Abstract
We have performed an expression cloning screen in Xenopus laevis with the aim of isolating novel gene activities from the neural plate. Out of 8064 clones screened we isolated 61 clones that affected either neural plate patterning or tadpole morphology. Of these, 20 clones encoded RNA binding proteins, and the majority of these are hnRNP or SR-proteins, which are associated with alternative splicing. All of these genes are expressed in the nervous system, and in several cases specific to neural tissue. Injecting mRNA encoding these proteins results in neural plate mispatterning and abnormal axial muscle segmentation. To initiate characterization of these proteins we selected Rbmx as a candidate for deeper analysis. Using morpholino mediated knockdown, we show that Rbmx is necessary for normal anterior neural plate patterning, neurogenesis, neural crest development, and axial muscle segmentation.
Expression cloning experiments in the African clawed frog Xenopus laevis have provided numerous insights into developmental mechanisms and have provided ways to identify genes that are involved in the process of neural induction and neural patterning. Examples include the organizer-expressed BMP antagonists that are essential for neural induction, and modulators of the Wnt pathway, that are required for anterior development (Smith and Harland, 1992; Smith et al., 1995; Glinka et al., 1998).
The majority of genes that have been isolated in expression cloning screens have brought focus to early events in embryogenesis such as neural and mesodermal induction and initial regional patterning. Expression cloning from libraries made from whole embryos has tended to identify potent factors expressed in the mesoderm. However, much of the patterning of the neural plate involves local interactions within the tissue. It was with the assumption that there likely are many other types of genes encoding intracellular signaling, scaffolding, RNA-binding, adapter, cytoskeletal, and functionally yet uncharacterized proteins involved in development that we constructed a neural plate cDNA library from the gastrula suitable for expression cloning. After injecting 8064 transcribed cDNAs in sets of 48, we isolated 61 active single clones that caused changes in neural gene expression or tadpole morphology. Phenotypes ranged from mispatterning of the nervous system, defects in mesoderm formation, defects in morphogenesis, secondary axis induction, and cell death. Surprisingly, and in contrast to experiments with whole embryo RNA, one third of the active clones were previously identified or putative RNA-binding proteins. This prevalence of RNA-binding factors suggests that selective RNA splicing or metabolism may be particularly important in the early development of the central nervous system.
Among the RNA-binding proteins we identified in our screen, we selected one, Rbmx (also known as hnRNP-G), for deeper characterization. This gene has been studied in zebrafish where knock-down of Rbmx leads to brain defects, impaired axial muscle segmentation, and defects in jaw cartilage formation (Tsend-Ayush et al., 2005). In Xenopus, the transcript is found more broadly in the embryo. Loss of function in X. laevis therefore provides information about the similarities and differences in function across vertebrate species and by extension RNA metabolism as a biological regulator. Here we show that not only does overexpression cause a phenotype, but MO mediated knockdown causes defects in anterior neural patterning, neural differentiation, and axial muscle segmentation. Our results support the idea that RNA-binding proteins and the processes they regulate, notably pre-mRNA splicing, are likely to be important for proper embryogenesis, and particularly neural development.
Materials and Methods
Embryo culture and microinjections
Xenopus laevis eggs were collected, fertilized, and embryos cultured and microinjected by standard procedures (Sive et al., 2000). Embryos were staged according to the normal table (Nieuwkoop and Faber, 1994).
Neural plate library construction and screening
Neural plate tissue from stage 11.5–12.0 embryos was excised and placed in 10 volumes of Trizol and frozen in liquid N2. RNA was isolated using a standard Trizol reagent (Invitrogen) protocol with an additional chloroform extraction and multiple 75% ethanol washes. Purity and integrity of the RNA was assessed using spectrophotometric analysis at A260/A280 and Northern analysis. PolyA RNA was isolated using the FastTrack 2.0 kit (Invitrogen). We used the Superscript Plasmid System for cDNA Synthesis (Invitrogen) with 3 μg polyA+ RNA as starting material. Radiolabeling was used to quantify both 1st strand cDNA and 2nd strand cDNA yield and a 30 cm column with sepharose CL-4B resin was used to size fractionate the cDNA. Double stranded cDNA was cloned into the NotI and SalI sites of pCS108 vector and used for transformation by electroporation. After plating, approximately 37,000 clones were arrayed into 384-well plates at the DOE Joint Genome Institute (Walnut Creek, CA). For screening, sets of 48 clones were picked using a 48-pin replicator and grown on LB/ampicillin dishes for 24 hours. Bacteria were harvested, plasmid mini-prepped and linearized with AscI restriction endonuclease. Capped mRNA was synthesized using the mMessage mMachine SP6 kit (Ambion). Two-cell stage embryos were injected with 2.5 ng of pooled synthetic mRNA, corresponding to approximately 50 pg from each clone. Active pools were split into 6 sets of 8 mRNAs, and ultimately, active single clones were selected. The identity of active clones was determined by sequencing and BLAST searches against databases. Single synthetic mRNAs (50pg) were injected with 100 pg of nuclear lacZ mRNA as a tracer (from nuc-beta-gal-CS2+; (Turner and Weintraub, 1994)).
Whole mount RNA in situ hybridization
Staining for lacZ tracer preceded staining for RNA by in situ hybridization (Sive et al. 2000). After fixation for 30 minutes in MEMFA with 3.7% formaldehyde and washing in PBS + 0.1% tween-20, tracer was visualized using Red-Gal (Research Organics; Sive et al. 2000); after staining, embryos were refixed for 2 hours and dehydrated in methanol. The following probes have been described previously: Xbra (Smith et al., 1991), MyoD (Hopwood et al., 1989), Pax2 (Heller and Brandli, 1997), Slug (Richter et al., 1988), N-tubulin (Richter et al., 1990), Pax6 (Hirsch and Harris, 1997), Sox2 (Grammer et al., 2000), Noggin (Smith and Harland, 1992), Xrx1 (Casarosa et al., 1997), En1 (Eizema et al., 1994), Lim1 (Taira et al., 1994), and Lbx1 (Martin and Harland, 2006). Probe for Otx5 was synthesized from clone 6O13 (neural plate library; this study) linearized with SalI and transcribed with T7.
Immunohistochemistry
Embryos were collected, fixed in MEMFA with 3.7% formaldehyde, and processed as described previously (Sive et al., 2000). Primary mouse monoclonal antibodies used were 6F11 (Lamb et al., 1993) diluted 1:10, and 12–101 (Kintner and Brockes, 1984) undiluted. Secondary HRP-conjugated donkey anti-mouse antibody (Jackson Labs) was used diluted 1:500 and visualized using diaminobenzidine (Sigma) as substrate.
Morpholino knockdown of Rbmx
For knockdown of Rbmx (hnRNP-G) we designed a morpholino oligonucleotide (MO) (GeneTools), with the sequence 5′-GGGCGGTCAGCTTCCACCATGGTGA-3′, that binds to the translation start site of the two X. laevis alloalleles of Rbmx. One or two blastomeres at the 2-cell stage was injected with 30–40 ng MO together with a flourescein labeled standard control MO as lineage tracer. Before fixation, the embryos were evaluated by fluorescence from the control MO and, if unilaterally injected, sorted according to which side was injected.
RESULTS
We used an early neural plate cDNA library to screen for genes with ability to affect embryonic development. We chose to construct a neural plate library with the primary aim to identify genes involved in neural patterning, but also to avoid the many potent factors that have previously been identified and are expressed in the mesoderm. Initially we screened for active pools by performing in situ hybridization for regional neural markers as well as differentiation markers (probes used were: Xrx1, N-tubulin, Pax3, Nkx6.2, Krox20, Dbx1, Slug, Shh, and Pax2). However, we realized that essentially all active clones that could be isolated using this approach could also be detected by changes in morphology if the embryos were cultured to stage 39. All of the active pools caused defects such as prominent kinking of the axis, spina bifida, or defects in head morphology, such as reduction of eye pigment. Such phenotypes serve as a simple initial reporter of strong bioactivity, but by themselves do not yield specific insight into the function of the genes, which must be dissected in more detail using gene specific probes, or observations of cell behavior. Nonetheless, this straightforward approach was effective for isolation of active clones. A number of pools resulted in apoptosis, cell death, edema, or epithelial disruptions. These were not investigated further and are not included in this study.
