Abstract
The conserved oligomannose epitope, Man9GlcNAc2, recognized by the broadly neutralizing human mAb 2G12 is an attractive prophylactic vaccine candidate for the prevention of HIV-1 infection. We recently reported total chemical synthesis of a series of glycopeptides incorporating one to three copies of Man9GlcNAc2 coupled to a cyclic peptide scaffold. Surface plasmon resonance studies showed that divalent and trivalent, but not monovalent, compounds were capable of binding 2G12. To test the efficacy of the divalent glycopeptide as an immunogen capable of inducing a 2G12-like neutralizing antibody response, we covalently coupled the molecule to a powerful immune-stimulating protein carrier and evaluated immunogenicity of the conjugate in two animal species. We used a differential immunoassay to demonstrate induction of high levels of carbohydrate-specific antibodies; however, these antibodies showed poor recognition of recombinant gp160 and failed to neutralize a panel of viral isolates in entry-based neutralization assays. To ascertain whether antibodies produced during natural infection could recognize the mimetics, we screened a panel of HIV-1-positive and -negative sera for binding to gp120 and the synthetic antigens. We present evidence from both direct and competitive binding assays that no significant recognition of the glycopeptides was observed, although certain sera did contain antibodies that could compete with 2G12 for binding to recombinant gp120.
Keywords: molecular mimicry, neutralizing antibody
The development of an effective prophylactic vaccine against HIV-1 remains an unrealized goal in the effort to contain the current pandemic. Such a vaccine would be defined by its ability to raise broadly neutralizing antibodies against multiple virus clades and isolates. A number of rarely occurring human mAbs, isolated from infected individuals, have demonstrated this desirable neutralization profile (1–3). For several of these mAbs, identification and extensive characterization of the neutralizing epitopes have been realized (4–7). This structural knowledge has been used to design antigens intended to direct the primary immune response toward the neutralizing epitope (8–10). However, in all immunization studies performed to date these vaccine candidates have failed to elicit broadly neutralizing antibodies. One mAb, 2G12, recognizes a clustered, high mannose carbohydrate epitope presented on the gp120 receptor-binding glycoprotein of the virus (11, 12). Crystallographic studies of Fab 2G12 bound to a Man9GlcNAc2 oligosaccharide revealed a unique interlocked VH domain-swapped dimer, which presents an extended high-affinity binding surface optimized for interaction with clustered carbohydrates (13). Although several reports have confirmed the critical role of a Manα1–2Man disaccharide in the 2G12 epitope, the optimal form of a synthetic carbohydrate mimetic of the epitope remains undefined (14–19).
Recently, we have described synthesis of a series of compounds in which Man9GlcNAc2 (1; Fig. 1) was coupled via Lansbury aspartylation to a modular cyclic peptide scaffold 2 designed to afford variability in the number of carbohydrate chains that can be incorporated (20). At the same time, the semirigid nature of the macrocycle serves to position the carbohydrate chains at distances approximating those defined from the crystal studies. Glycopeptides containing zero, one, two, or three oligosaccharides 2-5 were prepared and characterized with regard to their ability to bind 2G12. Only the divalent 4 and trivalent 5 compounds were recognized by the antibody, confirming the importance of multivalent presentation. The peptide scaffold contained a single cysteine residue that was used to effect directed covalent coupling of 4 to a maleimidated carrier protein complex. Subsequent ELISA analysis confirmed recognition of the conjugate by 2G12. Here, we report evaluation of the conjugate as a vaccine immunogen in guinea pigs and rhesus macaques. Robust carbohydrate-specific antibody responses were elicited in both species; however, the immune-sera lacked neutralizing activity and was incapable of effectively blocking binding of 2G12 to recombinant gp120. To assess whether polyclonal sera obtained from HIV-1-infected donors contained antibodies that recognized the mimetics we immobilized glycopeptides 2–4 to microspheres and used these in the context of a multiplexed immunoassay. We discuss the implications of these findings for the design of future carbohydrate-based HIV-1 vaccines.
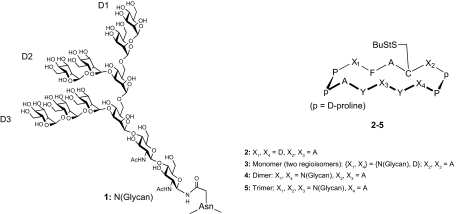
Fig. 1.
Structure of 2G12 epitope Man9GlcNAc2 1 and synthetic glycopeptide derivatives 2-5. The D1–D3 arms of Man9GlcNAc2 are labeled as in ref. 18. The actual composition of 3 was an isomeric mix with alternative placement of the oligomannan on either the X1 or X4 aspartate residue, as indicated.
Results and Discussion
Preparation of Glycopeptide–Outer Membrane Protein Complex (OMPC) Conjugate.
