Abstract
Sexual selection theory traditionally considers choosiness for mates to be negatively related to intra-sexual competition. Males were classically considered to be the competing, but not the choosy, sex. However, evidence of male choosiness is now accumulating. Male choosiness is expected to increase with an individual's competitive ability, and to decrease as intra-sexual competition increases. However, such predictions have never been tested in field conditions. Here, we explore male mate choice in a spider by studying size-assortative pairing in two natural sites that strongly differ in the level of male–male competition. Unexpectedly, our results demonstrate that mate choice shifts from opportunism to high selectivity as competition between males increases. Males experiencing weak competition did not exhibit size-related mating preferences. By contrast, when competition was intense we found strong size-assortative pairing due to male choice: while larger, more competitive males preferentially paired with larger, more fecund females, smaller males chose smaller females. Thus, we show that mating preferences of males vary with their competitive ability. The distinct preferences exhibited by males of different sizes seem to be an adaptive response to the lower reproductive opportunities arising from increased competition between males.
Keywords: sexual selection, male mate choice, intra-sexual competition, assortative mating, spider
1. Introduction
Mate choice and intra-sexual competition are the two components of sexual selection (Darwin 1871). Males often have a higher potential reproductive rate than females, and are thus considered to be the sex competing for access to mates, while females are assumed to be the choosy sex (Trivers 1972; Parker 1983; Clutton-Brock & Vincent 1991; Andersson 1994). This basic statement received further support from rare reversed sex-role species where the operational sex ratio (OSR, the ratio of ready-to-mate males to ready-to-mate females; Emlen & Oring 1977) is female biased. In this context, females compete for access to choosy males (Gwynne 1981). The degree of choosiness for mates, and of within-sex competition, can be tightly linked with changes in OSR (Forsgren et al. 2004). However, in conventional sex-role species, evidence of male choosiness is accumulating (Parker 1983; Schwagmeyer & Parker 1990; Olsson 1993; Cunningham & Birkhead 1998; Amundsen 2000; Bonduriansky 2001; Dunn et al. 2001; Preston et al. 2005). Both theoretical and empirical evidence suggested that males should be most choosy when mating is costly and when the quality of individual females varies greatly (Parker 1983; Johnstone et al. 1996; Kvarnemo & Simmons 1998; Wong & Jennions 2003). Male choosiness should be weak when competition for mates is intense due to very few mating opportunities (Lawrence 1986; Crowley et al. 1991; Berglund 1994; Kokko & Johnstone 2002). Furthermore, good competitors are expected to be choosier than poor ones that should instead mate opportunistically (Burley 1977; Ptacek & Travis 1997; Amundsen & Forsgren 2003; Fawcett & Johnstone 2003; Shine et al. 2003). However, no field study has ever tested these predictions. This may be due to two major reasons: (i) it is often difficult to accurately measure the competition level among males within a population (Kokko & Monaghan 2001; Shuster & Wade 2003; Forsgren et al. 2004; Nyman et al. 2006) and (ii) male mate choice usually cannot be inferred from observed mating patterns that may also be caused by contest competition between males, female choice or spatial and/or temporal heterogeneity of mate quality (Crespi 1989; Rowe & Arnqvist 1996).
In this study, we explored male mate choice under natural conditions in an annual orb-weaving spider, Zygiella x-notata (Araneae: Araneidae), with respect to the males' competitive ability and the strength of competition between them. In this species, mating occurs in summer when reproductive males are present. Adult males perform pre-copulatory mate guarding exclusively of immature females near their adult moult, with no more than one male per female at a time (Bel-Venner & Venner 2006). Male takeovers may occur before the female's adult moult. Whenever a female is moulting, only the male guarding her can be the first to court her and mate at once (Bel-Venner & Venner 2006). Males may obtain great fitness rewards from copulating with their recently moulted virgin mates owing to the first male sperm priority pattern in this species (Austad 1984). Nevertheless, mate guarding is both time consuming (Komdeur 2001; Bel-Venner & Venner 2006; Segoli et al. 2006) and energetically costly due to fierce fights between rival males (Plaistow et al. 2003; Low 2006). Males should thus benefit from selecting their mates depending on their own competitive ability and the variation in mate quality (Burley 1977; Parker 1983; Huber 2005; Preston et al. 2005), both of which are reflected by body size in the populations studied here. Large females laid both more and heavier eggs than did small ones in the field (see §2). As for males, competitive ability increased with body size, because large males were more likely than small ones to take over females from previous guardians (Bel-Venner & Venner 2006).