Using this strategy we injected 168 pools of 48 clones (8064 total) and screened for changes in neural plate patterning. This identified 61 different clones representing 56 different genes whose overexpression significantly affect X. laevis development. These genes can be classified based on their domain structure or biological activity (Table 1). In contrast to previous screens we only identified one cDNA clone that encoded a secreted protein (Table 1; Group A; Derriere, clone 21L16). In contrast we isolated 12 transcriptional regulators (Group B), all known, of which 10 were transcription factors and the remaining 2 transcriptional co-factors (Groucho4; 5D11 and NRcoACT5; 3H18). We also isolated cDNAs encoding proteins involved in intracellular signaling (Dishevelled; 18P21, MARCKS-like; 5F7, Anp32b; 3E20) or cell architecture (Claudin7L1; 1G22, Tubulin folding cofactor B, 3I20; NECAP2; 5L7). We also isolated 5 cDNAs that encoded proteins with no known function or homology to proteins with known activity (e.g. clones 5B3, 3K20, and 1E18).
Table 1.
Summary of isolated clones and overexpression phenotypes. Phenotype categories: AD: Anterior Defects, CED: Convergent Extension Defects, DD: Dorsal Defects, GD: Gastrulation Defects, K: Kinked, NM: Neural Mispatterning, PD: Pigmentation Defects, SA: Secondary Axis, SB: Spina Bifida, TD: Trunk Defects, V: Ventralized.
| Group | Gene name | Clone ID | BLASTn | Phenotype |
|---|---|---|---|---|
| A. Secreted | Derriere | 21L16 | BC073508 | V |
|
| ||||
| B. Transcription | Dbx1 | 5H19 | AF253504 | NM, K |
| Groucho4 | 5D11 | BC077582 | GD, PD | |
| Irx3 | 10D1 | AF027175 | SB, NM | |
| Kaiso | 5L24 | AF420316 | AD | |
| Not | 6E19 | Z19577 | AD, PD | |
| NRcoACT5 | 3H18 | BC042244 | NM, SB | |
| Oct91 | 21C4 | M60077 | PD, DD, NM | |
| Otx5 | 6O13 | AB034702 | CED | |
| OtxA | 4L2 | BC077357 | GD, TD, SB | |
| Sox2 | 18F19 | AF005476 | AD, GD | |
| SoxD | 20O19 | NM_001087732 | GD | |
| YB3 | 6A23 | BC042217 | TD, NM | |
|
| ||||
| C. mRNA splicing | hnRNPA1a | 8G20, 18D19 | BC045260 | NM |
| hnRNPA1 | 1I22 | BC072090 | K, NM, PD | |
| hnRNPA/B | 11N7 | BC043814 | K, AD | |
| hnRNPC(C1/C2) | 21M4 | BC041534 | K, AD, TD | |
| hnRNPD-like/JKTBP | 6D15, 14F20 | BC045124 | TD | |
| hnRNPG/RbmX | 2M5, 3A24 | BC070649 | K, AD, NM | |
| hnRNPL | 3E24 | BC077493 | NM | |
| SF-RS2/SF-SC35 | 5F17, 8A9 | BC045229 | AD, GD | |
| SF-RS3/SRp20 | 5L3 | BC046661 | GD, NM | |
| SRrp35/SR-repressor protein | 18L21 | BC084231 | K, AD, TD | |
| SF-RS10/Transformer2 | 14D18 | BC108873 | K, NM | |
| SFrs130/SRrp130 | 4D6 | BC072160 | PD | |
| YTH-domain family 2 protein | 14L18 | BC068959 | AD, TD | |
|
| ||||
| D. RNA binding | CIRP1/cold-inducible RNA binding protein-1 | 14J20 | AF278702 | DD |
| CIRP2/cold inducible RNA-binding protein-2 | 5P11,18E20 | BC054250 | GD | |
| DAZAP2 | 18E1 | BC042215 | GD | |
| FUS/TLS/pigpen | 6A17 | BC044319 | AD, ND | |
| KH-domain RNA-binding protein/Sam68 | 3N16 | AY260734 | NM, K, GD | |
| RBM4 | 3I22 | CR942584.2 | GD, NM | |
| Survival motor neuron protein (SMN) | 3G20 | BC068721 | NM | |
|
| ||||
| E. DNA binding | Histone H2A.Zl2 | 5H3 | BC044011 | NM |
| Pol delta interacting protein 3 | 18J21 | BC073024 | NM | |
| POLD3 p66 subunit | 10P1 | NM_001089291.1 | K, AD | |
|
| ||||
| F. Intracellular | Acidic nuclear phosphoprotein B (Anp32b) | 3E20,14B20 | BC110946.1 | TD, NM |
| Adaptin ear-binding coat-associated protein 2 (NECAP-2) | 5D7 | BC079728 | GD, K | |
| Adenine nucleotide translocase | 5L7 | BC043821 | GD | |
| Anaphase promoting complex subunit 11 | 5P7 | BC088720 | NM, AD, GD | |
| Claudin7L1 | 1G22 | AB072910 | GD, AD | |
| Dishevelled | 18P21 | BC090218.1 | DD, SA, PD | |
| MARCKS-like | 5F7 | AF187864 | GD | |
| Par6 | 1K22 | BC073237 | GD | |
| Phosphoribosyl pryophosphate synthetase 2 | 3C20 | BC045049 | NM | |
| Scaffold attachment factor A | 5J7 | BC084742 | NM, GD | |
| Strabismus | 14J18 | AF387816 | SB | |
| Tubulin folding cofactor B | 3I20 | BC106702 | NM | |
| Tyrosine phosphatase, Cdc25A | 14H20 | NM_001088487.1 | GD | |
| Uroplakin 1B | 18G1 | BC075147 | GD | |
| Yipf4 (yip1 domain containing protein) | 5J11 | BC070817 | GD, PD | |
|
| ||||
| G. Unknown | Small EDRK-rich factor 2/4F5 | 6K23 | BC097837 | GD, K |
| Predicted protein with 2 HMG domains | 5B3 | BC108499 | AD, NM | |
| Immediate early response-5-like (IER5L) | 14B18 | BC106540 | SB, DD | |
| Similar to KIAA1826 isoform 2 | 1E18 | BC076739 | K, AD | |
| Predicted small nuclear phosphoprotein; DC13 | 3K20 | BC060385 | NM | |
Remarkably, the largest single group of cDNAs that we isolated in our screen encoded genes involved in RNA binding or pre-mRNA processing. The majority of these were heterogeneous nuclear ribonucleoproteins (hnRNPs) or Serine/Arginine-rich proteins (SR-proteins), which are involved in multiple aspects of mRNA biology including exon definition, alternative splicing, nuclear export of RNA, and mRNA stability (Black, 2003). We also isolated several active cDNAs that encoded other types of RBPs, including Rbm4 (3I22) and Sam68 (3N16). These added to previously identified genes involved in Xenopus embryogenesis, such as Srp38 (Liu and Harland, 2005).
Regulation of RNA function, including mRNA stability and location, as well as alternative splicing of pre-mRNA is important for embryogenesis and function of the adult organism, but has not been studied in a developmental context to the same extent as that of many other biological regulatory mechanisms. Based on the prominence of RBPs in our screen, the relatively poor understanding of this aspect of embryogenesis, and their presumed biological significance, we initiated an effort to determine the expression pattern and function of these proteins during development.
Expression of RBPs during embryogenesis
The expression patterns for the transcripts isolated in the screen range from ubiquitous patterns with enrichment in the neural tube and somites to others with specific patterns in the neural tube, neural crest, and somites (Figure 1). Several of the RNA-binding molecules are expressed in both the neural tube and the somitic domain (Rbmx/hnRnpG, Rbm4, SRrp35, Sam68, hnRnpC, hnRnpA1)(Figure 1A–G,K–P,T–Y). Others, such as Sfrs3 showed ubiquitous expression, but enriched in neural and somitic tissues (Figure 1H–J). Rbmx is expressed along the entire anterior to posterior extent of the neural tube including strong expression in the forebrain (Figure 1A–C). Rbmx is also expressed in the somites and neural crest (Figure 1A–C). Expression of hnRnpL is specific to the neural tube and is not seen around the somites (Figure 1Q–S). The expression of hnRnpC is ubiquitous early (Figure 1T,U) but is restricted to the brain and neural tube as development proceeds (Figure 1V). Some other genes identified in the screen had very restricted expression domains; for example, Otx5, which has been described previously and is expressed in the forebrain, eye, and pineal gland (Vignali et al., 2000).
Figure 1.
