Abstract
Although the rhesus macaque brain is an excellent model system for the study of neurological diseases and their responses to treatment, its small size requires much higher spatial resolution, motivating use of ultra-high-field (B0) imagers. Their weaker radio-frequency fields, however, dictate longer pulses; hence longer TE localization sequences. Due to the shorter transverse relaxation time (T2) at higher B0s, these longer TEs subject metabolites to T2-weighting, that decrease their quantification accuracy. To address this we measured the T2sof N-acetylaspartate (NAA), choline (Cho), and creatine (Cr) in several gray matter (GM) and white matter (WM) regions of four healthy rhesus macaques at 7T using three-dimensional (3D) proton MR spectroscopic imaging at (0.4 cm)3 = 64 μl spatial resolution. The results show that macaque T2s are in good agreement with those reported in humans at 7T: 169 ± 2.3 ms for NAA (mean ± SEM), 114 ± 1.9 ms for Cr, and 128 ± 2.4 ms for Cho, with no significant differences between GM and WM. The T2 histograms from 320 voxels in each animal for NAA, Cr, and Cho were similar in position and shape, indicating that they are potentially characteristic of “healthy” in this species.
Keywords: animal models; MR spectroscopic imaging; rhesus macaque; transverse relaxation time; brain metabolites, 7 Tesla
Since the brain of nonhuman primates is biochemically, morphologically, and functionally similar to its human counterpart, rhesus macaques are increasingly used as advanced models for disease and treatment studies, e.g., in neuroAIDS (1), ischemic stroke (2), and Parkinson's and Huntington's diseases (3). Due to the need for repeated measurements, these studies favor nondestructive means: MRI for morphology and function, and proton MR spectroscopy (1H-MRS) for assessment of neuronal cells, cell energetics, and membrane turnover, through the levels of their surrogate markers, N-acetylaspartate (NAA), creatine (Cr), and choline (Cho) (4,5).
Unlike MRI, in which anatomy or contrast can be evaluated visually, 1H-MRS requires measurement of additional parameters for quantitative assessment. While instrumental factors (e.g., static, B0, and radio frequency [RF] field [B1] inhomogeneity) can be handled by field mapping (6), line fitting (7), and internal water referencing (8), molecular environment factors require knowledge of local longitudinal (T1) and transverse (T2) relaxation times (4). Although T1- and T2-weighting can be reduced with long repetition and short echo-times (TR ⪢ T1 and TE ⪡ T2) intermediate- and long-TE spectra are often preferred for their flatter baseline, decreased lipid contamination, and simpler peak structure (4). Consequently, reliable estimation of the metabolites’ T2 values is needed for their accurate quantification, especially at high fields (9,10).
MRS in animal models is most relevant if voxels can be assigned to analogous human structures. Given that the ∼80 cm3 macaque brain is 16-fold smaller than the average human's 1250-cm3 brain (11,12), isotropic (1.0 cm)3 voxels in the latter scale to (0.4 cm)3 = 64 μl in the former and since the signal-to-noise-ratio (SNR) depends only on voxel size and acquisition time (13), such resolution favors higher B0s. Unfortunately, the lower RF power available at the higher B0s (typically 5−8 kW at 7T vs. up to 30 kW at 3T) also produce less B1 (half as much at 7T than at 4T) per Watt (14), limiting the power that can be delivered to the coil before voltage breakdown or power deposition (specific absorption rate [SAR]) limits are exceeded, even in animals. A simple way to avoid both issues is to extend the duration of the RF pulses (and consequently the TE) in order to reduce their peak amplitude. The shorter T2s at higher B0, however (15,16), may render such sequences “intermediate” or “long” TE that subject metabolites to unknown T2-weighting (4).
Regional in vivo metabolite level variations make three-dimensional (3D) MR spectroscopic imaging (MRSI) the localization method of choice (5). To facilitate correction for the adverse effect of T2-weighting on the accuracy of metabolic quantification we measured the T2s of NAA, Cho, and Cr in several brain regions of four rhesus macaques at 7T with 3D MRSI at 64 μl spatial resolution using a two-point protocol optimized for precision per unit time (17).