We explored mate-guarding strategies of males at two distinct sites with different levels of competition between males (see below). Mating preferences of males were assessed, first, by comparing body size of guarded versus non-guarded females, and second, by the pattern of size-assortative pairing between guarded females and their first guardian (see §2). This mate-guarding system allowed us to test two predictions from classical sexual selection theory: (i) males should pair opportunistically when competition is intense, and they should be choosy when competition is weak, and (ii) in the latter case, males should preferentially guard the largest and most fecund females and large males should be more selective than small ones.
2. Material and methods
(a) General procedure
We conducted daily observations throughout one breeding season on spiders found along the glass walls of two similar buildings of the university campus (Nancy, France). The buildings were at least 100 m apart, and were separated by asphalt roads, making the movements of adult spiders between the two sites unlikely. We never recorded any migration of marked individuals between the two sites. One sampled area was 1600 cm long×200 cm high, and the other was 2050 cm long×200 cm high.
For visual identification, we marked each adult male or female, and each sub-adult female, found on either site by means of a unique combination of one to two colour dots on their legs. We sized all adults to the nearest 0.1 mm and used prosoma width as a body size indicator in our analyses, as it was strongly correlated with leg length (Bel-Venner & Venner 2006). In addition, we found a positive relationship between the body size of sub-adult and adult wild-born laboratory-reared females (prosoma width, linear regression: sub-adult size=0.671+0.435×adult size, n=123, R2=0.43, F1,121=91.47, p<0.0001).
At both the sites, we recorded the day of males' and females' adult moult, whenever feasible; we also recorded the location (to the nearest centimetre) and the pairing status of every adult male and sub-adult female daily. This systematic daily observation was combined with a nocturnal survey of roaming males. This field study allowed us to identify the first male that settled down near each female, and that became her first guardian, without first interacting with any rival male (see electronic supplementary material).
(i) Female body size and fecundity
We checked for egg laying daily until the last adult female died. We removed each clutch after visual maternal assignment, counted the fertile eggs and weighed them after they were dried for 24 hours at 60°C (balance Sartorius Basic BA 110S±0.1 mg). Although Z. x-notata females are iteroparous, 34 out of 47 egg-laying females laid only one clutch (72.3%). Consequently, we considered only the first clutch of each female in the analysis. Large females laid both more and heavier eggs than did small ones in the field (linear regression: clutch size=61.20×female size-69.47, N=47, R2=0.55; female size effect: Student's t-test, t45=7.56, p<0.0001; dry weight/egg=0.045×female size+0.103, N=47, R2=0.08; female size effect: Student's t-test, t45=2.25, p=0.029).
(b) Quantifying competition between males at both the sites
Variance and mean body size did not significantly differ either among males or among females, between the two sites named low-competition (LC) and high-competition (HC) sites, respectively (table 1); males: nLC=68, nHC=188, , , variance ratio: F67,187=1.276, p=0.23, Student's t-test for unpaired samples: Student's t-test, t254=0.862, p=0.390; females: nLC=72, nHC=85, , , variance ratio: F71,84=1.317, p=0.23, Student's t-test, t155=0.960, p=0.338). The two sites were thus homogeneous regarding quality of females and competitive ability of males.
Table 1.
Between-site differences in the level of competition between males. Competition was characterized by five distinct parameters: (i) the percentage of females that were guarded at least once among females that moulted as adults; (ii) the percentage of males, among those that were the first to guard any female, that succeeded in guarding their mate until her adult moult, and the remaining males failed due to takeover by rival males; (iii) male turnover, computed as the total number of males that successively guarded each female in the male removal experiment; (iv) the OSR, the daily ratio of adult males to sub-adult females; (v) the tertiary sex ratio, the ratio of males to females that both moulted as adults at each site.
| statistics | ||||||
|---|---|---|---|---|---|---|
| variable | high competition (HC) | low competition (LC) | Χ2 | d.f. | p | |
| (i) | percentage of guarded females (%) | 88.0 | 68.9 | 6.1 | 1 | 0.014 |
| no. of females | 50 | 74 | ||||
| (ii) | success rate of first guarding males (%) | 58.0 | 83.1 | 5.1 | 1 | 0.025 |
| no. of males | 31 | 59 | ||||
| (iii) | male turnover per female | 4 (2–5)a | 1 (1–2) | 9.8 | 1 | 0.002 |
| no. of females | 9 | 15 | ||||
| (iv) | operational sex ratio | 4.42b | 1.13b | 20.3c | 1 | <0.0001 |
| (v) | tertiary sex ratio | 0.50 | 0.25 | 20.9 | 1 | <0.0001 |
| no. of adult moulting | 121 | 189 | ||||
Median value (lower–upper quartiles).
