Abstract
We compared life-history traits and extinction risk of chondrichthyans (sharks, rays and chimaeras), a group of high conservation concern, from the three major marine habitats (continental shelves, open ocean and deep sea), controlling for phylogenetic correlation. Deep-water chondrichthyans had a higher age at maturity and longevity, and a lower growth completion rate than shallow-water species. The average fishing mortality needed to drive a deep-water chondrichthyan species to extinction (Fextinct) was 38–58% of that estimated for oceanic and continental shelf species, respectively. Mean values of Fextinct were 0.149, 0.250 and 0.368 for deep-water, oceanic and continental shelf species, respectively. Reproductive mode was an important determinant of extinction risk, while body size had a weak effect on extinction risk. As extinction risk was highly correlated with phylogeny, the loss of species will be accompanied by a loss of phylogenetic diversity. Conservation priority should not be restricted to large species, as is usually suggested, since many small species, like those inhabiting the deep ocean, are also highly vulnerable to extinction. Fishing mortality of deep-water chondrichthyans already exploited should be minimized, and new deep-water fisheries affecting chondrichthyans should be prevented.
Keywords: deep sea, Chondrichthyes, elasmobranch, conservation, shark fisheries
1. Introduction
The extinction risk of a species is strongly related to its life-history traits (Hutchings 2002). Species with low productivity, i.e. small litters, slow growth rates, late sexual maturity and long interbirth interval, are less able to compensate for increased mortality and are therefore more vulnerable to extinction (MacArthur & Wilson 1967). Life histories are shaped by the energy available in the environment for allocation to different biological processes and by the interactions that influence allocation decisions. The energy available for allocation depends ultimately on the primary productivity of the ecosystem (Arendt & Reznick 2005), while the strength of biological interactions depends on many ecosystem characteristics, including physical structure (Gotceitas & Colgan 1989; Almany 2004) and diversity (Sinclair et al. 2003; Duffy & Stachowicz 2006).
Three major marine environments can be distinguished in the oceans of the world: continental shelves, the open ocean and the deep sea (Briggs 1974; Helfman et al. 1997; Jones et al. 2002). The continental shelves extend from the shoreline to 200 m depth, include both pelagic and benthic habitats, and they are highly productive, variable and structured environments, comprising many different types of ecosystems (Jones et al. 2002). The open ocean extends, beyond the limit of the continental shelves, from the surface to 200 m depth. It is mostly devoid of refuges as it is transparent and bottomless, includes only pelagic habitats and its primary production is very low and highly patchy, with few places where most of the primary production occurs (Helfman et al. 1997; Jones et al. 2002). The deep sea extends from the limits of the continental shelves and the open ocean at 200 m depth to the maximum depths of the sea. It is a permanently dark and cold environment that depends on imports of organic matter from the other two ecosystems due to its null primary productivity (excepting hydrothermal vent communities; Gage & Tyler 1991; Robison 2004). Given these differences, some generalities regarding life-history traits of fishes living in these environments have been reported.
When compared with shallow-water fishes, deep-water fishes tend to have slower growth rate, later sexual maturity (Clarke et al. 2003), longer lifespan (Cailliet et al. 2001) and lower metabolic rates (Childress et al. 1980; Childress & Somero 1990), which result in longer turnover times, i.e. less productive populations (Merrett & Haedrich 1997). Predation (Childress 1995) and food availability hypotheses (Gordon et al. 1995) were proposed to explain this pattern. A high predation rate selects for a higher metabolism and fast turnover rates in the open ocean, a transparent and refuge-less environment (Childress & Somero 1990). In turn, as a result of more refuge opportunities and an increase in turbidity, predation pressure is relaxed in the shallow habitats of continental shelves, producing more variable life histories. In the deep sea, the predation pressure is even more relaxed since darkness limits the hunting abilities of visual predators. This relaxation makes possible the appearance of lower turnover rates, relying on low metabolic rates (Childress et al. 1980, 1990; Childress & Somero 1990), which might also be selected due to the low food availability of this habitat (Gordon et al. 1995). As a consequence of their life history, fishes living in these different environments vary in their capacity to compensate for increased mortality. Deep-water bony fishes are remarkably unproductive and highly vulnerable to overfishing (Koslow et al. 2000).
Cartilaginous fishes are even less productive and inherently more vulnerable to extinction than bony fishes (Myers & Worm 2005). If the low productivity of deep-water bony fishes can be applied to chondrichthyans, then deep-water chondrichthyans should be more sensitive to increased mortality than their shallow-water counterparts (Camhi et al. 1998; Musick et al. 2000; Stevens et al. 2000). Recent estimations of age and growth of deep-water chondrichthyans (e.g. Machado & Figueiredo 2000; Clarke et al. 2002; Sion et al. 2002; Moura et al. 2004; Irvine et al. 2006) revealed low turnover rates and also substantial variability in age at maturity and longevity among them, suggesting that their life histories may also be variable. To distinguish any life-history strategy common to deep water, which may predispose them to human-induced extinction, it is necessary to use appropriate statistical analyses.
