Abstract
The success of antiretroviral therapy has reduced the incidence of severe neurological complication resulting from human immunodeficiency virus (HIV) infection. However, increased patient survival has been associated with an increased prevalence of protracted forms of HIV encephalitis leading to moderate cognitive impairment. NeuroAIDS remains a great challenge to patients, their families, and our society. Thus development of preclinical models that will be suitable for testing promising new compounds with neurotrophic and neuroprotective capabilities is of critical importance. The simian immunodeficiency virus (SIV)-infected macaque is the premiere model to study HIV neuropathogenesis. This model was central to the seminal work of Dr. Opendra “Bill” Narayan. Similar to patients with HIV encephalitis, in the SIV model there is injury to the synaptodendritic structure of excitatory pyramidal neurons and inhibitory calbindin-immunoreactive interneurons. This article, which is part of a special issue of the Journal of NeuroVirology in honor of Dr. Bill Narayan, discusses the most important neurodegenerative features in preclinical models of neuroAIDS and their potential for treatment development.
Keywords: encephalitis, gp120, HIV, macaque, SIV, transgenic
Introduction
The control and eradication of the neurological complications associated with acquired immunodeficiency syndrome (AIDS) continues to be an important goal in efforts toward improving the well being of patients with human immunodeficiency virus (HIV). Bill Narayan dedicated his professional life with great determination and passion to better understand the pathogenesis of HIV infection in the simian immunodeficiency virus (SIV) model. His group (Narayan et al, 1995), as well as others (Lackner et al, 1991), elegantly demonstrated that in the simian model, SIV-infected perivascular macrophages enter the central nervous system (CNS) early in the progression of the disease, leading to a spectrum of neuroinflammatory and neurodegenerative alterations characteristic of this condition (Bissel et al, 2002; Lackner et al, 1991; Orandle et al, 2002; Westmoreland et al, 1998). Similarly, mononuclear cells infected with HIV-1 traffic into the CNS shortly after acquisition (Gendelman et al, 1997; Gonzalez-Scarano and Martin-Garcia, 2005) and can cause a progressive neurologic disease that translates into HIV-associated cognitive impairment (HACI) (Heaton et al, 1995). The cognitive deficits in patients with HIV profoundly affect the quality of life of people living with this condition and have often been linked to HIV encephalitis (HIVE) (Cherner et al, 2002). This neuroinflammatory condition (Gendelman et al, 1997; Wiley and Achim, 1994) is characterized by the presence of HIV-infected microglial cells, formation of microglial nodules, multinucleated giant cells, astrogliosis, myelin loss, and neurodegeneration (Bell, 2004; Budka et al, 1987; Everall et al, 2005).
With the advent of highly active antiretroviral therapies (HAART), the abundance of HIV in the brain and overt dementia has declined; however, as the number of treated subjects with chronic HIV infection increases, the prevalence of HACI is actually rising despite HAART (Gray et al, 2003; Maschke et al, 2000; McArthur et al, 2003; Sacktor et al, 2002). It is now becoming apparent that these patients may be suffering from protracted forms of HIVE (Bell, 2004; Gray et al, 2003) that might lead to more subtle cognitive alterations rather than to overt dementia (Cherner et al, 2002; Diesing et al, 2002; Gonzalez-Scarano and Martin-Garcia, 2005; Lawrence and Major, 2002). Patients with protracted mild forms of HIVE sometimes display more severe neurodegenerative pathology characterized by the simplification of the synaptodendritic structure of pyramidal neuronal populations in the neocortex, indicating that HIVE has transitioned from a subacute to a chronic condition (Everall et al, 2005).
Thus, future therapeutic developments for neuroAIDS might require the implementation of neuroprotective approaches in addition to the antiretroviral regimes. Development of such therapies involves the availability of adequate preclinical models. Bill Narayan made important contributions in characterizing the SIV model of neuroAIDS (Narayan et al, 1995; Stephens et al, 1995). This model is considered the premiere model to study the pathogenesis of HIVE. Other models include the HIV transgenic (tg) rat (Reid et al, 2001) and the HIV-gp120 tg mice (Toggas et al, 1994). Similar to models where gp120 (Sundar et al, 1991) or tat (Bansal et al, 2000; Jones et al, 1998) proteins were injected into the brains of mice, the tg model is representative of the toxic effects of the HIV proteins. However, whereas the injection models mimic the acute toxic effects of the disease, the tg models illustrate the chronic effects. A more recent model includes the injection of HIV-infected macrophages into the striatum of nude mice (Anderson et al, 2003). These models are being utilized both to understand the pathogenesis of the disease and mechanisms of neurodegeneration and for the testing of potentially neuroprotective treatments.
In this context, this review in honor of Bill Narayan will be focused on describing the most important neurodegenerative features in preclinical models of neuroAIDS, with a special emphasis on the SIV model.
Mechanisms of neurodegeneration and cognitive impairment in patients with HIVE
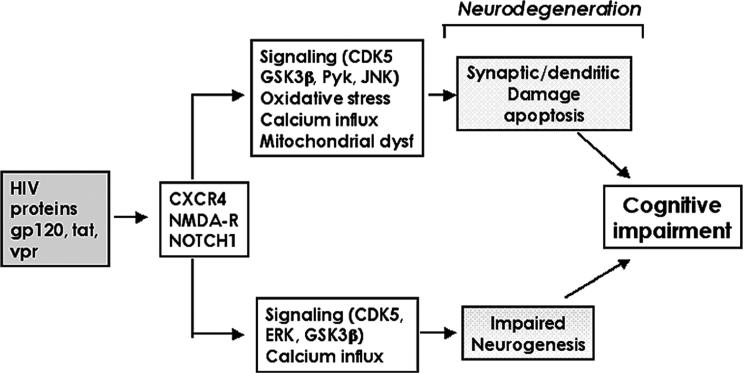
The mechanisms leading to cognitive impairment and dementia in AIDS patients are not completely understood. However, studies suggest that when HIV-infected monocytes/macrophages activate neuroinflammatory cells such as microglia and astrocytes (Gendelman et al, 1994; Langford and Masliah, 2001; Minagar et al, 2002; Mirra and del Rio, 1989; Nath, 1999; Pulliam et al, 1991; Speth et al, 2005; Wiley, 2003), these cells produce chemokines, cytokines, and neurotoxins that, in conjunction with secreted HIV proteins, damage the synaptodendritic arbor of neurons (Bellizzi et al, 2005) (Figure 1). This in turn leads to neuronal dysfunction and cell death probably via apoptosis (Brandimarti et al, 2004; Giulian et al, 1990; Kaul and Lipton, 1999; Martin-Garcia et al, 2002; Meucci et al, 1998; Nath, 2002; Pulliam et al, 1994, 1998; Sanders et al, 1998; Wang et al, 2004). This model predicts that levels of HIV in the CNS might reflect the extent of the structural and functional pathology in the brain (Brew et al, 1995; Glass et al, 1995; McArthur et al, 1997).
Figure 1.
Diagrammatic representation of the proposed mechanisms of HIV induced neuronal damage.
The neurodegenerative process in patients with HIVE is characterized by synaptic and dendritic damage (Masliah et al, 1997) to pyramidal neurons (Ellis et al, 2007; Masliah et al, 1997), loss of calbindinimmunoreactive interneurons (Masliah et al, 1995), and myelin loss (Langford et al, 2002) (Figure 2). Although disruption of the corticocortical connections might result in learning and attention deficits, hippocampal pathology is linked to memory loss and corticostriatal damage, resulting in motor alterations (Moore et al, 2006). The neuronal populations most severely affected in these regions include large pyramidal neurons in the neocortex (Figure 2) (Budka et al, 1987; Everall et al, 1991; Fox et al, 1997; Masliah et al, 1992a; Weis et al, 1993; Wiley et al, 1991a), spiny neurons in the putamen (Masliah et al, 1992b, 1996), medium-sized neurons in the globus pallidus, and interneurons in the hippocampus (Fox et al, 1997; Masliah et al, 1992b, 1995).
Figure 2.
Patterns of selective neuronal damage and myelin loss in the brains of patients with HIVE. (A–C) Decrease in the MAP2-immunoreactive dendritic arbor in HIVE. (D–F) Reduction in the numbers of calbindin-positive interneurons in HIVE. (G–I) White matter pallor and loss of myelin in the HIVE cases.
The severity of the cognitive impairment in patients with HIVE is associated with the extent of the synaptodendritic damage to pyramidal neurons in the neocortex (Cherner et al, 2002). In HIV patients with a history of methamphetamine (METH) abuse, damage to calbindin-immunoreactive neurons correlates with the severity of the cognitive impairment (Chana et al, 2006; Langford et al, 2003). In both cases, macrophage infiltration in the CNS is also a good predictor of the cognitive impairment (Achim and Wiley, 1996; Glass et al, 1995; Wiley et al, 1991b); however, the levels of HIV burden in the CNS are less predictive of the cognitive and mood deficits in these patients (Glass et al, 1995; Wiley et al, 1994). Consistent with these neuropathological studies, analyses of the brains of AIDS patients and observations in animal models show similar alterations in neuronal markers such as N-acetylaspartate (NAA) by nuclear magnetic resonance (NMR) spectroscopy (Gonzalez et al, 2000; Marcus et al, 1998; Wilkinson et al, 1997).