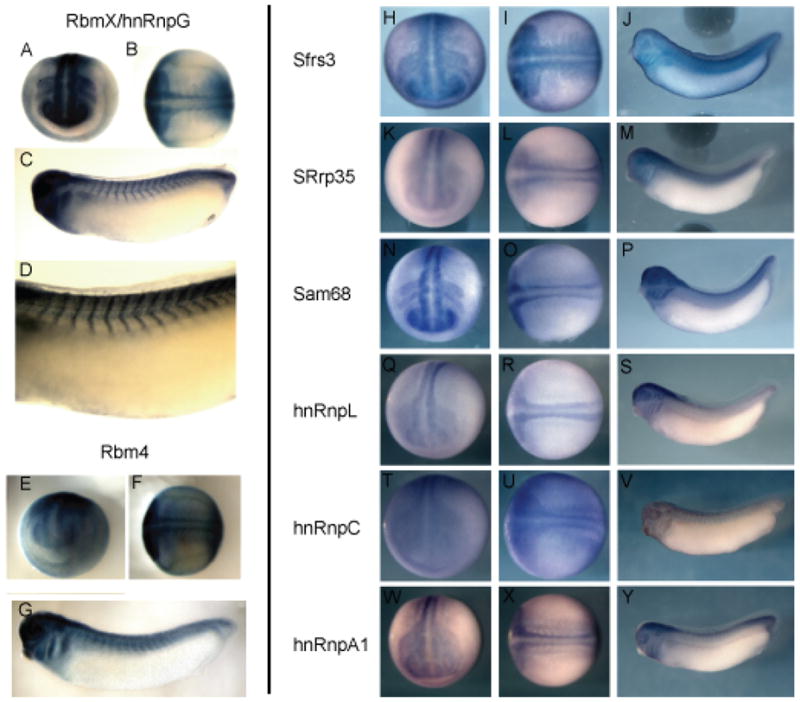
Expression pattern of select RNA binding proteins during development. (A,B,E,F,H,I,K,L,N,O,Q,R,T,U,W,X) stage 20 embryos from dorso-anterior and dorsal views. (C,D,G) stage 25 tailbud embryos; (J,M,P,S,V,Y) stage 32 tadpoles. (A–G) were cleared in BB/BA. (A–D), Rbmx/hnRnpG; (A,B) stage 20 embryos show hnRnpG expression all along the anterior-posterior extent of the neural tube with strong expression in the anterior neural domains, and placodal regions; (C,D) stage 25 tailbud embryos; Rbmx/HnRnpG is expressed in the fore-, mid-, and hindbrain, neural crest tissue of the branchial arches, and in the somites. (E,F) stage 20 embryos; Rbm4 is expressed in the neural tube, and at the tailbud stage. (G) Rbm4 is expressed in the brain and eye. (H–J) Sfrs3 is expressed ubiquitously, but expression is enriched in the neural tube and somites at the neural tube stage, and this pattern is maintained into the tadpole stage. (K–M) SRrp35 is expressed in the neural tube and weakly in the somites along the entire anterior-posterior axis. (N–P) Sam68 is expressed along the entire anterior-posterior axis of the neural tube and in the neural crest adjacent to the hindbrain. (Q–R) hnRnpL expression is present in the neural tube at stage 20 but is restricted to more anterior neural regions in the tadpole (S). (T–V) hnRnpC is expressed at low levels throughout the embryo early and is slightly enriched in the neural tube and somites at stage 20 (T,U), but is restricted primarily to the neural tube and somites by the tadpole stage (V). Expression of hnRnpA1 is enriched in the neural tube and somites at stage 20, and this expression is maintained into the tadpole stage (W–Y).
Effect of overexpression of RBPs during development
The effects of the overexpression of three RBPs, Rbmx, Sfrs3, Sam68, during neural plate stages are shown in Figure 2. Elevated expression of these RBPs by injection of 50 pg mRNA did not significantly alter the shape or size of the neural plate as evaluated by Sox2 expression (Figure 2A,F,K,P). However, mRNA injection frequently resulted in loss of primary neurogenesis in the injected part of the neural plate (Figure 2B,G,L,Q). To determine the effect on neural plate patterning we examined the expression of Pax2, which is expressed in the forebrain, midbrain-hindbrain boundary (MHB) and differentiating neurons of the spinal cord (Nornes et al., 1990; Heller and Brandli, 1997). Injection of Rbmx mRNA caused a reduction in the FB domain, but the MHB domain was intact, though misshapen (Figure 2H). In contrast, injection of 50 pg of Sfrs3 or Sam68 mRNA did not change Pax2 expression in the brain (Figure 2M,R). To asses the effect on neural crest development we analyzed changes in Slug expression in response to mRNA injection: Rbmx and Sam68 mRNA injection both caused an inhibition of neural crest formation as evaluated by Slug expression. In contrast, Sfrs3 overexpression changed the shape of the Slug expression domain, but did not affect the level of expression (Figure 2N). To determine if misexpression of these proteins also had effects on mesoderm development we analyzed MyoD expression and found Sfrs3 able to suppress MyoD expression, whereas Rbmx and Sam68 at this dose and stage had no effect (Figure 2E,J,O,T). Together these results show that misexpression of individual RBPs can cause specific changes in neural, neural crest, and muscle patterning and differentiation, but the differences between activities also suggest that there is considerable specificity in RBP function during development.
Figure 2.
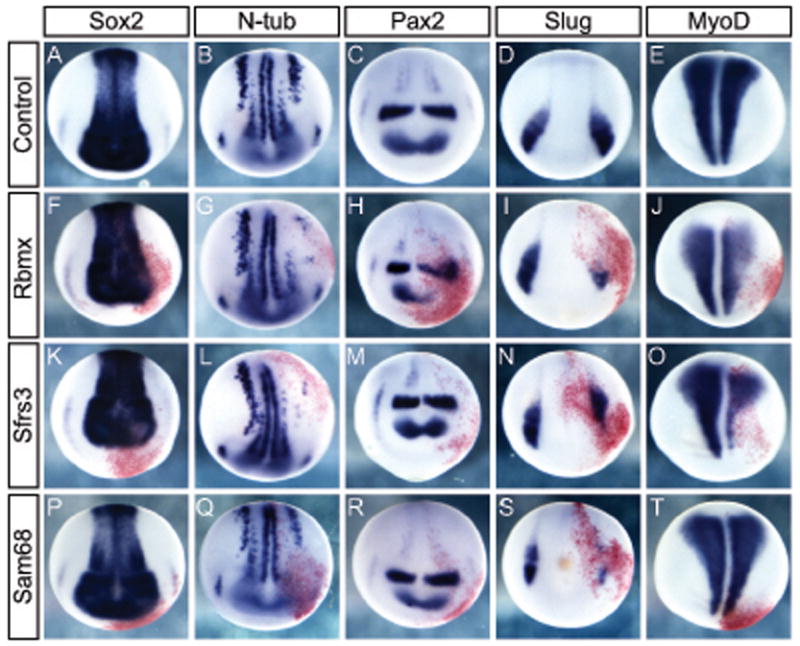
Effect of overexpression of RNA binding proteins on midneurula development. Whole mount in situ hybridization for gene expression during neurula stages on uninjected control embryos or embryos injected with 50 pg mRNA. Uninjected control embryos showing expression of Sox2 marking all neural tissue (A); N-tubulin in differentiating neurons (B); Pax2 in the forebrain, MHB, and differentiating neurons of the spinal cord (C); Slug marking neural crest (D); and MyoD marking axial mesoderm (E). RBMX mRNA injected embryos have normal neural plate morphology (F), but inhibited neural differentiation (G), and perturbed expression of Pax2 (H) and Slug (I), whereas axial mesoderm is largely unaffected (J). SFRS3 mRNA injection does not affect Sox2 expression (K), but neurogenesis is inhibited (L) though Pax2 expression in the brain (M) and Slug expression in the neural crest is intact (N). MyoD expression is reduced as response to SFRS3 (O). Sam68 injection does not alter Sox2 (P), Pax2 (R), or MyoD expression (T), but inhibit neurogenesis (Q) and Slug expression in neural crest (S). Embryos were injected with 50 pg mRNA in one cell at the 2-cell stage. Coinjected beta-galactosidase mRNA developed with red-gal was used as a lineage tracer. Embryo orientation is dorsal up, anterior facing.