Enhancement of carbohydrate and peptide immunogenicity can be achieved through covalent coupling to antigenic protein carriers. The OMPC carrier used for the current study is a component of a licensed pediatric vaccine, and its ability to promote Ig class switching has been demonstrated (21). In addition, OMPC displays potent adjuvant capabilities as a result of its ability to activate toll-like receptor types 2 and 4 (22, 23). We used 4 as a candidate for vaccine evaluation because quantities of 5 were limiting, and previous surface plasmon resonance data had shown similar binding profiles of 4 and 5 to 2G12 (20). Conjugation of 4 to OMPC was effected via a thiol-maleimide coupling strategy. Incorporation of the activating reagent, sulfosuccinimidyl 4-(N-maleimidomethyl)-cyclohexane-1-carboxylate (sSMCC), was determined by quantitation of tranexamic acid, released upon acid hydrolysis of maleimidated OMPC or conjugate using amino acid analysis. The observed activation level ranged from ≈5,000 to 6,000 mol sSMCC per mol carrier complex. Formation of a covalent bond between cysteinylated peptide and maleimidated carrier produced, upon acid hydrolysis, dicarboxyethylcysteine, which was quantitated as a measure of peptide loading. These values ranged from ≈2,000 to 3,000 mol peptide per mol carrier complex, indicating that ≈50% of available maleimide groups were derivatized.
Animal Immunizations.
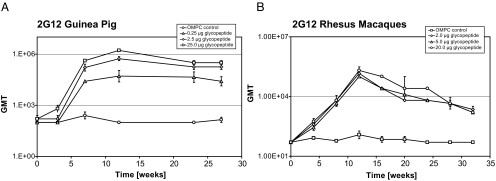
Conjugate or OMPC-only control were adsorbed to aluminum hydroxyphosphate and formulated with QS21 adjuvant for use in vaccination studies. Guinea pigs and rhesus macaques were immunized in a dose-escalating protocol, and bleeds were collected as outlined in Table 1. Antibody response was assessed by ELISA using 4 as coating antigen (Fig. 2). A dose-dependent response was observed in guinea pigs at 0.25- and 2.5-μg doses, whereas a less pronounced increase in titer was observed at 25 μg. At all three doses a boosting effect was observed after the second and third vaccinations. The immune response appeared to be well sustained with little decline in titer out to 27 weeks. These findings agree well with published data for other OMPC-based conjugates (24, 25). In contrast, macaque responses yielded equivalent geometric mean titer (GMT) at all three dosing levels, suggesting that maximum titer was elicited with ≤2 μg conjugated glycopeptide. Interestingly, whereas effective boosting was observed at 4 and 8 weeks, antibody levels appeared to fall off quickly after the third dose and no increase in titer was seen at the 6-month administration, although titers stabilized briefly before starting to again decline at week 28. It had previously been shown that polysaccharide-specific antibody titers elicited by immunization of infant rhesus monkeys with a pneumococcal capsular polysaccharide OMPC conjugate were reduced with increasing doses of conjugate and that this reduction was directly attributable to polysaccharide-induced suppression (26). It is conceivable that a similar repressive response is evidenced in the current study.
Table 1.
Vaccine administration protocols for animal immunogenicity studies
| Vaccine dose | Guinea pig |
Monkey |
||
|---|---|---|---|---|
| Time after prime,weeks | Bleed, weeks | Time after prime,weeks | Bleed, weeks | |
| Prime | 0 | 3 | 0 | 3 |
| Boost | 4 | 7 | 4 | 8 |
| Boost | 8 | 12 | 8 | 12 |
| – | 23 | – | 16 | |
| – | 27 | – | 20 | |
| Boost | NA | NA | 24 | 28 |
| – | 32 | |||
NA, not applicable.
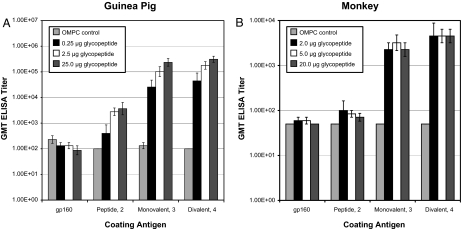
Fig. 2.
Antibody response to glycopeptide–OMPC conjugate vaccine. Serum antibody titers from guinea pigs (A) and rhesus macaques (B) immunized with conjugate or control were measured by ELISA using divalent glycopeptide 4 as coating antigen. Data points correspond to bleed schedules indicated in Table 1. For macaques, T = 0 data were not collected, so the GMT was set at the assay reciprocal dilution limit value of 50.