In addition to the damage to mature neuronal circuitries, recent studies have shown that HIV proteins might contribute to the neurodegenerative process by interfering with neurogenesis in the hippocampus (Krathwohl and Kaiser, 2004; Lawrence et al, 2004; Tran and Miller, 2005; van Marle et al, 2005). Neurogenesis in the dentate gyrus is an active process in the mature CNS and plays a role in synaptic plasticity, memory, and learning (Gage et al, 1998). Environmental enrichment has been shown to stimulate neurogenesis and improve performance in memory tasks in mice (Brown et al, 2003; Bruel-Jungerman et al, 2005; Olson et al, 2006). The wnt (Lie et al, 2005) and probably the cyclin-dependent kinase (CDK) signaling pathways, as we have begun to explore, play an important role in this process. Therefore the neurodegenerative process leading to cognitive alterations in HIV patients includes both (1) damage to the mature synaptodendritic apparatus of developed neurons and (2) impaired ability of neuronal progenitor cells (NPCs) in the hippocampal dentate gyrus to generate new neurons (Figure 3).
Figure 3.
Combined contribution of neuronal loss and defects of neurogenesis to the neurodegenerative process in HIVE.
The mechanisms leading to neurodegeneration in HIVE might involve a variety of pathways, including excitotoxicity (Haughey et al, 2001; Kaul et al, 2001), oxidative stress (Nath, 2002), mitochondrial dysfunction (Maragos et al, 2002; Turchan et al, 2003), and calcium dysregulation (Haughey and Mattson, 2002; Mattson, 2002). In addition, several lines of investigation have found that interference with signaling pathways mediating neuroprotection might also play an important role. Among them, previous studies have shown that HIV proteins abnormally activate the glycogen synthase kinase-3β (GSK3β) (Maggirwar et al, 1999) and extracellular-regulated kinase (ERK) (Lannuzel et al, 1997; Rusnati et al, 2001) signaling pathways, which otherwise are regulated by fibroblast growth factors (FGFs) (Hashimoto et al, 2002; Langford et al, 2005). Furthermore, HIV proteins trigger neurodegeneration by activating signaling pathways involved in apoptosis, such as Pyk2 (Del Corno et al, 2001), p38 and JNK (Kaul and Lipton, 1999; Lannuzel et al, 1997; Yi et al, 2004), and the RNA-activated protein kinase (Alirezaei et al, 2007).
More recently, and as part of a gene array study, we found that several components of the CDK5 signaling pathway are altered in patients with HIVE (Masliah et al, 2004). The CDK family is involved in regulating the cell cycle in dividing cells. To date, a total of nine CDKs and a number of cyclins (A to T) have been identified. Cyclin D activates CDK2, CDK4, and CDK6 during the G1 phase of cell division (Schwartz and Shah, 2005). The association between cyclin E and CDK2 is active at the G1/S transition and directs entry into S phase. The S phase is regulated by the cyclin A/CDK2 complex, and the G2 phase is modulated by the cyclin A/CDK1 (also known as cdc2) complex. Finally the cyclin B/CDK1 complex is necessary for mitosis to occur. Although in dividing peripheral tissues, CDKs play a role in modulating the progression of the cell cycle, in the mature CNS, CDKs are involved in synaptic plasticity and neuronal differentiation (Schwartz and Shah, 2005). Interestingly, in the nervous system, CDK5 is the predominant CDK, is highly expressed in neurons, and plays an important role in the mature and developing brain by regulating the phosphorylation of cytoskeletal and synaptic proteins (Fischer et al, 2005; Johansson et al, 2005).
Neuronal injury and NMR spectroscopy in SIVE models
Simian immunodeficiency virus is the closest known relative to HIV and like HIV, it infects CD4+ T lymphocytes, cells of monocyte/macrophage lineage, and brain macrophages (Lackner et al, 1991, 1994; Zink et al, 1998). SIV is a lentivirus with extensive sequence homology to HIV (Desrosiers, 1990a, 1990b; Gao et al, 1999); in rhesus macaques, SIV produces a clinical syndrome similar to that of human AIDS patients (Simon et al, 1992). The SIV-infected macaque is the premiere model of HIV neuropathogenesis; however, similar to HIV-infected humans, the low percentage (∼25%) of animals developing SIVE and the prolonged progression (1 to 3 years) to AIDS somewhat limit the usefulness of this model (Westmoreland et al, 1998). However, this traditional model does permit close examination of the complex metabolic and histopathologic changes that occur in the days and weeks after SIV infection.
Recent advances in noninvasive neuroimaging using 1H magnetic resonance spectroscopy (MRS) of HIV-infected individuals allow for the detection of brain abnormalities prior to the onset of neurological symptoms, and the reversal of abnormalities with antiretroviral therapy (Chang et al, 1999; Tracey et al, 1998). Metabolites most commonly studied in the CNS with HIV infection include the neuron-associated metabolite NAA, which decreases with neuron injury and death; choline (Cho), which is associated with cell membrane turnover and reactive gliosis; and myo-inositol (MI), a marker associated with inflammation and gliosis (Barker et al, 1995; Chang et al, 1999; Lee et al, 2003; Meyerhoff et al, 1993, 1999; Tracey et al, 1996). Changes in these metabolites have been studied in HIV infection, but a difficulty with such studies is lack of knowledge of the timing of infection and the nonuniform progression of CNS disease between patients. Thus, MRS studies in the SIV model present an excellent opportunity to probe the relationship between these resonances and the ongoing pathological changes during this time period.
In MRS studies of SIV-infected macaques, we observed decreases in the NAA/creatine (Cr) ratio (Greco et al, 2002), but these changes were subtle and rapidly resolved with control of viremia. However, we demonstrated that these transient changes in NAA/Cr best correlated with changes in synaptophysin during acute SIV infection in the macaque and reflect reversible neuronal injury during primary SIV infection (Lentz et al, 2005) (Figure 4). 1H MRS has been widely used to study HIV-infected patients, and although early studies reported profound loss in NAA/Cr (Barker et al, 1995; Chang et al, 1999; Chong et al, 1993; Jarvik et al, 1993; Menon et al, 1992; Tracey et al, 1996), such drops are less dramatic in the current HAART era. Improvement in the brain MR spectrum along with neurocognitive improvement has been reported following treatment with HAART (Chang et al, 1999; Stankoff et al, 2001), which parallels the effect of treatment in the model reported here. Still, a recent multicenter study reported an 8% decrease in NAA/Cr compared to controls in the centrum semiovale in patients undergoing HAART (Lee et al, 2003).
Figure 4.
MRS correlates of neuronal injury in the SIV model of HIV in the brain. (A, B) Comparison of the loss of MAP2-immunoreactive dendritic arbor and synaptophysin stained nerve terminals in SIVE. (C, D) Example of the alterations in calbindin interneurons in SIVE. (E, F) Comparison of the control neuronal distribution in SIV– and of microglial nodules with giant cells in SIVE. (G, H) Increased astrogliosis in SIVE.
In addition to the alterations in NAA in the SIV-infected rhesus macaque model of neuroAIDS, significant changes in the levels of Cho/Cr and MI/Cr are detected by 1H MRS during the first month of infection. For this study, animals were intravenously infected with SIVmac251 (Jarvik et al, 1993; Kodama et al, 1993) as previously described (Greco et al, 2002; Greco et al, 2004). In vivo 1H MRS studies were performed in animals imaged before inoculation with SIVmac251, and at 11 and 25 days post inoculation (d.p.i.), and the second cohort was imaged before inoculation and at 13 and 27 d.p.i. A profound, yet transient, astrogliosis that correlated highly with viremia was observed in these animals. We also found that in vivo Cho/Cr levels tended to follow a similar temporal trend as plasma virus levels and cortical astrogliosis for the first 2 weeks after infection but diverged subsequently. The in vivo MI/Cr ratio increased with peak viremia, but remained elevated despite control of plasma virus. MRS studies performed at 1.5 T of the frontal lobes of HIV-infected patients have demonstrated increased Cho/Cr (Barker et al, 1995; Chang et al, 1999), increased MI/Cr (Chang et al, 1999; Lopez-Villegas et al, 1997), and decreased NAA/Cr (Barker et al, 1995; Chang et al, 1999; Lopez-Villegas et al, 1997). Additionally, several MRS studies of HIV-infected individuals have demonstrated early elevations of Cho/Cr and/or MI/Cr, with decreases in NAA/Cr more commonly observed later in the progression of the disease (Laubenberger et al, 1996; Lopez-Villegas et al, 1997; Tarasow et al, 2003; Tracey et al, 1996). Interestingly, the temporal courses of Cho/Cr and MI/Cr are distinct, and the former better follows the course of astrogliosis.
