scales (Reznick et al. 1997; Hendry & Kinnison 1999; Huey et al. 2000; Losos et al. 2004; Phillips & Shine 2004; Carroll et al. 2005; Hendry 2005; Reznick & Ghalambor 2005). Whereas most of these studies consider the morphological end products of divergence by comparing contemporary populations that have diverged from a common ancestor, only a few studies have analysed morphological change over time, mostly in humans (e.g. Cole 2000; Jantz & Meadows Jantz 2000; Wescott & Jantz 2005). In these studies, a whole range of factors such as nutrition, health care and population composition change in ways that are hard to quantify, and a causal interpretation of the observed morphological changes is therefore difficult or impossible (Wescott & Jantz 2005). Because ecological factors were studied simultaneously, the processes that drive morphological change are better understood in Darwin's finches (Grant & Grant 2002, 2006). Nevertheless, long-term morphological time series with information on the selective regime are exceedingly rare.
Domestic dogs (Canis familiaris) are a unique system for the study of phenotypic evolution, because not only is there a considerable amount of morphological variation, but the history of breeds and the breed standards also provide a documented record of the selection regime that has been applied by breeders (e.g. Fondon & Garner 2004; Kemp et al. 2005; Lindblad-Toh et al. 2005; American Kennel Club 2006; Young & Bannasch 2006). Dog breeds are maintained by breeders as distinct lineages, and therefore provide a considerable control of genetic composition (Lindblad-Toh et al. 2005). In particular, for the St Bernard breed, preserved skeletal material is available from the past 120 years, which roughly corresponds to the time period since the establishment of the breed standard (Nussbaumer 2000; American Kennel Club 2006, p. 321). A previous study of historical change in the skull shape of St Bernards over time covered the period of the establishment of the breed to the 1940s (Huber 1947).
Here we use the methods of geometric morphometrics to study shape change in the skull of St Bernard dogs in the last 120 years. These methods permit a rigorous quantification of shape change and the results can be visualized and interpreted directly in their anatomical context. Because previous studies have emphasized the possible role of heterochrony and allometry in the evolution of dog breeds (Wayne 1986), we also examine the association of historical shape change with ontogenetic and static allometry of skull shape.
2. History of the St Bernard breed
The origins of the St Bernard date back to the mid-1600s when dogs were first kept at the monastery at Great Saint Bernard Pass in the Swiss Alps (Nussbaumer 2000; American Kennel Club 2006). It is thought that the dogs originated from the large ‘cow-herding’ dogs found throughout the countryside on farms. Originally guardians and companion animals, the dogs were extensively used to accompany the monks on their patrols for travellers in need of assistance (Nussbaumer 2000).
Systematic breeding of St Bernards with pedigree records began in the late 1850s with stock from the hospice of St Bernard pass. Some breeders aimed to maintain the type of dog found at the hospice while others favoured bulkier animals with larger heads and more pronounced ‘stops’ (the angle between the forehead and the muzzle; Dalziel 1888; Huber 1947; Nussbaumer 2000). The Swiss St Bernard Club was established in 1884, and in 1887 the Swiss Standard for St Bernards was adopted as the international breed standard (Siber 1884; Nussbaumer 2000; American Kennel Club 2006). These events marked a drastic change in the selective regime shaping the morphology and behaviour of the St Bernard. Because they were bred as pets and show dogs, breeders no longer selected for function but rather for form; the breed standard describes the perfect St Bernard in terms of its appearance, but not its behaviour (American Kennel Club 2006).
The breed standard specifies the shape of the head in considerable detail. In addition to many specifications concerning the skin and other soft parts, the breed standard also contains many details relevant to skull morphology. The desired headshape is imposing, massive and wide, with cheek bones (zygomatic arches) that are strongly developed and high. The ridges over the orbits are supposed to be prominent and to form nearly a right angle with the long axis of the head. The transition from the muzzle to the skull vault is to be abrupt and rather steep. The muzzle must be relatively short, wide and high, with a straight upper profile. These stipulations have remained similar from the first published breed standard to the current version (Siber 1884; American Kennel Club 2006).