MATERIALS AND METHODS
Nonhuman Primates
A total of four (two male, two female) healthy, 6- to 10-kg, 9- to 13-year-old, adult rhesus macaques (Macaca mulatta) were studied. Each was tranquilized with 15−20 mg/kg ketamine hydrochloride (intramuscular) and intubated to ensure a patent airway during the experiment (no mechanical ventilation was needed). Intravenous injection of 0.4 mg/kg atropine was administered to prevent bradycardia. Continuous infusion of 0.25 mg/kg/min propofol was maintained throughout via catheter in a saphenous vein. Heart rate, oxygen saturation, end-tidal CO2, and respiratory rate were continuously monitored. A heated water blanket and rectal temperature monitor were used to prevent hypothermia. Animals were under constant veterinary supervision and the protocol was approved by the Harvard Medical School and Massachusetts General Hospital (MGH) Institutional Animal Care and Utilization Committee (IACUC).
Instrumentation and MRI
All experiments were done in a 7T Magnetom imager (Siemens AG, Erlangen, Germany) with 18 cm × 18 cm diameter × depth transmit-receive head-coil (MR Instruments, Minneapolis, MN, USA). The coil produces up to 1 kHz B1 at 297 MHz with ∼2.4 kW reaching it from the 5-kW power amplifier. To image-guide the 1H-MRS volume of interest (VOI), sagittal, coronal, and axial turbo spin-echo MRI images were acquired at: TE/TR = 13 ms/5000 ms, matrix size = 512 × 521, 160 × 160 mm2, and slice thickness = 2 mm, as shown in Fig. 1.
FIG. 1.
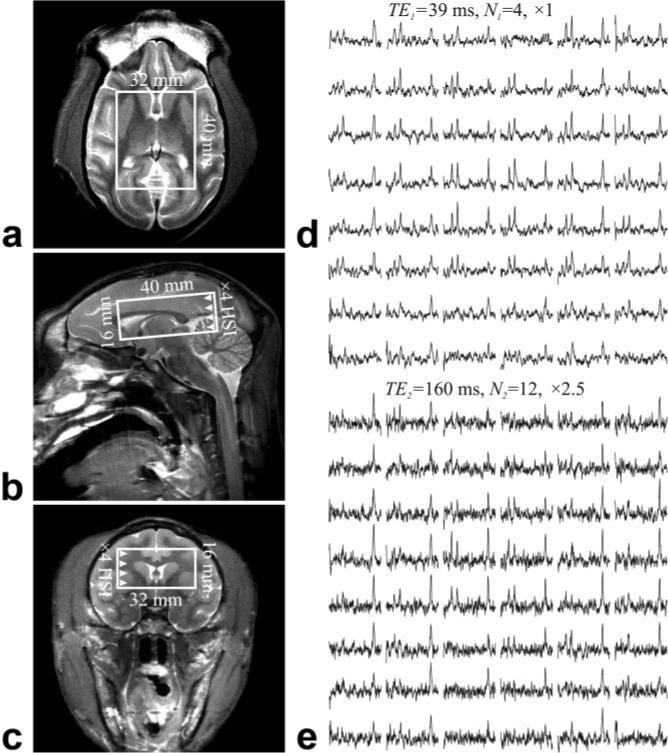
Left: (a) Axial, (b) sagittal, and (c) coronal T2-weighted MRI and typical position of the single-oblique 4.0 × 3.2 × 1.6 cm3 (AP × LR × IS) VOI in the ∼80-cm3 macaque brain. Right: Axial matrices of the real part of the spectra from the VOI over slice (a), from (d) the short TE1 = 39 ms, N1 = 4 data set and (e) the long TE2 = 165 ms, N2 = 12 data set acquisitions. All spectra are on common 3.5− 1.8 ppm and intensity scale at each TE. Long TE spectra are on a vertical scale × 2.5 times smaller than the short TE. Note the spectral resolution and SNR obtained from these isotropic (0.4 cm3) = 64-μl voxels and the substantial T2-weighting incurred from (d) to (e).