Deep-water fisheries are seen as alternatives to already exhausted shallow-water fisheries (Moore 1999; Morato et al. 2006). Chondrichthyans affected by deep-water fisheries are usually easily overexploited. After only 20 years of fishery exploitation, dramatic declines are evident in once-common deep-water chondrichthyans off southeast Australia (Graham et al. 2001) and in the deep-water skate Bathyraja spinicauda from the North Atlantic (Devine et al. 2006). An accurate assessment of extinction risk of deep-water chondrichthyans relative to shallow-water species will have an applied importance to assess the future prospects of deep-water fisheries catching chondrichthyans.
In this paper we first test the hypothesis that deep-water chondrichthyans have a longer turnover time (i.e. individuals reproduce at a slow pace making their populations grow or recovery slow) than shallow-water chondrichthyans, by comparing the growth completion rate, age at maturity and longevity of chondrichthyans from continental shelves, the open ocean and the deep sea. Second, we quantify the effect of the habitat and of life-history traits (body size and reproductive mode) in determining the extinction risk of sharks, rays and chimaeras. Importantly, we tested these hypotheses using comparative analysis techniques that control for the non-independence of species that results from their common phylogenetic history.
2. Material and methods
(a) Data sources
Data on maximum body size, smax; size at maturity, smat; longevity (maximum observed age), amax; age at maturity, amat; growth completion rate from the von Bertalanffy growth model, k; litter size, l; interbirth interval, i; reproductive mode, mrep; and habitat were obtained from 127 populations of 105 species (table S1 in the electronic supplementary material). For species with all parameters available for more than one population, the median value of each parameter was used. The size and age at which 50% of individuals are sexually mature and average fecundity were the preferred measurements of smat, amat and l, respectively. If these values were not available, the middle point of the minimum and maximum values given for each variable was used. Reproductive mode was categorized as oviparous or viviparous, with viviparity further classified into its variants according to whether the embryos are nourished by unfertilized eggs (oophagy), other embryos (adelphophagy), lipid-rich maternal secretion (histotrophy), placental connections (placentotrophy) or yolk from their yolk sac without any further maternal supply (lecithotrophy; Wourms 1981). For each species, habitat was categorized as oceanic, shelf or deep water according to Bigelow & Schroeder (1953), Last & Stevens (1994), Menni & Stehmann (2000), Ebert (2003) and Compagno et al. (1989, 2005).
We used the fishing mortality necessary to drive a species to extinction (Fextinct) as a measure of extinction risk (Mace 1994; Myers & Mertz 1998). Following Myers & Mertz (1998), Fextinct was calculated iteratively from the following equation:
where is the annual reproductive rate corrected by embryonic sex ratio (i.e. =l/i×0.5); asel is the age at which fishes enter the fishery; and M is natural mortality. When asel is set equal to 1 Fextinct is equivalent to the maximum intrinsic rate of population increase (rmax), which is a standard measurement of population productivity and extinction risk (Dulvy et al. 2004). An asel of 1 year was used in this study since most chondrichthyan species are large enough to be caught by age 1, either as target or by-catch. Fextinct was also calculated with asel set equal to 0 (i.e. fishing starts at birth) and amat (i.e. fishing starts at age at maturity) in order to test the sensitivity of the models (see below) to extreme values of asel. Direct estimates of M are available for only five species of chondrichthyans, so two indirect approaches (Hoenig (1983); Jensen (1996)) were initially tested. As values of Fextinct using either approach were highly correlated (r=0.995) and did not differ (slope =1.008), we used Jensen's (1996) estimation (M=1.65/amat).
(b) Data analysis
A correlogram analysis on Moran's I index of autocorrelation was performed to assess the importance of phylogeny on our response variables such as age at maturity, growth completion rate, longevity and Fextinct (Gittleman & Kot 1990). This analysis reveals how the phylogenetic correlation of each life-history trait and the extinction risk was distributed among taxonomic levels.
Since a phylogenetic tree that includes all the chondrichthyan species considered in our analysis is not available, we used two approaches to control for the phylogenetic correlation among species. First, we used the taxonomic classification as a proxy for phylogenetic relatedness according to the Compagno's (1999) classification. The taxonomic arrangement (species nested in genera, genera in families, families in orders, orders in cohorts and cohorts in subclasses) was included as a random effect in a mixed-effect linear model (herein called the taxonomy model) in order to correct for any phylogenetic effect reflected in the taxonomy. A stepwise model selection procedure based on minimization of Akaike's information criterion was conducted on the full model to find the best minimum adequate model.
Second, we fitted a model using generalized estimating equations (GEEs; Venables & Ripley 2002). This approach takes into account the phylogenetic correlation in life-history traits among species by constructing a correlation matrix using a phylogenetic tree (Paradis & Claude 2002). GEE models (herein called the phylogeny model) allow for the inclusion of multiple discrete or continuous variables, which cannot be done using other methods for controlling phylogenetic correlation (Paradis & Claude 2002). The tree for the 105 species considered here was built from topologies taken from different studies (figure S1 in the electronic supplementary material).
To test for differences in turnover time among deep-water, continental shelf and oceanic chondrichthyans, age at maturity, longevity and growth completion rates were included as dependent variables in the taxonomy and phylogeny models, with size at maturity and habitat as independent variables.