3. Material and methods
This study is based primarily on a historical series of 47 adult St Bernard dogs dating from 1885 to 2001. In addition, a series of nine juveniles was combined with the skulls of adults from 1980 to present to form a full ontogenetic series. All specimens are from the Albert Heim collection at the Natural History Museum in Berne, Switzerland. These skulls are from purebred St Bernards with known pedigree information, and therefore are representative of the historical change of the breed.
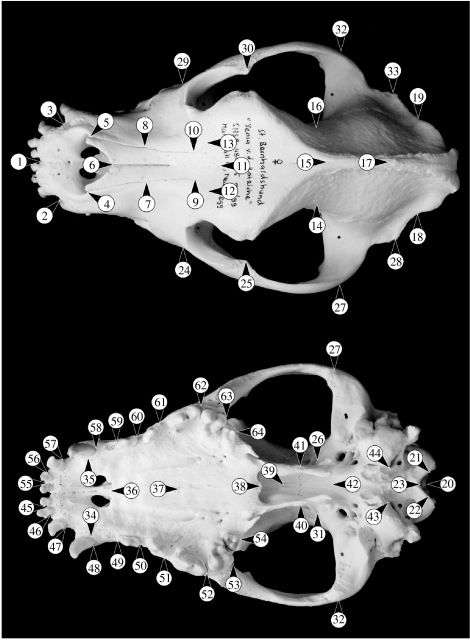
Three-dimensional coordinates was captured for 64 cranial landmarks (figure 1) with a Microscribe digitizer in dorsal and ventral views of the skulls. Twelve landmarks are in the median plane and the remaining landmarks form 26 bilateral pairs. Centroid size was used as a measure of cranial size and the information about variation of shape was extracted using generalized least-squares Procrustes superimposition separately for the historical and ontogenetic series (Dryden & Mardia 1998). For the comparison between the two datasets, the two coordinate systems were aligned so that the average shapes shared the same median plane and the same orientation of the anterior–posterior axis of the skull (landmarks 1 and 23). Because the skull is a bilaterally symmetric structure (object symmetry; e.g. Klingenberg et al. 2002), the total shape variation contains a component of asymmetry, differences between the left and right sides, as well as a component of symmetric variation corresponding to the average of the left and right sides. Because asymmetry is not of interest in this study, we consider only the symmetric component of variation (for details see Klingenberg et al. 2002). All analyses were carried out with a pre-release version of the MorphoJ software package (C.P. Klingenberg 2007, unpublished).
Figure 1.
Landmarks used in the morphometric analyses.
For the analysis of shape change over time and of allometry, we used multivariate regression of the Procrustes coordinates on the respective variables (e.g. Loy et al. 1998; Monteiro 1999). The amount of variation for which the regression model accounted was quantified as a percentage of the total shape variation, computed using the Procrustes metric (Goodall 1991; Klingenberg & McIntyre 1998). The statistical significance of the regressions was tested with permutation tests against the null hypothesis of independence (Good 2000). The vectors of regression coefficients from these analyses can be visualized as shape changes per unit of time or per unit of size increase, which allows a direct interpretation in their anatomical context. To illustrate the form of interdependence, we defined a shape score by projecting the shape data onto a line in the direction of the regression vector for each independent variable. If the regression model is written as y=βx+ϵ (where y is the row vector of shape variables; β is the regression vector; x is the independent variable; and ϵ is the row vector of error terms), the shape score s can be computed as s=yβ′(ββ′)−0.5. This score is the shape variable associated with the shape changes predicted by the regression model, but also includes the residual variation in that direction of shape space. This score therefore provides a graphical means to examine the strength of association and possible evidence of a nonlinear dependence (e.g. whether change over time is constant or slowing down).
For comparisons between historical change and allometries, we computed the angles between the corresponding regression vectors. These angles were computed as the arccosines of the signed inner products between the regression vectors (after standardization of both vectors to unit length). The resulting angles were compared to the distribution of angles from a Monte Carlo simulation of 100 000 pairs of random vectors in 98-dimensional space (the dimensionality of the symmetric component of shape variation; Klingenberg et al. 2002).
