Abstract
Failure of the human embryo to implant into the uterine wall during the early stages of pregnancy is a major cause of infertility. Implantation involves embryo apposition and adhesion to the endometrial epithelium followed by penetration through the epithelium and invasion of the embryonic trophoblast through the endometrial stroma. Although gene-knockdown studies have highlighted several molecules that are important for implantation in the mouse, the molecular mechanisms controlling implantation in the human are unknown. Here, we demonstrate in an in vitro model for human implantation that the Rho GTPases Rac1 and RhoA in human endometrial stromal cells modulate invasion of the human embryo through the endometrial stroma. We show that knockdown of Rac1 expression in human endometrial stromal cells inhibits human embryonic trophoblast invasion into stromal cell monolayers, whereas inhibition of RhoA activity promotes embryo invasion. Furthermore, we demonstrate that Rac1 is required for human endometrial stromal cell migration and that the motility of the stromal cells increases at implantation sites. This increased motility correlates with a localized increase in Rac1 activation and a reciprocal decrease in RacGAP1 levels. These results reveal embryo-induced and localized endometrial responses that may govern implantation of the human embryo.
Keywords: human trophoblast invasion, implantation in vitro, Rac-1 activation, RacGAP1, Rho GTPases
Implantation of the human embryo into the endometrium is a pivotal step in the establishment of successful pregnancy. In the human, implantation takes place between days 20 and 24 of the menstrual cycle, a period known as the window of implantation (1, 2). During the window of implantation the embryo reaches a stage of attachment competence (3), and the endometrium simultaneously reaches a stage of receptivity (4). The subsequent molecular dialogue between the implanting embryo and the receptive endometrium is key for the initiation and progression of implantation (5).
The implantation process itself is a complex and multistep event (6). The attachment-competent embryo initially orientates itself and apposes to the endometrial epithelium. Once positioned correctly, the embryo sheds its protective zona pellucida and comes into direct contact with the epithelium, forming initial contacts that are subsequently translated into firm adhesion sites. Finally, the embryonic trophoblast penetrates the epithelium and its underlying basement membrane, and the embryo invades the underlying stromal cells. Trophoblast proliferation, differentiation, and invasion through the stroma follow, allowing the formation of a connection with the maternal vasculature and, ultimately, the formation of a functional placenta.
Studies of human embryo implantation have been limited, and the precise mechanisms that govern implantation in the human are unknown, largely because human implantation sites are inaccessible in vivo. Mouse models have provided valuable insights into the molecular mechanisms that occur during embryo implantation (7); however, these models do not necessarily translate to the human because the reproductive physiology of mice and humans is different (8). We have developed an in vitro model that recapitulates the early steps of human embryonic trophoblast invasion into the stroma during embryo implantation (9). In this model, human embryos are cocultured with decidualized primary human endometrial stromal cells (hESCs). The embryos implant into the hESCs, and the implantation sites can be analyzed as whole mounts, revealing penetration and invasion of the trophoblast through the hESC monolayer. At the end of the culture period, the invading trophoblast is in direct contact with the growth surface and is completely covered by stromal cells. We have used this model here to determine the function of Rho GTPases in hESCs in implantation.
Rho GTPases are a family of proteins that act coordinately to regulate dynamic cellular processes by modulating actin and microtubule dynamics, myosin activity, and cell adhesion (10). In particular, RhoA and Rac1 have been shown to act in concert to regulate cell migration and motility in a variety of cell types: Rac1 promotes lamellipodial protrusion at the front of migrating cells, whereas RhoA is required predominantly for retraction at the rear (11). Rac1 and RhoA often reciprocally regulate each other; for example, Rac1 can act via its target the serine/threonine kinase p21-activated kinase (PAK) to reduce RhoA activation (12), and the RhoA target Rho-kinase (ROCK) can inhibit Rac1 activation (13).
Although the dynamic nature and remodeling of the endometrium during the menstrual cycle has been well documented (14), the control of endometrial cell remodeling during embryo implantation has not been addressed. Furthermore, the presence of RhoA in human endometrial stromal and decidual tissue, and in decidual cells cultured in vitro, has been reported (15, 16), however, its function has not been directly investigated. Here, we show that the Rho GTPases RhoA and Rac1 have opposing roles in regulating hESC motility and together act to regulate implantation of the human embryo in vitro.
Results
Rho GTPases Are Present in hESCs and Are Required During Embryo Implantation.
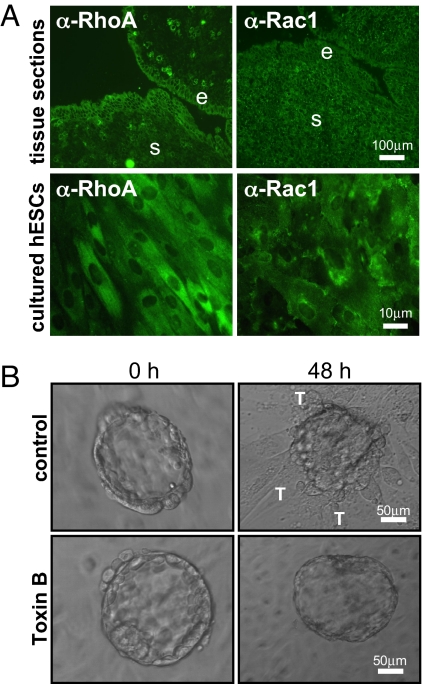
We first examined the expression of the Rho GTPases RhoA and Rac1 in human endometrial cells. Immunohistochemistry of endometrial tissue sections obtained from the midsecretory stage of the menstrual cycle, corresponding to the window of implantation, revealed the presence of RhoA and Rac1 in the endometrial stroma and in epithelial cells at the luminal edge of the endometrium (Fig. 1A). In addition, hESCs isolated from endometrial tissue and cultured in vitro displayed positive immunostaining for both RhoA and Rac1.