To determine the importance of habitat and life-history traits in determining extinction risk, we built a taxonomy model with size at maturity, reproductive mode and habitat as fixed effects, and log(Fextinct) as the response variable. These independent variables were also included in a phylogeny model, with Fextinct as the response variable. A Gamma error distribution was specified for the phylogeny model due to the multiplicative structure and non-constant variance of the model (Firth 1988); and a log link was used because the response variable takes only positive values. An analysis of condition indices and variance decomposition proportions (Belsley et al. 1980) revealed no multicollinearity among the independent variables (all condition indices were ≪10).
To identify the most vulnerable to extinction chondrichthyan orders, we ran a GEE model with Fextinct as the response variable and the factor order as the independent variable. The orders Orectolobiformes, Hexanchiformes and Pristiformes were grouped because data for each of them were restricted to only one species. This was preferred over removing these species from the analysis in order to maintain the same correlation structure as in the other models of Fextinct.
All models were also run with maximum size instead of size at maturity, which were not included simultaneously owing to their very high correlation (r=0.98). We also reran the models with a correction for the litter size in oviparous species since the egg stage adds an extra mortality in these individuals. We applied a 24% reduction to the litter size of oviparous species, according to the predation rate that eggs experience from their main predators, gastropod borers (Lucifora & García 2004).
All analyses were carried out using the R statistical software, v. 2.3.0 (R Development Core Team 2006). Taxonomy models were built using the lme function in the R package nlme (Venables & Ripley 2002), and correlograms and phylogeny models using the functions correlogram and compar.gee, respectively, in the ape package (Paradis 2006). Codes for analyses are given in the electronic supplementary material.
3. Results
(a) Phylogenetic correlation patterns
The response variables differed in the degree of correlation with phylogeny (figure S2 in the electronic supplementary material). Age at maturity was significantly and positively correlated at the lowest taxonomic ranks (genus, family and order) followed by a lack of correlation or a slight negative correlation at higher levels. Growth completion rate was significantly and positively correlated at the genus level, but no correlation was found at higher levels, except for a very low correlation (Moran's I=−0.048, p=0.0475) at the cohort level (figure S2 in the electronic supplementary material). The phylogenetic correlation of longevity was higher at the order level and decreased in higher taxonomic ranks (figure S2 in the electronic supplementary material).
There was a significant positive phylogenetic correlation of extinction risk within genera, families and orders and a negative phylogenetic correlation at the cohort and subclass levels (figure S2 in the electronic supplementary material). That is, species in the same genus, genera in the same family and families in the same order tend to have similar values of Fextinct. By contrast, orders of the same cohort and cohorts of the same subclass tended to differ in Fextinct, though with a lower correlation.
(b) Differences in turnover time among habitats
Deep-water chondrichthyans grow more slowly, mature later and live longer than continental shelf or oceanic chondrichthyans (figure S3 in the electronic supplementary material). Depending on the model, the growth completion rate in deep-water chondrichthyans was 46–60% and 55–63% of oceanic and continental shelf species, respectively (table 1). Age at maturity was 2.39–2.60 and 1.61–2.07 times higher in deep-water chondrichthyans than in oceanic and continental shelf species, respectively. Longevity was 1.00–1.75 times higher in deep-water species than in oceanic and continental shelf species, but only significantly different in the taxonomy model (table 1).
Table 1.
Coefficients (standard error within brackets) of linear models relating age at maturity, longevity and growth completion rate with habitat and body size. Habitat coefficients are relative to deep-sea species. The taxonomy model is a mixed-effects linear model with the taxonomic hierarchy included as a random effect, and the phylogeny model is a generalized estimating equation model with a log link which corrects for phylogenetic correlation using a phylogenetic tree; *p≤0.05, **p≤0.01, ***p≤0.001.
| age at maturity | longevity | growth completion rate | ||||
|---|---|---|---|---|---|---|
| variables | taxonomy | phylogeny | taxonomy | phylogeny | taxonomy | phylogeny |
| intercept | 2.289 (0.176)*** | 1.627 (0.330)*** | 3.103 (0.136)*** | 2.510 (0.301)*** | −2.430 (0.227)*** | −1.633 (0.374)*** |
| habitat (oceanic) | −0.955 (0.250)*** | −0.873 (0.142)*** | −0.561 (0.210)** | −0.007 (0.130) | 0.776 (0.287)** | 0.504 (0.161)** |
| (shelf) | −0.729 (0.180)*** | −0.478 (0.135)** | −0.562 (0.141)*** | −0.005 (0.123) | 0.671 (0.190)*** | 0.362 (0.153)* |
| size at maturity | 0.003 (<0.001)*** | 0.006 (<0.001)*** | 0.002 (<0.001)*** | 0.005 (<0.001)*** | −0.003 (<0.001)*** | −0.004 (<0.001)*** |
(c) Determinants of extinction risk
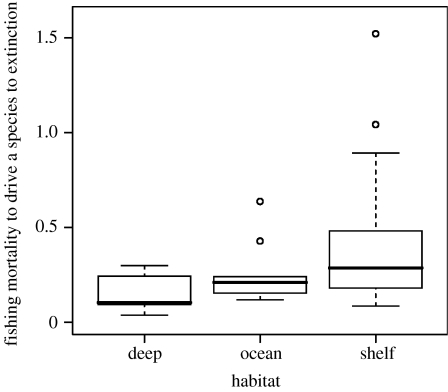
The mean value of Fextinct was 0.149, 0.250 and 0.368 for deep-water, oceanic and continental shelf species, respectively. Both taxonomy and phylogeny models showed that the extinction risk was significantly associated with habitat and reproductive modes. Maturity size was not included in the best taxonomy model and had a weak effect in the phylogeny model. Deep-water chondrichthyans were 1.6–1.9 and 2.4–2.9 times more vulnerable to extinction than continental shelf and oceanic chondrichthyans, respectively (figure 1). Oviparity and lecithotrophic viviparity were associated with a lower extinction risk (table 2). In spite of the small effect of size at maturity on the extinction risk, the large variation in body size of chondrichthyans (20–10 000 cm) results in a substantial effect of body size at the extremes of the range (table 2). In all models, similar results were obtained when maximum size was used instead of size at maturity and after applying the mortality correction to the litter size of oviparous species.