Estimates from the generalized linear model.
Site effect is given after fixing the season effect, as OSR increased throughout the season at both the sites (Χ42=17.60, p=0.0015; site–season interaction, Χ42=4.90, p=0.30).
In all our analyses, we removed the temporal covariation in size of males and females using residuals computed from polynomial regression models instead of absolute body size values (see electronic supplementary material). This allowed us to determine the actual competitive ability of a male relative to other males of the site, and the actual quality of a female about to moult relative to females available in the site.
(i) Success rate of first pairs
For each female whose moulting date was known, we identified her first guardian (see electronic supplementary material) and checked to see whether he was still present at her moult; if so we considered that he had successfully mated (Bel-Venner & Venner 2006). Whenever this male had obviously been replaced by another male before the female's moulting, we considered him to have failed due to takeover. We then computed the proportion of first guardians that successfully mated. To avoid pseudo-replications due to possible repeated sampling of males within sites, we randomly selected only one guarding event per male. We tested the influence of the site and males' residual size on the probability for a male to successfully guard his mate with a logistic regression through a generalized linear model fitted on a binomial error.
(ii) Male removal experiment and male turnover
We conducted a parallel experiment at both the sites to test (i) the difference in male–male competition level between sites and (ii) the link between size and attractiveness of females. We lowered the local competition between males near randomly chosen females by daily and permanently removing males found guarding experimental females (Bel-Venner & Venner 2006). Male turnover (the total number of successive guardians) thus reflected attractiveness of a female independent of the competitive ability of her mates. We tested the influence of the site and females' residual size on male turnover using a generalized linear model fitted on a Poisson error.
(iii) Operational sex ratio
To estimate the OSR at both the sites, we computed the daily ratio of adult males present at one site to females reaching sexual maturity within the following 7 days (the mean delay for males to start guarding females; Bel-Venner & Venner 2006). For consistency in our results within each site, we considered OSR on days when at least 10 individuals of either sex were sampled. Therefore, we analysed OSR over 46 and 42 consecutive days, starting from 2 August and 10 July at LC and HC sites, respectively. To avoid dependent data occurring day to day, we selected five OSR values per site, sampled every 10 days after day 1, so that no females, and very few males, were sampled twice. We compared OSR between the two sites using a generalized linear model fitted on a binomial error distribution. We also tested the effects of the time of the year and site–time interaction in a backward stepwise procedure, and fitted the difference of deviance between two successive models on a Χ2 distribution (table 1). All statistical analyses were run with R (R Development Core Team 2005, http://CRAN.R-project.org).
(c) Size-assortative pairing as an unbiased result of male mate choice
To exclude possible competitive interactions between rival males when mate guarding (see electronic supplementary material), we analysed size-assortative pairing considering only pairs formed by a female and the first male to guard her. To avoid pseudo-replications due to possible repeated sampling of males within sites, we randomly selected only one guarding event per male. The classical alternatives, other than male mate choice, that might also explain assortative pairing (i.e. temporal or spatial heterogeneity of male and female body size, contest competition between males, female choice, female density, sampling bias) could be excluded (see electronic supplementary material). We analysed size-assortative pairing using a linear model to simultaneously test the effects of site, females' residual size and their interaction on residual size of males.
3. Results
(a) Competition level at both the sites
The two sites under study differed drastically according to the level of competition between males for access to the pre-receptive females. These sites (HC and LC) differed with respect to five distinct parameters related to male–male competition (table 1) as follows. (i) The rate of guarding was higher at HC than LC because HC females were more likely to be guarded by at least one male before their adult moult. (ii) Males were less likely to successfully guard their mates at HC than LC owing to a higher rate of takeover by rival males. The issue of guarding did not depend on their size at any site (probability of success; effect of male residual size: Χ12=2.54, p=0.11; effect of male size–site interaction: Χ12=0.91, p=0.34). (iii) The male removal experiment performed on randomly selected females revealed a higher male turnover, and thus a higher rate of intrusion by rival males, at HC than LC. This field experiment further demonstrated that local competition at any site was higher near large females than small ones because large females experienced a greater male turnover (effect of female size on male turnover: Χ12=4.43, p=0.03; effect of the female size–site interaction: Χ12=0.39, p=0.53). (iv) The OSR remained highly male biased throughout the season at HC, whereas it was much more balanced at LC. (v) This difference of OSR pre-existed for spiders in their penultimate instar, as the ratio of males to females moulting as adults was twice as high at HC as at LC.