4. Results
(a) Historical change
The St Bernard skull has changed shape considerably since systematic breeding and selection began in the late 1800s (figure 2). The multivariate regression of shape on time and centroid size was highly significant statistically (p<0.0001), and accounted for 22.1% of the total amount of shape variation. Similarly, the multivariate regression on time alone was statistically significant (p<0.0001) and accounted for 18.8% of the variation of shape. In contrast, the regression of shape on centroid size alone was not statistically significant (p=0.11) and accounted for only 3.4% of the shape variation. The differences between the results (significance test, amounts of shape variation explained and regression vectors) of regression analyses of shape on time with and without centroid size as an additional independent variable were negligible.
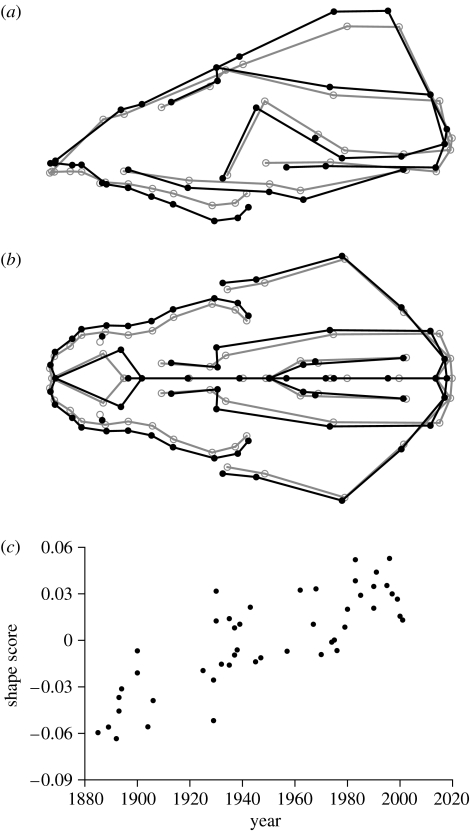
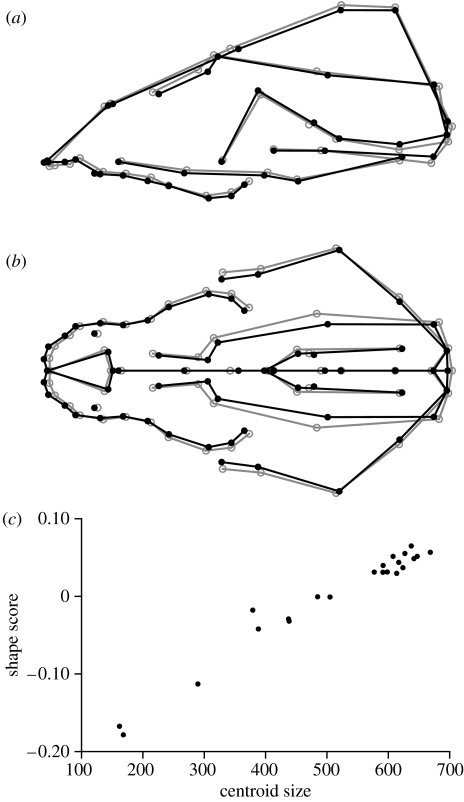
Figure 2.
Historical change of skull shape in St Bernard dogs. (a,b) Regression vectors of shape on time. The grey circles show the average shape and the change from the grey circles to the black dots indicates the landmark shift corresponding to the predicted shape change over 100 years. The grey and black lines are to indicate the contours of selected anatomical units in the same two shapes. (c) The corresponding shape scores for the dogs included in the study as a function of time.
The shape features associated with the historical change are primarily an upward and posterior shift of the landmarks of the nose, a tilting of the palate and maxillae that increases their inclination relative to the long axis of the skull, and an upward shift of the landmarks at the anterior margin of the frontal bone and on the occipital crest (figure 2a). Moreover, a broadening of the skull, especially of the snout, is clearly apparent (figure 2b).