RBP mRNA injection results in muscle mispatterning
Frequently, injection of RBP mRNA followed by culturing to swimming tadpole stages resulted in disruption of axial muscle or kinking of the embryo. To investigate the basis of this phenotype further we stained injected embryos with the muscle-specific antibody 12–101 (Kintner and Brockes, 1984). In uninjected stage 36 embryos, the axial musculature is divided into discrete and highly regular segments along the A-P axis (Figure 3A–C). When we injected mRNA encoding RBPs this pattern was damaged to a varying degree depending on the mRNA. Injection of 50 pg RnpA1a mRNA caused minor defects in division of the individual muscle segments (Figure 3D–F). In contrast, two other RBPs, RnpC1/C2 (Figure 3G–I) and Rbm4 (Figure 5J–L), almost completely prevented segregation of the axial muscle into individual segments. A similar effect was observed when embryos were injected with Sam68, which also prevented segment division (Figure 3M–O). In the latter case the overall staining intensity of the muscle was weaker, suggesting that less differentiated muscle mass was being formed. Lastly, when we injected SRrp35 mRNA the embryos displayed a strong kinked phenotype, which was accompanied by a severe disruption of the regular pattern of the segments (Figure 3P–R). Injection of RnpL mRNA caused no effect on muscle formation or segment segregation (Figure 3S–U) indicating that it is not simply elevated levels of RBP expression in general that caused the defects, but instead that there is specificity of action between the different RBPs.
Figure 3.
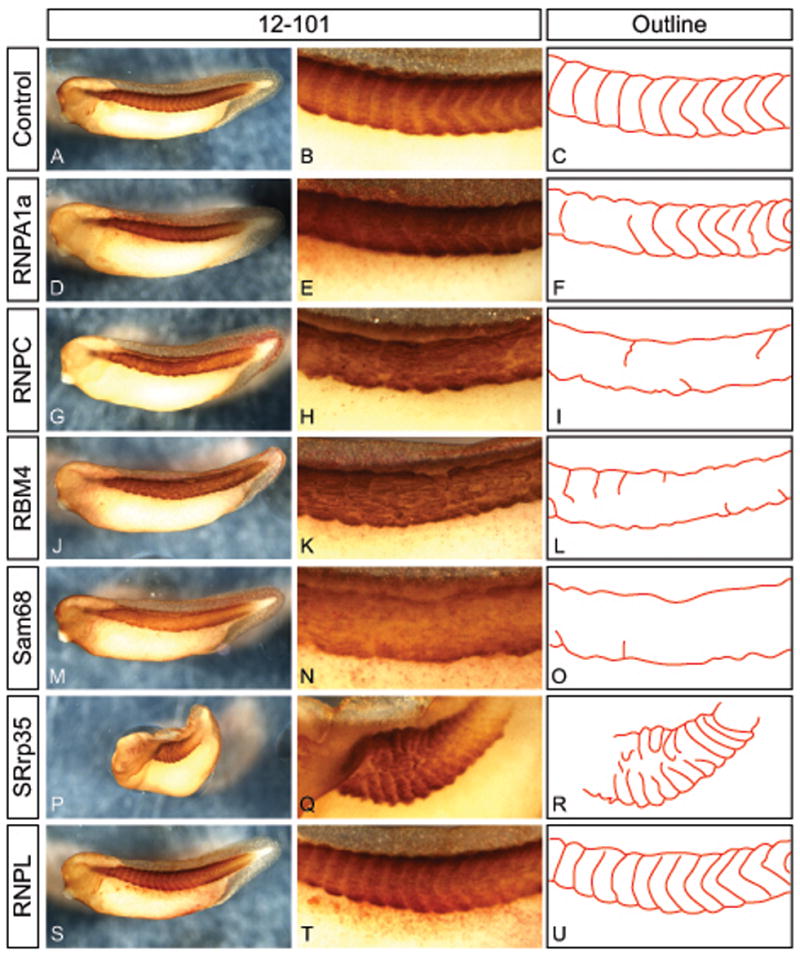
Effect of overexpression of RNA binding proteins on axial muscle structure. Embryos were injected with 50 pg mRNA and betal-gal lineage tracer into one cell at the 2-cell stage and cultured to stage 37 followed by immunostaining with 12/101 to mark skeletal muscle. Uninjected controls in whole view (A), magnified view of axial muscle (B), and schematic outline of the segmentation pattern (C). Embryos injected with mRNA for RNPA1a (D–F), RNPC1/C2 (G–I), or Rbm4 (J–L) have normal overall amount of muscle (D,G,J), but show irregular segmentation (E,F,H,I,K,L). Injection of Sam68 mRNA stains weaker for 12/101 (M) and show almost completely absent segmentation (N,O). Misexpression of SRrp35 caused severe lateral kinking of the embryo (P) and highly irregular segmentation (Q,R). Injection of RNPL mRNA as a control did not affect overall muscle amount (S) or muscle segmentation (T,U). Embryos were injected with 50 pg mRNA in one cell at the 2-cell stage. Coinjected beta-galactosidase mRNA developed with red-gal was used as a lineage tracer.
Figure 5.
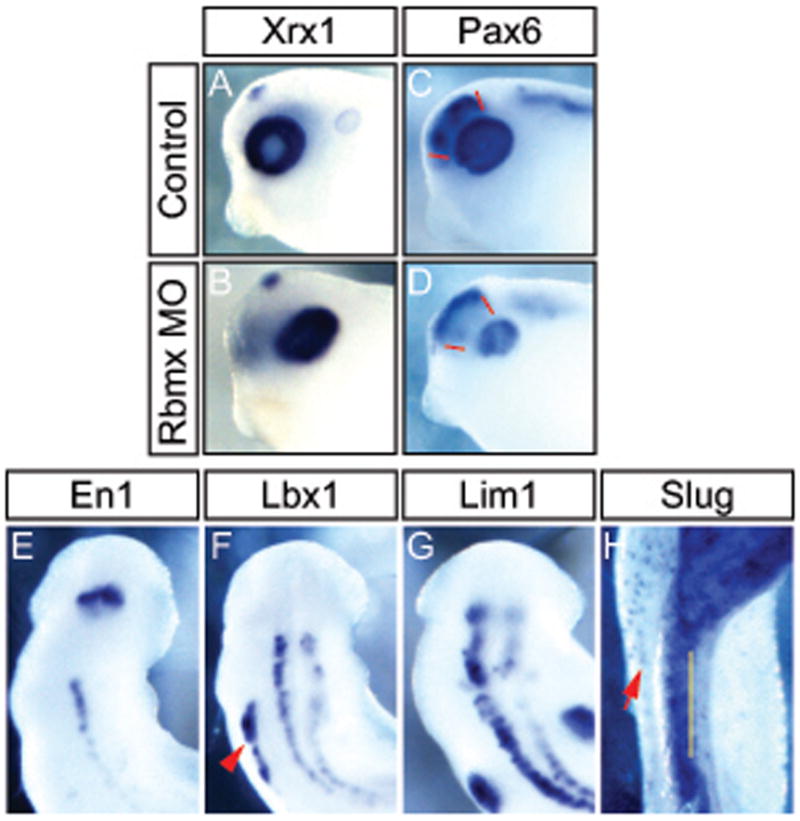
Rbmx is required for normal eye development and neuronal differentiation. Embryos injected unilaterally with Rbmx MO and cultured to stage 31 (A–D), stage 28 (E–F), or stage 35 (H). (A,B) In situ hybridization for Xrx1. (C,D) In situ hybridization for Pax6; red lines indicate Pax6 expression domain in normal forebrain (C), which is absent in the Rbmx MO injected side (D). (E–H) dorsal view of head and anterior trunk of Rbmx morphants. Injected side is to the right. (E–H) In situ hybridization using antisense probe for En1 (E), Lbx1 (F), Lim1 (G), and Slug (H). Red arrowhead in (F) indicates Lbx1 expression in hypaxial muscle on the uninjected side. Yellow line in (H) indicates the midline and red arrow indicates melanocytes on the uninjected side. Notably Slug expression is absent in the neural tube and trunk on the injected side.
Knockdown of RBMX inhibits neurogenesis and causes mispatterning of the anterior neural plate
In an attempt to evaluate the physiological requirements for RBPs during development we selected Rbmx for a loss-of-function study. We designed a MO that targeted the translation start site of both X. laevis alleles and studied its effect on development. Rbmx morphants were morphologically normal through neurulation but had shortened trunks and head defects at tadpole stages (data not shown). Unilateral knockdown resulted in severe kinking and deformed eyes on the injected side.