More recently, two novel, accelerated AIDS macaque models have been developed that demonstrate rapid disease progression and high rates of incidence of SIVE (Schmitz et al, 1999a, 1999b; Williams et al, 2001; Zink and Clements, 2002). One model uses a combination of viruses, one which results in CD8+ T-lymphocyte depletion, and thus immune system suppression, in combination with a second virus that is macrophage-tropic that replicates efficiently within CNS macrophages (Clements et al, 2002; Zink and Clements, 2002). The other model uses monoclonal antibody (mAb) treatment to deplete systemic CD8+ lymphocytes, which results in early and significant monocyte/macrophage accumulation in the CNS, rapid disease progression, and a high incidence of SIVE (Schmitz et al, 1999a, 1999b; Williams et al, 2001). Both models and studies by others using SIV-infected rhesus macaques support the role of the peripheral immune system in indirectly contributing to CNS disease progression and severity (Marcondes et al, 2001; Sopper et al, 1998).
We recently reported the effects of non-CNS penetrating, antiretroviral agents in controlling and reversing neuronal injury (Gonzalez et al, 2006; Williams et al, 2005) in an accelerated AIDS model. Utilizing the mAb-induced depletion of CD8+ cells in the SIV-macaque model, infected animals that did not receive therapy became moribund with AIDS within 8 to 12 weeks. Histopathological examination of brain tissues from these animals revealed severe inflammation, robust macrophage accumulation, multinucleated giant cell (MNGC) formation, productive viral replication, morphologic alterations of cortical neurons, and damage to the synaptodendritic neuronal arbor. However, animals that underwent daily therapy were found to have a reduction in peripheral activated/infected monocytes, a lack of active viral replication or MNGC formation in brain tissues, and a near complete reversal of neuronal injury, even though peripheral viral load levels remained high (105 to 106 copies/ml). MRS allowed for noninvasive, in vivo monitoring of neuronal injury as measured by the ratio of NAA/Cr in both treated and untreated animals. All macaques were found to have large decreases in NAA/Cr levels during the first 4 weeks of infection; however, those receiving treatment thereafter underwent a nearly complete recovery to that of preinfection NAA/Cr levels. These results not only underscore the role of activated/infected peripheral blood monocytes in neuroAIDS, but also provide a plausible explanation for the clinical success of antiretroviral therapy in reducing the incidence of overt HIV-associated dementia since the 1990s despite the lack of CNS penetration by many of these drugs.
To summarize, in the classic SIV macaque model of neuroAIDS, the brain undergoes a profound but transient astrogliosis as quantified by glial fibrillary acidic protein (GFAP) immunohistochemistry during the first month of infection. In vivo 1H MRS during this period demonstrates that Cho/Cr more closely tracks changes in GFAP than MI/Cr, although both attain peak levels at the same time as GFAP peaks. Subtle changes in NAA closely reflect the synaptodendritic pathology observed in this model. Using the rapidly progressing SIV-infected macaque model, substantial neuronal injury along with profound SIVE is observed within weeks of infection. This new model coupled with MRS permits an efficient testing of hypotheses of the pathogenesis of neuroAIDS through studies of antiretroviral therapies, inflammation modulators, inhibitors of cell trafficking, and neuroprotective and neurotrophic agents.
Selective neuronal injury mediated by HIV proteins and comorbid factors in rodent models
In addition to the neurotoxic chemokines and cytokines produced by HIV-infected macrophages/microglia (Kaul and Lipton, 1999; Li et al, 2005; Ryan et al, 2002; Xiong et al, 2000), these cells have been also shown to release HIV proteins such as gp120 and Tat (Mattson et al, 2005; Xiong et al, 2000). The acute neurotoxic effects of HIV proteins have been modeled in vivo by injecting nanomolar amounts into the neocortex, limbic system, and striatum (Bansal et al, 2000; Lipton, 1992a, 1992b; Lipton et al, 1995). The chronic effects have been investigated in tg models overexpressing gp120 (Toggas et al, 1994) or Tat (Bansal et al, 2000) under constitutive astroglial promoters such as GFAP or under the control of viral vectors. Overall, these models have shown that gp120 is neurotoxic to excitatory pyramidal neurons, resulting in synaptodendritic injury in the neocortex and limbic system (Toggas et al, 1994, 1996). As a result of the synaptic damage, these mice develop alterations in long term potentiation (LTP) as well as behavioral deficits in the water maze (Anderson et al, 2003; D'Hooge et al, 1999).
Expression of Tat from astrocytes delivered into the brain has been shown to target synapses and to disrupt axonal functioning (Bruce-Keller et al, 2003). Tat also has been shown to cooperate with comorbid factors in HIVE patients such as METH (Langford et al, 2004; Maragos et al, 2002). Combined Tat and METH injected into the striatum is toxic to spiny neurons (Maragos et al, 2002). HIV proteins and METH in combination are also especially toxic to γ-aminobutyric acid (GABA)-ergic interneurons in the neocortex and striatum (Figure 5). METH is an important comorbid factor to consider because the drug abuser population is among the fastest growing HIV-infected group (Nath et al, 2001).
Figure 5.
Synergistic toxic effects of HIV proteins and METH in a mouse model. (A–D) Loss of calbindin interneurons in the neocortex of GFAP-gp120 tg mice challanged with methamphetamine. (E–H) Enhanced damage to calbindin neurons in the basal ganglia of GFAP-gp120 tg mice challanged with methamphetamine.
The selective neurotoxic effects of other HIV proteins are currently under investigation. Recent studies have suggested that HIV-gp120 might disrupt neurogenesis by promoting cell-cycle withdrawal of NPCs (Okamoto et al, 2007). In gp120 tg mice, neurogenesis is reduced by affecting the cyclin kinase pathway component cdc25c (Okamoto et al, 2007).
However, the mechanisms of toxicity of gp120 and Tat are complex and might involve cooperation with other molecules including excitotoxins such as glutamate (Kaul and Lipton, 2006), platelet-activating factor (PAF) (Gelbard et al, 1994; Westmoreland et al, 1996), and tumor necrosis factor alpha (TNFα) (Buscemi et al, 2007). Although restricted in nature, these models offer an excellent opportunity for testing and development of neuroprotective agents for the treatment of patients with HIV cognitive impairment (Everall et al, 2002; Toggas et al, 1996).
Neuronal dysfunction in recent models of HIV-associated neuropathology
Two new models have been developed in recent years, the nude mice grafted with HIV-infected macrophages in the striatum (Anderson et al, 2003) and the HIV tg rat (Reid et al, 2001). A number of studies in the engrafted nude model have shown that the synaptodendritic structure in the striatum of the mice is damaged (Anderson et al, 2003). This was associated with alterations in LTP and behavioral performance (Persidsky and Gendelman, 2002; Zink et al, 2002) that can be partially reverted with neuroprotective agents such as lithium chloride and valproic acid (Dou et al, 2003, 2005). Similarly, we have shown that lithium is neuroprotective in the gp120 tg model (Everall et al, 2002) of neurodegeneration as well as in a model of Alzheimer's disease (Rockenstein et al, 2007) by reducing the activity of GSK3β, a kinase that regulates cell fate and synaptic plasticity (Hashimoto et al, 2002). Based on these preclinical studies, we conducted a pilot study with lithium in patients with HIV and cognitive alterations and showed that this neuroprotective agent is capable of reducing the deficits in this patients (Letendre et al, 2006). Therefore, the preclinical models simulate some important aspects of HIV-mediated neurotoxicity that might serve as targets for neuroprotective therapy development.
A novel, noninfectious HIV-1 tg rat expresses an HIV-1 provirus with a deletion of functional gag and pol genes (Reid et al, 2001). This tg rat model reportedly develops clinical manifestations of human HIV disease, and mimics the persistent infection that results from the presence of HIV viral proteins in the host. In the water maze behavioral test, HIV-1 tg rats showed a deficit in learning how to swim to the location of the hidden platform but did not show a deficit in their memory of the general location of the hidden platform (Vigorito et al, 2007). It is yet not clear which neuronal populations (if any) are affected in this model and what are the selective patterns of neurodegeneration. However, this model offers an interesting alternative for the study of HIV pathogenesis and the development of neuroprotective therapies.
In summary, damage to neuronal circuitries, similar to what it is observed in patients with HIVE, has been documented in animal models ranging from the SIV macaque models to the tg rat and mouse. Better understanding and characterizing the patterns of neuronal damage in these models is important in progressing towards the goal of developing neuroprotective therapies for HIVE.
Acknowledgments
This work was supported by NIH grants NS050041 (R.G.G.), NS051129 (M.R.L.), NS34626 (R.G.G.), RR13213 (R.G.G.), NS34626 (R.G.G.), MH45294 (E.M.), MH59745 (E.M.), MH58164 (E.M.), and DA12065 (E.M.).
Footnotes
Dedicated to the memory of Opendra “Bill” Narayan.