1H-MRSI
Our chemical-shift imaging (CSI)-based procedure adjusted the first and second order shims to consistent 65 ± 5 Hz whole-head water linewidth. A 40 × 32 × 16 mm3 anterior-posterior × left-right × inferior-superior (AP × LR × IS) VOI was image-guided onto the anatomy of interest and aligned along the splenium-genu axis of the corpus callosum (see Fig. 1). Manual shimming reduced the water linewidth to 50 ± 5 Hz. The VOI was excited using point-resolved spectroscopy sequence (PRESS) (TR = 1600 ms, see choice of TEs below) with two interleaved second-order Hadamard-encoded slabs (four slices) for optimal duty-cycle and SNR (18). This also allows us to double the Hadamard slice-select gradient to 12 mT/m for minimal 0.7 mm (17.5% slice width) chemical-shift displacement between NAA and Cho. These slices were 16 × 16 (LR × AP) 2D CSI-encoded in a 64 × 64 × 16 mm3 (AP × LR × IS) FOV to yield 320 isotropic (0.4 cm3) voxels in the VOI. Signals were digitized for 256 ms with 2048 complex points at a bandwidth = 4 kHz. Each 3D 1H-MRSI “block” was acquired in 13.7 min.
Choice of TE and Acquisition Strategy
The desire for higher spatial resolution and T2 precision in the noisy MRSI experiment makes for long acquisition times. To maximize its efficiency we employed two strategies: 1) 3D 1H-MRSI that yields the same SNR per given time as single-voxel methods (13) yet covers a much larger volume (18); and 2) a two-point scheme that optimizes not just the usual two TEs, but also the number of averages (N1 and N2) at each TE, for the best T2 estimate/unit time (9,17). Using the literature human Cr of ≈ 100 ms (15) as the initial “guess” for the T2s sought, has led to TE1 = 39 ms (minimum for our setup), N1 = 4, and TE2 = 165 ms (), and N2 = 12 (17). The error in the resultant T2 remains similar over a −25% to +40% range about , as shown in Fig. 2 of Ref. 9. This strategy led to a 3.6-h protocol: 55 and 164 min at TE1 and TE2.
FIG. 2.
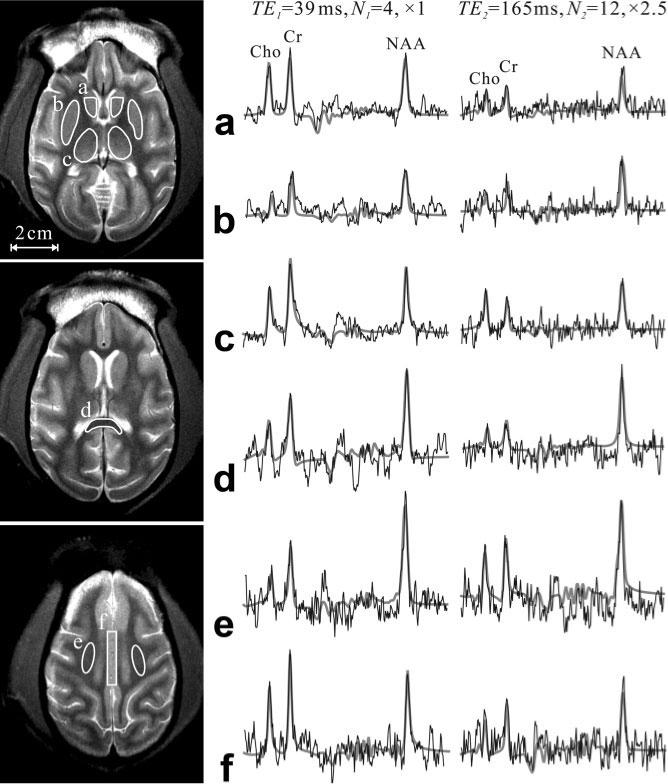
Axial brain T2-weighted images showing the ROIs where the voxels’ T2 were averaged (solid lines): (a) caudate nucleus, (b)putamen, (c) thalamus, (d) splenium of the corpus callosum, (e) centrum semiovale, and (f) cingulate gyrus. Right: Spectra at the two TEs from one 64-μl voxel within the each of the corresponding ROIs (thin black line) superimposed with the FITT (7) lineshapes used for T2 estimation (thick gray lines). Note its good estimate of the experimental spectra at each of the two echo times and the substantial T2-weighting incurred between them.
1H-MRSI Postprocessing
The 1H-MRSI data were processed offline with in-house software. Residual water signals were removed in the time domain, followed by apodization with a 6-Hz Lorentzian filter. Voxel-shift to align the CSI grid with the NAA VOI was followed by Fourier transform in the time and two spatial dimensions (LR, AP) and Hadamard transform along the IS direction. Automatic frequency and zero-order phase corrections were done in reference to the NAA peak in each voxel, as shown in Fig. 1. Relative levels of NAA, Cr, and Cho were estimated from their peak areas using parametric spectral modeling and least-squares optimization, as shown in Fig. 2 (7).