This change in shape over time appears linear and continues through the entire period for which data are available (figure 2c). In other words, there is no evidence for variation in the rate of change; in particular, the trend does not appear to slow down. The magnitude of the change can be expressed as the length of the regression vector (the square root of the sum of squared regression coefficients of the shape coordinates on time). This results in an estimated rate of 0.000697 units of Procrustes distance per year.
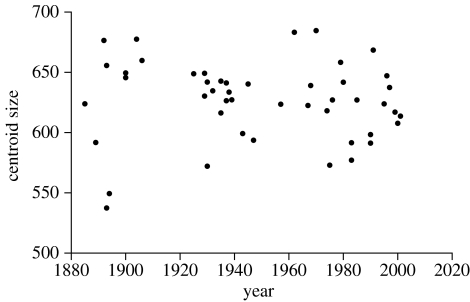
In contrast, there appears to be no change in cranial size through the study period (figure 3). The linear regression of centroid size on time accounts for only 0.12% of the variance of centroid size, and the permutation test indicates that this is not statistically significant (p=0.59). The constancy of the size of the skull demonstrates that historical change of St Bernard skull shape cannot be ascribed simply to allometry.
Figure 3.
Trend of cranial centroid size of St Bernard dogs through the study period.
(b) Allometry
Although the regression of shape on centroid size of adult dogs accounted for only 3.4% of the shape variation and was not statistically significant at the conventional 5% level, the p-value of 0.11 still suggests that there is some weak evidence against the null hypothesis of independence. Given the small sample size (n=47) and the high dimensionality of the data (98 dimensions for the symmetric component of variation; Klingenberg et al. 2002), it is also to be expected that the power of the test is low. We therefore present the results of the regression, but we urge readers to interpret them with caution.
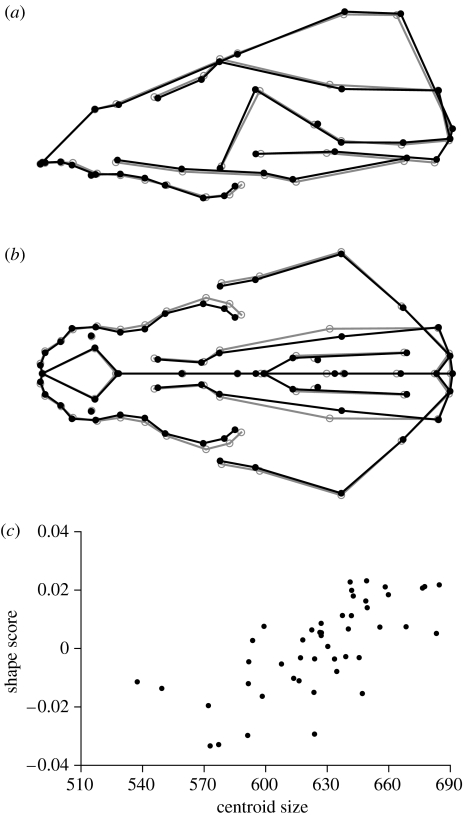
The regression of skull shape on centroid size, which represents the pattern of static allometry (e.g. Klingenberg 1996), shows a lengthening and narrowing of the skull that particularly affects the posterior part of the maxillae and portions of the braincase (figure 4a,b). There is a considerable amount of scatter about the relationship between this shape change and centroid size (figure 4c), which indicates that static allometry is fairly weak. The angle between this regression vector for static allometry and the regression vector for historical change was 105.2°. Although the Monte Carlo simulation suggested that the deviation from a right angle, as expected under the null hypothesis of independence, was significant (p=0.008), the problems of the regression for static allometry (see above) raise doubts whether this relatively small deviation can be interpreted.
Figure 4.
Static allometry of adult St Bernard dogs. (a,b) Shape change associated with static allometry, estimated from regression of shape on centroid size in the historical data set. The grey circles show the average shape and the change from the grey circles to the black dots indicates the predicted landmark shift corresponding to an increase of centroid size by 100 mm. The grey and black lines are to indicate the contours of selected anatomical units in the same two shapes. (c) The corresponding shape scores for the dogs included in the study as a function of centroid size.