References
- Achim CL, Wiley CA. Inflammation in AIDS and the role of the macrophage in brain pathology. Curr Opin Neurol. 1996;9:221–225. doi: 10.1097/00019052-199606000-00013. [DOI] [PubMed] [Google Scholar]
- Alirezaei M, Watry DD, Flynn CF, Kiosses WB, Masliah E, Williams BR, Kaul M, Lipton SA, Fox HS. Human immunodeficiency virus-1/surface glycoprotein 120 induces apoptosis through RNA-activated protein kinase signaling in neurons. J Neurosci. 2007;27:11047–11055. doi: 10.1523/JNEUROSCI.2733-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ER, Boyle J, Zink WE, Persidsky Y, Gendelman HE, Xiong H. Hippocampal synaptic dysfunction in a murine model of human immunodeficiency virus type 1 encephalitis. Neuroscience. 2003;118:359–369. doi: 10.1016/s0306-4522(02)00925-9. [DOI] [PubMed] [Google Scholar]
- Bansal AK, Mactutus CF, Nath A, Maragos W, Hauser KF, Booze RM. Neurotoxicity of HIV-1 proteins gp120 and Tat in the rat striatum. Brain Res. 2000;879:42–49. doi: 10.1016/s0006-8993(00)02725-6. [DOI] [PubMed] [Google Scholar]
- Barker PB, Lee RR, McArthur JC. AIDS dementia complex: evaluation with proton MR spectroscopic imaging. Radiology. 1995;195:58–64. doi: 10.1148/radiology.195.1.7892496. [DOI] [PubMed] [Google Scholar]
- Bell JE. An update on the neuropathology of HIV in the HAART era. Histopathology. 2004;45:549–559. doi: 10.1111/j.1365-2559.2004.02004.x. [DOI] [PubMed] [Google Scholar]
- Bellizzi MJ, Lu SM, Masliah E, Gelbard HA. Synaptic activity becomes excitotoxic in neurons exposed to elevated levels of platelet-activating factor. J Clin Invest. 2005;115:3185–3192. doi: 10.1172/JCI25444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissel SJ, Wang G, Ghosh M, Reinhart TA, Capuano S, 3rd, Stefano Cole K, Murphey-Corb M, Piatak M, Jr, Lifson JD, Wiley CA. Macrophages relate presynaptic and postsynaptic damage in simian immunodeficiency virus encephalitis. Am J Pathol. 2002;160:927–941. doi: 10.1016/S0002-9440(10)64915-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandimarti R, Khan MZ, Fatatis A, Meucci O. Regulation of cell cycle proteins by chemokine receptors: A novel pathway in human immunodeficiency virus neuropathogenesis? J Neuro Virol. 2004;10(Suppl 1):108–112. doi: 10.1080/753312761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew B, Rosenblum M, Cronin K, Price R. AIDS dementia comples and HIV-1 brain infection: clinical-virological correlations. Ann Neurol. 1995;38:563–570. doi: 10.1002/ana.410380404. [DOI] [PubMed] [Google Scholar]
- Brown J, Cooper-Kuhn CM, Kempermann G, Van Praag H, Winkler J, Gage FH, Kuhn HG. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J Neurosci. 2003;17:2042–2046. doi: 10.1046/j.1460-9568.2003.02647.x. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Chauhan A, Dimayuga FO, Gee J, Keller JN, Nath A. Synaptic transport of human immunodeficiency virus-Tat protein causes neurotoxicity and gliosis in rat brain. J Neurosci. 2003;23:8417–8422. doi: 10.1523/JNEUROSCI.23-23-08417.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Laroche S, Rampon C. New neurons in the dentate gyrus are involved in the expression of enhanced long-term memory following environmental enrichment. Eur J Neurosci. 2005;21:513–521. doi: 10.1111/j.1460-9568.2005.03875.x. [DOI] [PubMed] [Google Scholar]
- Budka H, Costanzi G, Cristina S, Lechi A, Parravicini C, Trabattoni R, Vago L. Brain pathology induced by infection with the human immunodeficiency virus (HIV). A histological, immunocytochemical, and electron microscopical study of 100 autopsy cases. Acta Neuropathol (Berl) 1987;75:185–198. doi: 10.1007/BF00687080. [DOI] [PubMed] [Google Scholar]
- Buscemi L, Ramonet D, Geiger JD. Human immunodeficiency virus type-1 protein Tat induces tumor necrosis factor-alpha-mediated neurotoxicity. Neurobiol Dis. 2007;26:661–670. doi: 10.1016/j.nbd.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chana G, Everall IP, Crews L, Langford D, Adame A, Grant I, Cherner M, Lazzaretto D, Heaton R, Ellis R, Masliah E. Cognitive deficits and degeneration of interneurons in HIV+ methamphetamine users. Neurology. 2006;67:1486–1489. doi: 10.1212/01.wnl.0000240066.02404.e6. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Leonido-Yee M, Witt M, Speck O, Walot I, Miller EN. Highly active antiretroviral therapy reverses brain metabolite abnormalities in mild HIV dementia. Neurology. 1999;53:782–789. doi: 10.1212/wnl.53.4.782. [DOI] [PubMed] [Google Scholar]
- Cherner M, Masliah E, Ellis RJ, Marcotte TD, Moore DJ, Grant I, Heaton RK. Neurocognitive dysfunction predicts postmortem findings of HIV encephalitis. Neurology. 2002;59:1563–1567. doi: 10.1212/01.wnl.0000034175.11956.79. [DOI] [PubMed] [Google Scholar]
- Chong WK, Sweeney B, Wilkinson ID, Paley M, Hall-Craggs MA, Kendall BE, Shepard JK, Beecham M, Miller RF, Weller IV, et al. Proton spectroscopy of the brain in HIV infection: correlation with clinical, immunologic, and MR imaging findings. Radiology. 1993;188:119–124. doi: 10.1148/radiology.188.1.8099750. [DOI] [PubMed] [Google Scholar]
- Clements JE, Babas T, Mankowski JL, Suryanarayana K, Piatak M, Jr, Tarwater PM, Lifson JD, Zink MC. The central nervous system as a reservoir for simian immunodeficiency virus (SIV): steady-state levels of SIV DNA in brain from acute through asymptomatic infection. J Infect Dis. 2002;186:905–913. doi: 10.1086/343768. [DOI] [PubMed] [Google Scholar]
- Del Corno M, Liu QH, Schols D, de Clercq E, Gessani S, Freedman BD, Collman RG. HIV-1 gp120 and chemokine activation of Pyk2 and mitogen-activated protein kinases in primary macrophages mediated by calcium-dependent, pertussis toxin-insensitive chemokine receptor signaling. Blood. 2001;98:2909–2916. doi: 10.1182/blood.v98.10.2909. [DOI] [PubMed] [Google Scholar]
- Desrosiers RC. HIV-1 origins. A finger on the missing link. Nature. 1990a;345:288–289. doi: 10.1038/345288a0. [DOI] [PubMed] [Google Scholar]
- Desrosiers RC. The simian immunodeficiency viruses. Annu Rev Immunol. 1990b;8:557–578. doi: 10.1146/annurev.iy.08.040190.003013. [DOI] [PubMed] [Google Scholar]
- D'Hooge R, Franck F, Mucke L, De Deyn PP. Age-related behavioural deficits in transgenic mice expressing the HIV-1 coat protein gp120. Eur J Neurosci. 1999;11:4398–4402. doi: 10.1046/j.1460-9568.1999.00857.x. [DOI] [PubMed] [Google Scholar]
- Diesing TS, Swindells S, Gelbard H, Gendelman HE. HIV-1-associated dementia: a basic science and clinical perspective. AIDS Read. 2002;12:358–368. [PubMed] [Google Scholar]
- Dou H, Birusingh K, Faraci J, Gorantla S, Poluektova LY, Maggirwar SB, Dewhurst S, Gelbard HA, Gendelman HE. Neuroprotective activities of sodium valproate in a murine model of human immunodeficiency virus-1 encephalitis. J Neurosci. 2003;23:9162–9170. doi: 10.1523/JNEUROSCI.23-27-09162.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou H, Ellison B, Bradley J, Kasiyanov A, Poluektova LY, Xiong H, Maggirwar S, Dewhurst S, Gelbard HA, Gendelman HE. Neuroprotective mechanisms of lithium in murine human immunodeficiency virus-1 encephalitis. J Neurosci. 2005;25:8375–8385. doi: 10.1523/JNEUROSCI.2164-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007;8:33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- Everall I, Luthert P, Lantos P. Neuronal loss in the frontal cortex in HIV infection. Lancet. 1991;337:1119–1121. doi: 10.1016/0140-6736(91)92786-2. [DOI] [PubMed] [Google Scholar]
- Everall IP, Bell C, Mallory M, Langford D, Adame A, Rockestein E, Masliah E. Lithium ameliorates HIV-gp120-mediated neurotoxicity. Mol Cell Neurosci. 2002;21:493–501. doi: 10.1006/mcne.2002.1196. [DOI] [PubMed] [Google Scholar]
- Everall IP, Hansen LA, Masliah E. The shifting patterns of HIV encephalitis neuropathology. Neurotox Res. 2005;8:51–61. doi: 10.1007/BF03033819. [DOI] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Pang PT, Lu B, Tsai LH. Opposing roles of transient and prolonged expression of p25 in synaptic plasticity and hippocampus-dependent memory. Neuron. 2005;48:825–838. doi: 10.1016/j.neuron.2005.10.033. [DOI] [PubMed] [Google Scholar]