Fig. 1.
Expression of RhoA and Rac1 in hESCs and their involvement during human embryo implantation. (A) Immunohistochemistry of frozen tissue sections of midsecretory human endometrium and of cultured hESCs demonstrates the presence of RhoA and Rac1. Endometrial stromal (s) cells and epithelial (e) cells within the tissue are indicated. (B) Toxin B pretreatment of hESCs (0.1 ng/ml, 1 h) before coculture inhibits embryo invasion and trophoblast (T) spreading.
We investigated the function of Rho GTPases in hESCs during implantation by the use of Toxin B from Clostridium difficile. This bacterial toxin enters the cell via endocytosis and irreversibly glucosylates members of the Rho GTPase family, rendering them functionally inactive (17). Treatment of hESCs with Toxin B for 1 h caused a dose-dependent disruption of the actin cytoskeleton [supporting information (SI) Fig. S1A], and analysis of RhoA activity with a RhoA pulldown assay confirmed inhibition of the basal levels of RhoA activity (Fig. S1B). Pretreatment of hESCs with Toxin B resulted in a dramatic decrease in embryo invasion in an in vitro implantation model (Fig. 1B), indicating a requirement for Rho GTPase signaling in hESCs during this stage of implantation. Toxin B pretreatment of hESCs did not affect embryo attachment, and all embryos observed were able to attach but not spread out and invade into the stromal monolayer (Fig. 1B).
Silencing of hESC Rac1 Inhibits Trophoblast Invasion Whereas RhoA Silencing Potentiates Invasion.
C. difficile Toxin B inhibits all classes of Rho GTPases; therefore, to determine which member of the Rho family was inhibiting embryo implantation we used RNAi-mediated silencing to knock down specifically the expression of either RhoA or Rac1 in hESCs. Human ESCs were transfected with siRNAs, and embryos were added to transfected hESC monolayers 24 h after transfection. Cocultures were fixed after 48 h, and embryo attachment and invasion were analyzed. In addition, the extent of RhoA and Rac1 silencing was confirmed by Western blotting of duplicate cell monolayers and by counterstaining transfected hESCs with anti-RhoA and anti-Rac1 antibodies (Fig. S2).
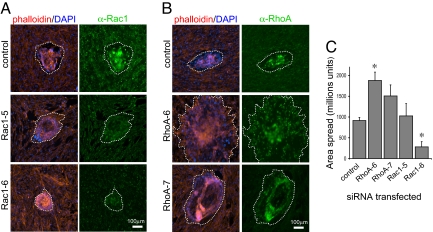
Embryo invasion into hESCs transfected with Rac1 siRNAs was inhibited significantly (Fig. 2A), although attachment was not affected. In contrast, silencing of RhoA expression in hESCs caused a significant increase in the subsequent levels of embryo invasion (Fig. 2B). Quantification of the extent of trophoblast spreading (Fig. 2C) revealed that, for the case of embryos cocultured with hESCs transfected with the Rac1–6 siRNA, the trophoblast spread area was >3-fold less than that shown by embryos cocultured with hESCs transfected with a control, nonsilencing siRNA. Furthermore, the effects on the extent of trophoblast spreading correlated directly with the degree of silencing, with the more effective Rac1 siRNA, Rac1-6 (Fig. S2), having a more pronounced effect on invasion. Conversely, silencing of hESC RhoA by using the RhoA-6 siRNA caused a significant ≈2-fold increase the subsequent extent of trophoblast invasion (Fig. 2C).
Fig. 2.
Effects of silencing hESC RhoA and Rac1 on embryo invasion. (A) Silencing of hESC Rac1 with siRNAs (Rac1-5 and Rac1-6) inhibits embryo invasion (dotted lines indicate approximate area of trophoblast spread). Human ESCs were transfected with 25 nM each Rac1 siRNA or 25 nM nonsilencing (control) siRNA 24 h before the addition of human blastocysts. (B) Silencing of hESC RhoA by using siRNAs (RhoA-6 and RhoA-7) enhances embryo invasion. Cells were transfected as above before coculture. (C) RhoA silencing in hESCs significantly increases the area of embryo spread, whereas Rac1 silencing inhibits spreading. Results shown are mean areas ± SEM. Significant differences between control cells are indicated by * (P < 0.05).
Inhibition of Rho-Kinase Signaling in hESCs Increases Trophoblast Invasion and Viability.