(b) Male mate choice
At the LC site, the guarding males did not select the highest quality females, since females' residual size did not differ between guarded and unguarded individuals (guarded females: mean residual size±s.e.=0.012±0.028 mm, n=51; unguarded females=−0.026±0.033 mm, n=23; Student's t-test, t72=0.782, p=0.44). It was not possible to test for a similar relationship at the HC site because we observed only six unguarded females.
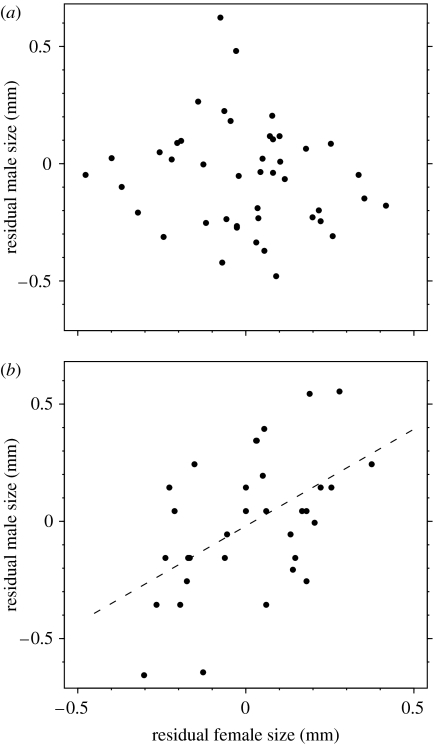
After randomly selecting only one guarding event per male, we found a strong difference in size-assortative pairing between the two sites (effect of the female size–site interaction on male size: two-way analysis of variance (ANOVA); F1,56=8.357, p=0.005). Contrary to our predictions, the first pairs were not assorted by size at the LC site, but they were at the HC site (figure 1; LC: 44 females paired with 28 distinct males, Student's t-test, t56=1.088, p=0.28; HC: 34 females paired with 31 distinct males, estimate±s.e.=0.709±0.234; Student's t-test, t56=3.034, p=0.003).
Figure 1.
Size-related pairing patterns between the females and the first males guarding them at the (a) LC and (b) HC sites. Residual sizes (prosoma width, mm) are shown and used in analysis: they reflect the competitive ability of a male relative to other males of his site, and the relative fecundity of a female among pre-moulting females of her site. After randomly selecting only one guarding event per male, we found a strong difference in size-assortative pairing between the two sites (effect of the female size–site interaction on male size: two-way ANOVA; F1,56=8.357, p=0.005). The first pairs were not assorted by size at the LC site ((a), 44 females paired with 28 distinct males, Student's t-test, t56=1.088, p=0.28) but they were at the HC site ((b), 34 females paired with 31 distinct males, estimate±s.e.=0.709±0.234; Student's t-test, t56=3.034, p=0.003).
4. Discussion
This is, to our knowledge, the first study that unambiguously examined male mate choice in field conditions in a conventional sex-role species. We quantified between-male competition levels through both direct observations and a male removal experiment. We simultaneously studied two sites that strongly differed according to the competition levels between males, while they were homogeneous regarding female density and spatial distribution of male and female sizes. Since all these conditions were met, this provides an unusually appropriate field design for analysing variations in male mate choice according to intra-sexual competition. Unlike previous laboratory experiments (Lawrence 1986; Berglund 1994) and classical mate choice theory (Clutton-Brock & Vincent 1991; Crowley et al. 1991; Jennions & Petrie 1997; Kokko & Johnstone 2002), our results show that males are pairing opportunistically under weak intra-sexual competition and are highly selective when competition for guarding females is high.
When competition between them was weak, guarding males did not select the highest quality females, and we found no size-assortative pairing (figure 1a). Because males are mobile, and may wander out of the study sites, we could not rigorously quantify the number of matings per male during his lifetime. However, we found that paired males were more successful at guarding under low than high competition (table 1). Guarding males under low competition could thus successfully mate with several partners. They could benefit more from pairing opportunistically (to maximize the number of mates) than by being selective, which could be time consuming. By contrast, when competition for mates was high, males were selective, and their preferences differed according to their own competitive ability. We found size-assortative mating of first pairs based on male choice, which reflected the preference of both larger males for larger females and smaller males for smaller mates (figure 1b).