Previously we showed that 4, when conjugated to OMPC, retained its ability to bind 2G12, suggesting that the critical divalent carbohydrate epitope was not adversely compromised by the coupling reaction (20). To qualitatively ascertain the proportion of the immune response that was directed toward this conformation, we performed a differential ELISA in which the highest titer sera was analyzed independently against 2, 3, 4, and gp160 (Fig. 3). The week-23 response in guinea pigs (Fig. 3A) showed measurable titer against the peptide backbone 2 relative to the OMPC-only control at the 2.5- and 25-μg dosing levels. The bulk of the response, however, was carbohydrate-directed as judged by specific titers to glycopeptides 3 and 4. At the 0.25-μg dose the GMT against either 3 or 4 was ≈10-fold higher relative to the peptide-specific response induced by immunization with 25 μg of conjugated glycopeptide, whereas titers at 2.5 and 25 μg were nearly 100-fold higher. A statistical analysis (Student's t test) of the aggregate data revealed a significant difference between either monovalent or divalent oligosaccharide relative to the peptide backbone (P < 0.0001). However, the difference was not statistically significant in the response measured for 3 versus 4 (P = 0.1036). Given that 2G12 exhibits preferential binding for the divalent carbohydrate antigen, this finding suggests that a significant “2G12-like” response was not mounted in the guinea pig. This conclusion is further corroborated by the observation that the immune sera did not specifically recognize gp160. In macaques (Fig. 3B), the week-28 response to 2 appeared to be nearly absent, perhaps masked by the presence of carbohydrate in a manner analogous to the physiologic role of immune evasion often mediated by oligosaccharides (27). Here, too, the magnitude of response to the carbohydrate as presented on 3 or 4 was robust; and although qualitatively there is a suggestion of a divalent-specific response, the difference in titers did not achieve statistical significance (P = 0.0819). In contrast, the induction of carbohydrate-specific titers relative to peptide-specific titers achieved significance (P = 0.0009) at all dose levels. As was seen in the guinea pigs, the sera did not bind to gp160, suggesting that the synthetic epitope-mimetics did not authentically represent the 2G12 epitope.
Fig. 3.
Specificity analysis of antibody response to glycopeptide–OMPC conjugate vaccine. Serum collected at the indicated time point after final booster administration was assessed by differential ELISA for its ability to bind recombinant purified gp160 or synthetic glycopeptides 2–4. (A) Guinea pig sera collected at week 23, postdose 3. (B) Macaque sera collected at week 28, postdose 4.
Neutralization of Virus.
Sera from high-titer guinea pig (week 23) and macaque (weeks 12, 16, 20, and 24) was tested in in vitro neutralization assays as both neat sera or fractionated total IgG. Neither guinea pig or macaque sera showed any ability to neutralize clade B isolate HXB2 in a p4–2/R5 entry inhibition assay. Macaque sera were further tested in a TZM-bl pseudovirion entry inhibition assay against two clade B strains with known sensitivity to 2G12. No specific neutralization was observed in any group (data not shown).
Serum Antibody Analysis by Multiplexed Immunoassay.
To evaluate whether the polyclonal antibody repertoire generated in response to natural HIV-1 infection contained antibodies that recognized our synthetic antigens, we developed a bead-based multiplexed immunoassay format to test a panel of sera obtained from HIV-1-positive and -negative donors in a direct binding assay. We first confirmed the ability of phycoerythrin (PE)-labeled 2G12 to differentially recognize immobilized divalent and trivalent glycopeptides [supporting information (SI) Fig. S1]. PE-2G12 bound immobilized 4 with ≈5-fold and 20-fold higher affinity than 3 and 2, respectively. It is interesting to note that in this format 3 shows weak binding, whereas earlier results reported that the same oligosaccharide structure on a linear cysteinylated peptide was not recognized by 2G12 until dimerized via oxidation of the unprotected thiol (14). One explanation may be that loading density on the microspheres can bring certain carbohydrate chains into proximity to present a pseudoextended binding surface that is capable of recognition by 2G12. However, the antibody's preference for gp120 is readily evidenced by the 3.5-fold higher response relative to 4. The observation that partial gp120 mimicry is realized only by 4 is confirmed by a competition binding study in the multiplexed format (Fig. S2) where soluble gp120 prevents binding of PE-2G12 to 4 in a dose-dependent manner.
There are several ways of interpreting the aggregate results from immunization and antigen-binding studies. In terms of direct binding, the strong preference for gp120 can reflect either enhanced avidity or affinity for carbohydrates presented on the protein relative to those on the synthetic scaffold. Glycosylation on gp120 can account for >50% of the molecular mass (28), and it is known that multiple N-linked glycosylation sites are associated with 2G12 binding (11, 29). 2G12-resistant viruses have been shown to associate with mutations in these glycosylation sites (30, 31), and a recent study using such viruses has suggested that the antibody's antiviral efficacy across clades may be narrower than originally claimed (32). Sequence analysis indicated that three glycosylation sites (N295, N332, and N392) were critical for interaction while several others contributed to binding (12). Thus, although the presence of dimeric Man9GlcNAc2 may be sufficient for engagement of 2G12, overall epitope density on gp120 may make it a far more effective competitor. Conversely, if the enhanced binding to gp120 reflects a true affinity difference, it can be postulated that 4 is an insufficient mimotope of the native structure, most likely because of a lack of conformational constraint. Intriguingly, an “induced-fit” model of binding can be postulated to explain why the epitope-mimetics bind 2G12 but do not induce a similar class of antibody when used as an immunogen. In this model, the structure of the oligomannan chains on the mimetic resemble but are not exactly the same as the authentic 2G12 epitope on gp120. It is conceivable that their extended conformation is sufficient to engage 2G12 weakly after which binding induces a conformational change toward a more authentic and stable structure. However, in the OMPC–glycopeptide conjugate there is no external pressure to assume the correct conformation, and, as a result, the majority of the polyclonal response is directed against nonrelevant epitope conformations.