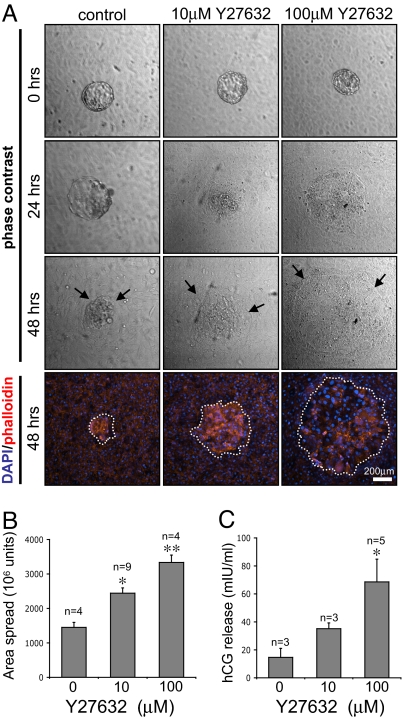
We extended our observations of the effects of RhoA silencing, by inhibiting signaling downstream of RhoA in hESCs by using a cell-permeable ROCK inhibitor, Y27632 (18). ROCK is one of the downstream effectors of RhoA, and its activation is implicated in cell movement and the formation of stress fibers and focal adhesions (19). Human ESCs were treated with Y27632 before being cocultured with human embryos in in vitro implantation assays. Analysis of embryo attachment and trophoblast spreading (Fig. 3A) showed that, similar to the effect seen after RhoA silencing, embryo invasion into hESCs treated with Y27632 was markedly increased compared with embryo invasion into control cells. The temporal dynamics of implantation were also affected, in that embryos that were cocultured with 100 μM Y27632-treated hESCs exhibited a high degree of trophoblast spreading after only 24 h of coculture. Counterstaining of cells with DAPI and phalloidin highlighted the extent of trophoblast spreading, and quantification (Fig. 3B) showed that the area of trophoblast spreading in embryos cocultured with 100 μM Y27632-treated hESCs was >2-fold higher compared with those cultured with untreated cells. Furthermore, analysis of human chorionic gonadotrophin (hCG) levels in the coculture supernatants (Fig. 3C) demonstrated that embryos cocultured with Y27632-treated hESCs secreted significantly higher levels (> 5-fold) of hCG, confirming enhanced trophoblast viability and development when ROCK is inhibited in the stroma.
Fig. 3.
Effects of hESC ROCK inhibition on human embryo invasion. (A) Treatment of hESCs with the ROCK inhibitor Y27632 before embryo coculture increases the extent of trophoblast invasion (indicated by arrows). DAPI/phalloidin staining after 48 h of coculture is also shown (dotted lines indicate trophoblast spreading). (B) Y27632 pretreatment of hESCs increases the area of trophoblast spread. Values are mean levels ± SEM. Significant differences between control and Y27632-treated cells are indicated by * (P < 0.05) and ** (P < 0.01). (C) Y27632 pretreatment of hESCs leads to enhanced hCG release by embryos. Values displayed are mean levels of hCG detected in the culture supernatant after 48 h of coculture ± SEM. P values are displayed as above.
Human ESCs Are Motile During Embryo Implantation.
The results above indicate that inhibition of hESC Rac1 signaling causes inhibition of embryo invasion whereas inhibition of hESC RhoA signaling leads to enhanced embryo invasion. We analyzed the effects of RhoA and Rac1 inhibition on stromal cell migration to determine whether changes in hESC motility underpin the observed effects on embryo implantation.
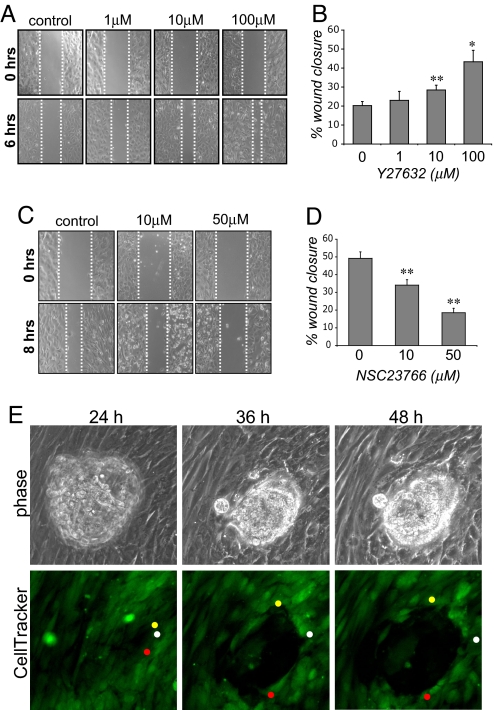
Treatment of hESCs with Y27632 caused a significant increase in cell motility in wound-healing assays (Fig. 4A). Quantification of wound closure (Fig. 4B) demonstrated that hESCs pretreated with 100 μM Y27632 displayed a ≈2-fold increase in wound closure compared with control untreated hESCs. In contrast, and as shown for other cell types (20), inhibition of Rac1 signaling with the Rac1 inhibitor, NSC23766 (21) resulted in a significant decrease in cell motility (Fig. 4 C and D).
Fig. 4.
Regulation of hESC motility by RhoA, Rac1, and the human embryo. (A) Treatment of hESCs with Y27632 increases cell motility in wound-healing assays. Cells were treated with the indicated amounts of Y27632 before wounding. Wound width was analyzed 0 and 6 h after wounding. (B) Wound closure, expressed as a percentage of the width of the initial wound, is increased after Y27632 treatment. Significant differences between control and Y27632-treated cells are indicated by * (P < 0.05) and ** (P < 0.01). (C) NSC23766 treatment decreases hESC motility in wound-healing assays. Cells were treated with the indicated amounts of NSC23766 before wounding. Wound width was analyzed 0 and 8 h after wounding. (D) Wound healing (displayed as above) is significantly reduced after NSC23766 treatment. (E) Increased motility of hESCs at the implantation site is observed during time-lapse imaging of human embryos implanting into CellTracker Green-labeled hESCs. The movements of three individual cells are tracked by colored circles.