Since Rbmx is expressed in the neural plate we analyzed the effect of Rbmx knock-down on neural plate patterning and primary neurogenesis. Embryos injected with Rbmx MO at the 1-cell stage formed few primary neurons as judged by N-tubulin expression compared to uninjected embryos (Figure 4A). This was not due to general loss of neural tissue since Sox2 expression was unaffected (Figure 4C,D). Similarly, the early mesodermal markers MyoD and Xbra were normal in Rbmx MO injected embryos, indicating that mesoderm specification and initial patterning were normal (Figure 4E–H). Because Rbmx is expressed highly in the brain and spinal cord regions and since zebrafish Rbmx morphants show brain defects, we also analyzed markers specific for different regions of the neural plate. Xenopus Rbmx morphants showed anterior neural patterning defects such as a ectopic expression of Otx5 in the prospective forebrain (Figure 4I,J). Normally, Otx5 expression is strong in the isthmus and the anterior neural ridge (ANR) (Vignali et al., 2000). However, Rbmx morphants expressed high levels of Otx5 throughout the anterior neural plate, whereas the normal strong expression restricted to the ANR was weak and fuzzy. To test whether gene expression in the ANR was generally perturbed, we examined the expression of Noggin which, during midneurula stages, is expressed in the ANR as well as the notochord (Knecht and Harland, 1997). In situ hybridization showed that noggin expression in the ANR was completely absent, whereas expression in the notochord was normal (Figure 4K,L). To characterize the extent of neural defects further, we analyzed expression of Pax2, which is expressed in several regions of the neural plate including the forebrain, MHB, trigeminal ganglion, and some differentiated interneurons in the spinal neural plate (Nornes et al., 1990; Heller and Brandli, 1997). In Rbmx morphants that had been unilaterally injected, the trigeminal ganglion and Pax2 expressing interneurons were absent, but Pax2 expression in the MHB and forebrain appeared normal (Figure 4M,N). We also analyzed the expression of several genes involved in AP as well as D-V patterning of the neural plate, including Pax6, Pax3, Dbx1, and HoxB9, however their expression was spatially normal but slightly reduced in intensity (Figure 4Q,R and data not shown). Together these observations indicate that Rbmx is required for normal neurogenesis and anterior patterning during neural plate stages but is dispensable for regional patterning of more posterior neural structures. Since zebrafish morphants showed jaw deformities, which often reflects neural crest defects, we analyzed Slug expression to determine if initial neural crest formation was normal. Rbmx morphants analyzed for Slug expression at stage 17 showed a decrease of neural crest tissue on the injected side that extended throughout the A-P axis (Figure 4O,P).
Figure 4.
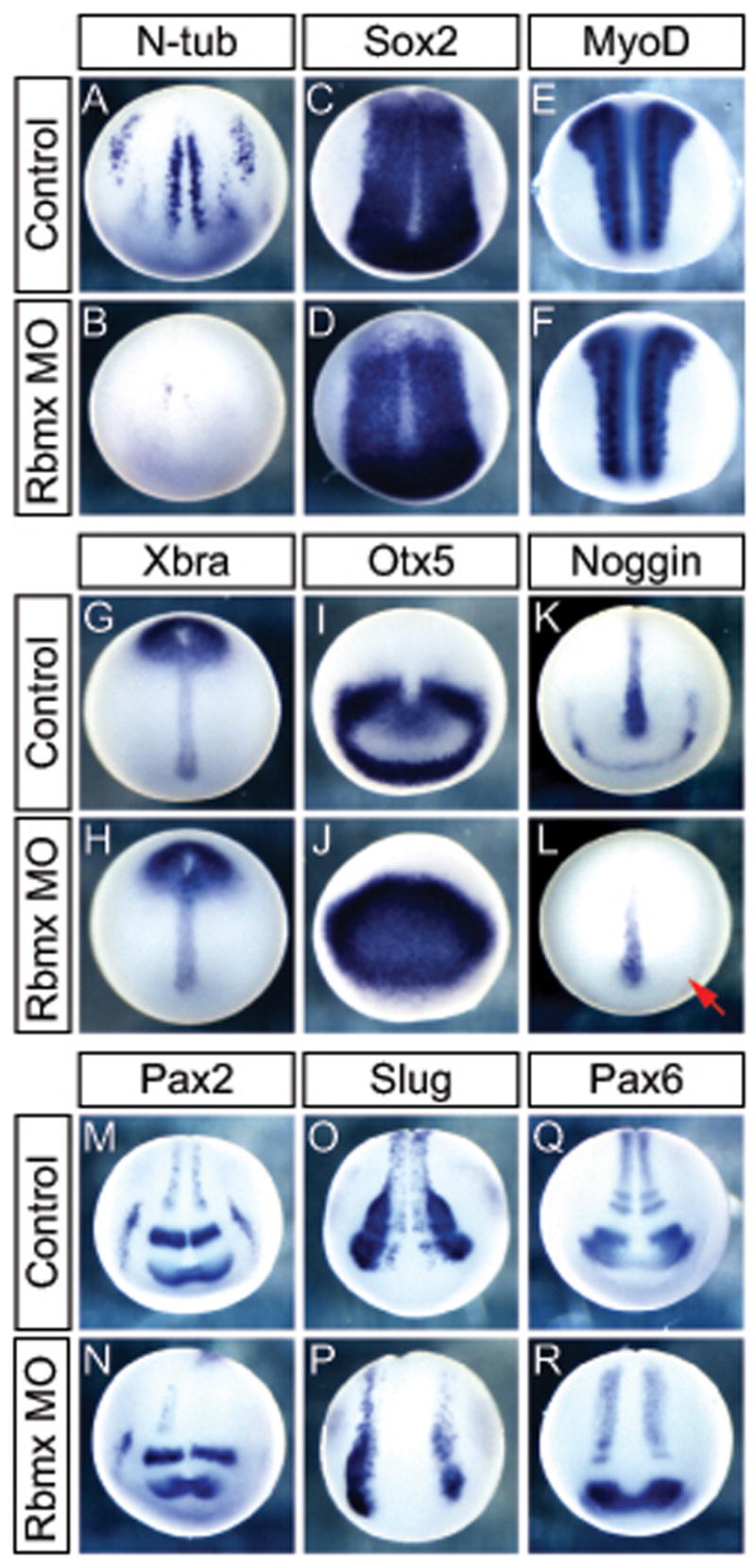
Rbmx is necessary for normal neural development independent of neural induction and mesoderm formation. Embryos are either uninjected controls, injected with Rbmx MO in one cell at the 1-cell stage (B,D,F,H,J,L) or one side at the 2-cell stage Injected (N,M,P; injected side to the right). Embryos were analyzed at stage 14 (A–L) or 17 (M–R). (A,B) N-tubulin positive primary neurons do not form in Rbmx MO injected embryos. (C,D) Sox2 expression shows normal morphology of the neural plate. (E–H) early mesoderm formation is normal as judged by MyoD (E,F) and Xbra (G,H) expression. (I,J) Otx5 expression in the anterior neural plate is not excluded from the presumptive forebrain. (K,L) Noggin expression is absent in the anterior neural ridge (red arrow) indicating perturbed anterior neural development. (M,N) Pax2 expressing interneurons in the spinal neural plate fail to form on the injected side. (O,P) Reduced Slug expression on the injected side indicate defect in neural crest formation. (Q,R) medio-lateral patterning of the spinal neural plate proper is normal judged by Pax6 expression.
Rbmx knockdown inhibits neurogenesis and causes brain defects at tadpole stages
To determine how the early patterning defects affected later stages, we analyzed the phenotype of Rbmx morphants after hatching. To determine the molecular basis for the head and eye defects we analyzed the expression of two regulators of eye development – Xrx1 and Pax6. Xrx1 expression at tadpole stages is restricted to the retinal layer of the eye and was unchanged in Rbmx morphants (Figure 5A,B). However, the center of the eye, where the lens forms and Xrx1 is normally excluded, expressed high levels of Xrx1 in Rbmx morphants indicating that the lens probably did not form properly. Pax6 was also expressed in Rbmx morphant eyes at seemingly normal levels, though in a smaller domain reflecting the reduced eye size (Figure 5C,D). Similarly, Pax6 expression in the forebrain was also reduced(Figure 5C,D; red lines), indicating that the early anterior patterning defects resulted in permanent developmental defects in the brain.