- Fox L, Mallory M, Achim C, Masliah E. Neurode-generation od somatostatin-immunoreactive neurons in HIV encephalitis. J Neuropathol Exp Neurol. 1997;56:360–368. doi: 10.1097/00005072-199704000-00004. [DOI] [PubMed] [Google Scholar]
- Gage FH, Kempermann G, Palmer TD, Peterson DA, Ray J. Multipotent progenitor cells in the adult dentate gyrus. J Neurobiol. 1998;36:249–266. doi: 10.1002/(sici)1097-4695(199808)36:2<249::aid-neu11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Gao F, Bailes E, Robertson DL, Chen Y, Rodenburg CM, Michael SF, Cummins LB, Arthur LO, Peeters M, Shaw GM, Sharp PM, Hahn BH. Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature. 1999;397:436–441. doi: 10.1038/17130. [DOI] [PubMed] [Google Scholar]
- Gelbard H, Nottet H, Swindells S, Jett M, Dzenko K, Genis P, White R, Wang L, Choi Y-B, Zhang D, Lipton S, Tourtellotte W, Epstein L, Gendelman H. Platelet-activating factor: a candidate human immunodeficiency virus type 1-induced neurotoxin. J Virol. 1994;68:4628–4635. doi: 10.1128/jvi.68.7.4628-4635.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendelman H, Lipton S, Tardieu M, Bukrinsky M, Nottet H. The neuropathogenesis of HIV-1 infection. J Leukoc Biol. 1994;56:389–398. doi: 10.1002/jlb.56.3.389. [DOI] [PubMed] [Google Scholar]
- Gendelman HE, Persidsky Y, Ghorpade A, Limoges J, Stins M, Fiala M, Morrisett R. The neuropathogenesis of the AIDS dementia complex. AIDS. 1997;11(Suppl A):S35–S45. [PubMed] [Google Scholar]
- Giulian D, Vaca K, Noonan C. Secretion of neurotoxins by mononuclear phagocytes infected with HIV-1. Science. 1990;250:1593–1596. doi: 10.1126/science.2148832. [DOI] [PubMed] [Google Scholar]
- Glass J, Fedor H, Wesselingh S, McArthur J. Immunocytochemical quantification of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol. 1995;38:755–762. doi: 10.1002/ana.410380510. [DOI] [PubMed] [Google Scholar]
- Gonzalez RG, Cheng LL, Westmoreland SV, Sakaie KE, Becerra LR, Lee PL, Masliah E, Lackner AA. Early brain injury in the SIV-macaque model of AIDS. AIDS. 2000;14:2841–2849. doi: 10.1097/00002030-200012220-00005. [DOI] [PubMed] [Google Scholar]
- Gonzalez RG, Greco JB, He J, Lentz MR, O'Neil S, Pilkenton SJ, Ratai EM, Westmoreland S. New insights into the neuroimmunity of SIV infection by magnetic resonance spectroscopy. J Neuroimmune Pharmacol. 2006;1:152–159. doi: 10.1007/s11481-006-9016-4. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- Gray F, Chretien F, Vallat-Decouvelaere AV, Scaravilli F. The changing pattern of HIV neuropathology in the HAART era. J Neuropathol Exp Neurol. 2003;62:429–440. doi: 10.1093/jnen/62.5.429. [DOI] [PubMed] [Google Scholar]
- Greco JB, Sakaie KE, Aminipour S, Lee PL, Chang LL, He J, Westmoreland S, Lackner AA, Gonzalez RG. Magnetic resonance spectroscopy: an in vivo tool for monitoring cerebral injury in SIV-infected macaques. J Med Primatol. 2002;31:228–236. doi: 10.1034/j.1600-0684.2002.02009.x. [DOI] [PubMed] [Google Scholar]
- Greco JB, Westmoreland SV, Ratai EM, Lentz MR, Sakaie K, He J, Sehgal PK, Masliah E, Lackner AA, Gonzalez RG. In vivo 1H MRS of brain injury and repair during acute SIV infection in the macaque model of neuro AIDS. Magn Reson Med. 2004;51:1108–1114. doi: 10.1002/mrm.20073. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Sagara Y, Everall IP, Mallory M, Everson A, Langford D, Masliah E. Fibroblast growth factor 1 regulates signaling via the GSK3beta pathway: implications for neuroprotection. J Biol Chem. 2002;277:32985–32991. doi: 10.1074/jbc.M202803200. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Mattson MP. Calcium dysregulation and neuronal apoptosis by the HIV-1 proteins Tat and gp120. J Acquir Immune Defic Syndr. 2002;31(Suppl 2):S55–S61. doi: 10.1097/00126334-200210012-00005. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Nath A, Mattson MP, Slevin JT, Geiger JD. HIV-1 Tat through phosphorylation of NMDA receptors potentiates glutamate excitotoxicity. J Neurochem. 2001;78:457–467. doi: 10.1046/j.1471-4159.2001.00396.x. [DOI] [PubMed] [Google Scholar]
- Heaton R, Grant I, Butters N, White D, Kirson D, Atkinson J, McCutchan J, Taylor M, Kelly M, Ellis R, Wolfson T, Velin R, Marcotte T, Hesselink J, Jernigan T, Cahndler J, Wallace M, Abramson I, Group H. The HNRC 500—neuropsychology of HIV infection at different disease stages. J Int Neuropsych Soc. 1995;1:231–251. doi: 10.1017/s1355617700000230. [DOI] [PubMed] [Google Scholar]
- Jarvik JG, Lenkinski RE, Grossman RI, Gomori JM, Schnall MD, Frank I. Proton MR spectroscopy of HIV-infected patients: characterization of abnormalities with imaging and clinical correlation. Radiology. 1993;186:739–744. doi: 10.1148/radiology.186.3.8430182. [DOI] [PubMed] [Google Scholar]
- Johansson JU, Lilja L, Chen XL, Higashida H, Meister B, Noda M, Zhong ZG, Yokoyama S, Berggren PO, Bark C. Cyclin-dependent kinase 5 activators p35 and p39 facilitate formation of functional synapses. Brain Res Mol Brain Res. 2005;138:215–227. doi: 10.1016/j.molbrainres.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Jones M, Olafson K, Del Bigio MR, Peeling J, Nath A. Intraventricular injection of human immunodeficiency virus type 1 (HIV-1) tat protein causes inflammation, gliosis, apoptosis, and ventricular enlargement. J Neuropathol Exp Neurol. 1998;57:563–570. doi: 10.1097/00005072-199806000-00004. [DOI] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kaul M, Lipton SA. Chemokines and activated macrophages in HIV gp120-induced neuronal apoptosis. Proc Natl Acad Sci U S A. 1999;96:8212–8216. doi: 10.1073/pnas.96.14.8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Lipton SA. Mechanisms of neuronal injury and death in HIV-1 associated dementia. Curr HIV Res. 2006;4:307–318. doi: 10.2174/157016206777709384. [DOI] [PubMed] [Google Scholar]
- Kodama T, Mori K, Kawahara T, Ringler DJ, Desrosiers RC. Analysis of simian immunodeficiency virus sequence variation in tissues of rhesus macaques with simian AIDS. J Virol. 1993;67:6522–6534. doi: 10.1128/jvi.67.11.6522-6534.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krathwohl MD, Kaiser JL. HIV-1 promotes quiescence in human neural progenitor cells. J Infect Dis. 2004;190:216–226. doi: 10.1086/422008. [DOI] [PubMed] [Google Scholar]
- Lackner A, Smith M, Munn R, Martfeld D, Gardner M, Marx P, Dandekar S. Localization of simian immunodeficiency virus in the central nervous system of rhesus monkeys. Am J Pathol. 1991;139:609–621. [PMC free article] [PubMed] [Google Scholar]
- Lackner AA, Vogel P, Ramos RA, Kluge JD, Marthas M. Early events in tissues during infection with pathogenic (SIVmac239) and nonpathogenic (SIV-mac1A11) molecular clones of simian immunodeficiency virus. Am J Pathol. 1994;145:428–439. [PMC free article] [PubMed] [Google Scholar]
- Langford D, Adame A, Grigorian A, Grant I, McCutchan JA, Ellis RJ, Marcotte TD, Masliah E. Patterns of selective neuronal damage in methamphetamine-user AIDS patients. J Acquir Immune Defic Syndr. 2003;34:467–474. doi: 10.1097/00126334-200312150-00004. [DOI] [PubMed] [Google Scholar]
- Langford D, Grigorian A, Hurford R, Adame A, Crews L, Masliah E. The role of mitochondrial alterations in the combined toxic effects of human immunodeficiency virus Tat protein and methamphetamine on calbindin-positive neurons. J Neuro Virol. 2004;10:327–337. doi: 10.1080/13550280490520961. [DOI] [PubMed] [Google Scholar]