In contrast to static allometry among adults, there is very clear evidence for ontogenetic allometry in St Bernards. The regression of skull shape on centroid size in the ontogenetic series accounted for 56.3% of the shape variation in the ontogenetic series, and the permutation test indicated that it was statistically significant (p<0.0001).
The pattern of shape variation for ontogenetic allometry, estimated from the regression of shape on centroid size in the ontogenetic series, is a trend towards a more slender skull shape with increasing size (figure 5a,b). This pattern is similar to that for static allometry but shows size-associated variation in a number of additional landmarks at the nose and in the posterior part of the skull. This similarity was also reflected in the angle between the regression vectors for static and ontogenetic allometry, which was 55.8° and thus smaller than expected for pairs of random vectors (p<0.00001). In contrast, the angle between the regression vectors for ontogenetic allometry and for historical change was 87.5° and not significantly different from the expected right angle for pairs of random vectors (p=0.66). The scatter plot for ontogenetic allometry clearly shows that the association of the shape score with centroid size is quite strong in the ontogenetic series (figure 5c).
Figure 5.
Ontogenetic allometry of St Bernard dogs. (a,b) Shape change associated with ontogenetic allometry, estimated from the regression of shape on centroid size in the ontogenetic series. The grey circles show the average shape and the change from the grey circles to the black dots indicates the predicted landmark shift corresponding to an increase of centroid size by 100 mm. The grey and black lines are to indicate the contours of selected anatomical units in the same two shapes. (c) The corresponding shape scores for the dogs included in the study as a function of centroid size.
5. Discussion
Our results show that the skulls of St Bernard dogs have undergone a considerable morphological transformation in the last 120 years, a trend that may still be continuing (figure 2c). The shape changes associated with this trend correspond to the features specified in the breed standard for St Bernards (American Kennel Club 2006, p. 322 ff.). The upper jaw and palate have tilted, raising the anterior and lowering the posterior part, which contributes to the shortened and relatively high muzzle. This tilting of the upper jaw and palate, together with the upward shifts of landmarks on the frontal bone, contribute to the pronounced stop, the angle between the muzzle and the forehead, which the breed standard specifies as desirable. The shifts of the landmarks on the anterior margin of the frontal bone also reflect the pronounced supraorbital ridge that is another desired feature for the breed. In addition, the skull has become wider and the height of the occipital crest increased. Both the width of the head and its general ‘very powerful and imposing’ appearance are emphasized in the breed standard (American Kennel Club 2006, p. 322). The close agreement between the observed changes and the features described as desirable in the breed standard, and therefore favoured by breeders, suggests that the observed change was brought about by selective breeding.
The more or less linear trend in the shape variable that corresponds to historical change (figure 2c) indicates a sustained response of skull shape to the selection imposed by breeding. There is no evidence for a slowing down of the trend, as it has been found in many artificial selection experiments (Roff 1997). Unfortunately, the available data do not allow us to decide whether a sufficient amount of genetic variation still persists from the initial, heterogeneous breeding stock (Huber 1947; Nussbaumer 2000) or whether genetic variation is replenished continuously by new mutation (e.g. Fondon & Garner 2004).
Interestingly, there appears to be no trend for centroid size of the skull (figure 3), even though the breed standard specifies that the head should be ‘massive’ (American Kennel Club 2006, p. 322) and the size of the head is presumably under selection. Because body size and the sizes of structures such as the head usually are associated with heritable variation that provides the potential for a response to selection (e.g. Roff 1997), the apparent lack of a response raises the question whether some constraint may have prevented an increase of skull size in St Bernards.