These results suggest that the spreading and invasion of the embryonic trophoblast require motility of maternal hESCs. We examined hESC movement during implantation by using time-lapse microscopy of human embryos implanting into fluorescently labeled hESC monolayers. Images taken from time-lapse microscopy movies (Fig. 4E, see also Movie S1) show that human day 6 embryos attach to monolayers of hESCs after 24 h of coculture. At this stage, the hESCs are present as an intact and confluent monolayer. Over the next 24 h of coculture, however, the hESCs at the embryo–endometrial interface become increasingly motile and migrate away from the implantation site, allowing the embryo to spread out across the monolayer. Tracking of single cells within the implantation site shows hESCs migrating away from the implanting embryo (Fig. 4E, colored dots).
Increased Levels of Rac1 Activity Are Observed in hESCs at the Implantation Site.
We explored the possibility that hESC migration at the implantation site correlates with increased Rac1 activity. We did so by using an in situ binding assay to monitor endogenous Rac1 activity in hESC–embryo cocultures. This assay, based on a similar RhoA activation assay (22), utilizes a PAK-GST fusion protein that binds only to the GTP-bound active form of Rac1. Such binding can then be detected by using anti-GST antibodies. This assay was used successfully to detect exogenously expressed active GFP-Rac1 in hESCs (Fig. S3).
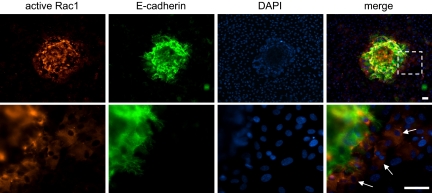
In embryo–hESC cocultures, high levels of active Rac1 were detected in the hESCs that were adjacent to implanting embryos (Fig. 5). Basal levels of active Rac1 in hESCs distal to the implantation site were otherwise low, and levels of total hESC Rac1 were not spatially restricted (as seen in Fig. 2A). Rac1 activity was particularly high in hESCs that were in immediate contact with trophoblast cells (Fig. 5, arrows), suggesting a localized, embryo-induced increase in the levels of Rac1 activity, and hence hESC motility, during implantation.
Fig. 5.
Rac1 activation in hESCs during embryo implantation. (Upper) In situ staining for active Rac1 in embryo–hESC cocultures demonstrates that cells adjacent to the implanting embryo (which is stained positive for E-cadherin) have higher levels of active Rac1 than those further away. Nuclei are counterstained with DAPI. (Lower) Higher resolution, arrows indicate cells with high levels of active Rac1. (Scale bars, 30 μm.)
Levels of hESC RacGAP1 Are Decreased Locally in Response to the Implanting Embryo.
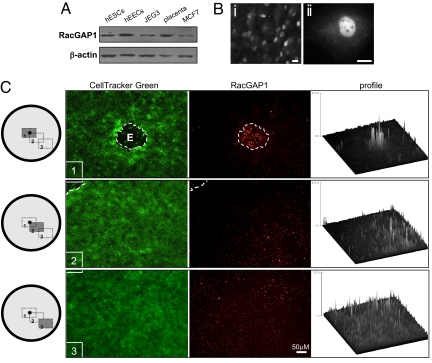
The activity of Rac1 and other Rho GTPases can be regulated by guanine nucleotide exchange factors and dissociation inhibitors, which control the exchange of GDP for GTP, and by GTPase-activating proteins (GAPs) that promote GTP hydrolysis. Previous microarray data demonstrated that RacGAP1 (also known as MgcRacGAP) is down-regulated in hESCs after exposure to trophoblast-conditioned medium (23). We analyzed RacGAP1 expression in hESCs to determine whether the embryo-induced increase in hESC Rac1 activity that we observed in vitro resulted from localized inhibition or down-regulation of RacGAP1, which, in other cell types, inactivates Rac1 (24). Western blot analysis showed that RacGAP1 is expressed in hESCs and in human endometrial epithelial cells, JEG3 choriocarcinoma cells, and placental tissue (Fig. 6A).
Fig. 6.
Regulation of RacGAP1 expression in hESCs in response to a human embryo. (A) Western blots demonstrate RacGAP1 expression in hESCs, human endometrial epithelial cells (hEECs), JEG3 choriocarcinoma cells, placenta, and MCF7 breast cancer cells. A loading control (β-actin) is also shown. (B) Immunohistochemistry of RacGAP1 showing localization in hESCs. Low (i) and high (ii) magnification images are shown. (Scale bars, 10 μm.) (C) RacGAP1 expression in CellTracker Green-labeled hESCs cocultured with human embryos is lowest in the region surrounding the embryo. Images were obtained from three different regions (1, 2, and 3) across the coculture monolayer as indicated in the schematic. Intensity profiling of the images shown demonstrates a gradient of RacGAP1 expression.
Immunohistochemistry of hESC monolayers demonstrated that, as seen for other cell types (25, 26), RacGAP1 localized to the nucleus and cytosolic structures of hESCs (Fig. 6B). Furthermore, after RNAi-mediated knockdown of RacGAP1 expression in hESCs, we found an increase in Rac1 activity (Fig. S4), confirming the role of RacGAP1 as a regulator of Rac1 activity in hESCs.
Analysis of hESC implantation whole mounts revealed that RacGAP1 staining was dramatically reduced in hESCs cocultured with human embryos, and this decrease was most obvious in the region of the hESC monolayer adjacent to the implanting embryo (Fig. 6C). Quantitative profiling of the staining intensity revealed an increasing gradient of RacGAP1 from the proximal to the distal areas of the hESCs relative to the embryo. The localized decrease in the levels of RacGAP1 correlated with the localized increase in the levels of active Rac1 in regions adjacent to the implantation site, suggesting that soluble factors released by the embryo modulate RacGAP1 expression, and hence Rac1 activity and hESC motility, during implantation.