To determine if the defects in primary neurogenesis that we observed at early stages extended to secondary neurogenesis at tadpole stages, we analyzed several genes expressed in differentiated neurons. The homeobox genes En1 and Lbx1 are expressed in different classes of interneurons in the spinal cord and hindbrain (Matise and Joyner, 1997; Gross et al., 2002). In embryos unilaterally injected with Rbmx MO, neurons expressing En1 completely failed to develop in the spinal cord (Figure 5E). En1 expression in the MHB was normal in Rbmx morphants showing that specification of the isthmus is independent of Rbmx function. Similarly, Lbx1 expressing neurons were severely reduced particularly in the anterior region of the spinal cord, whereas some Lbx1 expressing interneurons formed in the trunk region (Figure 5F). To test if motor neurons developed normally we analyzed expression of Lim1, a LIM-homeodomain transcription factor. Again, we observed a dramatic decrease in the number of Lim1 expressing neurons in the spinal cord and hindbrain (Figure 5G). The failure to form several distinct classes of the neurons was further substantiated by reduced expression of Hb9 (motor neurons), Isl1 (sensory and motor neurons), and Pax2 (interneurons) (data not shown). Together, these results indicate that Rbmx function is required for normal development of multiple classes of neurons.
Lastly, we analyzed Slug expression in the neural crest at tadpole stages to test if the early decrease of Slug expression resulted in permanent changes. Indeed, Rbmx knockdown on one side resulted in a clear reduction of Slug expressing neural crest derived cells on the injected side (Figure 5H). This change in gene expression, was confirmed by a reduction in the number of neural crest-derived melanocytes in the trunk (5H and data not shown).
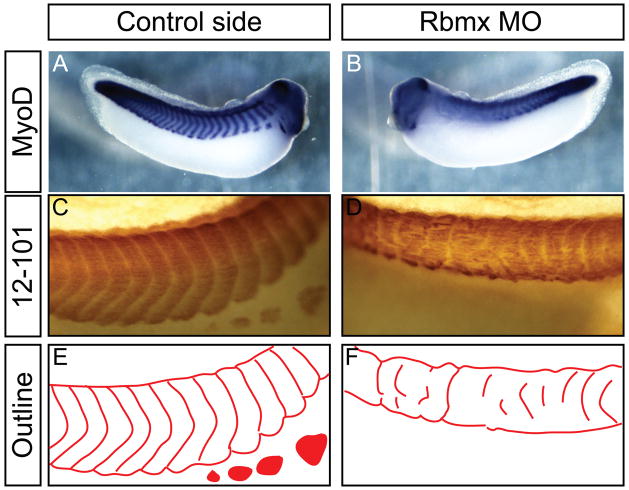
Rbmx knockdown cause irregular axial muscle segmentation
Since Rbmx is expressed outside of the nervous system, most notably in the circumblastoporal tissue and a narrow domain of what appears to be axial muscle segments, we investigated the effects of Rbmx knock-down on axial muscle formation. Mesoderm induction and initial patterning appeared normal as judged by MyoD and Xbra expression (Figure 4E–H). At tadpole stages, however, MyoD expression was weak and diffuse, without the regular striped pattern observed in normal embryos (Figure 6A,B). Using 12–101 immunohistochemistry we also confirmed that the defects in MyoD expression were followed by defects in axial muscle organization. Indeed, in embryos unilaterally injected with Rbmx MO the muscle tissue was severely reduced along the dorsal-ventral axis on the injected side and the segmentation was highly erratic (Figure 6C,D; outlined in Figure 6E,F). In addition, the hypaxial muscles that separate from the ventral axial muscle block and migrate to populate the ventral side of the embryo, failed to form (Figure 6C–F). The absence of recognizable hypaxial muscle could be either a failure to form altogether, or a defect in separation from the axial domain. To distinguish between these two possibilities, we investigated expression of Lbx1, a transcription factor marking hypaxial muscle in Xenopus and other vertebrates (Dietrich et al., 1998; Martin and Harland, 2006). Embryos injected unilaterally with Rbmx MO showed a strong reduction or complete failure to express Lbx1 in the hypaxial muscle (Figure 5F, red arrowhead). The reduction of Lbx1 expression suggests that hypaxial muscle is not being specified in the absence of Rbmx.
Figure 6.
Rbmx is required for normal muscle formation and segmentation. Embryos shown are injected with Rbmx MO in one cell at the 2-cell stage and cultured to stage 38. (A,B) in situ hybridazation for MyoD. (C,D) 12–101 antibody staining for skeletal muscle. (E,F) outline of muscle segmentation pattern shown in (C,D). Hypaxial muscle migrated from the axial muscle block in (E, colored solid) is absent on the injected side (F).
DISCUSSION
In this study we have used expression cloning to identify novel genes expressed in the frog neural plate that are involved in frog embryogenesis. We chose early neural plate tissue as starting material since the majority of other expression cloning screens have used either cDNA isolated from early embryos, such as blastulae or whole gastrulae, or later tadpole stages (Smith and Harland, 1992; Smith et al., 1995; Glinka et al., 1998; Grammer et al., 2000). The largest single class of molecules isolated was a set likely to be involved in regulation of RNA metabolism.
With the exception of the TGF-beta family member Derriere, we did not isolate any secreted factors in our screen. The reason for this could be that the they are not represented in our library, or at least the part that we screened, or that those present require a higher amount of mRNA than the 50 pg we have used in our screen for the initial pools. In contrast we have isolated twelve transcriptional regulators, all of which have been described in Xenopus previously, though, to our knowledge, not all as having effects on neural development, e.g. Not, Oct91, and YB3. We have also isolated several proteins that we pooled in the broad category “intracellular proteins” (group F, table 1). Several members of this category have been described to be components of the versatile Wnt/planar cell polarity signaling (Dishevelled, Strabismus; Klingensmith et al., 1994; Darken et al., 2002) and MAP kinase pathway (MARCKS-like, Par6 (Joberty et al., 2000; Zhao et al., 2001)); these pathways interact extensively and are known to have profound effects on development. The mechanism by which the other members of group F alter development is not clear and merit future investigations. We also isolated 5 genes whose function is entirely unknown (group G).
The most striking result from our screen is the isolation of 20 clones, out of 56 total, that encode putative RBPs of which the majority (13 clones, group C) are hnRNPs or SR-proteins. These proteins are known to be the principal regulators of alternative splicing. The importance of this class of proteins is supported by a previous study on the function of SRp38 on neural differentiation (Liu and Harland, 2005). In their effect on splicing they act by binding to specific sequences present in the pre-mRNA and either recruit, in the case of hnRNPs, or suppress, for SR-proteins, spliceosome components. Recruitment of the spliceosome then results in inclusion or prevention of nearby exons in the mature transcript (Black, 2003). Exon selection during pre-mRNA splicing is not the sole function of SR-proteins and hnRNPs. They are also known to have important roles in mRNA export from the nucleus, mRNA stability in the cytoplasm, as well as control of translation rates. It is therefore possible that at least some of the effects we observe are not caused by changes in splicing but rather transport or translation of mature transcripts.
The genome sequencing projects show that relatively simple animals such as worms and sea anemones have similar number of genes when compared to more complex vertebrates. While differential activation and repression of transcription through multiple enhancer elements is undoubtedly one mechanism to generate complexity, mRNA splicing, localization, translation and stability are also powerful ways of controlling gene expression (Dreyfuss et al., 1993; Black, 2003; Reed and Cheng, 2005). In addition, mRNA localization is a common regulatory theme for initiating differences in cell type in embryos, (Melton, 1987; Berleth et al., 1988), and regulation of mRNA stability or translation through micro RNA is also an emerging theme (Martello et al., 2007). Alternative splicing has been proposed as a mechanism for expanding the proteomic complexity from a limited number of genes in vertebrates. In support of this hypothesis it has been shown that the frequency of alternative splicing increases along the metazoan phyla. The proportion of genes that are subject to alternative splicing in Drosophila is 15%, in mice it is one third, whereas in humans it is estimated that from 40–74% of all transcripts are alternatively spliced (Brett et al., 2002; Modrek and Lee, 2002; Johnson et al., 2003). Although alternative splicing can in principle lead to an increase in the proteomic repertoire of the genome, it is still not clear what proportion have functional significance. Among the clear examples is the case of alternative splicing of the FGF receptor 2, where differences in the extracellular domain change the affinity for different FGF ligands (Orr-Urtreger et al., 1993; Ornitz et al., 1996). Importantly, the different splice forms are expressed in mutually exclusive cell types, effectively making several functionally independent transcripts from a single gene.