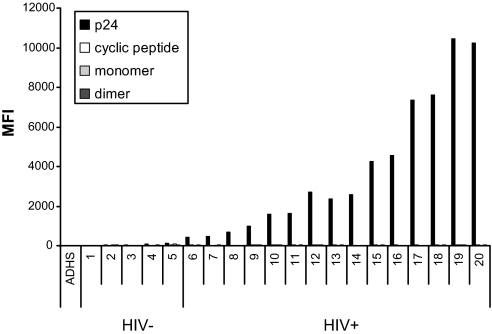
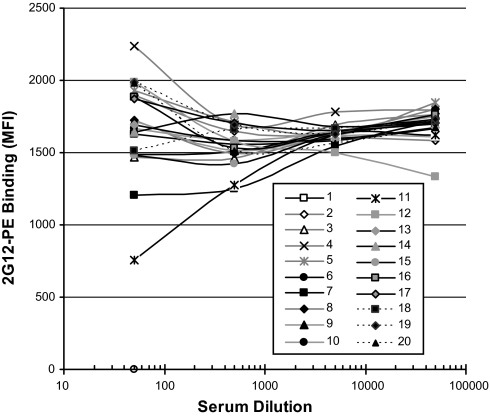
Analysis of the human sera panel is presented in Fig. 4. HIV-1 positivity was confirmed by detection of antibodies against p24 in donors 6–20, and these are ranked based on increasing titer. In contrast, no serum from either HIV-1-negative or -positive donors showed any binding to the synthetic mimetics. To confirm the expected low seroprevalence of 2G12-like antibodies, we tested the panel in a competition binding experiment with PE-2G12 (Fig. 5). Of 15 HIV-1-positive sera, only two (donors 7 and 11) appeared to contain low titers of antibodies that competed with PE-2G12. This result is in good agreement with previously published seroprevalence data (11) and correlates with the binding results discussed above.
Fig. 4.
Antibodies from HIV-1-positive individuals do not bind synthetic 2G12 epitope mimetics. A panel of HIV-1-negative (n = 5) and HIV-1-positive (n = 15) sera was incubated with a four-plex microsphere mixture containing immobilized HIV-1 p24 protein, and synthetic compounds 2–4. Bound antibody was detected by using a PE-labeled mouse anti-human IgG.
Fig. 5.
Detection of 2G12-like antibodies in HIV-1 patient sera. HIV-1-negative and -positive sera was tested in a competitive binding multiplexed immunoassay to determine whether any component of the antibodies elicited during natural infection could compete with PE-labeled 2G12 for binding to immobilized gp120. In this format, a decrease in median fluorescence intensity indicates the presence of competitive-binding antibodies.
Our results suggest that presentation of Man9GlcNAc2 on the constrained cyclic scaffold herein described is insufficient to induce a polyclonal response that recognizes the native 2G12 epitope. Inability to induce a response to the oligomannan is not the reason for this failure because we clearly show that the conjugate elicited a robust carbohydrate-specific antibody response. Our results corroborate recently published work (19) in which synthetic oligomannans were coupled to a variety of carriers and tested as potential vaccine candidates. Although in this study no attempt was made to mimic the proposed epitope structure, the conjugates showed some ability to bind 2G12. However, carbohydrate-specific titers in immunized rabbits were low, likely because of an immunodominant response to the chemical linkers used in coupling, and the sera lacked any neutralization potency. In contrast, we show by differential ELISA that a major component of the immune response can be directed toward the carbohydrate portion of the glycopeptide, although the lack of gp160 recognition strongly suggests that the majority of this response is to irrelevant conformations of the oligosaccharide. This hypothesis is further strengthened by the observation that divalent glycopeptide 4 is unable to effectively displace gp120 bound to PE-2G12. Our serum panel assessment confirmed the relative rarity of 2G12-like responses among infected patients, but, more importantly, it showed that the mimetics were not recognized even by the small subset of individuals who elicited antibodies capable of competing with 2G12. This finding suggests that the synthetic structures, while able to bind 2G12 in in vitro assays, do not accurately mimic the carbohydrate epitope on an infectious HIV-1 virion. Earlier work dissecting the 2G12 epitope specificity suggested that the size of the oligomannan necessary for 2G12 recognition could be reduced and that truncated oligosaccharides such as Manα1–2Manα1–2Man or Manα1–2Manα1–6Man might form the basis of a less complex immunogen (18). Our present study would appear to suggest the opposite; that is, incorporation of Man9GlcNAc2, even in clustered patterns that provide good binding surfaces for 2G12, is, by itself, insufficient to function as an effective immunogen. Several potential explanations for this observation may be offered. The failure to induce carbohydrate-specific responses has already been addressed. Second, the animal species in which immunization studies are performed may be incapable of generating the unique domain-exchanged structure of 2G12. Although we did not address this possibility directly, the comparable results obtained in rodent and nonhuman primates suggests that this is unlikely. A more plausible cause is that conformational flexibility in the oligomannan arms is still high, and the immune response is “diluted” by recognition of improper structures within the chemical space. This complicating factor has been proposed as a reason for the inability to mimic the broad neutralizing characteristics of other HIV-1-directed mAbs (4, 10, 33).