Discussion
We have demonstrated that the process of human embryonic trophoblast invasion into hESCs in vitro (i) is regulated by both the embryo and the stroma; (ii) requires Rac1-dependent hESC migration and inhibition of hESC RacGAP1 expression; and (iii) is inhibited by hESC RhoA. Overall, these studies indicate that the embryo itself has a crucial function in modulating hESC gene expression and function to allow its subsequent invasion into the endometrial stroma.
It is known that the formation of a functional placenta requires extensive, although tightly controlled, invasion of the trophoblast through the stroma (27). Thus, the mechanisms that control trophoblast invasion must involve both signals that promote invasion and those that act to restrict it. The results presented here suggest that controlled trophoblast invasion may be mediated by a delicate balance between stromal RhoA and Rac1 activities, that in turn regulate hESC motility, in response to the implanting embryo. The migration of hESCs away from the implantation site may facilitate trophoblast invasion and implantation of the embryo into the stromal compartment. Impairment of hESC motility, either directly through Rac1 inhibition as demonstrated here or through alternative pathways could therefore be an important factor in impeding successful implantation in implantation failure. Alternatively, regulated inhibition of motility may be an important mechanism in restricting the degree of invasion during the normal implantation process.
An intricate balance between RhoA and Rac1 signaling has been observed in a variety of cell types (28). It was shown that Y27632 treatment of Swiss 3T3 cells can enhance Rac1 activation, leading to increased cell motility, and that this effect is likely to be mediated by inhibition of a suppressive effect of ROCK on Rac1 activity (13). In addition, a RhoGAP that antagonizes Rac1 activity in a ROCK-dependent manner has recently been identified (29), further supporting the notion of mutual antagonism between RhoA and Rac1. Analysis of Rac1 activity in Y27632-treated hESCs by using a Rac1 pulldown assay (Fig. S4) demonstrated that activity of Rac1, but not that of Cdc42, increased with increasing concentrations of Y27632 treatment. This, together with the observed opposing roles of RhoA and Rac1 on hESC migration, thus suggests that a delicate balance between the RhoA and Rac1 pathways does exist in hESCs to regulate their motility and hence their response to an implanting embryo.
The concept of cross-talk at the embryo–endometrial interface is widely accepted and is believed to be important for mediating the priming, apposition, and adhesion of the embryo during implantation. For example, previous studies with human blastocysts have demonstrated the importance of embryo-derived signals in regulating protein expression in human endometrial epithelial cells. The levels of endometrial epithelial proteins, including β3 integrin (30), leptin and its receptor (31), MUC1 (32), and the chemokine receptors CXCR1 and CXCR4 (33), have been shown to be modulated in vitro by the presence of a human blastocyst.
More recently, gene expression profiling of hESCs has shown that levels of many hESC mRNAs are modulated in response to trophoblast-conditioned medium (23) or after coculture with first-trimester trophoblast explants (34), further corroborating the concept of embryo-mediated modulation of endometrial cell gene expression. Here, we have demonstrated that in vitro, the human embryo induces changes in hESC RacGAP1 expression levels.
RacGAP1 is a GTPase-activating protein that has been implicated in controlling cell growth and differentiation (35) and cytokinesis (25). Studies have shown that RacGAP1 may exert its GAP activity on RhoA and Cdc42 and on Rac1 (24, 36), and this may depend on its phosphorylation status (37). In contrast, some studies suggest that RacGAP1 is able to mediate some of its functions independently of its GAP activity (38). Recently, RacGAP1 has also been implicated in regulating the localization and transcriptional activity of signal transducer and activation of transcription (STAT) proteins (39, 40), a family of proteins that have been strongly implicated in the stromal decidualization response and embryo implantation (41). Here, we have shown that hESC RacGAP1 levels are down-regulated in response to human embryos and that this correlates with an increase in Rac1 activity observed in hESCs. In addition, RNAi-mediated silencing of RacGAP1 in hESCs decreased the GAP activity of Rac1, confirming its role as a regulator of Rac1 function in these cells.
The precise role of RacGAP1 in hESCs, in particular its involvement in mediating the balance between RhoA and Rac1 activities, is now open to investigation. The specific embryo-derived factor mediating the observed reduction in hESC RacGAP1 levels also remains to be characterized. The gradient of RacGAP1 levels observed around the implantation site suggests that the embryo-induced modulation of hESC is highly localized and needs be spatially restricted during the invasion process. This phenomenon of localized modulation of genes by the embryo has been demonstrated in the stroma around implantation sites in the mouse for a number of different molecules, for example IL-11Rα (42), but here it is demonstrated for human embryos.
Our results demonstrate an important function for the Rho family of GTPases in regulating human embryonic trophoblast invasion into the stroma, a key stage of early embryo implantation. We suggest that the cell surface receptors upstream of the Rho family of GTPases as well as the molecules that regulate Rho GTPase activity in the endometrium are potential candidate therapeutic targets for improving the rate of embryo implantation in in vitro fertilization and for the treatment of infertility, or, conversely, as targets for contraceptives.
Materials and Methods
Tissue Samples and Cell Culture.
Endometrial tissues were obtained from fertile patients aged 20–46 years undergoing sterilization or hysterectomy for benign conditions. Samples were obtained with informed consent in accordance with the requirements of the Central Oxford Research Ethics Committee. Patients had regular 26- to 33-day menstrual cycles and had received no hormonal medication in the preceding 3 months. Samples were obtained during the midsecretory stage of the menstrual cycle (days 20–22), as confirmed by histological examination.