Size-assortative mating and/or pairing is commonly reported among various taxa (Olsson 1993; Jormalainen et al. 1994; Harari et al. 1999; Hume et al. 2002; Kolm 2002; Hoefler 2007), and is generally explained as the outcome of competition between males or of mutual mate choice. These two mechanisms might co-occur, and both imply that choosy males uniformly prefer high-quality mates. Only large males can exclude their smaller rivals from large females (Fairbairn 1988; Crespi 1989), and/or large females, preferring larger males, can successfully reject smaller males (Rowe & Arnqvist 1996; Johnstone 1997; Harari et al. 1999; Kolm 2002). However, these hypotheses can be ruled out in our study because the pairing pattern of guarding pairs resulted neither from any female resistance nor from competition between males. Our results suggest instead that the positive size-assortative pairing observed directly results from distinct mating preferences exhibited by males of various competitive abilities.
In our study system, paired males incur a greater risk of being dislodged by takeover before they fertilize their mate when competition is high (table 1). Consequently, the number of mates should be low and their quality should strongly influence a male's lifetime reproductive success. In this highly competitive context, males exhibit different mating preferences according to their own competitive ability. While large and competitive males can afford to select high-quality females, weak competitors seem to reduce local competition, primarily by focusing on less fecund, and also less attractive, females that represent their only chance to mate with virgin females. In accordance with this hypothesis, we found that under high competition, the risk of takeover was higher near large females because they attracted more males than did small ones. Moreover, despite the large size advantage of males engaged in dyads (Bel-Venner & Venner 2006), mating success of a male was independent of his size, suggesting that smaller males, by focusing on less attractive females, get as many mating opportunities as large males.
Since competition between males is commonly considered one major determinant of males' lifetime reproductive success (Andersson 1994), investigations on male mate choice have long been neglected. However, male choosiness for mates is now well documented in a number of conventional sex-role species (Lawrence 1986; Fairbairn 1988; Verrell 1989; Amundsen & Forsgren 2003; Mathews 2003; Shine et al. 2003; Wong & Jennions 2003; Basolo 2004; Preston et al. 2005). Among theoretical and empirical studies that explored male mate choice, most concluded one of the two alternatives: either males mate indiscriminately or they select high-quality females. Such a mating preference should strongly increase variability in the level of local mate competition between males, with competition peaking for the highest quality, most attractive females. Our study, in accordance with theoretical work (Fawcett & Johnstone 2003), suggests that this heterogeneous local competition could favour distinct mating preferences among males according to their own competitive ability. Low-quality males could avoid the most attractive females and mate with lower quality ones, thus locally reducing competition and increasing their chances of mating successfully. A laboratory experiment revealing distinct mating preferences among males of different sizes in a cichlid fish also supports this hypothesis (Basolo 2004).
Our study confirms that within-sex competition and choosiness for mates can co-occur. It also suggests that a competition-driven decrease in breeding opportunities for males not only increases their choosiness but also leads to differential mating preferences among males depending on their competitive ability. This mechanism, which could be widespread yet still largely ignored, should influence the evolution of sexual traits (Andersson & Simmons 2006), and could take part in sympatric speciation that may imply assortative pairing (Dieckmann & Doebeli 1999; McKinnon et al. 2004). Competition between females in conventional sex-role species has received little attention; however, recent field studies revealed that it could strongly affect females' breeding opportunities, as well as the quality of their mates (Bro-Jørgensen 2002; Clutton-Brock et al. 2006). Similarly, our fieldwork shows the need for considering male choosiness for mates, especially in the context of high competition, as well as inter-individual variations in male mating preferences.
Acknowledgments
We thank T. H. Clutton-Brock, E. Desouhant, A. Pasquet, R. Leborgne and two anonymous reviewers for their helpful comments on earlier drafts of this manuscript. Financial support was received from CNRS (France).