Several recent publications have attempted to address the question of optimal epitope presentation. Wang et al. (34) reported use of a cyclic peptide scaffold similar to the one we describe for presentation of the D1 arm of the 2G12 epitope. Surface plasmon resonance studies of 2G12 binding to this construct yielded results nearly identical to ours in that the effect of clustering on enhanced antibody recognition was clearly evident. Similarly, synthetic multivalent dendrons presenting Man4 or Man9 oligosaccharides were shown to induce good binding of 2G12 and have been proposed as potential vaccine components (35). Although such approaches to “direct” the immune response to more relevant antibody generation are important, a successful strategy will likely need to encompass a method to “lock” the optimal oligosaccharide conformation in a preferred orientation. Continuing advances in synthetic carbohydrate chemistry offer several routes to such structures in which cross-links of defined length and rigidity can be introduced between two or more oligomannose chains, wherein the spacing can be informed by crystallographic data.
Conclusion
Despite the lack of a functional immune response to our candidate vaccine, we believe the work herein described is important in that: (i) immunogen design was informed by crystallographic data of 2G12 bound to its epitope; and (ii) competition-binding data definitively shows that although multivalent mimetics can bind 2G12 they are poor competitors for binding of the mAb to native, gp120. This finding strongly suggests that even in a clustered presentation there is sufficient flexibility for the glycan chains to adopt a suboptimal conformation. (iii) Our vaccine induced a robust carbohydrate-specific response higher in magnitude relative to nonspecific scaffold or linker responses. The observation that no significant specificity for the divalent oligomannan was observed relative to the monomeric construct suggests a reason for the failure to elicit a neutralizing response. Importantly, it also points a way toward future attempts to improve immunogen design.
Materials and Methods
Glycopeptide Synthesis.
Synthesis of glycopeptides 2–5 has been described (20). Briefly, glycosylamine 1-NH2 was aspartylated with peptides containing either two or three aspartate side chains in a convergent protocol to yield the corresponding divalent and trivalent glycopeptides 4 and 5. Monovalent glycopeptide 3 was isolated as a minor incomplete coupling product from the reaction that yielded 4.
Preparation of OMPC–Glycopeptide Conjugate.
Purified OMPC of Neisseria meningitidis serotype B (Merck) was maleimidated on a portion of its surface-accessible lysine residues by reaction with sSMCC as described (10). The cysteinyl form of 4 was generated by treatment with excess mercaptoethanesulfonic acid and sodium salt, and the purified lyophilized product was stored under vacuum. Cysteinyl glycopeptide was solubilized in Ar-degassed 0.1 M Hepes (pH 7.3), 2 mM EDTA at 2 mg/ml and mixed with maleimidated OMPC at a 1.5:1 thiol-to-maleimide mole ratio. Reaction proceeded overnight at 4°C in the dark after which residual carrier maleimide groups were quenched with 2-mercaptoethanol. Unreacted glycopeptide and reagents were removed by dialysis. A portion of the maleimidated OMPC was carried through the process without addition of glycopeptide to serve as a reaction control. The amount of covalently incorporated glycopeptide was determined by quantitation of dicarboxyethylcysteine using amino acid analysis (36).
Animal Immunizations.
All animal experiments were approved by Merck Research Laboratories' Institutional Animal Care and Use Committee. OMPC-only control and conjugate were adsorbed onto aluminum hydroxyphosphate (Merck alum), and the saponin-based QS21 adjuvant (Antigenics) was added to a final concentration of 100 μg/ml. Female Duncan Harley guinea pigs (Covance Research Laboratories) were maintained in accordance with the institutional animal care protocols of Merck Research Laboratories. Vaccines were delivered i.m., in 0.2-ml aliquots into both quadriceps according to the specified schedule. Dose ranging was performed at 0, 0.25, 2.5, and 25 μg of glycopeptide with six animals per group. Sera were prepared from blood samples collected as specified in Table 1. For nonhuman primate studies, Indian rhesus macaques were maintained in accordance with the institutional animal care protocols of Merck Research Laboratories and New Iberia Research Center (New Iberia, LA). Vaccines were delivered i.m., in 0.5-ml aliquots into both deltoids at the specified schedule. Dose ranging was performed at 0, 2, 5, and 20 μg of glycopeptide with four animals per group. Sera were prepared from blood samples collected as specified in Table 1. All animals were monitored and cared for under guidelines established by the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Serum ELISA.