Metabolite T2 Determination
Proton T2 relaxation times of the NAA, Cr, and Cho were assessed in vivo in each voxel using,
| [1] |
where S1 and S2 are the metabolite's peak areas at the short, TE1, and long, TE2, in that voxel. A total of six different structures/regions were examined: the caudate nucleus, thalamus, putamen, and cingulate gyrus in the gray matter (GM); and the splenium of the corpus callosum and centrum semiovale in the white matter (WM). Each was outlined manually on the axial MRI (c.f., Fig. 2) and our software averaged the metabolites’ T2s in all voxels that fell entirely or partially within the outline. No cerebrospinal fluid (CSF) partial volume correction was made to the estimated metabolite levels since its contribution is the same at either TE and cancels out in the S1/S2 ratio of Eq. [1].
RESULTS
Sample spectra at the short and long echo times are shown in Figs. 1 and 2. They demonstrate the SNR and spectral resolution obtained at 7T from 64-μl voxels at these TEs. The average metabolite SNRs, defined as peak-height divided by twice the root-mean-square of the noise (see 4.3.14 in Ref. 19) for the short TE1 were 9.8 ± 1.0, 10.0 ± 1.0, and 8.9 ± 1.2 (mean ± SEM) for NAA, Cr, and Cho, respectively. Comparison of the spectra at the short and long TEs in Figs. 1 and 2 demonstrate the substantial T2 weighting incurred. The T2 values of the regions shown in Fig. 2 and obtained as described above, are compiled in Table 1. Note that the NAA, Cr, and Cho T2s are similar among the GM and WM regions studied.
Table 1.
Mean ± SEM Values of Proton T2 Relaxation Times (ms) at 7T of N-Acetylaspartate (NAA), Creatine (Cr), and Choline (Cho) in the Various GM and WM Brain Regions Shown in Fig. 2, from All Four Macaques Studied
| NAA T2 (ms) | Cr T2 (ms) | Cho T2 (ms) | |
|---|---|---|---|
| Gray matter (GM) structures | |||
| Caudate | 173 ± 13 | 109 ± 13 | 132 ± 16 |
| Thalamus | 184 ± 14 | 118 ± 4 | 131 ± 8 |
| Putamen | 174 ± 12 | 116 ± 6 | 122 ± 10 |
| Cingulate gyrus | 169 ± 6 | 126 ± 4 | 133 ± 3 |
| Average of GM structures | 175 ± 3 | 118 ± 7 | 129 ± 3 |
| White matter (WM) structures | |||
| Splenium of CC | 180 ± 3 | 112 ± 4 | 133 ± 8 |
| Centrum semiovale | 167 ± 4 | 119 ± 6 | 122 ± 5 |
| Average of WM structures | 173 ± 6 | 116 ± 4 | 127 ± 6 |
| Global WM + GM average | 169 ± 2.3 | 114 ± 1.9 | 128 ± 2.4 |
CC = corpus callosum.
The T2 histograms for the NAA, Cr, and Cho levels from all 320 VOI voxels from each of the four animals studied are shown in Fig. 3. Note the overall histogram shape for each of the three metabolites is similar among the four animals, indicating good interanimal reproducibility.
FIG. 3.