Figure 1.
Fextinct (the fishing mortality needed to drive a species to extinction) for sharks, rays and chimaeras (class Chondrichthyes) of the three main marine habitats. Bold line, median; box, interquartile range; whiskers, range (excluding outliers) and open circles, outliers.
Table 2.
Coefficients (standard error within brackets) of linear models relating the fishing mortality required to drive a population to extinction (Fextinct) with habitat and life-history traits. (Habitat and reproductive mode coefficients are relative to deep-sea species and adelphophagic species, respectively. *p≤0.05, **p≤0.01, ***p≤0.001.)
| variables | taxonomy | phylogeny |
|---|---|---|
| intercept | −3.108 (0.532)*** | −2.479 (0.559)** |
| habitat (oceanic) | 0.863 (0.241)*** | 1.050 (0.160)*** |
| (shelf) | 0.640 (0.179)*** | 0.467 (0.154)* |
| size at maturity | — | −0.003 (<0.001)*** |
| reproductive mode (histotrophic) | 0.765 (0.533) | 0.455 (0.577) |
| (lecitotrophic) | 1.048 (0.517)* | 1.208 (0.477)* |
| (oophagic) | 0.419 (0.573) | 0.379 (0.406) |
| (placental) | 1.318 (0.527)* | 1.177 (0.483) |
| (oviparous) | 1.899 (0.531)*** | 1.629 (0.505)** |
Extinction risk differed among orders with Squaliformes and Lamniformes being the most vulnerable to extinction (p=0.028 and 0.024, respectively) and Rajiformes (p=0.027) the least vulnerable. When compared with Rajiformes, Squaliformes and Lamniformes were 4.73 and 4.45 times more vulnerable, respectively.
Similar qualitative results were obtained when Fextinct was calculated with asel set equal to 0 or amat (tables S2 and S3 in the electronic supplementary material).
4. Discussion
Regardless of the model used, our results indicate that the life-history traits and the extinction risk of chondrichthyans are highly associated with habitat. Deep-water chondrichthyans have longer turnover times (i.e. slower growth, later age at maturity and higher longevity) and, as a consequence, higher extinction risk than oceanic and continental shelf chondrichthyans. Also, chondrichthyans tend to have a higher extinction risk if they are matrotrophically viviparous (i.e. embryos are nourished by their mothers during development) and, less importantly, have large body size. As extinction risk, as well as age at maturity, was highly correlated with phylogeny the loss of species will be accompanied with a loss of phylogenetic diversity.
Extinction risk was significantly affected by reproductive mode; it was lowest for oviparity, increased with lecithotrophic viviparity and was highest in adelphophagic, oophagic, histotrophic and placental viviparity. This sequence implies that non-matrotrophic modes have the lowest extinction risk. Matrotrophy increases the energetic cost of reproduction to females and makes them less able to reproduce more often or have larger litters than non-matrotrophic modes (Wourms & Lombardi 1992). Thus, an increased reproductive output by non-matrotrophic females results in a lower extinction risk at the population level.
Our estimate indicates that an average fishing mortality approximately 58 and 38% of that applied to continental shelf and oceanic species, respectively, is sufficient to drive deep-sea chondrichthyans to extinction. Remarkably, the pattern is apparent without incorporating the putatively less productive deep-water chondrichthyans in the analysis (i.e. sleeper sharks Somniosus spp., bramble sharks Echinorhinus spp., sixgill shark Hexanchus griseus, bigeye sand tiger shark Odontaspis noronhai, goblin shark Mitsukurina owstoni, false catshark Pseudotriakis microdon, some longnose skates Dipturus spp. and giant stingaree Plesiobatis daviesi) for which no data on age at maturity are available (Kyne et al. 2006).
We acknowledge that life-history variation among species within the same major marine habitat may exist, as documented for other taxa. Mesopelagic, bathypelagic and seamount-associated fishes differ in their growth, maturity and longevity (Childress et al. 1980) and a similar situation may occur between benthic and pelagic continental shelf fishes. Future information on chondrichthyan life history will make possible to group species in a more fine-scale habitat categorization representing the wide range of chondrichthyan ecological variation (Compagno 1990).