A daunting problem in elucidating the biological role of these proteins is to determine their target genes and the precise effects they have. Recent advances in DNA chip technology have made it possible to detect individual splice events on a genomic scale. In Drosophila, removal of individual splice factors has remarkably restricted effects, ranging from a few dozens to a few hundred targets affected (Blanchette et al., 2005). Similarly, a genetic mouse knockout of the brain specific splice factor Nova2 showed that less than 7% of alternatively spliced exons were affected when Nova2 was removed (Ule et al., 2005). Strikingly, the majority of the affected genes were involved in synapse function, suggesting that alternative splicing can act on a genomic scale to regulate gene expression involved particular functions. The example of Nova2 is likely only the tip of the iceberg as demonstrated by a recently study on the expression of RBPs in fetal mouse brain (McKee et al., 2005). In this study, the expression pattern of all known 380 RNPs in the mouse genome was studied during mouse brain development and 85% showed restricted expression within specific domains of the brain. Our results together with the mouse embryological studies and the genome scale microarray analysis argue for a prominent role of alternative splicing in the development and function of the vertebrate nervous system.
To test specifically how RBPs/splice factors may influence embryogenesis, we chose Rbmx for further investigation. We selected Rbmx partly because its role in zebrafish embryogenesis has been described which would allow us to evaluate the evolutionarily conserved functions of this protein (Tsend-Ayush et al., 2005). The phenotype observed in zebrafish with MO mediated knockdown of Rbmx resembles in several respects the one we observe in Xenopus. In both species Rbmx knockdown results in brain and somite defects indicating that at least some functions of RBMX are conserved. The somitic defects observed in zebrafish was attributed to non-cell autonomous effects since RBMX does not seem to be expressed in the somites or their derivatives (Tsend-Ayush et al., 2005). In Xenopus, by contrast, Rbmx is expressed in the somites and narrow stripes of the axial muscle segments, suggesting that the expression here is important for proper muscle formation. Interestingly, we consistently observed increasingly severe phenotypes when embryos were injected on both sides compared to unilateral injections. This observation could be consistent with Rbmx regulating one or several secreted factors that can partly rescue the embryo when injected in one side only.
Rbmx morphants show a near-complete loss of several classes of differentiated neurons in the spinal cord and parts of the hindbrain, although gene expression marking the progenitor domains along the dorso-ventral axis was normal. Similarly, patterning along the anterior-posterior axis of the spinal cord appears normal. In contrast, the anterior neural plate requires Rbmx function for proper regionalization and eye development. These results suggest that Rbmx function in the nervous system is required for patterning of anterior regions as well as neural differentiation in the spinal cord, but is dispensable for patterning the spinal cord. Although the early anterior neural defects are most likely a consequence of mispatterning events, it is possible that other, particularly later, defects are caused by changes in proliferation and/or apoptosis.
Our findings that Rbmx is necessary for brain patterning and neurogenesis closely recapitulate the knockdown phenotype that has been described in zebrafish (Tsend-Ayush et al., 2005), and our results highlight the importance of Rbmx during embryogenesis and its evolutionary conserved function in embryonic development. Not much is known about this gene in humans, but the genomic region that contains the human orthologue of Rbmx correlates with several human retardation syndromes and eye diseases (Zucchi et al., 1999), making further study of the mechanism of Rbmx function interesting.
The exact molecular function of RBMX is not entirely clear. In vitro experiments have demonstrated that RBMX is capable of influencing alternative splicing event arguing that this is one molecular activity of RBMX (Venables et al., 2000; Wang et al., 2007). Recently and surprisingly, it was argued that RBMX could function as a transcriptional cofactor in hepatocytes and interact with SREBP-1c (Takemoto et al., 2007). Other types of RNPs often have roles in mRNA localization and stability apart from their ability to change splicing. However, similar functions have not been described for this protein.
To fully understand the biological function of Rbmx during development it is essential to determine its direct target genes. Improvements in biochemical techniques combined with genomic analysis have dramatically improved the prospects of addressing this important question, and will enable the study of regulated alternative splicing in early development.
Acknowledgments
D.S.D. was a recipient of an Alfred Benzon Fellowship. This work was supported by NIH grant GM 42341 to R.M.H.
References
- Berleth T, Burri M, Thoma G, Bopp D, Richstein S, Frigerio G, Noll M, Nusslein-Volhard C. The role of localization of bicoid RNA in organizing the anterior pattern of the Drosophila embryo. Embo J. 1988;7:1749–1756. doi: 10.1002/j.1460-2075.1988.tb03004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black DL. Mechanisms of alternative pre-messenger RNA splicing. Annual Review in Biochemistry. 2003;72:291–336. doi: 10.1146/annurev.biochem.72.121801.161720. [DOI] [PubMed] [Google Scholar]
- Blanchette M, Green RE, Brenner SE, Rio DC. Global analysis of positive and negative pre-mRNA splicing regulators in Drosophila. Genes & Development. 2005;19:1306–1314. doi: 10.1101/gad.1314205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett D, Pospisil H, Valcarcel J, Reich J, Bork P. Alternative splicing and genome complexity. Nat Genet. 2002;30:29–30. doi: 10.1038/ng803. [DOI] [PubMed] [Google Scholar]
- Casarosa S, Andreazzoli M, Simeone A, Barsacchi G. Xrx1, a novel Xenopus homeobox gene expressed during eye and pineal gland development. Mech Dev. 1997;61:187–198. doi: 10.1016/s0925-4773(96)00640-5. [DOI] [PubMed] [Google Scholar]
- Darken RS, Scola AM, Rakeman AS, Das G, Mlodzik M, Wilson PA. The planar polarity gene strabismus regulates convergent extension movements in Xenopus. Embo J. 2002;21:976–985. doi: 10.1093/emboj/21.5.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich S, Schubert FR, Healy C, Sharpe PT, Lumsden A. Specification of the hypaxial musculature. Development. 1998;125:2235–2249. doi: 10.1242/dev.125.12.2235. [DOI] [PubMed] [Google Scholar]
- Dreyfuss G, Matunis MJ, Pinol-Roma S, Burd CG. hnRNP proteins and the biogenesis of mRNA. Annu Rev Biochem. 1993;62:289–321. doi: 10.1146/annurev.bi.62.070193.001445. [DOI] [PubMed] [Google Scholar]
- Eizema K, Koster JG, Stegeman BI, Baarends WM, Lanser PH, Destree OH. Comparative analysis of Engrailed-1 and Wnt-1 expression in the developing central nervous system of Xenopus laevis. Int J Dev Biol. 1994;38:623–632. [PubMed] [Google Scholar]
- Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- Grammer TC, Liu KJ, Mariani FV, Harland RM. Use of large-scale expression cloning screens in the Xenopus laevis tadpole to identify gene function. Dev Biol. 2000;228:197–210. doi: 10.1006/dbio.2000.9945. [DOI] [PubMed] [Google Scholar]
- Gross MK, Dottori M, Goulding M. Lbx1 specifies somatosensory association interneurons in the dorsal spinal cord. Neuron. 2002;34:535–549. doi: 10.1016/s0896-6273(02)00690-6. [DOI] [PubMed] [Google Scholar]
- Heller N, Brandli AW. Xenopus Pax-2 displays multiple splice forms during embryogenesis and pronephric kidney development. Mech Dev. 1997;69:83–104. doi: 10.1016/s0925-4773(97)00158-5. [DOI] [PubMed] [Google Scholar]
- Hirsch N, Harris WA. Xenopus Pax-6 and Retinal Development. Journal of Neurobiology. 1997;32:45–61. [PubMed] [Google Scholar]