- Langford D, Hurford R, Hashimoto M, Digicaylioglu M, Masliah E. Signalling crosstalk in FGF2-mediated protection of endothelial cells from HIV-gp120. BMC Neurosci. 2005;6:8. doi: 10.1186/1471-2202-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford D, Masliah E. Crosstalk between components of the blood brain barrier and cells of the CNS in microglial activation in AIDS. Brain Pathol. 2001;11:306–312. doi: 10.1111/j.1750-3639.2001.tb00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford TD, Letendre SL, Marcotte TD, Ellis RJ, Mc-Cutchan JA, Grant I, Mallory ME, Hansen LA, Archibald S, Jernigan T, Masliah E. Severe, demyelinating leukoencephalopathy in AIDS patients on antiretroviral therapy. AIDS. 2002;16:1019–1029. doi: 10.1097/00002030-200205030-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannuzel A, Barnier JV, Hery C, Huynh VT, Guibert B, Gray F, Vincent JD, Tardieu M. Human immunodeficiency virus type 1 and its coat protein gp120 induce apoptosis and activate JNK and ERK mitogen-activated protein kinases in human neurons. Ann Neurol. 1997;42:847–856. doi: 10.1002/ana.410420605. [DOI] [PubMed] [Google Scholar]
- Laubenberger J, Haussinger D, Bayer S, Thielemann S, Schneider B, Mundinger A, Hennig J, Langer M. HIV-related metabolic abnormalities in the brain: depiction with proton MR spectroscopy with short echo times. Radiology. 1996;199:805–810. doi: 10.1148/radiology.199.3.8638009. [DOI] [PubMed] [Google Scholar]
- Lawrence DM, Durham LC, Schwartz L, Seth P, Maric D, Major EO. Human immunodeficiency virus type 1 infection of human brain-derived progenitor cells. J Virol. 2004;78:7319–7328. doi: 10.1128/JVI.78.14.7319-7328.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence DM, Major EO. HIV-1 and the brain: connections between HIV-1-associated dementia, neuropathology and neuroimmunology. Microbes Infect. 2002;4:301–308. doi: 10.1016/s1286-4579(02)01542-3. [DOI] [PubMed] [Google Scholar]
- Lee PL, Yiannoutsos CT, Ernst T, Chang L, Marra CM, Jarvik JG, Richards TL, Kwok EW, Kolson DL, Simpson D, Tang CY, Schifitto G, Ketonen LM, Meyerhoff DJ, Lenkinski RE, Gonzalez RG, Navia BA. A multi-center 1H MRS study of the AIDS dementia complex: validation and preliminary analysis. J Magn Reson Imaging. 2003;17:625–633. doi: 10.1002/jmri.10295. [DOI] [PubMed] [Google Scholar]
- Lentz MR, Kim JP, Westmoreland SV, Greco JB, Fuller RA, Ratai EM, He J, Sehgal PK, Halpern EF, Lack-ner AA, Masliah E, Gonzalez RG. Quantitative neuropathologic correlates of changes in ratio of N-acetylaspartate to creatine in macaque brain. Radiology. 2005;235:461–468. doi: 10.1148/radiol.2352040003. [DOI] [PubMed] [Google Scholar]
- Letendre SL, Woods SP, Ellis RJ, Atkinson JH, Masliah E, van den Brande G, Durelle J, Grant I, Everall I. Lithium improves HIV-associated neurocognitive impairment. Aids. 2006;20:1885–1888. doi: 10.1097/01.aids.0000244208.49123.1b. [DOI] [PubMed] [Google Scholar]
- Li W, Galey D, Mattson MP, Nath A. Molecular and cellular mechanisms of neuronal cell death in HIV dementia. Neurotox Res. 2005;8:119–134. doi: 10.1007/BF03033824. [DOI] [PubMed] [Google Scholar]
- Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR, Gage FH. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- Lipton S. HIV-related neurotoxicity. Brain Pathol. 1992a;1:193–199. doi: 10.1111/j.1750-3639.1991.tb00659.x. [DOI] [PubMed] [Google Scholar]
- Lipton S. Requirement for macrophages in neuronal injury induced by HIV envelope protein gp120. Neuro Report. 1992b;3:913–915. doi: 10.1097/00001756-199210000-00023. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Brenneman DE, Silverstein FS, Masliah E, Mucke L. gp120 and neurotoxicity in vivo. Trends Pharmacol Sci. 1995;16:122. doi: 10.1016/s0165-6147(00)88998-1. [DOI] [PubMed] [Google Scholar]
- Lopez-Villegas D, Lenkinski RE, Frank I. Biochemical changes in the frontal lobe of HIV-infected individuals detected by magnetic resonance spectroscopy. Proc Natl Acad Sci U S A. 1997;94:9854–9859. doi: 10.1073/pnas.94.18.9854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggirwar SB, Tong N, Ramirez S, Gelbard HA, Dewhurst S. HIV-1 Tat-mediated activation of glycogen synthase kinase-3beta contributes to Tat-mediated neurotoxicity. J Neurochem. 1999;73:578–586. doi: 10.1046/j.1471-4159.1999.0730578.x. [DOI] [PubMed] [Google Scholar]
- Maragos WF, Young KL, Turchan JT, Guseva M, Pauly JR, Nath A, Cass WA. Human immunodeficiency virus-1 Tat protein and methamphetamine interact synergistically to impair striatal dopaminergic function. J Neurochem. 2002;83:955–963. doi: 10.1046/j.1471-4159.2002.01212.x. [DOI] [PubMed] [Google Scholar]
- Marcondes MC, Burudi EM, Huitron-Resendiz S, Sanchez-Alavez M, Watry D, Zandonatti M, Henriksen SJ, Fox HS. Highly activated CD8(+) T cells in the brain correlate with early central nervous system dysfunction in simian immunodeficiency virus infection. J Immunol. 2001;167:5429–5438. doi: 10.4049/jimmunol.167.9.5429. [DOI] [PubMed] [Google Scholar]
- Marcus CD, Taylor-Robinson SD, Sargentoni J, Ainsworth JG, Frize G, Easterbrook PJ, Shaunak S, Bryant DJ. 1H MR spectroscopy of the brain in HIV-1-seropositive subjects: evidence for diffuse metabolic abnormalities. Metab Brain Dis. 1998;13:123–136. doi: 10.1023/a:1020609213664. [DOI] [PubMed] [Google Scholar]
- Martin-Garcia J, Kolson DL, Gonzalez-Scarano F. Chemokine receptors in the brain: their role in HIV infection and pathogenesis. AIDS. 2002;16:1709–1730. doi: 10.1097/00002030-200209060-00003. [DOI] [PubMed] [Google Scholar]
- Maschke M, Kastrup O, Esser S, Ross B, Hengge U, Hufnagel A. Incidence and prevalence of neurological disorders associated with HIV since the introduction of highly active antiretroviral therapy (HAART). J Neurol Neurosurg Psychiatry. 2000;69:376–380. doi: 10.1136/jnnp.69.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Achim C, Ge N, DeTeresa R, Terry R, Wiley C. Spectrum of human immunodeficiency virus-associated neocortical damage. Ann Neurol. 1992a;32:321–329. doi: 10.1002/ana.410320304. [DOI] [PubMed] [Google Scholar]
- Masliah E, Ge N, Achim C, DeTeresa R, Wiley C. Patterns of neurodegeneration in HIV encephalitis. Neuro AIDS. 1996;1:161–173. doi: 10.1300/j128v01n01_08. [DOI] [PubMed] [Google Scholar]
- Masliah E, Ge N, Achim C, Hansen L, Wiley C. Selective neuronal vulnerability in HIV encephalitis. J Neuropathol Exp Neurol. 1992b;51:585–593. doi: 10.1097/00005072-199211000-00003. [DOI] [PubMed] [Google Scholar]
- Masliah E, Ge N, Achim C, Wiley C. Differential vulnerability of calbindin-immunoreactive neurons in HIV encephalitis. J Neuropathol Exp Neurol. 1995;54:350–357. doi: 10.1097/00005072-199505000-00008. [DOI] [PubMed] [Google Scholar]
- Masliah E, Heaton RK, Marcotte TD, Ellis RJ, Wiley CA, Mallory M, Achim CL, McCutchan JA, Nelson JA, Atkinson JH, Grant I. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC Group. The HIV Neurobehavioral Research Center. Ann Neurol. 1997;42:963–972. doi: 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- Masliah E, Roberts ES, Langford D, Everall I, Crews L, Adame A, Rockenstein E, Fox HS. Patterns of gene dysregulation in the frontal cortex of patients with HIV encephalitis. J Neuroimmunol. 2004;157:163–175. doi: 10.1016/j.jneuroim.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Oxidative stress, perturbed calcium homeostasis, and immune dysfunction in Alzheimer's disease. J Neuro Virol. 2002;8:539–550. doi: 10.1080/13550280290100978. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Haughey NJ, Nath A. Cell death in HIV dementia. Cell Death Differ. 2005;12(Suppl 1):893–904. doi: 10.1038/sj.cdd.4401577. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Haughey N, Gartner S, Conant K, Pardo C, Nath A, Sacktor N. Human immunodeficiency virus-associated dementia: an evolving disease. J Neuro Virol. 2003;9:205–221. doi: 10.1080/13550280390194109. [DOI] [PubMed] [Google Scholar]
- McArthur JC, McClernon DR, Cronin MF, Nance-Sproson TE, Saah AJ, St Clair M, Lanier ER. Relationship between human immunodeficiency virus-associated dementia and viral load in cerebrospinal fluid and brain. Ann Neurol. 1997;42:689–698. doi: 10.1002/ana.410420504. [DOI] [PubMed] [Google Scholar]