The evidence linking body size to extinction risk in cartilaginous fishes is contradictory. Smith et al. (1998) estimated the rebound potential (a measure of resilience to exploitation) of 28 shallow-water Pacific sharks. They found that there was a continuum of resilience with age at maturity and body size, with the earliest-maturing and smallest species being more resilient than late-maturing and larger ones. Dulvy et al. (2000) studied changes in species abundance and species composition of skate communities of the Northeast Atlantic after prolonged exploitation and found that large species tended to decrease in abundance and to be replaced by smaller species. Finally, Dulvy & Reynolds (2002) showed that among body size, latitudinal and depth range, only body size predicted extinction risk in skates. By contrast, Cortés (2002) found no correlation between the intrinsic rate of increase and body size for 38 species of shallow-water sharks. Frisk et al. (2001) calculated the intrinsic rate of increase for 34 species of shallow-water sharks and skates, and found that body size was only loosely correlated to it (r2=0.17). These contrasting results regarding the relationship between body size and extinction risk are probably due to the correlation between body size and many other, more meaningful, life-history traits, such as age at maturity, litter size or longevity (Blueweiss et al. 1978; Purvis et al. 2000). Our results suggest that body size has a slight effect on extinction risk (probably due to a correlation with age at maturity) that may become apparent at the extremes of the body size range.
Our analysis identified the clades containing the order Lamniformes (mackerel sharks) and Squaliformes (dogfishes) as the most extinction-prone groups. Mackerel sharks comprise some of the largest chondrichthyans and some of the species most seriously threatened with extinction, such as the sand tiger shark Carcharias taurus, the basking shark Cetorhinus maximus, the porbeagle Lamna nasus and the white shark Carcharodon carcharias (Compagno et al. 2005). Dogfishes are generally small species (excluding sleeper sharks Somniosus spp.) that inhabit almost exclusively deep-sea ecosystems, the habitat associated with the highest extinction risk. Management and conservation measurements are usually set based on indirect proxies of extinction risk like body size, when information on life-history traits or population trends are unavailable (Reynolds et al. 2005). Nevertheless, we found vulnerable species at both extremes of the size continuum, from the big mackerel sharks to the small dogfishes. Focusing conservation efforts only on large species will leave highly vulnerable species without any protection. Given their high extinction risk, we recommend that all deep-water chondrichthyans should be given high conservation priority regardless of its size.
Deep-sea fisheries are expanding rapidly and the very low levels of fishing mortality needed to drive deep-sea chondrichthyans to extinction may have already been reached in some areas (Graham et al. 2001; Devine et al. 2006; Morato et al. 2006). In addition, deep-sea fisheries appear to have already reached the maximum depths attainable by chondrichthyans, leaving them without any depth refuges (Priede et al. 2006). Minimizing fishing mortality in deep-water habitats already exploited and preventing new deep-water ecosystems to be exploited are necessary to avoid the extinction of these species.
Acknowledgments
This study is part of the Pew Global Shark Assessment, supported by a grant from the Pew Institute for Ocean Science, and the Future of Marine Animal Populations project of the Sloan Census of Marine Life. We thank Wade Blanchard for statistical advice, and Julia Baum, Stephanie Boudreau, Daniel Boyce, Katherine Dunn, Roberto Menni, Coilin Minto and four anonymous reviewers for their comments and suggestions that greatly improved the paper. We dedicate this paper to the memory of Ransom Myers. His joy, generosity and encouragement are deeply missed.
Supplementary Material
Data with sources and code used for building the phylogenetic tree and data analysis
Phylogenetic tree of Chondrichthyes (sharks, rays and chimaeras) used in the generalized estimating equation model to control for phylogenetic correlation. The tree is a composite from published partial trees (see references for phylogenetic relationships)
Correlogram of normalized Moran's I autocorrelation index of age at maturity, growth completion rate (von Bertalanffy's k), longevity and extinction risk (Fextinct) for taxonomic groups of the class Chondrichthyes (sharks, rays and chimaeras). Filled and empty circles represent significant (p<0.05) and non significant correlations, respectively. G, genus; F, family; O, order; C, cohort and S, subclass