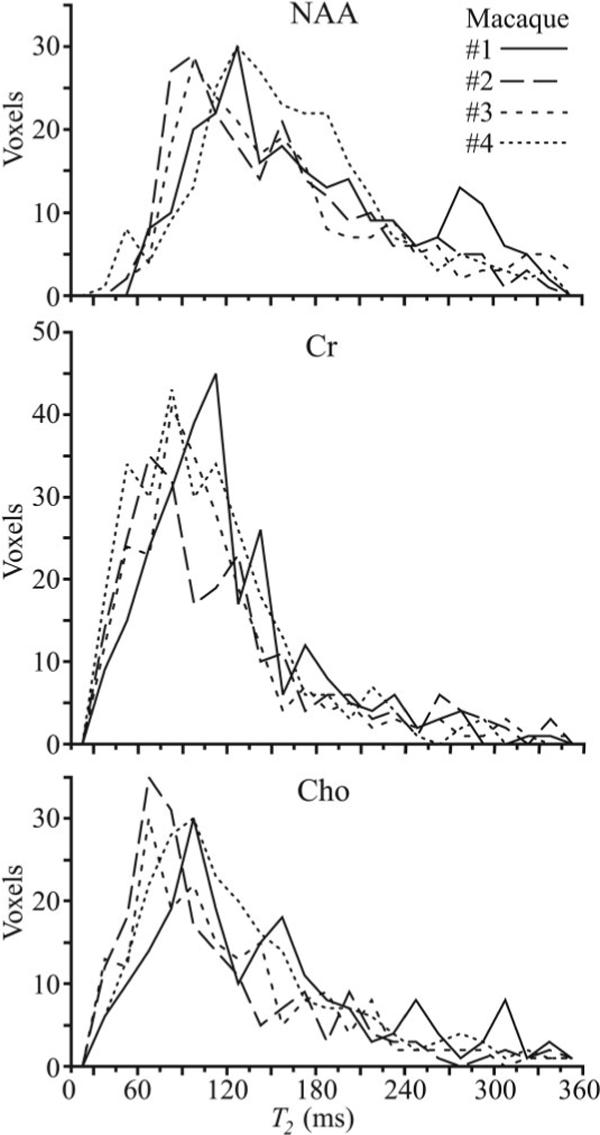
Histograms of the NAA, Cr, and Cho T2s from all 320 voxels in the VOI for each of the four macaques. Note the interprimate similarity of the histograms for each of the metabolites, indicating that: 1) a global T2 value for each would lead to less than ±10% variation in metabolic quantification; and 2) these T2 distributions are, therefore, probably characteristic of “healthy” in this species’ brain. Note that the histograms peak positions yield T2 values similar to those reported in the human brain at 7T, making the macaque a good model system in this respect.
DISCUSSION
Accurate values for metabolite relaxation times serve two main roles: they are required for reliable and reproducible quantification (10); and they are necessary to establish the optimal sequence acquisition parameters (18). Although several studies have shown that the T2s of water and metabolites progressively shorten with the increase of B0, only a few report in vivo values much above 3T (15,16). While they exhibit a consistent trend, the actual metabolite T2s or the extent of their variations in different macaque brain regions were hereto unknown.
The localization and two-point T2 techniques yielded in vivo 7T T2 maps at optimal precision for the available time, over an extensive (25%) of the macaque brain at spatial resolution that scales as the respective brain volumes. The results reveal that these T2 values are, as expected, much shorter than the 180−250 ms reported for these metabolites at 3T in the human brain (9,10). Comparing with single-voxel human T2 values at 7T reported by Michaeli et al. (15) shows that these T2 values are in agreement with the macaque's, i.e., 109 vs. 114 ms for Cr and 158 vs. 169 ms for NAA (Cho T2 was not reported in that study). This suggests that ultra-high-field T2s transcend species and that in this respect macaques are good model system for the human brain.
The spatial resolution and extensive coverage (20 cm3 of the ∼80-cm3 brain) also offer an opportunity to examine two assumptions often made in quantitative 1H-MRSI: that each metabolite has one global T2; and that it is the same in all subjects. Our results conveniently substantiate both. Specifically, the variation in T2 values among different regions within the GM or WM is less than 5% for NAA, Cr, and Cho. Such narrow distribution leads to under 5% metabolite concentration estimation variations in the short and intermediate TE < T2, regimes (9).
Although time and cost preclude test-retest experiments to ascertain the intra-animal reproducibility (precision) of these T2s explicitly, their coefficient of variation (CV) obtainable with this method cannot theoretically be better than 3.6/SNR16 (9,17). (SNR16 is achieved if the entire duration of the experiment were spent for 4 + 12 = 16 averages at TE1). This places the lower limit on what the instrumental noise contribution to the CV might be. Since for four averages at TE1 the SNR4 were ≈ 10 (see Results) extrapolating to 16 averages renders SNR16 = SNR4 × ≈ 20, indicating that the CVs in a single voxel cannot be smaller than 3.6/SNR16 ≈ 20%.