The rate at which the shape change has progressed in the study period is difficult to compare with other estimates of rates of shape evolution because Procrustes distance, the most widespread measure of shape difference, depends on the number and arrangement of landmarks and therefore cannot be easily be compared between studies. Nevertheless, it is possible to make a broad comparison of the magnitude of change. The rate of change computed from the regression of shape on time yields a change of approximately 0.084 units of Procrustes distance over the 120 years of the study period. This is comparable to the distances in average body shapes among fish populations separated by periods up to 4000 years (Monteiro & Gomes-Jr. 2005) or between tooth shape divergence of mammal taxa separated by thousands to millions of years (Polly 2001). Episodes of particularly strong selection can achieve even faster divergence, as suggested by selection experiments on wing shape in Drosophila (Houle et al. 2003) or observations on bill size and shape in Darwin's finches (Grant & Grant 2002, 2006). The results from our study of St Bernard dogs are remarkable in that they indicate that a sustained response to such selection can be maintained for many generations.
Previous research has suggested that morphological diversity in dogs may be due in large part to allometric shape changes (Wayne 1986). Our data indicate that this is not the case for the historical change in St Bernards. Not only was there no consistent trend of skull size in the time period covered by our study, but also there was a major discrepancy between the patterns of historical shape change (figure 2a,b) and those for size-related shape change (figures 4 and 5). The shape changes associated with static allometry (figure 4a,b) are similar to that for ontogenetic allometry (figure 5a,b) in that they both show a narrowing and lengthening of the skull, which indicates that shape responds similarly to changes of size, regardless of whether they are due to size differences among adults or to growth. Moreover, this similarity of the allometric shape change provides support for the estimate of static allometry, even though its statistical significance is somewhat doubtful. In contrast, the trend in historical shape change was for a wider skull with a relatively shorter and more angled muzzle. This discrepancy between the historical shape change and the allometry of skull shape suggests that allometry did not act as a constraint that would have impeded the selection of certain skull shape features by breeders. This parallels the findings of other studies where strong selection has overcome relative constraints by allometry or has even changed the allometry itself (Weber 1990; Beldade et al. 2002; Frankino et al. 2005).
The historical change in St Bernards shows that selection by breeders can produce sustained change of shape in one direction, and thus can produce morphological alterations comparable to the differences between taxa that have been diverging for much longer times. Morphological variation in dogs is comparable to diversification in higher taxa (Wayne 1986; Young & Bannasch 2006), and dogs can therefore serve as a model system for studying the mechanisms involved in the evolution of morphological disparity.
Acknowledgments
We wish to thank Marc Nussbaumer for the photos of the St Bernard skull and for assistance during data collection. We are indebted to the Albert Heim collection and the Natural History Museum Bern for access to the collection. This work was funded by the United States Department of Education, the Leverhulme Trust and the Royal Society.
Supplementary Material
References
- American Kennel Club. Ballatine Books; New York, NY: 2006. The complete dog book. [Google Scholar]