Supplementary Material
We show additional results showing how size-assortative pairing between a female and her first guardian unambiguously reflects his decision
References
- Amundsen T. Why are female birds ornamented? Trends Ecol. Evol. 2000;15:149–155. doi: 10.1016/s0169-5347(99)01800-5. doi:10.1016/S0169-5347(99)01800-5 [DOI] [PubMed] [Google Scholar]
- Amundsen T, Forsgren E. Male preference for colourful females affected by male size in a marine fish. Behav. Ecol. Sociobiol. 2003;54:55–64. doi:10.1007/s00265-003-0593-4 [Google Scholar]
- Andersson M. Princeton University Press; Princeton, NJ: 1994. Sexual selection. [Google Scholar]
- Andersson M, Simmons L.W. Sexual selection and mate choice. Trends Ecol. Evol. 2006;21:296–302. doi: 10.1016/j.tree.2006.03.015. doi:10.1016/j.tree.2006.03.015 [DOI] [PubMed] [Google Scholar]
- Austad S. Evolution of sperm priority patterns in spiders. In: Smith R.L, editor. Sperm competition and the evolution of animal mating systems. Academic Press; San Diego, CA: 1984. pp. 223–249. [Google Scholar]
- Basolo A.L. Variation between and within the sexes in body size preferences. Anim. Behav. 2004;68:75–82. doi:10.1016/j.anbehav.2003.07.019 [Google Scholar]
- Bel-Venner M.C, Venner S. Mate-guarding strategies and male competitive ability in an orb-weaving spider: results from a field study. Anim. Behav. 2006;71:1315–1322. doi:10.1016/j.anbehav.2005.08.010 [Google Scholar]
- Berglund A. The operational sex ratio influences choosiness in a pipefish. Behav. Ecol. 1994;5:254–258. doi:10.1093/beheco/5.3.254 [Google Scholar]
- Bonduriansky R. The evolution of male mate choice in insects: a synthesis of ideas and evidence. Biol. Rev. 2001;76:305–339. doi: 10.1017/s1464793101005693. doi:10.1017/S1464793101005693 [DOI] [PubMed] [Google Scholar]
- Bro-Jørgensen J. Overt female mate competition and preference for central males in a lekking antelope. Proc. Natl Acad. Sci. USA. 2002;99:9290–9293. doi: 10.1073/pnas.142125899. doi:10.1073/pnas.142125899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burley N. Parental investment, mate choice and mate quality. Proc. Natl Acad. Sci. USA. 1977;74:3476–3479. doi: 10.1073/pnas.74.8.3476. doi:10.1073/pnas.74.8.3476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock T.H, Vincent A.C.J. Sexual selection and the potential reproductive rates of males and females. Nature. 1991;351:58–59. doi: 10.1038/351058a0. doi:10.1038/351058a0 [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T.H, Hodge S.J, Spong G, Russell A.F, Jordan N.R, Bennett N.C, Sharpe L.L, Manser M.B. Intrasexual competition and sexual selection in cooperative mammals. Nature. 2006;444:1065–1068. doi: 10.1038/nature05386. doi:10.1038/nature05386 [DOI] [PubMed] [Google Scholar]
- Crespi B.J. Causes of assortative mating in arthropods. Anim. Behav. 1989;38:980–1000. doi:10.1016/S0003-3472(89)80138-1 [Google Scholar]
- Crowley P.H, Travers S.E, Linton M.C, Cohn S.L, Sih A, Sargent R.C. Mate density, predation risk, and the seasonal sequence of mate choices: a dynamic game. Am. Nat. 1991;137:567–596. doi:10.1086/285184 [Google Scholar]
- Cunningham E.J.A, Birkhead T.R. Sex roles and sexual selection. Anim. Behav. 1998;56:1311–1321. doi: 10.1006/anbe.1998.0953. doi:10.1006/anbe.1998.0953 [DOI] [PubMed] [Google Scholar]
- Darwin C. Murray; London, UK: 1871. The descent of man and selection in relation to sex. [Google Scholar]
- Dieckmann U, Doebeli M. On the origin of species by sympatric speciation. Nature. 1999;400:354–357. doi: 10.1038/22521. doi:10.1038/22521 [DOI] [PubMed] [Google Scholar]