The actual upper limit on the CV from the combination of instrumental and biological noise is estimated from the ∼30% half-width at half-maximum of the histograms in Fig. 3. Assuming that these two noise sources are independent and add as , we deduce that the biological intra-animal T2s variability is less than 22%. Since the biological variability is similar in its magnitude to the instrumental contribution in this experiment, comparisons of T2's in single voxels are unable to provide biologically significant information. Averaging several voxels within a region of interest (ROI) and across several macaques, however, can substantially reduce the instrumental component of the noise. This is reflected in the T2s reported in Table 1, where the 3% to 7% CVs are sufficiently small for the reported T2s to be considered characteristic of “healthy” in this species. This also suggests that additional T2 measurements on healthy animals, e.g., at study onset, are unnecessary since they are unlikely to yield significantly different results.
It is noteworthy that although a 3.6-h-long protocol may seem excessive, it is merely the consequence of the target spatial resolution and T2 precision. Since the sensitivity of 1H is not a function of the sample, but only of the voxel size and acquisition time (13), a 10% T2 precision in a human at 7T and 1-cm3 voxels will be (16/3)2 ≈ 25-fold shorter—a very feasible ∼8 min.
CONCLUSIONS
Combining 3D 1H-MRSI with an optimized two-point acquisition protocol makes for the most efficient use of ∼4h to estimate regional neurometabolites T2 in rhesus macaques at spatial resolution proportional to analogous structures in the human brain. These regional T2 values are in line with the inverse relationship with magnetic field strength and in excellent agreement with the human in vivo values, as indeed expected from a good model system. These T2s and their variations between several WM and GM structures indicate that for the purposes of metabolic quantification use of one value for each metabolite is sufficient for T2-weighting corrections for intermediate or longer TE sequences at 7T. The narrow, overlapping T2 histograms for these metabolites indicates that they are likely to be characteristic of “healthy” in this species.
ACKNOWLEDGMENTS
We thank Dr. Andrew Maudsley of the University of Miami for the use of the SITools-FITT spectral modeling software, Ms. Diane Raikowsky and Drs. Mike Duggan and Elisabeth Moeller for animal veterinary care, Mr. Andreas Potthast and Drs. Christopher Wiggins, Graham Wiggins, and Lawrence Wald for 7T technical support. The MGH A.A. Martinos Center for Biomedical Imaging is also supported by the National Center for Research Resources grant number P41RR14075 and the Mental Illness and Neuro-science Discovery (MIND) Institute.
Grant sponsor: National Institutes of Health (NIH); Grant numbers: EB01015, NS050520, NS050041, NS051129, AI028691; Grant sponsor: National Center for Research Resources; Grant number: P41RR14075; Grant sponsor: Mental Illness and Neuroscience Discovery (MIND) Institute.
REFERENCES
- 1.Greco JB, Westmoreland SV, Ratai EM, Lentz MR, Sakaie K, He J, Sehgal PK, Masliah E, Lackner AA, Gonzalez RG. In vivo 1H MRS of brain injury and repair during acute SIV infection in the macaque model of neuroAIDS. Magn Reson Med. 2004;51:1108–1114. doi: 10.1002/mrm.20073. [DOI] [PubMed] [Google Scholar]
- 2.Roitberg B, Khan N, Tuccar E, Kompoliti K, Chu Y, Alperin N, Kordower JH, Emborg ME. Chronic ischemic stroke model in cynomolgus monkeys: behavioral, neuroimaging and anatomical study. Neurol Res. 2003;25:68–78. doi: 10.1179/016164103101200950. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Cabezas MA, Rico B, Sanchez-Gonzalez MA, Cavada C. Distribution of the dopamine innervation in the macaque and human thalamus. Neuroimage. 2007;34:965–984. doi: 10.1016/j.neuroimage.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 4.Jansen JF, Backes WH, Nicolay K, Kooi ME. 1H MR spectroscopy of the brain: absolute quantification of metabolites. Radiology. 2006;240:318–332. doi: 10.1148/radiol.2402050314. [DOI] [PubMed] [Google Scholar]