- Menon DK, Ainsworth JG, Cox IJ, Coker RC, Sargentoni J, Coutts GA, Baudouin CJ, Kocsis AE, Harris JR. Proton MR spectroscopy of the brain in AIDS dementia complex. J Comput Assist Tomogr. 1992;16:538–542. doi: 10.1097/00004728-199207000-00007. [DOI] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci U S A. 1998;95:14500–14505. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhoff DJ, Bloomer C, Cardenas V, Norman D, Weiner MW, Fein G. Elevated subcortical choline metabolites in cognitively and clinically asymptomatic HIV+ patients. Neurology. 1999;52:995–1003. doi: 10.1212/wnl.52.5.995. [DOI] [PubMed] [Google Scholar]
- Meyerhoff DJ, MacKay S, Bachman L, Poole N, Dillon WP, Weiner MW, Fein G. Reduced brain N-acetylaspartate suggests neuronal loss in cognitively impaired human immunodeficiency virus-seropositive individuals: in vivo 1H magnetic resonance spectroscopic imaging. Neurology. 1993;43:509–515. doi: 10.1212/wnl.43.3_part_1.509. [DOI] [PubMed] [Google Scholar]
- Minagar A, Shapshak P, Fujimura R, Ownby R, Heyes M, Eisdorfer C. The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. J Neurol Sci. 2002;202:13–23. doi: 10.1016/s0022-510x(02)00207-1. [DOI] [PubMed] [Google Scholar]
- Mirra S, del Rio C. The fine structure of acquired immunodeficiency syndrome encephalopathy. Arch Pathol Lab Med. 1989;113:858–865. [PubMed] [Google Scholar]
- Moore DJ, Masliah E, Rippeth JD, Gonzalez R, Carey CL, Cherner M, Ellis RJ, Achim CL, Marcotte TD, Heaton RK, Grant I. Cortical and subcortical neurodegeneration is associated with HIV neurocognitive impairment. AIDS. 2006;20:879–887. doi: 10.1097/01.aids.0000218552.69834.00. [DOI] [PubMed] [Google Scholar]
- Narayan O, Joag S, Stephens E. Selected models of HIV-induced neurological disease. Curr Topics Microbiol Immunol. 1995;202:151–166. doi: 10.1007/978-3-642-79657-9_11. [DOI] [PubMed] [Google Scholar]
- Nath A. Pathobiology of human immunodeficiency virus dementia. Semin Neurol. 1999;19:113–127. doi: 10.1055/s-2008-1040830. [DOI] [PubMed] [Google Scholar]
- Nath A. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J Infect Dis. 2002;186(Suppl 2):S193–S198. doi: 10.1086/344528. [DOI] [PubMed] [Google Scholar]
- Nath A, Maragos WF, Avison MJ, Schmitt FA, Berger JR. Acceleration of HIV dementia with methamphetamine and cocaine. J Neuro Virol. 2001;7:66–71. doi: 10.1080/135502801300069737. [DOI] [PubMed] [Google Scholar]
- Okamoto S, Kang Y-J, Brechtel CW, Siviglia E, Russo R, Clemente A, Harrop A, McKercher S, Kaul M, Lipton SA. HIV/gp120 decreases adult neural progenitor cell proliferation via checkpoint kinase-mediated cell-cycle withdrawal and G1 arrest. Cell Stem Cell. 2007;1:230–236. doi: 10.1016/j.stem.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Olson AK, Eadie BD, Ernst C, Christie BR. Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus. 2006;16:250–260. doi: 10.1002/hipo.20157. [DOI] [PubMed] [Google Scholar]
- Orandle MS, MacLean AG, Sasseville VG, Alvarez X, Lackner AA. Enhanced expression of proinflammatory cytokines in the central nervous system is associated with neuroinvasion by simian immunodeficiency virus and the development of encephalitis. J Virol. 2002;76:5797–5802. doi: 10.1128/JVI.76.11.5797-5802.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persidsky Y, Gendelman HE. Murine models for human immunodeficiency virus type 1-associated dementia: the development of new treatment testing paradigms. J Neuro Virol. 2002;8(Suppl 2):49–52. doi: 10.1080/13550280290167993. [DOI] [PubMed] [Google Scholar]
- Pulliam L, Clarke JA, McGuire D, McGrath MS. Investigation of HIV-infected macrophage neurotoxin production from patients with AIDS dementia. Adv Neuroimmunol. 1994;4:195–198. doi: 10.1016/s0960-5428(06)80257-3. [DOI] [PubMed] [Google Scholar]
- Pulliam L, Herndier B, Tang N, McGrath M. Human immunodeficiency virus-infected macrophages produce soluble factors that cause histological and neurochemical alterations in cultured human brains. J Clin Invest. 1991;87:503–512. doi: 10.1172/JCI115024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulliam L, Zhou M, Stubblebine M, Bitler CM. Differential modulation of cell death proteins in human brain cells by tumor necrosis factor alpha and platelet activating factor. J Neurosci Res. 1998;54:530–538. doi: 10.1002/(SICI)1097-4547(19981115)54:4<530::AID-JNR10>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Reid W, Sadowska M, Denaro F, Rao S, Foulke J, Jr, Hayes N, Jones O, Doodnauth D, Davis H, Sill A, O'Driscoll P, Huso D, Fouts T, Lewis G, Hill M, Kamin-Lewis R, Wei C, Ray P, Gallo RC, Reitz M, Bryant J. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc Natl Acad Sci U S A. 2001;98:9271–9276. doi: 10.1073/pnas.161290298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockenstein E, Torrance M, Adame A, Mante M, Bar-on P, Rose JB, Crews L, Masliah E. Neuroprotective effects of regulators of the glycogen synthase kinase-3beta signaling pathway in a transgenic model of Alzheimer's disease are associated with reduced amyloid precursor protein phosphorylation. J Neurosci. 2007;27:1981–1991. doi: 10.1523/JNEUROSCI.4321-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusnati M, Urbinati C, Musulin B, Ribatti D, Albini A, Noonan D, Marchisone C, Waltenberger J, Presta M. Activation of endothelial cell mitogen activated protein kinase ERK(1/2) by extracellular HIV-1 Tat protein. Endothelium. 2001;8:65–74. doi: 10.3109/10623320109063158. [DOI] [PubMed] [Google Scholar]
- Ryan LA, Cotter RL, Zink WE, 2nd, Gendelman HE, Zheng J. Macrophages, chemokines and neuronal injury in HIV-1-associated dementia. Cell Mol Biol (Noisy-le-grand) 2002;48:137–150. [PubMed] [Google Scholar]
- Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, Stern Y, Albert S, Palumbo D, Kieburtz K, De Marcaida JA, Cohen B, Epstein L. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neuro Virol. 2002;8:136–142. doi: 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]
- Sanders VJ, Pittman CA, White MG, Wang G, Wiley CA, Achim CL. Chemokines and receptors in HIV encephalitis. AIDS. 1998;12:1021–1026. [PubMed] [Google Scholar]
- Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, Racz P, Tenner-Racz K, Dalesandro M, Scallon BJ, Ghrayeb J, Forman MA, Montefiori DC, Rieber EP, Letvin NL, Reimann KA. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999a;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- Schmitz JE, Simon MA, Kuroda MJ, Lifton MA, Ollert MW, Vogel CW, Racz P, Tenner-Racz K, Scallon BJ, Dalesandro M, Ghrayeb J, Rieber EP, Sasseville VG, Reimann KA. A nonhuman primate model for the selective elimination of CD8+ lymphocytes using a mouse-human chimeric monoclonal antibody. Am J Pathol. 1999b;154:1923–1932. doi: 10.1016/S0002-9440(10)65450-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz GK, Shah MA. Targeting the cell cycle: a new approach to cancer therapy. J Clin Oncol. 2005;23:9408–9421. doi: 10.1200/JCO.2005.01.5594. [DOI] [PubMed] [Google Scholar]
- Simon MA, Chalifoux LV, Ringler DJ. Pathologic features of SIV-induced disease and the association of macrophage infection with disease evolution. AIDS Res Hum Retroviruses. 1992;8:327–337. doi: 10.1089/aid.1992.8.327. [DOI] [PubMed] [Google Scholar]
- Sopper S, Sauer U, Hemm S, Demuth M, Muller J, Stahl-Hennig C, Hunsmann G, ter Meulen V, Dorries R. Protective role of the virus-specific immune response for development of severe neurologic signs in simian immunodeficiency virus-infected macaques. J Virol. 1998;72:9940–9947. doi: 10.1128/jvi.72.12.9940-9947.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speth C, Dierich MP, Sopper S. HIV-infection of the central nervous system: the tightrope walk of innate immunity. Mol Immunol. 2005;42:213–228. doi: 10.1016/j.molimm.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Stankoff B, Tourbah A, Suarez S, Turell E, Stievenart JL, Payan C, Coutellier A, Herson S, Baril L, Bricaire F, Calvez V, Cabanis EA, Lacomblez L, Lubetzki C. Clinical and spectroscopic improvement in HIV-associated cognitive impairment. Neurology. 2001;56:112–115. doi: 10.1212/wnl.56.1.112. [DOI] [PubMed] [Google Scholar]