Relationship between age at maturity, growth completion rate and longevity (estimated as maximum observed age) with maximum body size in chondrichthyans from continental shelves (open circles), open ocean (grey circles) and deep sea (black circles). The axis for maximum size has a log scale to improve visualization
References
- Almany G.R. Differential effects of habitat complexity, predators and competitors on abundance of juvenile and adult coral reef fishes. Oecologia. 2004;141:105–113. doi: 10.1007/s00442-004-1617-0. doi:10.1007/s00442-004-1617-0 [DOI] [PubMed] [Google Scholar]
- Arendt J.D, Reznick D.N. Evolution of juvenile growth rates in female guppies (Poecilia reticulata): predator regime or resource level? Proc. R. Soc. B. 2005;272:333–337. doi: 10.1098/rspb.2004.2899. doi:10.1098/rspb.2004.2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsley D.A, Kuh E, Welsch R.E. Wiley; New York, NY: 1980. Regression diagnostics: identifying influential data and sources of collinearity. [Google Scholar]
- Bigelow H.P, Schroeder W.C. Sawfishes, guitarfishes, skates and rays. In: Tee-Van J, Breder C.M, Parr A.E, Schroeder W.C, Schultz L.P, editors. Fishes of the western north Atlantic, part 2. Memoirs of the Sears Foundation for Marine Research; New Haven, CT: 1953. pp. 1–514. [Google Scholar]
- Blueweiss L, Fox H, Kudzma V, Nakashima D, Peters R, Sams S. Relationships between body size and some life history parameters. Oecologia. 1978;37:257–272. doi: 10.1007/BF00344996. doi:10.1007/BF00344996 [DOI] [PubMed] [Google Scholar]
- Briggs J.C. McGraw-Hill; New York, NY: 1974. Marine zoogeography. [Google Scholar]
- Cailliet G.M, Andrews A.H, Burton E.J, Watters D.L, Kline D.E, Ferry-Graham L.A. Age determination and validation studies of marine fishes: do deep-dwellers live longer? Exp. Gerontol. 2001;36:739–764. doi: 10.1016/s0531-5565(00)00239-4. doi:10.1016/S0531-5565(00)00239-4 [DOI] [PubMed] [Google Scholar]
- Camhi M, Fowler S, Musick J, Bräutigam A, Fordham S. IUCN; Gland, Switzerland; Cambridge, UK: 1998. Sharks and their relatives: ecology and conservation. [Google Scholar]
- Childress J.J. Are there physiological and biochemical adaptations of metabolism in deep-sea animals? Trends Ecol. Evol. 1995;10:30–36. doi: 10.1016/s0169-5347(00)88957-0. doi:10.1016/S0169-5347(00)88957-0 [DOI] [PubMed] [Google Scholar]
- Childress J.J, Somero G.N. Metabolic scaling: a new perspective based on scaling of glycolitic enzyme activities. Am. Zool. 1990;30:161–173. [Google Scholar]
- Childress J.J, Taylor S.M, Cailliet G.M, Price M.H. Patterns of growth, energy utilization and reproduction in some meso- and bathypelagic fishes off southern California. Mar. Biol. 1980;61:27–40. doi:10.1007/BF00410339 [Google Scholar]
- Childress J.J, Price M.H, Favuzzi J, Cowles D. Chemical composition of midwater fishes as a function of depth of occurrence off the Hawaiian islands: food availability as a selective factor? Mar. Biol. 1990;105:235–246. doi:10.1007/BF01344292 [Google Scholar]
- Clarke M.W, Connolly P.L, Bracken J.J. Age estimation of the exploited deepwater shark Centrophorus squamosus from the continental slopes of the Rockall trough and Porcupine bank. J. Fish. Biol. 2002;60:501–514. doi:10.1111/j.1095-8649.2002.tb01679.x [Google Scholar]
- Clarke M.W, Kelly C.J, Connolly P.L, Molloy J.P. A life history approach to the assessment and management of deepwater fisheries in the northeast Atlantic. J. Northwest Atl. Fish. Sci. 2003;31:401–411. [Google Scholar]
- Compagno L.J.V. Alternative life-history styles of cartilaginous fishes in time and space. Environ. Biol. Fish. 1990;28:33–75. doi:10.1007/BF00751027 [Google Scholar]
- Compagno L.J.V. Checklist of living elasmobranchs. In: Hamlett W.C, editor. Sharks, skates, and rays: the biology of elasmobranch fishes. Johns Hopkins University Press; Baltimore, MD: 1999. pp. 471–498. [Google Scholar]
- Compagno L.J.V, Ebert D.A, Smale M.J. Struik Publishers; Cape Town, Republic of South Africa: 1989. Guide to the sharks and rays of southern Africa. [Google Scholar]
- Compagno L, Dando M, Fowler S. Harper Collins; London, UK: 2005. A field guide to the sharks of the world. [Google Scholar]
- Cortés E. Incorporating uncertainty into demographic modeling: application to shark populations and their conservation. Conserv. Biol. 2002;16:1048–1062. doi:10.1046/j.1523-1739.2002.00423.x [Google Scholar]
- Dulvy N.K, Reynolds J.D. Predicting extinction vulnerability in skates. Conserv. Biol. 2002;16:440–450. doi:10.1046/j.1523-1739.2002.00416.x [Google Scholar]
- Duffy J.E, Stachowicz J.J. Why biodiversity is important to oceanography: potential roles of genetic, species, and trophic diversity in pelagic ecosystem processes. Mar. Ecol. Prog. Ser. 2006;311:179–189. doi:10.3354/meps311179 [Google Scholar]