- Beldade P, Koops K, Brakefield P.M. Developmental constraints versus flexibility in morphological evolution. Nature. 2002;416:844–847. doi: 10.1038/416844a. doi:10.1038/416844a [DOI] [PubMed] [Google Scholar]
- Carroll S.P, Loye J.E, Dingle H, Mathieson M, Famula T.R, Zalucki M.P. And the beak shall inherit—evolution in response to invasion. Ecol. Lett. 2005;8:944–951. doi: 10.1111/j.1461-0248.2005.00800.x. doi:10.1111/j.1461-0248.2005.00800.x [DOI] [PubMed] [Google Scholar]
- Cole T.J. Secular trends in growth. Proc. Nutr. Soc. 2000;59:317–324. doi: 10.1017/s0029665100000355. [DOI] [PubMed] [Google Scholar]
- Dalziel H. L. Upcot Gill; London, UK: 1888. The St Bernard; its history, points, breeding and rearing. [Google Scholar]
- Dryden I, Mardia K. Wiley; Chichester, UK: 1998. Statistical shape analysis. [Google Scholar]
- Fondon J.W, III, Garner H.R. Molecular origins of rapid and continuous morphological evolution. Proc. Natl Acad. Sci. USA. 2004;101:18 058–18 063. doi: 10.1073/pnas.0408118101. doi:10.1073/pnas.0408118101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankino W.A, Zwaan B.J, Stern D.L, Brakefield P.M. Natural selection and developmental constraints in the evolution of allometries. Science. 2005;307:718–720. doi: 10.1126/science.1105409. doi:10.1126/science.1105409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good P. Springer; New York, NY: 2000. Permutation tests: a practical guide to resampling methods for testing hypotheses. [Google Scholar]
- Goodall C.R. Procrustes methods in the statistical analysis of shape. J. R. Stat. Soc. B. 1991;53:285–339. [Google Scholar]
- Grant P.R, Grant B.R. Unpredictable evolution in a 30-year study of Darwin's finches. Science. 2002;296:707–711. doi: 10.1126/science.1070315. doi:10.1126/science.1070315 [DOI] [PubMed] [Google Scholar]
- Grant P.R, Grant B.R. Evolution of character displacement in Darwin's finches. Science. 2006;313:224–226. doi: 10.1126/science.1128374. doi:10.1126/science.1128374 [DOI] [PubMed] [Google Scholar]
- Hendry A.P. Evolutionary biology—the power of natural selection. Nature. 2005;433:694–695. doi: 10.1038/433694a. doi:10.1038/433694a [DOI] [PubMed] [Google Scholar]
- Hendry A.P, Kinnison M.T. Perspective: the pace of modern life: measuring rates of contemporary microevolution. Evolution. 1999;53:1637–1653. doi: 10.1111/j.1558-5646.1999.tb04550.x. doi:10.2307/2640428 [DOI] [PubMed] [Google Scholar]
- Houle D, Mezey J.G, Galpern P, Carter A. Automated measurement of Drosophila wings. BMC Evol. Biol. 2003;3:25. doi: 10.1186/1471-2148-3-25. doi:10.1186/1471-2148-3-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber W. Die Veränderung der St. Bernhardsrasse in den vergangenen 140 Jahren. Schweizer Hunde-Sport. 1947;21:389–394. [Google Scholar]
- Huey R.B, Gilchrist G.W, Carlson M.L, Berrigan D, Serra L. Rapid evolution of a geographic cline in size in an introduced fly. Science. 2000;287:308–309. doi: 10.1126/science.287.5451.308. doi:10.1126/science.287.5451.308 [DOI] [PubMed] [Google Scholar]
- Jantz R.L, Meadows Jantz L. Secular change in craniofacial morphology. Am. J. Hum. Biol. 2000;12:327–338. doi: 10.1002/(SICI)1520-6300(200005/06)12:3<327::AID-AJHB3>3.0.CO;2-1. doi:10.1002/(SICI)1520-6300(200005/06)12:3<327::AID-AJHB3>3.0.CO;2-1 [DOI] [PubMed] [Google Scholar]
- Kemp T.J, Bachus K.N, Nairn J.A, Carrier D.R. Functional trade-offs in the limb bones of dogs selected for running versus fighting. J. Exp. Biol. 2005;208:3475–3482. doi: 10.1242/jeb.01814. doi:10.1242/jeb.01814 [DOI] [PubMed] [Google Scholar]
- Klingenberg C.P. Multivariate allometry. In: Marcus L.F, Corti M, Loy A, Naylor G.J.P, Slice D.E, editors. Advances in morphometrics. vol. 284. Plenum Press; New York, NY: 1996. pp. 23–49. [Google Scholar]
- Klingenberg C.P, McIntyre G.S. Geometric morphometrics of developmental instability: analyzing patterns of fluctuating asymmetry with Procrustes methods. Evolution. 1998;52:1363–1375. doi: 10.1111/j.1558-5646.1998.tb02018.x. doi:10.2307/2411306 [DOI] [PubMed] [Google Scholar]