- Stephens EB, Liu ZQ, Zhu GW, Adany I, Joag SV, Foresman L, Berman NE, Narayan O. Lymphocyte-tropic simian immunodeficiency virus causes persistent infection in the brains of rhesus monkeys. Virology. 1995;213:600–614. doi: 10.1006/viro.1995.0032. [DOI] [PubMed] [Google Scholar]
- Sundar S, Cierpial M, Kamaraju L, Long S, Hsieh S, Lorenz C, Aaron M, Ritchie J, Weiss J. Human immunodeficiency virus glycoprotein (gp120) infused into rat brain induces interleukin 1 to elevate pituitary-adrenal activity and decreased peripheral cellular immune responses. Proc Natl Acad Sci U S A. 1991;88:11246–11250. doi: 10.1073/pnas.88.24.11246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarasow E, Wiercinska-Drapalo A, Kubas B, Dzienis W, Orzechowska-Bobkiewicz A, Prokopowicz D, Walecki J. Cerebral MR spectroscopy in neurologically asymptomatic HIV-infected patients. Acta Radiol. 2003;44:206–212. doi: 10.1080/j.1600-0455.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- Toggas S, Masliah E, Mucke L. Prevention of HIV-1 gp120-induced neuronal damage in the central nervous system of transgenic mice by the NMDA receptor atagonist memantine. Brain Res. 1996;706:303–307. doi: 10.1016/0006-8993(95)01197-8. [DOI] [PubMed] [Google Scholar]
- Toggas S, Masliah E, Rockenstein E, Mucke L. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature. 1994;367:188–193. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- Tracey I, Carr CA, Guimaraes AR, Worth JL, Navia BA, Gonzalez RG. Brain choline-containing compounds are elevated in HIV-positive patients before the onset of AIDS dementia complex: a proton magnetic resonance spectroscopic study. Neurology. 1996;46:783–788. doi: 10.1212/wnl.46.3.783. [DOI] [PubMed] [Google Scholar]
- Tracey I, Hamberg LM, Guimaraes AR, Hunter G, Chang I, Navia BA, Gonzalez RG. Increased cerebral blood volume in HIV-positive patients detected by functional MRI. Neurology. 1998;50:1821–1826. doi: 10.1212/wnl.50.6.1821. [DOI] [PubMed] [Google Scholar]
- Tran PB, Miller RJ. HIV-1, chemokines and neurogenesis. Neurotox Res. 2005;8:149–158. doi: 10.1007/BF03033826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turchan J, Pocernich CB, Gairola C, Chauhan A, Schifitto G, Butterfield DA, Buch S, Narayan O, Sinai A, Geiger J, Berger JR, Elford H, Nath A. Oxidative stress in HIV demented patients and protection ex vivo with novel antioxidants. Neurology. 2003;60:307–314. doi: 10.1212/01.wnl.0000042048.85204.3d. [DOI] [PubMed] [Google Scholar]
- van Marle G, Antony JM, Silva C, Sullivan A, Power C. Aberrant cortical neurogenesis in a pediatric neuro AIDS model: neurotrophic effects of growth hormone. AIDS. 2005;19:1781–1791. doi: 10.1097/01.aids.0000189854.06194.87. [DOI] [PubMed] [Google Scholar]
- Vigorito M, LaShomb AL, Chang SL. Spatial learning and memory in HIV-1 transgenic rats. J Neuroimmune Pharmacol. 2007;2:319–328. doi: 10.1007/s11481-007-9078-y. [DOI] [PubMed] [Google Scholar]
- Wang Z, Trillo-Pazos G, Kim SY, Canki M, Morgello S, Sharer LR, Gelbard HA, Su ZZ, Kang DC, Brooks AI, Fisher PB, Volsky DJ. Effects of human immunodeficiency virus type 1 on astrocyte gene expression and function: potential role in neuropathogenesis. J Neuro Virol. 2004;10(Suppl 1):25–32. doi: 10.1080/753312749. [DOI] [PubMed] [Google Scholar]
- Weis S, Haug H, Budka H. Neuronal damage in the cerebral cortex of AIDS brains: a morphometric study. Acta Neuropathol. 1993;85:185–189. doi: 10.1007/BF00227766. [DOI] [PubMed] [Google Scholar]
- Westmoreland SV, Halpern E, Lackner AA. Simian immunodeficiency virus encephalitis in rhesus macaques is asociated with rapid disease progression. J Neuro Virol. 1998;4:260–268. doi: 10.3109/13550289809114527. [DOI] [PubMed] [Google Scholar]
- Westmoreland SV, Kolson D, Gonzalez-Scarano F. Toxicity of TNF alpha and platelet activating factor for human NT2N neurons: a tissue culture model for human immunodeficiency virus dementia. J Neuro Virol. 1996;2:118–126. doi: 10.3109/13550289609146545. [DOI] [PubMed] [Google Scholar]
- Wiley C, Achim C. HIV encephalitis is the pathologic correlate of dementia in AIDS. Ann Neurol. 1994;36:673–676. doi: 10.1002/ana.410360422. [DOI] [PubMed] [Google Scholar]
- Wiley C, Masliah E, Morey M, Lemere C, DeTeresa R, Grafe M, Hansen L, Terry R. Neocortical damage during HIV infection. Ann Neurol. 1991a;29:651–657. doi: 10.1002/ana.410290613. [DOI] [PubMed] [Google Scholar]
- Wiley CA. Detection of HIV-1 DNA in microglia/macrophages, astrocytes and neurons isolated from brain tissue with HIV-1 encephalitis by laser capture microdissection. Brain Pathol. 2003;13:415. doi: 10.1111/j.1750-3639.2003.tb00040.x. author reply 415–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley CA, Masliah E, Achim CL. Measurement of CNS HIV burden and its association with neurologic damage. Adv Neuroimmunol. 1994;4:319–325. doi: 10.1016/s0960-5428(06)80272-x. [DOI] [PubMed] [Google Scholar]
- Wiley CA, Schrier RD, Morey M, Achim C, Venable JC, Nelson JA. Pathogenesis of HIV encephalitis. Acta Pathol Jpn. 1991b;41:192–196. doi: 10.1111/j.1440-1827.1991.tb01646.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson ID, Lunn S, Miszkiel KA, Miller RF, Paley MN, Williams I, Chinn RJ, Hall-Craggs MA, Newman SP, Kendall BE, Harrison MJ. Proton MRS and quantitative MRI assessment of the short term neurological response to antiretroviral therapy in AIDS. J Neurol Neurosurg Psychiatry. 1997;63:477–482. doi: 10.1136/jnnp.63.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K, Westmoreland S, Greco J, Ratai E, Lentz M, Kim WK, Fuller RA, Kim JP, Autissier P, Sehgal PK, Schinazi RF, Bischofberger N, Piatak M, Lifson JD, Masliah E, Gonzalez RG. Magnetic resonance spectroscopy reveals that activated monocytes contribute to neuronal injury in SIV neuroAIDS. J Clin Invest. 2005;115:2534–2545. doi: 10.1172/JCI22953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KC, Corey S, Westmoreland SV, Pauley D, Knight H, deBakker C, Alvarez X, Lackner AA. Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. J Exp Med. 2001;193:905–915. doi: 10.1084/jem.193.8.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong H, Zeng YC, Lewis T, Zheng J, Persidsky Y, Gendelman HE. HIV-1 infected mononuclear phagocyte secretory products affect neuronal physiology leading to cellular demise: relevance for HIV-1-associated dementia. J Neuro Virol. 2000;6(Suppl 1):S14–S23. [PubMed] [Google Scholar]
- Yi Y, Lee C, Liu QH, Freedman BD, Collman RG. Chemokine receptor utilization and macrophage signaling by human immunodeficiency virus type 1 gp120: Implications for neuropathogenesis. J Neuro Virol. 2004;10(Suppl 1):91–96. doi: 10.1080/753312758. [DOI] [PubMed] [Google Scholar]
- Zink MC, Clements JE. A novel simian immunodeficiency virus model that provides insight into mechanisms of human immunodeficiency virus central nervous system disease. J Neuro Virol. 2002;8(Suppl 2):42–48. doi: 10.1080/13550280290101076. [DOI] [PubMed] [Google Scholar]
- Zink MC, Spelman JP, Robinson RB, Clements JE. SIV infection of macaques—modeling the progression to AIDS dementia. J Neuro Virol. 1998;4:249–259. doi: 10.3109/13550289809114526. [DOI] [PubMed] [Google Scholar]
- Zink WE, Anderson E, Boyle J, Hock L, Rodriguez-Sierra J, Xiong H, Gendelman HE, Persidsky Y. Impaired spatial cognition and synaptic potentiation in a murine model of human immunodeficiency virus type 1 encephalitis. J Neurosci. 2002;22:2096–2105. doi: 10.1523/JNEUROSCI.22-06-02096.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]