- Dulvy N.K, Metcalfe J.D, Glainville J, Pawson M, Reynolds J.D. Fishery stability, local extinctions, and shifts in community structure in skates. Conserv. Biol. 2000;14:283–293. doi:10.1046/j.1523-1739.2000.98540.x [Google Scholar]
- Dulvy N.K, Ellis J.R, Goodwin N.B, Grant A, Reynolds J.D, Jennings S. Methods of assessing extinction risk in marine fishes. Fish Fish. 2004;5:255–276. [Google Scholar]
- Devine J.A, Baker K.D, Haedrich R.L. Deep-sea fishes qualify as endangered. Nature. 2006;439:29. doi: 10.1038/439029a. doi:10.1038/439029a [DOI] [PubMed] [Google Scholar]
- Ebert D.A. University of California Press; Berkeley, CA: 2003. Sharks, rays, and chimaeras of California. [Google Scholar]
- Firth D. Multiplicative errors: log-normal or gamma? J. R. Statist. Soc. B. 1988;50:266–268. [Google Scholar]
- Frisk M.G, Miller T.J, Fogarty M.J. Estimation and analysis of biological parameters in elasmobranch fishes: a comparative life history study. Can. J. Fish. Aquat. Sci. 2001;58:969–981. doi:10.1139/cjfas-58-5-969 [Google Scholar]
- Gage J.D, Tyler P.A. Cambridge University Press; Cambridge, UK: 1991. Deep-sea biology: a natural history of organisms at the deep-sea floor. [Google Scholar]
- Gittleman J.L, Kot M. Adaptation: statistics and a null model for estimating phylogenetic effects. Syst. Zool. 1990;39:227–241. doi:10.2307/2992183 [Google Scholar]
- Gordon J.D.M, Merrett N.G, Haedrich R.L. Environmental and biological aspects of slope-dwelling fishes of the North Atlantic. In: Hopper A.G, editor. Deep-water fisheries of the North Atlantic oceanic slope. Kluwer Academic Publishers; Dordrecht, The Netherlands: 1995. pp. 1–26. [Google Scholar]
- Gotceitas V, Colgan P. Predator foraging success and habitat complexity: quantitative test of the threshold hypothesis. Oecologia. 1989;80:158–166. doi: 10.1007/BF00380145. [DOI] [PubMed] [Google Scholar]
- Graham K.J, Andrew N.L, Hodgson K.E. Changes in relative abundance of sharks and rays on Australian south east fishery trawl grounds after twenty years of fishing. Mar. Freshw. Res. 2001;52:549–561. doi:10.1071/MF99174 [Google Scholar]
- Helfman G.S, Collette B.B, Facey D.E. Blackwell Science; Malden, MA: 1997. The diversity of fishes. [Google Scholar]
- Hoenig J.M. Empirical use of longevity data to estimate mortality rates. Fish. Bull. 1983;82:898–903. [Google Scholar]
- Hutchings J.A. Life histories of fish. In: Hart P.J.B, Reynolds J.D, editors. Handbook of fish biology and fisheries. Fish biology. vol. 1. Blackwell Science; Oxford, UK: 2002. pp. 149–174. [Google Scholar]
- Irvine S.B, Stevens J.D, Laurenson L.J.B. Surface bands of deepwater squalid dorsal-fin spines: an alternative method for ageing Centroselachus crepidater. Can. J. Fish. Aquat. Sci. 2006;63:617–627. doi:10.1139/f05-237 [Google Scholar]
- Jensen A.L. Beverton and Holt life history invariants result from optimal trade-off of reproduction and survival. Can. J. Fish. Aquat. Sci. 1996;53:820–822. doi:10.1139/cjfas-53-4-820 [Google Scholar]
- Jones, K. M. M., Fitzgerald, D. G. & Sale, P. F. 2002 Comparative ecology of marine fish communities. In Handbook of fish biology and fisheries, vol. 1, Fish biology (eds P. Hart & J. D. Reynolds), pp. 341–358. Oxford, UK: Blackwell Science.
- Koslow J.A, Boehlert G.W, Gordon J.D.M, Haedrich R.L, Lorance P, Parin N. Continental slope and deep-sea fisheries: implications for a fragile ecosystem. ICES J. Mar. Sci. 2000;57:548–557. doi:10.1006/jmsc.2000.0722 [Google Scholar]
- Kyne P, Cavanagh R, Fowler S, Valenti S. IUCN; Gland, Switzerland; Cambridge, UK: 2006. IUCN shark specialist group red list assessments. [Google Scholar]
- Last P.R, Stevens J.D. CSIRO; Hobart, Australia: 1994. Sharks and rays of Australia. [Google Scholar]
- Lucifora L.O, García V.B. Gastropod predation on egg cases of skates (Chondrichthyes Rajidae) in the southwestern Atlantic: quantification and life history implications. Mar. Biol. 2004;145:917–922. doi:10.1007/s00227-004-1377-8 [Google Scholar]
- MacArthur R.H, Wilson E.O. Princeton University Press; Princeton, NJ: 1967. The equilibrium theory of island biogeography. [Google Scholar]
- Mace P.M. Relationships between common biological reference points used as thresholds and targets of fisheries management strategies. Can. J. Fish. Aquat. Sci. 1994;51:110–122. [Google Scholar]
- Machado P.B, Figueiredo I. A technique for aging the birdbeak dogfish (Deania calcea Lowe 1839) from dorsal spines. Fish. Res. 2000;45:93–98. doi:10.1016/S0165-7836(99)00100-9 [Google Scholar]
- Menni R.C, Stehmann M.F.W. Distribution, environment and biology of batoid fishes off Argentina, Uruguay and Brazil. A review. Rev. Mus. Argentino Cienc. Nat. (N. Ser.) 2000;2:69–109. [Google Scholar]