The isolation and culture of hESCs were performed as described in ref. 9, and decidualization was induced by culturing confluent hESCs in the presence of 0.5 mM 8-Br-cAMP (Sigma) for 3 days. Cells were used between passages 2 and 6 and were ≈98% pure as assessed by Thy-1 and vimentin expression. These cells have also been shown to be positive for progesterone and estrogen receptors and CD10, and negative for cytokeratin 18, CD45, and CD68, confirming their purity and physiological status (43, 44).
Embryo Collection and Co-Culture.
Embryos were donated for research with informed consent by patients attending the Oxford Fertility Unit at the John Radcliffe Hospital, Oxford. Experiments were performed with ethical approval from the Oxfordshire Research Ethics Committee, and a research license was granted by the Human Fertilization and Embryology Authority. Ovarian stimulation, oocyte retrieval, and in vitro fertilization (IVF) were performed as described in ref. 45. Embryos were cultured in Sydney IVF Blastocyst medium (Cook Medical Ltd.) overlaid with light paraffin oil (MediCult U.K. Ltd.). Blastocysts were scored according to the Gardner and Schoolcraft system (46) and were transferred into coculture experiments. Assisted hatching was performed if necessary as described in ref. 47. Blastocysts of grade 4 or above were used were distributed equally among experimental conditions according to their grade.
Hatched blastocysts were cocultured with confluent monolayers of decidualized hESCs for 48 h as described in ref. 9. Embryo attachment and morphology were by light or immunofluorescence microscopy. Secretion of hCG by blastocysts into the culture medium was measured by using a fluorimmunometric assay (Delfia) according to the manufacturer's instructions.
C. difficile Toxin B, Y27632, and NSC23766 were obtained from Calbiochem. Decidualized hESCs were cultured in the presence of Toxin B (1 h), Y27632 or NSC23766 (both 16 h), and were then washed extensively before analysis or the addition of embryos.
Wound-Healing Assays.
Human ESCs were seeded onto coverslips, grown to confluence, and decidualized. Cells were then pretreated with ROCK or Rac1 inhibitors as above. Each coverslip was scratched with a sterile 10-μl pipette tip, washed with PBS, and placed into fresh medium. Wound width was analyzed by phase-contrast microscopy immediately and after 6 or 8 h. Wound closure was calculated and expressed as a percentage of the initial wound width. Data shown represent the mean ± SEM of triplicate sets of measurements taken from three independent experiments.
Immunohistochemistry and Tissue Staining.
Cells or whole mounts were fixed, permeabilized, and blocked as described in ref. 9. Cells were incubated with primary antibody diluted in 0.1% BSA overnight at 4°C followed by the appropriate conjugated secondary antibody for 1 h at room temperature. The following primary antibodies were used: anti-RhoA mouse monoclonal antibody and anti-E-cadherin rabbit polyclonal antibody (Santa Cruz Biotechnology); anti-Rac1 mouse monoclonal antibody (Upstate); and anti-RacGAP1 (AbCam Plc.). Secondary antibodies used were: donkey anti-mouse FITC and donkey anti-rabbit FITC (Jackson ImmunoResearch Laboratories) or goat anti-mouse Alexa Fluor 594 (Molecular Probes). Texas Red phalloidin (Molecular Probes) was added during the secondary incubation to visualize actin. Cells were mounted in Vectashield mounting medium containing DAPI (Vector Laboratories).
Western Blotting.
Proteins were separated on SDS/polyacrylamide gels and transferred to PVDF membranes that were then blocked in 5% nonfat dry milk. Membranes were incubated in primary antibody overnight at 4°C followed by a 1-h incubation with the appropriate horseradish peroxidase-conjugated secondary antibody. Immunoreactive bands were visualized with an ECL detection kit (Amersham).
Pulldown and in Situ Rho GTPase Activation Assays.
RhoA and Rac1 pulldown assays were carried out by using commercially available kits (Upstate) according to the manufacturer's guidelines. After pulldown, eluted RhoA, Rac1, and Cdc42 were detected by immunoblotting. Total cell lysates were also analyzed, and Coomassie blue staining of the eluted fractions confirmed equal loading of GST fusion proteins. Active Rac1 was detected in situ in fixed hESCs by permeabilization of cells with 0.05% Triton X-100 for 5 min at 4°C followed by incubation with 1% BSA for 1 h. Cells were incubated with 50 μg/ml PAK-GST (Cytoskeleton) for 1 h followed by anti-GST antibody (Sigma) overnight at room temperature followed by the appropriate secondary antibodies for 1 h.
Plasmid and siRNA Transfections.
Human ESCs were transfected with pCB6-GFP-Rac1L61 by using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Short-interfering RNAs directed against human RhoA (RhoA-6 and RhoA-7) and Rac1 (Rac1-5 and Rac1-6) control, nonsilencing siRNAs, and HiPerfect transfection reagent (all from Qiagen) were used according to the manufacturer's instructions.
Microscopy and Time-Lapse Imaging.
Cells were viewed with a Leica DMRBE microscope, and images were captured with a Hamamatsu Orca C4742-95 digital camera and Openlab software (Improvision). For time-lapse microscopy, cells were placed in a microscope incubator (Solenta Scientific) and maintained at 37°C with 5% CO2. Images were captured every 30 min with a Leica DMIRB inverted microscope connected to a Hamamatsu Orca C4742-80 camera (Improvision) and were processed by using the Openlab software. ImageJ (National Institutes of Health) was used to obtain intensity profiles of images. CellTracker Green CMFDA (Invitrogen) was used for fluorescent labeling of live hESCs before embryo addition, according to the manufacturer's instructions.
Supplementary Material
Acknowledgments.