Maxisorp 96-well plates (NUNC) were incubated with 10 ng (2, 3, or 4) in PBS at 4°C overnight. Plates were washed with PBS containing 0.05% Tween-20 (WB) and blocked with 1% BSA in WB for 2 h at room temperature. Plates were washed as above, and 100 μl of a 4-fold immuneserum dilution series was added and the plates were incubated for 2 h at room temperature. After washing, the appropriate HRP-conjugated secondary antibody was added and plates were incubated for 1 h at room temperature. Plates were washed and 100 μl of 1,2 phenylenediamine dihydrochloride solution was added and incubated for 10 min at room temperature followed by 100 μl of 0.5 M H2SO4. The absorbance at 492 nm was read with an ELISA plate reader.
Neutralization Assay.
The p4–2/R5 single-cycle entry inhibition assay using HIV-1 clade B strain HXB2 has been described (33). Selected macaque sera were also tested in a TZM-bl reporter cell entry inhibition assay (37) against HIV-1 clade B strains SF162 and TRO. The IgG fraction was purified from neat sera by using batch protein A Sepharose affinity chromatography, buffer-exchanged into DMEM cell culture, and concentrated 5-fold. Total protein was determined by using a commercial bicinchoninic acid assay (Pierce).
Immobilization of Antigens on Microspheres.
Commercially purchased HIV-1 p24, gp41, and gp120 were conjugated to carboxylated Xmap microspheres (Radix) as described (38). Lyophilized mimetics 2–5 (750 μg) were solubilized in 0.5 ml of dimethylformamide, diluted to 1.0 ml with 50 mM Mes, pH 6.5, and incubated with maleimidated microspheres for 3 h in the dark. Residual maleimide sites were quenched with 0.1 M cysteine for 2 h. The beads were washed twice with PBS and stored in PBS containing 1% BSA. 2G12 antibody was purchased from Polymun Scientific and was PE-labeled (PE-2G12) by standard protocols.
Multiplexed Epitope Mapping Serology.
Fifteen confirmed HIV-1-positive sera and five confirmed HIV-1-negative sera were randomly chosen from a panel purchased from serum brokers. Sera were diluted 1:10, 1:100, 1:1,000, and 1:10,000 in antibody-depleted human serum (ADHS) and incubated for 2 h at room temperature with a four-plex mixture of immobilized p24, 2, 3, and 4 at 2,500 microspheres per well, per antigen. Beads were washed and a secondary human anti-IgG-PE mAb (HP6043, Biotrend) was added to a final concentration of 2.5 μg/ml. Beads were incubated 30 min at room temperature and washed, and bound fluorescence was quantitated on a BioPlex100 instrument (Bio-Rad).
2G12 Competitive Immunoassay.
2G12-PE was added at a final concentration of 2.5 μg/ml to a dilution series of HIV-1-positive and -negative sera (1:50, 1:500, 1:5,000, and 1:50,000) in ADHS. Immobilized gp120 microspheres (5,000) were added to the mixture and incubated overnight at room temperature. Beads were washed, and bound fluorescence was quantitated on a BioPlex100 instrument.
Supplementary Material
Acknowledgments.
We thank Dr. D Montefiori (Duke University, Durham, NC) for neutralization testing of immunesera, R. Hepler (Merck Research Laboratories) for serum purifications, M. Davies (Merck Research Laboratories) for rhesus macaque immunizations, and L. Stansberry (Merck Research Laboratories) for statistical analysis. Financial support for this research was provided by the National Institutes of Health Grant CA103823 (to S.J.D.).
Footnotes
Conflict of interest statement: This paper is a joint contribution from the Merck, Sharpe, and Donne Research Laboratories and the Memorial Sloan-Kettering Research Institute. S.J.D. is a consultant in organic chemistry at Merck. However, his consultantship is in medicinal chemistry and does not extend to the matters in this program. J.G.J., H.C.S., D.W.O., K.M.G., D.D.N., M.T.E., R.H., M.F., V.Y.D., M.C., and J.W.S. are all current or former employees of Merck and Company and were employed by Merck when the research was performed.
This article contains supporting information online at www.pnas.org/cgi/content/full/0807837105/DCSupplemental.