- Merrett N.R, Haedrich R.L. Chapman and Hall; New York, NY: 1997. Deep-sea demersal fish and fisheries. [Google Scholar]
- Moore J.A. Deep-sea finfish fisheries: lessons from history. Fisheries. 1999;24:16–21. doi:10.1577/1548-8446(1999)024<0016:DFFLFH>2.0.CO;2 [Google Scholar]
- Morato T, Watson R, Pitcher T.J, Pauly D. Fishing down the deep. Fish Fish. 2006;7:24–34. [Google Scholar]
- Moura T, Figueiredo I, Machado P.B, Gordo L.S. Growth pattern and reproductive strategy of the holocephalan Chimaera monstrosa along the Portuguese continental slope. J. Mar. Biol. Assoc. UK. 2004;84:801–804. doi:10.1017/S002531540400997Xh [Google Scholar]
- Musick J.A, Burgess G, Cailliet G, Camhi M, Fordham S. Management of sharks and their relatives (Elasmobranchii) Fisheries. 2000;25:9–13. doi:10.1577/1548-8446(2000)025<0009:MOSATR>2.0.CO;2 [Google Scholar]
- Myers R.A, Mertz G. The limits of exploitation: a precautionary approach. Ecol. Appl. 1998;8:S165–S169. doi:10.2307/2641375 [Google Scholar]
- Myers R.A, Worm B. Extinction, survival or recovery of large predatory fishes. Phil. Trans. R. Soc. B. 2005;360:13–20. doi: 10.1098/rstb.2004.1573. doi:10.1098/rstb.2004.1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis E. Springer; New York, NY: 2006. Analysis of phylogenetics and evolution with R. [Google Scholar]
- Paradis E, Claude J. Analysis of comparative data using generalized estimating equations. J. Theor. Biol. 2002;218:175–185. doi: 10.1006/jtbi.2002.3066. doi:10.1006/jtbi.2002.3066 [DOI] [PubMed] [Google Scholar]
- Priede I.G, Froese R, Bailey D.M, Bergstad O.A, Collins M.A, Dyb J.E, Henriques C, Jones E.G, King N. The absence of sharks from abyssal regions of the world's oceans. Proc. R. Soc. B. 2006;273:1435–1441. doi: 10.1098/rspb.2005.3461. doi:10.1098/rspb.2005.3461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purvis A, Gittleman J.L, Cowlishaw G, Mace G.M. Predicting extinction risk in declining species. Proc. R. Soc. B. 2000;267:1947–1952. doi: 10.1098/rspb.2000.1234. doi:10.1098/rspb.2000.1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R Foundation for Statistical Computing; Vienna, Austria: 2006. R: a language and environment for statistical computing. [Google Scholar]
- Reynolds J.D, Dulvy N.K, Goodwin N.B, Hutchings J.A. Biology of extinction risk in marine fishes. Proc. R. Soc. B. 2005;272:2337–2344. doi: 10.1098/rspb.2005.3281. doi:10.1098/rspb.2005.3281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison B.H. Deep pelagic biology. J. Exp. Mar. Biol. Ecol. 2004;300:253–272. doi:10.1016/j.jembe.2004.01.012 [Google Scholar]
- Sinclair A.R.E, Mduma S, Brashares J.S. Patterns of predation in a diverse predator–prey system. Nature. 2003;425:288–290. doi: 10.1038/nature01934. doi:10.1038/nature01934 [DOI] [PubMed] [Google Scholar]
- Sion, L., D'Onghia, G. & Carlucci, R. 2002 A simple technique for aging the velvet belly, Etmopterus spinax (Squalidae). In Proc. 4th European Elasmobranch Association Meeting, Livorno, Italy, 2002, 135–139.
- Smith S.E, Au D.W, Show C. Intrinsic rebound potential of 26 species of Pacific sharks. Mar. Freshw. Res. 1998;49:663–678. doi:10.1071/MF97135 [Google Scholar]
- Stevens J.D, Bonfil R, Dulvy N.K, Walker P.A. The effects of fishing on sharks, rays, and chimaeras (chondrichthyans), and the implications for marine ecosystems. ICES J. Mar. Sci. 2000;57:476–494. doi:10.1006/jmsc.2000.0724 [Google Scholar]
- Venables W.N, Ripley B.D. 4th edn. Springer; New York, NY: 2002. Modern applied statistics with S. [Google Scholar]
- Wourms J.P. Viviparity: the maternal–fetal relationship in fishes. Am. Zool. 1981;21:473–515. [Google Scholar]
- Wourms J.P, Lombardi J. Reflections on the evolution of piscine viviparity. Am. Zool. 1992;32:276–293. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data with sources and code used for building the phylogenetic tree and data analysis
Phylogenetic tree of Chondrichthyes (sharks, rays and chimaeras) used in the generalized estimating equation model to control for phylogenetic correlation. The tree is a composite from published partial trees (see references for phylogenetic relationships)
Correlogram of normalized Moran's I autocorrelation index of age at maturity, growth completion rate (von Bertalanffy's k), longevity and extinction risk (Fextinct) for taxonomic groups of the class Chondrichthyes (sharks, rays and chimaeras). Filled and empty circles represent significant (p<0.05) and non significant correlations, respectively. G, genus; F, family; O, order; C, cohort and S, subclass
Relationship between age at maturity, growth completion rate and longevity (estimated as maximum observed age) with maximum body size in chondrichthyans from continental shelves (open circles), open ocean (grey circles) and deep sea (black circles). The axis for maximum size has a log scale to improve visualization