We thank Enda McVeigh, Karen Turner, the staff of the Oxford in Vitro Fertilization Unit, and all of the patients who donated their spare embryos for this research. The GFP-Rac1 plasmid was a generous gift from Michael Way (London Research Institute, Lincoln's Inn Fields Laboratories, U.K.). This work was supported by the Wellcome Trust and the Medical Research Council.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806219105/DCSupplemental.
References
- 1.Tabibzadeh S. Molecular control of the implantation window. Hum Reprod Update. 1998;4:465–471. doi: 10.1093/humupd/4.5.465. [DOI] [PubMed] [Google Scholar]
- 2.Kao LC, et al. Global gene profiling in human endometrium during the window of implantation. Endocrinology. 2002;143:2119–2138. doi: 10.1210/endo.143.6.8885. [DOI] [PubMed] [Google Scholar]
- 3.Watson AJ, Natale DR, Barcroft LC. Molecular regulation of blastocyst formation. Anim Reprod Sci. 2004;82–83:583–592. doi: 10.1016/j.anireprosci.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Achache H, Revel A. Endometrial receptivity markers, the journey to successful embryo implantation. Hum Reprod Update. 2006;12:731–746. doi: 10.1093/humupd/dml004. [DOI] [PubMed] [Google Scholar]
- 5.Paria BC, Song H, Dey SK. Implantation: Molecular basis of embryo–uterine dialogue. Int J Dev Biol. 2001;45:597–605. [PubMed] [Google Scholar]
- 6.Kimber SJ, Spanswick C. Blastocyst implantation: The adhesion cascade. Semin Cell Dev Biol. 2000;11:77–92. doi: 10.1006/scdb.2000.0154. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Dey SK. Road map to embryo implantation: Clues from mouse models. Nat Rev Genet. 2006;7:185–199. doi: 10.1038/nrg1808. [DOI] [PubMed] [Google Scholar]
- 8.Lee KY, DeMayo FJ. Animal models of implantation. Reproduction. 2004;128:679–695. doi: 10.1530/rep.1.00340. [DOI] [PubMed] [Google Scholar]
- 9.Carver J, et al. An in vitro model for stromal invasion during implantation of the human blastocyst. Hum Reprod. 2003;18:283–290. doi: 10.1093/humrep/deg072. [DOI] [PubMed] [Google Scholar]
- 10.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 11.Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Dev Biol. 2004;265:23–32. doi: 10.1016/j.ydbio.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Zenke FT, et al. p21-activated kinase 1 phosphorylates and regulates 14-3-3 binding to GEF-H1, a microtubule-localized Rho exchange factor. J Biol Chem. 2004;279:18392–18400. doi: 10.1074/jbc.M400084200. [DOI] [PubMed] [Google Scholar]
- 13.Tsuji T, et al. ROCK and mDia1 antagonize in Rho-dependent Rac activation in Swiss 3T3 fibroblasts. J Cell Biol. 2002;157:819–830. doi: 10.1083/jcb.200112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salamonsen LA. Tissue injury and repair in the female human reproductive tract. Reproduction. 2003;125:301–311. doi: 10.1530/rep.0.1250301. [DOI] [PubMed] [Google Scholar]
- 15.Shiokawa S, et al. Small guanosine triphosphatase RhoA and Rho-associated kinase as regulators of trophoblast migration. J Clin Endocrinol Metab. 2002;87:5808–5816. doi: 10.1210/jc.2002-020376. [DOI] [PubMed] [Google Scholar]
- 16.Shiokawa S, et al. Function of the small guanosine triphosphate-binding protein RhoA in the process of implantation. J Clin Endocrinol Metab. 2000;85:4742–4749. doi: 10.1210/jcem.85.12.7054. [DOI] [PubMed] [Google Scholar]
- 17.Voth DE, Ballard JD. Clostridium difficile toxins: Mechanism of action and role in disease. Clin Microbiol Rev. 2005;18:247–263. doi: 10.1128/CMR.18.2.247-263.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uehata M, et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 19.Riento K, Ridley AJ. Rocks: Multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4:446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- 20.Zuo Y, Shields SK, Chakraborty C. Enhanced intrinsic migration of aggressive breast cancer cells by inhibition of Rac1 GTPase. Biochem Biophys Res Commun. 2006;351:361–367. doi: 10.1016/j.bbrc.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 21.Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci USA. 2004;101:7618–7623. doi: 10.1073/pnas.0307512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berdeaux RL, Diaz B, Kim L, Martin GS. Active Rho is localized to podosomes induced by oncogenic Src and is required for their assembly and function. J Cell Biol. 2004;166:317–323. doi: 10.1083/jcb.200312168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hess AP, et al. Decidual stromal cell response to paracrine signals from the trophoblast: Amplification of immune and angiogenic modulators. Biol Reprod. 2007;76:102–117. doi: 10.1095/biolreprod.106.054791. [DOI] [PubMed] [Google Scholar]
- 24.Toure A, et al. MgcRacGAP, a new human GTPase-activating protein for Rac and Cdc42 similar to Drosophila rotundRacGAP gene product, is expressed in male germ cells. J Biol Chem. 1998;273:6019–6023. doi: 10.1074/jbc.273.11.6019. [DOI] [PubMed] [Google Scholar]
- 25.Hirose K, Kawashima T, Iwamoto I, Nosaka T, Kitamura T. MgcRacGAP is involved in cytokinesis through associating with mitotic spindle and midbody. J Biol Chem. 2001;276:5821–5828. doi: 10.1074/jbc.M007252200. [DOI] [PubMed] [Google Scholar]