- 5.Juchem C, Merkle H, Schick F, Logothetis N, Pfeuffer J. Region and volume dependencies in spectral line width assessed by 1H 2D MR chemical shift imaging in the monkey brain at 7T. Magn Reson Imaging. 2004;22:1373–1383. doi: 10.1016/j.mri.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Gruetter R, Tkac I. Field mapping without reference scan using asymmetric echo-planar techniques. Magn Reson Med. 2000;43:319–323. doi: 10.1002/(sici)1522-2594(200002)43:2<319::aid-mrm22>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 7.Soher BJ, Young K, Govindaraju V, Maudsley AA. Automated spectral analysis III: application to in vivo proton MR spectroscopy and spectroscopic imaging. Magn Reson Med. 1998;40:822–831. doi: 10.1002/mrm.1910400607. [DOI] [PubMed] [Google Scholar]
- 8.Dong Z, Dreher W, Leibfritz D. Toward quantitative short-echo-time in vivo proton MR spectroscopy without water suppression. Magn Reson Med. 2006;55:1441–1446. doi: 10.1002/mrm.20887. [DOI] [PubMed] [Google Scholar]
- 9.Zaaraoui W, Fleysher L, Fleysher R, Liu S, Soher BJ, Gonen O. Human brain-structure resolved T(2) relaxation times of proton metabolites at 3 Tesla. Magn Reson Med. 2007;57:983–989. doi: 10.1002/mrm.21250. [DOI] [PubMed] [Google Scholar]
- 10.Tsai SY, Posse S, Lin YR, Ko CW, Otazo R, Chung HW, Lin FH. Fast mapping of the T2 relaxation time of cerebral metabolites using proton echo-planar spectroscopic imaging (PEPSI). Magn Reson Med. 2007;57:859–865. doi: 10.1002/mrm.21225. [DOI] [PubMed] [Google Scholar]
- 11.Cheverud J, Falk D, Vannier M, Konigsberg L, Helmkamp R, Hildebolt C. Heritability of brain size and surface features in rhesus macaques (Macaca mulatta). J Hered. 1990;81:51–57. doi: 10.1093/oxfordjournals.jhered.a110924. [DOI] [PubMed] [Google Scholar]
- 12.Baare W, Hulshoff Pol H, Boomsma D, Posthuma D, de Geus E, Schnack H, van Haren N, van Oel C, Kahn R. Quantitative genetic modeling of variation in human brain morphology. Cereb Cortex. 2001;11:816–824. doi: 10.1093/cercor/11.9.816. [DOI] [PubMed] [Google Scholar]
- 13.Macovski A. Noise in MRI. Magn Reson Med. 1996;36:494–497. doi: 10.1002/mrm.1910360327. [DOI] [PubMed] [Google Scholar]
- 14.Vaughan JT, Garwood M, Collins CM, Liu W, DelaBarre L, Adriany G, Andersen P, Merkle H, Goebel R, Smith MB, Ugurbil K. 7T vs. 4T: RF power, homogeneity, and signal-to-noise comparison in head images. Magn Reson Med. 2001;46:24–30. doi: 10.1002/mrm.1156. [DOI] [PubMed] [Google Scholar]
- 15.Michaeli S, Garwood M, Zhu XH, DelaBarre L, Andersen P, Adriany G, Merkle H, Ugurbil K, Chen W. Proton T2 relaxation study of water, N-acetylaspartate, and creatine in human brain using Hahn and Carr-Purcell spin echoes at 4T and 7T. Magn Reson Med. 2002;47:629–633. doi: 10.1002/mrm.10135. [DOI] [PubMed] [Google Scholar]
- 16.Otazo R, Mueller B, Ugurbil K, Wald L, Posse S. Signal-to-noise ratio and spectral linewidth improvements between 1.5 and 7 Tesla in proton echo-planar spectroscopic imaging. Magn Reson Med. 2006;56:1200–1210. doi: 10.1002/mrm.21067. [DOI] [PubMed] [Google Scholar]
- 17.Fleysher L, Fleysher R, Liu S, Zaaraoui W, Gonen O. Optimizing the precision-per-unit-time of quantitative MR metrics: Examples for T(1), T(2), and DTI. Magn Reson Med. 2007;57:380–387. doi: 10.1002/mrm.21144. [DOI] [PubMed] [Google Scholar]
- 18.Goelman G, Liu S, Hess D, Gonen O. Optimizing the efficiency of high-field multivoxel spectroscopic imaging by multiplexing in space and time. Magn Reson Med. 2006;56:34–40. doi: 10.1002/mrm.20942. [DOI] [PubMed] [Google Scholar]
- 19.Ernst RR, Bodenhausen G, Wokaun A. Principles of nuclear magnetic resonance in one and two dimensions, The International Series of Monographs on Chemistry. Clarendon Press; Oxford: 1987. p. 152. [Google Scholar]