- Dunn D.W, Crean C.S, Gilburn A.S. Male mating preference for female survivorship in the seaweed fly Gluma musgravei (Diptera: Coelopidae) Proc. R. Soc. B. 2001;268:1255–1258. doi: 10.1098/rspb.2001.1642. doi:10.1098/rspb.2001.1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emlen S.T, Oring L.W. Ecology, sexual selection and the evolution of mating systems. Science. 1977;197:215–223. doi: 10.1126/science.327542. doi:10.1126/science.327542 [DOI] [PubMed] [Google Scholar]
- Fairbairn D.J. Sexual selection for homogamy in the Gerridae: an extension of Ridley's comparative approach. Evolution. 1988;42:1212–1222. doi: 10.1111/j.1558-5646.1988.tb04181.x. doi:10.2307/2409005 [DOI] [PubMed] [Google Scholar]
- Fawcett T.W, Johnstone R.A. Mate choice in the face of costly competition. Behav. Ecol. 2003;14:771–779. doi:10.1093/beheco/arg075 [Google Scholar]
- Forsgren E, Amundsen T, Borg A.A, Bjelvenmark J. Unusually dynamic sex roles in a fish. Nature. 2004;429:551–554. doi: 10.1038/nature02562. doi:10.1038/nature02562 [DOI] [PubMed] [Google Scholar]
- Gwynne D.T. Sexual difference theory: Mormon crickets show role-reversal in mate choice. Science. 1981;213:779–780. doi: 10.1126/science.213.4509.779. doi:10.1126/science.213.4509.779 [DOI] [PubMed] [Google Scholar]
- Harari A, Handler A, Landolt P. Size-assortative mating, male choice and female choice in the curculionid beetle Diaprepes abbreviatus. Anim. Behav. 1999;58:1191–1200. doi: 10.1006/anbe.1999.1257. doi:10.1006/anbe.1999.1257 [DOI] [PubMed] [Google Scholar]
- Hoefler C.D. Male mate choice and size-assortative pairing in a jumping spider, Phidippus clarus. Anim. Behav. 2007;73:943–954. doi:10.1016/j.anbehav.2006.10.017 [Google Scholar]
- Huber B.A. Sexual selection research on spiders: progress and biases. Biol. Rev. 2005;80:363–385. doi: 10.1017/s1464793104006700. doi:10.1017/S1464793104006700 [DOI] [PubMed] [Google Scholar]
- Hume K.D, Elwood R.W, Dick J.T.A, Connaghan K.M. Size-assortative pairing in Gammarus pulex (Crustacea: Amphipoda): a test of the timing hypothesis. Anim. Behav. 2002;2:239–244. doi:10.1006/anbe.2002.3045 [Google Scholar]
- Jennions M.D, Petrie M. Variation in male choice and mating preferences: a review of causes and consequences. Biol. Rev. 1997;72:283–327. doi: 10.1017/s0006323196005014. doi:10.1017/S0006323196005014 [DOI] [PubMed] [Google Scholar]
- Johnstone R.A. The tactics of mutual mate choice and competitive search. Behav. Ecol. Sociobiol. 1997;40:51–59. doi:10.1007/s002650050315 [Google Scholar]
- Johnstone R.A, Reynolds J.D, Deutsch J.C. Mutual mate choice and sex differences in choosiness. Evolution. 1996;50:1382–1391. doi: 10.1111/j.1558-5646.1996.tb03912.x. doi:10.2307/2410876 [DOI] [PubMed] [Google Scholar]
- Jormalainen V, Merilaita S, Tuomi J. Mate choice and male–male competition in Idotea baltica (Crustacea, Isopoda) Ethology. 1994;96:46–57. [Google Scholar]
- Kokko H, Johnstone R.A. Why is mutual mate choice not the norm? Operational sex ratios, sex roles and the evolution of sexually dimorphic and monomorphic signalling. Phil. Trans. R. Soc. B. 2002;357:319–330. doi: 10.1098/rstb.2001.0926. doi:10.1098/rstb.2001.0926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokko H, Monaghan P. Predicting the direction of sexual selection. Ecol. Lett. 2001;4:159–165. doi:10.1046/j.1461-0248.2001.00212.x [Google Scholar]
- Kolm N. Male size determines reproductive output in a paternal mouthbrooding fish. Anim. Behav. 2002;63:727–733. doi:10.1006/anbe.2001.1959 [Google Scholar]
- Komdeur J. Mate guarding in the Seychelles Warbler is energetically costly and adjusted to paternity risk. Proc. R. Soc. B. 2001;268:2103–2111. doi: 10.1098/rspb.2001.1750. doi:10.1098/rspb.2001.1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvarnemo C, Simmons L.W. Male potential reproductive rate influences mate choice in a bushcricket. Anim. Behav. 1998;55:1499–1506. doi: 10.1006/anbe.1998.0732. doi:10.1007/s002650000246 [DOI] [PubMed] [Google Scholar]