- 26.Kitamura T, et al. Role of MgcRacGAP/Cyk4 as a regulator of the small GTPase Rho family in cytokinesis and cell differentiation. Cell Struct Funct. 2001;26:645–651. doi: 10.1247/csf.26.645. [DOI] [PubMed] [Google Scholar]
- 27.van den Brule F, et al. Trophoblast invasion and placentation: Molecular mechanisms and regulation. Chem Immunol Allergy. 2005;88:163–180. doi: 10.1159/000087833. [DOI] [PubMed] [Google Scholar]
- 28.Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 29.Ohta Y, Hartwig JH, Stossel TP. FilGAP, a Rho- and ROCK-regulated GAP for Rac binds filamin A to control actin remodelling. Nat Cell Biol. 2006;8:803–814. doi: 10.1038/ncb1437. [DOI] [PubMed] [Google Scholar]
- 30.Simon C, et al. Embryonic regulation of integrins α3, α4, and α1 in human endometrial epithelial cells in vitro. J Clin Endocrinol Metab. 1997;82:2607–2616. doi: 10.1210/jcem.82.8.4153. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez RR, et al. Leptin and leptin receptor are expressed in the human endometrium, and endometrial leptin secretion is regulated by the human blastocyst. J Clin Endocrinol Metab. 2000;85:4883–4888. doi: 10.1210/jcem.85.12.7060. [DOI] [PubMed] [Google Scholar]
- 32.Meseguer M, et al. Human endometrial mucin MUC1 is up-regulated by progesterone and down-regulated in vitro by the human blastocyst. Biol Reprod. 2001;64:590–601. doi: 10.1095/biolreprod64.2.590. [DOI] [PubMed] [Google Scholar]
- 33.Dominguez F, et al. Hormonal and embryonic regulation of chemokine receptors CXCR1, CXCR4, CCR5, and CCR2B in the human endometrium and the human blastocyst. Mol Hum Reprod. 2003;9:189–198. doi: 10.1093/molehr/gag024. [DOI] [PubMed] [Google Scholar]
- 34.Popovici RM, et al. Gene expression profiling of human endometrial–trophoblast interaction in a coculture model. Endocrinology. 2006;147:5662–5675. doi: 10.1210/en.2006-0916. [DOI] [PubMed] [Google Scholar]
- 35.Kawashima T, et al. MgcRacGAP is involved in the control of growth and differentiation of hematopoietic cells. Blood. 2000;96:2116–2124. [PubMed] [Google Scholar]
- 36.Jantsch-Plunger V, et al. CYK-4: A Rho family GTPase-activating protein (GAP) required for central spindle formation and cytokinesis. J Cell Biol. 2000;149:1391–1404. doi: 10.1083/jcb.149.7.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Minoshima Y, et al. Phosphorylation by aurora B converts MgcRacGAP to a RhoGAP during cytokinesis. Dev Cell. 2003;4:549–560. doi: 10.1016/s1534-5807(03)00089-3. [DOI] [PubMed] [Google Scholar]
- 38.Yamada T, Hikida M, Kurosaki T. Regulation of cytokinesis by MgcRacGAP in B lymphocytes is independent of GAP activity. Exp Cell Res. 2006;312:3517–3525. doi: 10.1016/j.yexcr.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 39.Kawashima T, et al. Rac1 and a GTPase-activating protein, MgcRacGAP, are required for nuclear translocation of STAT transcription factors. J Cell Biol. 2006;175:937–946. doi: 10.1083/jcb.200604073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tonozuka Y, et al. A GTPase-activating protein binds STAT3 and is required for IL-6-induced STAT3 activation and for differentiation of a leukemic cell line. Blood. 2004;104:3550–3557. doi: 10.1182/blood-2004-03-1066. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura H, et al. Mouse model of human infertility: Transient and local inhibition of endometrial STAT-3 activation results in implantation failure. FEBS Lett. 2006;580:2717–2722. doi: 10.1016/j.febslet.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 42.Robb L, et al. Infertility in female mice lacking the receptor for interleukin 11 is due to a defective uterine response to implantation. Nat Med. 1998;4:303–308. doi: 10.1038/nm0398-303. [DOI] [PubMed] [Google Scholar]
- 43.Klemmt PA, Carver JG, Kennedy SH, Koninckx PR, Mardon HJ. Stromal cells from endometriotic lesions and endometrium from women with endometriosis have reduced decidualization capacity. Fertil Steril. 2006;85:564–572. doi: 10.1016/j.fertnstert.2005.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klemmt PA, Carver JG, Koninckx P, McVeigh EJ, Mardon HJ. Endometrial cells from women with endometriosis have increased adhesion and proliferative capacity in response to extracellular matrix components: Toward a mechanistic model for endometriosis progression. Hum Reprod. 2007;22:3139–3147. doi: 10.1093/humrep/dem262. [DOI] [PubMed] [Google Scholar]
- 45.Dokras A, Sargent IL, Barlow DH. Human blastocyst grading: An indicator of developmental potential? Hum Reprod. 1993;8:2119–2127. doi: 10.1093/oxfordjournals.humrep.a137993. [DOI] [PubMed] [Google Scholar]
- 46.Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: Toward a single blastocyst transfer. Fertil Steril. 2000;73:1155–1158. doi: 10.1016/s0015-0282(00)00518-5. [DOI] [PubMed] [Google Scholar]
- 47.Chobotova K, et al. Heparin-binding epidermal growth factor and its receptor ErbB4 mediate implantation of the human blastocyst. Mech Dev. 2002;119:137–144. doi: 10.1016/s0925-4773(02)00342-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.