References
- 1.Srivastava IK, Ulmer JB, Barnett SW. Role of neutralizing antibodies in protective immunity against HIV. Hum Vaccines. 2005;1:45–60. doi: 10.4161/hv.1.2.1764. [DOI] [PubMed] [Google Scholar]
- 2.Haigwood NL, Stamatatos L. Role of neutralizing antibodies in HIV infection. AIDS. 2003;17(Suppl 4):S67–S71. doi: 10.1097/00002030-200317004-00008. [DOI] [PubMed] [Google Scholar]
- 3.Stiegler G, Katinger H. Therapeutic potential of neutralizing antibodies in the treatment of HIV-1 infection. J Antimicrob Chemother. 2003;51:757–759. doi: 10.1093/jac/dkg172. [DOI] [PubMed] [Google Scholar]
- 4.Chakraborty K, et al. Design of immunogens that present the crown of the HIV-1 V3 loop in a conformation competent to generate 447–52D-like antibodies. Biochem J. 2006;399:483–491. doi: 10.1042/BJ20060588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardoso RM, et al. Broadly neutralizing anti-HIV antibody 4E10 recognizes a helical conformation of a highly conserved fusion-associated motif in gp41. Immunity. 2005;22:163–173. doi: 10.1016/j.immuni.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 6.Ofek G, et al. Structure and mechanistic analysis of the anti-human immunodeficiency virus type 1 antibody 2F5 in complex with its gp41 epitope. J Virol. 2004;78:10724–10737. doi: 10.1128/JVI.78.19.10724-10737.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darbha R, et al. Crystal structure of the broadly cross-reactive HIV-1-neutralizing Fab X5 and fine mapping of its epitope. Biochemistry. 2004;43:1410–1417. doi: 10.1021/bi035323x. [DOI] [PubMed] [Google Scholar]
- 8.Saphire EO, et al. Structure of a high-affinity “mimotope” peptide bound to HIV-1-neutralizing antibody b12 explains its inability to elicit gp120 cross-reactive antibodies. J Mol Biol. 2007;369:696–709. doi: 10.1016/j.jmb.2007.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franke R, Hirsch T, Eichler J. A rationally designed synthetic mimic of the discontinuous CD4-binding site of HIV-1 gp120. J Recept Signal Transduction. 2006;26:453–460. doi: 10.1080/10799890600923179. [DOI] [PubMed] [Google Scholar]
- 10.McGaughey GB, et al. HIV-1 vaccine development: constrained peptide immunogens show improved binding to the anti-HIV-1 gp41 MAb. Biochemistry. 2003;42:3214–3223. doi: 10.1021/bi026952u. [DOI] [PubMed] [Google Scholar]
- 11.Trkola A, et al. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J Virol. 1996;70:1100–1108. doi: 10.1128/jvi.70.2.1100-1108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scanlan CN, et al. The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of α1–>2 mannose residues on the outer face of gp120. J Virol. 2002;76:7306–7321. doi: 10.1128/JVI.76.14.7306-7321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calarese DA, et al. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science. 2003;300:2065–2071. doi: 10.1126/science.1083182. [DOI] [PubMed] [Google Scholar]
- 14.Dudkin VY, et al. Toward fully synthetic carbohydrate-based HIV antigen design: On the critical role of bivalency. J Am Chem Soc. 2004;126:9560–9562. doi: 10.1021/ja047720g. [DOI] [PubMed] [Google Scholar]
- 15.Geng X, Dudkin VY, Mandal M, Danishefsky SJ. In pursuit of carbohydrate-based HIV vaccines, part 2: The total synthesis of high-mannose-type gp120 fragments–evaluation of strategies directed to maximal convergence. Angew Chem Int Ed. 2004;43:2562–2565. doi: 10.1002/anie.200353626. [DOI] [PubMed] [Google Scholar]
- 16.Mandal M, Dudkin VY, Geng X, Danishefsky SJ. In pursuit of carbohydrate-based HIV vaccines, part 1: The total synthesis of hybrid-type gp120 fragments. Angew Chem Int Ed. 2004;43:2557–2561. doi: 10.1002/anie.200353625. [DOI] [PubMed] [Google Scholar]
- 17.Wang LX, Ni J, Singh S, Li H. Binding of high-mannose-type oligosaccharides and synthetic oligomannose clusters to human antibody 2G12: Implications for HIV-1 vaccine design. Chem Biol. 2004;11:127–134. doi: 10.1016/j.chembiol.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 18.Calarese DA, et al. Dissection of the carbohydrate specificity of the broadly neutralizing anti-HIV-1 antibody 2G12. Proc Natl Acad Sci USA. 2005;102:13372–13377. doi: 10.1073/pnas.0505763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ni J, Song H, Wang Y, Stamatos NM, Wang LX. Toward a carbohydrate-based HIV-1 vaccine: Synthesis and immunological studies of oligomannose-containing glycoconjugates. Bioconjugate Chem. 2006;17:493–500. doi: 10.1021/bc0502816. [DOI] [PubMed] [Google Scholar]
- 20.Krauss IJ, et al. Fully synthetic carbohydrate HIV antigens designed on the logic of the 2G12 antibody. J Am Chem Soc. 2007;129:11042–11044. doi: 10.1021/ja074804r. [DOI] [PubMed] [Google Scholar]