- Hopwood N, Pluck A, Gurdon J. MyoD expression in the forming somites is an early response to mesoderm induction in Xenopus embryos. Embo J. 1989;8:3409–3417. doi: 10.1002/j.1460-2075.1989.tb08505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joberty G, Petersen C, Gao L, Macara IG. The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat Cell Biol. 2000;2:531–539. doi: 10.1038/35019573. [DOI] [PubMed] [Google Scholar]
- Johnson JM, Castle J, Garrett-Engele P, Kan Z, Loerch PM, Armour CD, Santos R, Schadt EE, Stoughton R, Shoemaker DD. Genome-wide survey of human alternative pre-mRNA splicing with exon junction microarrays. Science. 2003;302:2141–2144. doi: 10.1126/science.1090100. [DOI] [PubMed] [Google Scholar]
- Kintner CR, Brockes JP. Monoclonal antibodies identify blastemal cells derived from dedifferentiating limb regeneration. Nature. 1984;308:67–69. doi: 10.1038/308067a0. [DOI] [PubMed] [Google Scholar]
- Klingensmith J, Nusse R, Perrimon N. The Drosophila segment polarity gene dishevelled encodes a novel protein required for response to the wingless signal. Genes Dev. 1994;8:118–130. doi: 10.1101/gad.8.1.118. [DOI] [PubMed] [Google Scholar]
- Knecht AK, Harland RM. Mechanisms of dorsal-ventral patterning in noggin-induced neural tissue. Development. 1997;124:2477–2488. doi: 10.1242/dev.124.12.2477. [DOI] [PubMed] [Google Scholar]
- Lamb TM, Knecht AK, Smith WC, Stachel SE, Economides AN, Stahl N, Yancopolous GD, Harland RM. Neural induction by the secreted polypeptide noggin. Science. 1993;262:713–718. doi: 10.1126/science.8235591. [DOI] [PubMed] [Google Scholar]
- Liu KJ, Harland RM. Inhibition of neurogenesis by SRp38, a neuroD-regulated RNA-binding protein. Development. 2005;132:1511–1523. doi: 10.1242/dev.01703. [DOI] [PubMed] [Google Scholar]
- Martello G, Zacchigna L, Inui M, Montagner M, Adorno M, Mamidi A, Morsut L, Soligo S, Tran U, Dupont S, Cordenonsi M, Wessely O, Piccolo S. MicroRNA control of Nodal signalling. Nature. 2007;449:183–188. doi: 10.1038/nature06100. [DOI] [PubMed] [Google Scholar]
- Martin BL, Harland RM. A novel role for lbx1 in Xenopus hypaxial myogenesis. Development. 2006;133:195–208. doi: 10.1242/dev.02183. [DOI] [PubMed] [Google Scholar]
- Matise MP, Joyner AL. Expression patterns of developmental control genes in normal and Engrailed-1 mutant mouse spinal cord reveal early diversity in developing interneurons. J Neurosci. 1997;17:7805–7816. doi: 10.1523/JNEUROSCI.17-20-07805.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AE, Minet E, Stern C, Riahi S, Stiles CD, Silver PA. A genome-wide in situ hybridization map of RNA-binding proteins reveals anatomically restricted expression in the developing mouse brain. BMC Dev Biol. 2005;5:14. doi: 10.1186/1471-213X-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton DA. Translocation of a localized maternal mRNA to the vegetal pole of Xenopus oocytes. Nature. 1987;328:80–82. doi: 10.1038/328080a0. [DOI] [PubMed] [Google Scholar]
- Modrek B, Lee C. A genomic view of alternative splicing. Nat Genet. 2002;30:13–19. doi: 10.1038/ng0102-13. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal table of Xenopus laevis (Daudin) : a systematical and chronological survey of the development from the fertilized egg till the end of metamorphosis. Vol. 252. New York: Garland Pub; 1994. p. 210. leaves of plates pp. [Google Scholar]
- Nornes HO, Dressler GR, Knapik EW, Deutsch U, Gruss P. Spatially and temporally restricted expression of Pax2 during murine neurogenesis. Development. 1990;109:797–809. doi: 10.1242/dev.109.4.797. [DOI] [PubMed] [Google Scholar]
- Ornitz DM, Xu J, Colvin JS, McEwen DG, MacArthur CA, Coulier F, Gao G, Goldfarb M. Receptor specificity of the fibroblast growth factor family. J Biol Chem. 1996;271:15292–15297. doi: 10.1074/jbc.271.25.15292. [DOI] [PubMed] [Google Scholar]
- Orr-Urtreger A, Bedford MT, Burakova T, Arman E, Zimmer Y, Yayon A, Givol D, Lonai P. Developmental localization of the splicing alternatives of fibroblast growth factor receptor-2 (FGFR2) Dev Biol. 1993;158:475–486. doi: 10.1006/dbio.1993.1205. [DOI] [PubMed] [Google Scholar]
- Reed R, Cheng H. TREX, SR proteins and export of mRNA. Curr Opin Cell Biol. 2005;17:269–273. doi: 10.1016/j.ceb.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Richter K, Good P, Dawid I. A developmentally regulated, nervous system-specific gene in Xenopus encodes a putative RNA-binding protein. New Biology. 1990;2:556–565. [PubMed] [Google Scholar]
- Richter K, Grunz H, Dawid I. Gene expression in the embryonic nervous system of Xenopus laevis. Proc Natl Acad Sci U S A. 1988;85:8086–8090. doi: 10.1073/pnas.85.21.8086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sive HL, Grainger RM, Harland RM. Early development of Xenopus laevis A laboratory manual. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- Smith JC, Price BM, Green JB, Weigel D, Herrmann BG. Expression of a Xenopus homolog of Brachyury (T) is an immediate-early response to mesoderm induction. Cell. 1991;67:79–87. doi: 10.1016/0092-8674(91)90573-h. [DOI] [PubMed] [Google Scholar]
- Smith WC, Harland RM. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell. 1992;70:829–840. doi: 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- Smith WC, McKendry R, Ribisi S, Jr, Harland RM. A nodal-related gene defines a physical and functional domain within the Spemann organizer. Cell. 1995;82:37–46. doi: 10.1016/0092-8674(95)90050-0. [DOI] [PubMed] [Google Scholar]
- Taira M, Otani H, Jamrich M, Dawid IB. Expression of the LIM class homeobox gene Xlim-1 in pronephros and CNS cell lineages of Xenopus embryos is affected by retinoic acid and exogastrulation. Development. 1994;120:1525–1536. doi: 10.1242/dev.120.6.1525. [DOI] [PubMed] [Google Scholar]
- Takemoto T, Nishio Y, Sekine O, Ikeuchi C, Nagai Y, Maeno Y, Maegawa H, Kimura H, Kashiwagi A. RBMX is a novel hepatic transcriptional regulator of SREBP-1c gene response to high-fructose diet. FEBS Lett. 2007;581:218–222. doi: 10.1016/j.febslet.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Tsend-Ayush E, O’Sullivan LA, Grutzner FS, Onnebo SM, Lewis RS, Delbridge ML, Marshall Graves JA, Ward AC. RBMX gene is essential for brain development in zebrafish. Dev Dyn. 2005;234:682–688. doi: 10.1002/dvdy.20432. [DOI] [PubMed] [Google Scholar]
- Turner DL, Weintraub H. Expression of achaete-scute homolog 3 in Xenopus embryos converts ectodermal cells to a neural fate. Genes Dev. 1994;8:1434–1447. doi: 10.1101/gad.8.12.1434. [DOI] [PubMed] [Google Scholar]
- Ule J, Ule A, Spencer J, Williams A, Hu JS, Cline M, Wang H, Clark T, Fraser C, Ruggiu M, Zeeberg BR, Kane D, Weinstein JN, Blume J, Darnell RB. Nova regulates brain-specific splicing to shape the synapse. Nat Genet. 2005;37:844–852. doi: 10.1038/ng1610. [DOI] [PubMed] [Google Scholar]
- Venables JP, Elliott DJ, Makarova OV, Makarov EM, Cooke HJ, Eperon IC. RBMY, a probable human spermatogenesis factor, and other hnRNP G proteins interact with Tra2beta and affect splicing. Hum Mol Genet. 2000;9:685–694. doi: 10.1093/hmg/9.5.685. [DOI] [PubMed] [Google Scholar]
- Vignali R, Colombetti S, Lupo G, Zhang W, Stachel S, Harland RM, Barsacchi G. Xotx5b, a new member of the Otx gene family, may be involved in anterior and eye development in Xenopus laevis. Mech Dev. 2000;96:3–13. doi: 10.1016/s0925-4773(00)00367-1. [DOI] [PubMed] [Google Scholar]
- Wang J, Tse SW, Andreadis A. Tau exon 6 is regulated by an intricate interplay of trans factors and cis elements, including multiple branch points. J Neurochem. 2007;100:437–445. doi: 10.1111/j.1471-4159.2006.04252.x. [DOI] [PubMed] [Google Scholar]
- Zhao H, Cao Y, Grunz H. Isolation and characterization of a Xenopus gene (XMLP) encoding a MARCKS-like protein. Int J Dev Biol. 2001;45:817–826. [PubMed] [Google Scholar]
- Zucchi I, Jones J, Affer M, Montagna C, Redolfi E, Susani L, Vezzoni P, Parvari R, Schlessinger D, Whyte MP, Mumm S. Transcription map of Xq27: candidates for several X-linked diseases. Genomics. 1999;57:209–218. doi: 10.1006/geno.1999.5768. [DOI] [PubMed] [Google Scholar]