- Klingenberg C.P, Barluenga M, Meyer A. Shape analysis of symmetric structures: quantifying variation among individuals and asymmetry. Evolution. 2002;56:1909–1920. doi: 10.1111/j.0014-3820.2002.tb00117.x. [DOI] [PubMed] [Google Scholar]
- Lindblad-Toh K, et al. Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature. 2005;438:803–819. doi: 10.1038/nature04338. doi:10.1038/nature04338 [DOI] [PubMed] [Google Scholar]
- Losos J.B, Schoener T.W, Spiller D.A. Predator-induced behaviour shifts and natural selection in field-experimental lizard populations. Nature. 2004;432:505–508. doi: 10.1038/nature03039. doi:10.1038/nature03039 [DOI] [PubMed] [Google Scholar]
- Loy A, Mariani L, Bertelletti M, Tunesi L. Visualizing allometry: geometric morphometrics in the study of shape changes in the early stages of the two-banded sea bream, Diplodus vulgaris (Perciformes, Sparidae) J. Morphol. 1998;237:137–146. doi: 10.1002/(SICI)1097-4687(199808)237:2<137::AID-JMOR5>3.0.CO;2-Z. doi:10.1002/(SICI)1097-4687(199808)237:2<137::AID-JMOR5>3.0.CO;2-Z [DOI] [PubMed] [Google Scholar]
- Monteiro L.R. Multivariate regression models and geometric morphometrics: the search for causal factors in the analysis of shape. Syst. Biol. 1999;48:192–199. doi: 10.1080/106351599260526. doi:10.1080/106351599260526 [DOI] [PubMed] [Google Scholar]
- Monteiro L.R, Gomes-Jr J.L. Morphological divergence rate tests for natural selection: uncertainty of parameter estimation and robustness of results. Genet. Mol. Biol. 2005;28:345–355. doi:10.1590/S1415-47572005000200028 [Google Scholar]
- Nussbaumer M. Naturhistorisches Museum der Burgergemeinde Bern; Berne, Switzerland: 2000. Barry vom Grossen St Bernhard. [Google Scholar]
- Phillips B.L, Shine R. Adapting to an invasive species: toxic cane toads induce morphological changes in Australian snakes. Proc. Natl Acad. Sci. USA. 2004;101:17 150–17 155. doi: 10.1073/pnas.0406440101. doi:10.1073/pnas.0406440101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polly P.D. On morphological clocks and paleophylogeography: towards a timescale for Sorex hybrid zones. Genetica. 2001;112–113:339–357. doi:10.1023/A:1013395907225 [PubMed] [Google Scholar]
- Reznick D.N, Ghalambor C.K. Selection in nature: experimental manipulations of natural populations. Integr. Comp. Biol. 2005;45:456–462. doi: 10.1093/icb/45.3.456. doi:10.1093/icb/45.3.456 [DOI] [PubMed] [Google Scholar]
- Reznick D.N, Shaw F.H, Rodd F.H, Shaw R.G. Evaluation of the rate of evolution in natural populations of guppies (Poecilia reticulata) Science. 1997;275:1934–1937. doi: 10.1126/science.275.5308.1934. doi:10.1126/science.275.5308.1934 [DOI] [PubMed] [Google Scholar]
- Roff D.A. Chapman and Hall; New York, NY: 1997. Evolutionary quantitative genetics. [Google Scholar]
- Siber, M. 1884 Der Sanct Bernhardshund. In Schweizerisches Hundestammbuch, vol. 1, pp. 28–39. Zurich, Switzerland: Orell Füssli & Co.
- Wayne R.K. Cranial morphology of domestic and wild canids: the influence of development on morphological change. Evolution. 1986;40:243–261. doi: 10.1111/j.1558-5646.1986.tb00467.x. doi:10.2307/2408805 [DOI] [PubMed] [Google Scholar]
- Weber K.E. Selection on wing allometry in Drosophila melanogaster. Genetics. 1990;126:975–989. doi: 10.1093/genetics/126.4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wescott D.J, Jantz R.L. Assessing craniofacial secular change in American blacks and whites using geometrics morphometry. In: Slice D.E, editor. Modern morphometrics in physical anthropology. Kluwer Academic/Plenum Press; New York, NY: 2005. pp. 231–245. [Google Scholar]
- Young A, Bannasch D. Morphological variation in the dog. In: Ostrander E.A, Giger U, Lindblad-Toh K, editors. The dog and its genome. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2006. pp. 47–65. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.