- 21.Donnelly JJ, Deck RR, Liu MA. Immunogenicity of a Hemophilus influenzae polysaccharide–Neisseria meningitidis outer membrane protein complex conjugate vaccine. J Immunol. 1990;145:3071–3079. [PubMed] [Google Scholar]
- 22.Latz E, Franko J, Golenbock DT, Schreiber JR. Hemophilus influenzae type b-outer membrane protein complex glycoconjugate vaccine induces cytokine production by engaging human toll-like receptor 2 (TLR2) and requires the presence of TLR2 for optimal immunogenicity. J Immunol. 2004;172:2431–2438. doi: 10.4049/jimmunol.172.4.2431. [DOI] [PubMed] [Google Scholar]
- 23.Mirlashari MR, Lyberg T. Expression and involvement of Toll-like receptors (TLR)2, TLR4, and CD14 in monocyte TNF-α production induced by lipopolysaccharides from Neisseria meningitidis. Med Sci Monit. 2003;9:BR316–B324. [PubMed] [Google Scholar]
- 24.Fan J, et al. Preclinical study of influenza virus A M2 peptide conjugate vaccines in mice, ferrets, and rhesus monkeys. Vaccine. 2004;22:2993–3003. doi: 10.1016/j.vaccine.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 25.Joyce J, et al. Immunogenicity and protective efficacy of Bacillus anthracis poly-γ-d-glutamic acid capsule covalently coupled to a protein carrier using a novel triazine-based conjugation strategy. J Biol Chem. 2006;281:4831–4843. doi: 10.1074/jbc.M509432200. [DOI] [PubMed] [Google Scholar]
- 26.McNeely TB, Liu X, Bringman T, Donnelly JJ. Effect of individual conjugate dose on immunogenicity of type 6B pneumococcal polysaccharide–N. meningitidis outer membrane protein complex conjugate vaccines in infant rhesus monkeys. Vaccine. 2000;18:2808–2816. doi: 10.1016/s0264-410x(00)00082-7. [DOI] [PubMed] [Google Scholar]
- 27.Vigerust DJ, Shepherd VL. Virus glycosylation: role in virulence and immune interactions. Trends Microbiol. 2007;15:211–218. doi: 10.1016/j.tim.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Botarelli P, et al. N-glycosylation of HIV-gp120 may constrain recognition by T lymphocytes. J Immunol. 1991;147:3128–3132. [PubMed] [Google Scholar]
- 29.Wyatt R, et al. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature. 1998;393:705–711. doi: 10.1038/31514. [DOI] [PubMed] [Google Scholar]
- 30.Balzarini J, et al. Marked depletion of glycosylation sites in HIV-1 gp120 under selection pressure by the mannose-specific plant lectins of Hippeastrum hybrid and Galanthus nivalis. Mol Pharmacol. 2005;67:1556–1565. doi: 10.1124/mol.104.005082. [DOI] [PubMed] [Google Scholar]
- 31.Nabatov AA, et al. Intrapatient alterations in the human immunodeficiency virus type 1 gp120 V1V2 and V3 regions differentially modulate coreceptor usage, virus inhibition by CC/CXC chemokines, soluble CD4, and the b12 and 2G12 monoclonal antibodies. J Virol. 2004;78:524–530. doi: 10.1128/JVI.78.1.524-530.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huskens D, Van Laethem K, Vermeire K, Balzarini J, Schols D. Resistance of HIV-1 to the broadly HIV-1-neutralizing, anticarbohydrate antibody 2G12. Virology. 2007;360:294–304. doi: 10.1016/j.virol.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 33.Joyce JG, et al. Enhancement of α-helicity in the HIV-1 inhibitory peptide DP178 leads to an increased affinity for human monoclonal antibody 2F5 but does not elicit neutralizing responses in vitro: Implications for vaccine design. J Biol Chem. 2002;277:45811–45820. doi: 10.1074/jbc.M205862200. [DOI] [PubMed] [Google Scholar]
- 34.Wang J, Li H, Zou G, Wang LX. Novel template-assembled oligosaccharide clusters as epitope mimics for HIV-neutralizing antibody 2G12: Design, synthesis, and antibody binding study. Org Biomol Chem. 2007;5:1529–1540. doi: 10.1039/b702961f. [DOI] [PubMed] [Google Scholar]
- 35.Wang SK, et al. Targeting the carbohydrates on HIV-1: Interaction of oligomannose dendrons with human monoclonal antibody 2G12 and DC-SIGN. Proc Natl Acad Sci USA. 2008;105:3690–3695. doi: 10.1073/pnas.0712326105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nahas DA, Palladino JS, Joyce JG, Hepler RW. Amino acid analysis of peptide loading ratios in conjugate vaccines: A comparison of direct electrochemical detection and 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate precolumn derivatization methods. Bioconjugate Chem. 2008;19:322–326. doi: 10.1021/bc700232z. [DOI] [PubMed] [Google Scholar]
- 37.Polonis VR, et al. Recent advances in the characterization of HIV-1 neutralization assays for standardized evaluation of the antibody response to infection and vaccination. Virology. 2008;375:315–320. doi: 10.1016/j.virol.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 38.Opalka D, et al. Analysis of the HIV-1 gp41 specific immune response using a multiplexed antibody detection assay. J Immunol Methods. 2004;287:49–65. doi: 10.1016/j.jim.2004.01.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.