- Lawrence W.S. Male choice and competition in Tetraopes tetraophthalmus: effects of local sex ratio variation. Behav. Ecol. Sociobiol. 1986;18:289–296. doi:10.1007/BF00300006 [Google Scholar]
- Low M. The energetic cost of mate guarding is correlated with territorial intrusions in the New Zealand stitchbird. Behav. Ecol. 2006;17:270–276. doi:10.1093/beheco/arj025 [Google Scholar]
- Mathews L.M. Tests of the mate-guarding hypothesis for social monogamy: male snapping shrimp prefer to associate with high-value females. Behav. Ecol. 2003;14:63–67. doi:10.1093/beheco/14.1.63 [Google Scholar]
- McKinnon J.S, Mori S, Blackman B.K, David L, Kingsley D.M, Jamieson L, Chou J, Schluter D. Evidence for ecology's role in speciation. Nature. 2004;429:294–298. doi: 10.1038/nature02556. doi:10.1038/nature02556 [DOI] [PubMed] [Google Scholar]
- Nyman A, Kvarnemo C, Svensson O. The capacity for additional matings does not affect male mating competition in the sand goby. Anim. Behav. 2006;71:865–870. doi:10.1016/j.anbehav.2005.06.020 [Google Scholar]
- Olsson M. Male preference for large females and assortative mating for body size in sand lizard (Lacerta agilis) Behav. Ecol. Sociobiol. 1993;32:337–341. doi:10.1007/BF00183789 [Google Scholar]
- Parker G.A. Mate quality and mating decisions. In: Bateson P, editor. Mate choice. Cambridge University Press; Cambridge, UK: 1983. pp. 141–166. [Google Scholar]
- Plaistow S, Bollache L, Cézilly F. Energetically costly precopulatory mate guarding in the amphipod Gammarus pulex: causes and consequences. Anim. Behav. 2003;65:683–691. doi:10.1006/anbe.2003.2116 [Google Scholar]
- Preston B, Stevenson I, Pemberton J, Coltman D, Wilson K. Male mate choice influences female promiscuity in Soay sheep. Proc. R. Soc. B. 2005;272:365–373. doi: 10.1098/rspb.2004.2977. doi:10.1098/rspb.2004.2977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptacek M.B, Travis J. Mate choice in the Sailfin Molly Poecilia latipinna. Evolution. 1997;51:1217–1231. doi: 10.1111/j.1558-5646.1997.tb03969.x. doi:10.2307/2411051 [DOI] [PubMed] [Google Scholar]
- Rowe L, Arnqvist G. Analysis of the causal components of assortative mating in water striders. Behav. Ecol. Sociobiol. 1996;38:279–286. doi:10.1007/s002650050243 [Google Scholar]
- Schwagmeyer P.L, Parker G.A. Male mate choice as predicted by sperm competition in thirteen-lined ground squirrels. Nature. 1990;348:62–64. doi:10.1038/348062a0 [Google Scholar]
- Segoli M, Harari A.R, Lubin Y. Limited mating opportunities and male monogamy: a field study of white widow spiders, Latrodectus pallidus (Theridiidae) Anim. Behav. 2006;72:635–642. doi:10.1016/j.anbehav.2005.11.021 [Google Scholar]
- Shine R, Phillips B, Waye H, LeMaster M, Mason R. The lexicon of love: what cues cause size-assortative courtship by male garter snakes? Behav. Ecol. Sociobiol. 2003;53:234–237. doi:10.1007/s00265-002-0568-x [Google Scholar]
- Shuster S.M, Wade M.J. Princeton University Press; Princeton, NJ: 2003. Mating systems and strategies. [Google Scholar]
- Trivers R. Parental investment and sexual selection. In: Campbell B, editor. Sexual selection and the descent of man. Aldine; Chicago, IL: 1972. pp. 136–179. [Google Scholar]
- Verrell P.A. Male mate choice for fecund females in a plethodontid salamander. Anim. Behav. 1989;38:1086–1088. doi:10.1016/S0003-3472(89)80150-2 [Google Scholar]
- Wong B.B.M, Jennions M.D. Costs influence male mate choice in a freshwater fish. Proc. R. Soc. B. 2003;270:S36–S38. doi: 10.1098/rsbl.2003.0003. doi:10.1098/rsbl.2003.0003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
We show additional results showing how size-assortative pairing between a female and her first guardian unambiguously reflects his decision