Abstract
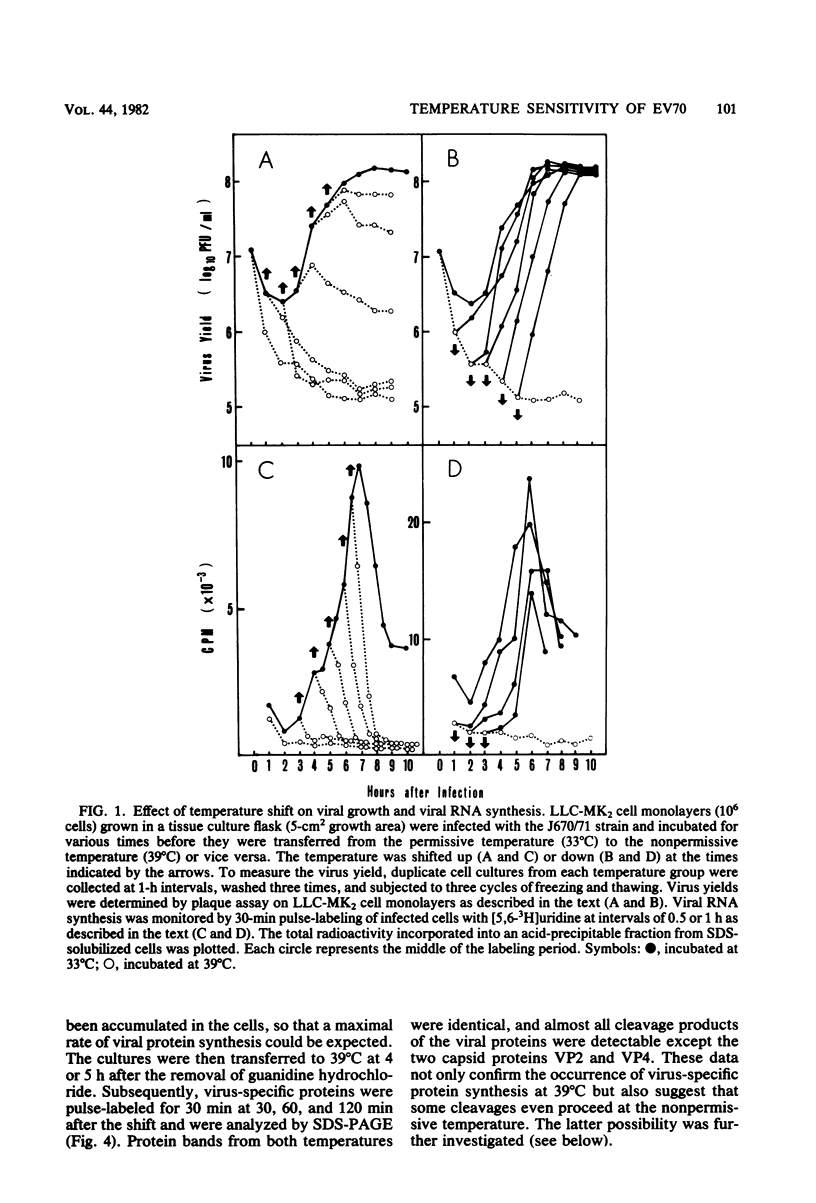
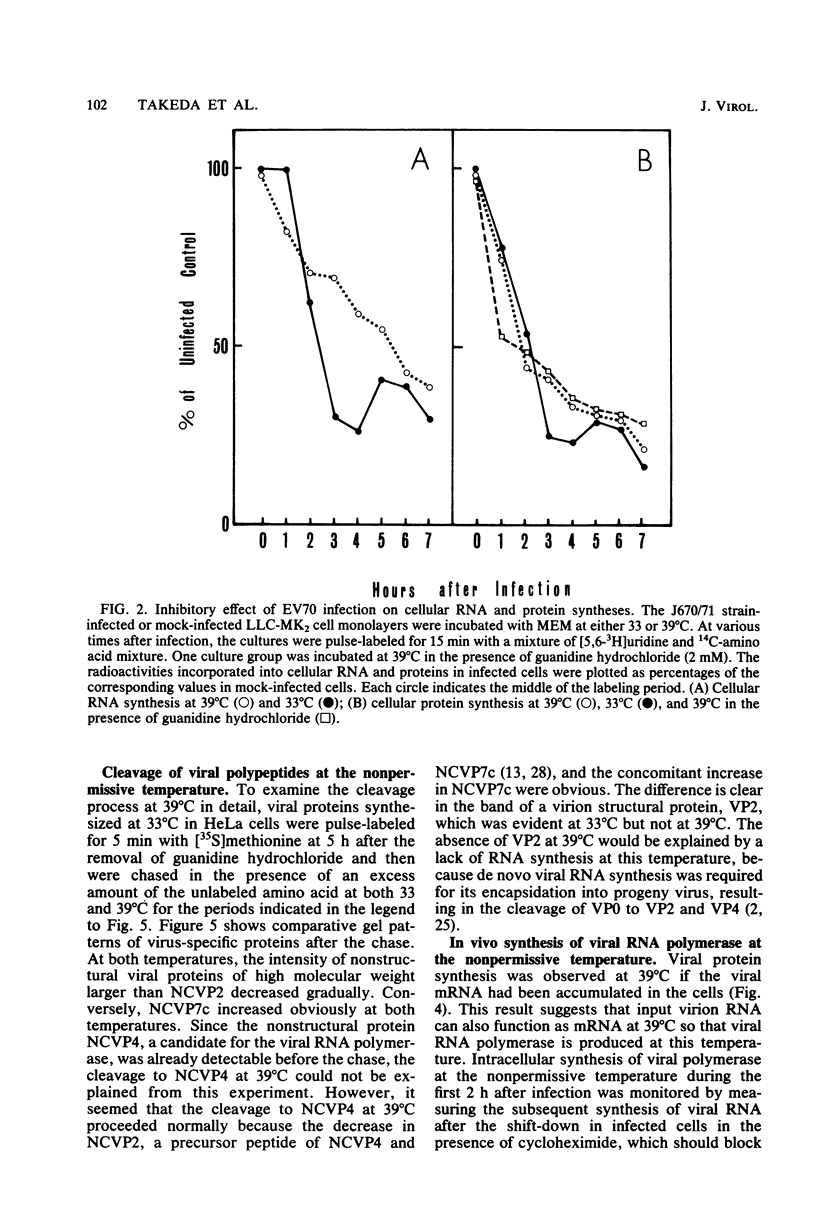
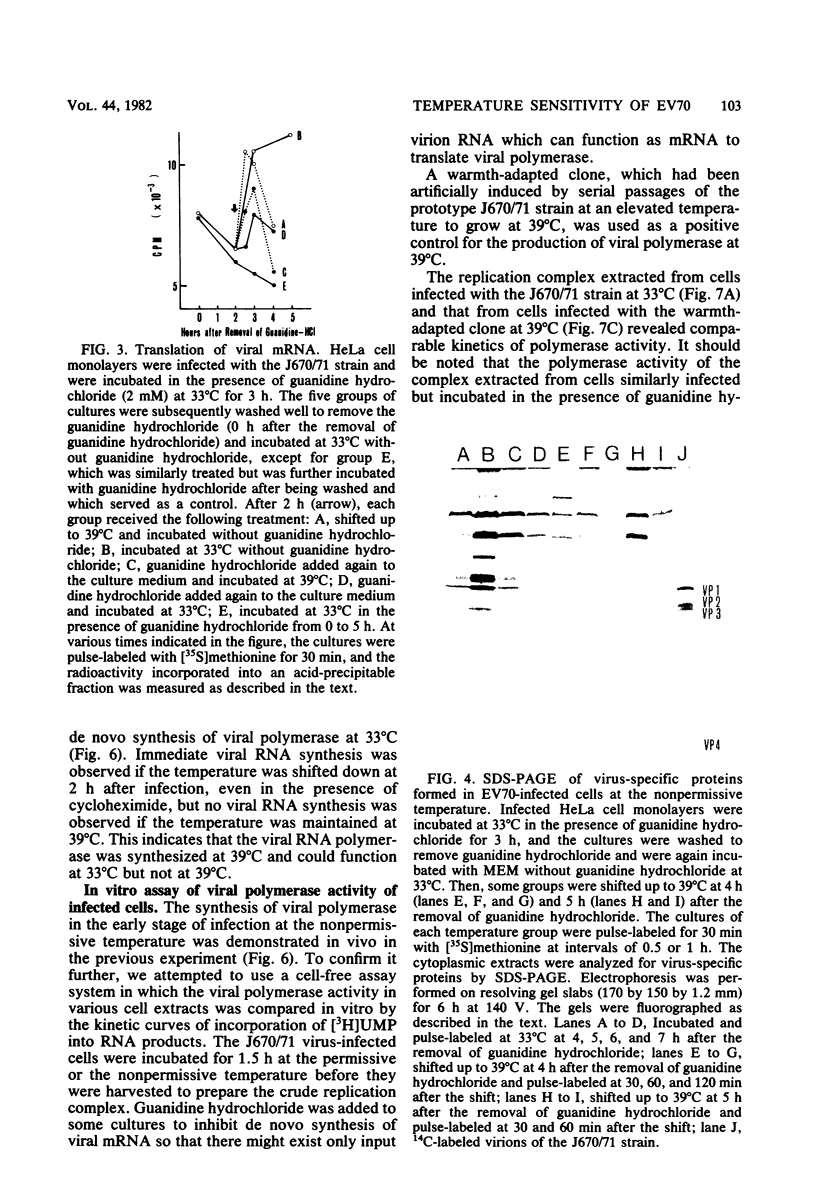
The mechanism of the failure of enterovirus type 70 to replicate at a nonpermissive temperature (39 degrees C) was investigated, and the following results were obtained. (i) Viral RNA synthesis was not observed at 39 degrees C in LLC-MK2 cells, in accordance with our previous findings with primary monkey kidney cells (Miyamura et al., Intervirology 9:206-213, 1978). (ii) Shutoff of host cell macromolecular synthesis by virus infection was as efficient at 39 degrees C as at a permissive temperature (33 degrees C). This inhibitory effect similarly occurred even in the presence of guanidine hydrochloride. (iii) Viral protein synthesis proceeded in vivo at the nonpermissive temperature, and the rate of the protein synthesis was higher than that at the permissive temperature under the conditions in which sufficient viral mRNA had been accumulated. This was also confirmed by analyzing the intracellular proteins synthesized at the nonpermissive temperature by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, which identified them as virus-specific proteins. (iv) When infected cells were incubated at 39 degrees C and then transferred to 33 degrees C, viral RNA synthesis took place even in the presence of cycloheximide. (v) Furthermore, in experiments performed with an in vitro cell-free assay system, viral polymerase activity was found in the membrane-bound preparation extracted from infected cells which had been incubated at 39 degrees C in the presence or absence of guanidine hydrochloride. These results indicate that early translation of mRNA proceeds normally at the nonpermissive temperature and that the temperature-sensitive defect resides in the transcriptional stage.
Full text
PDF


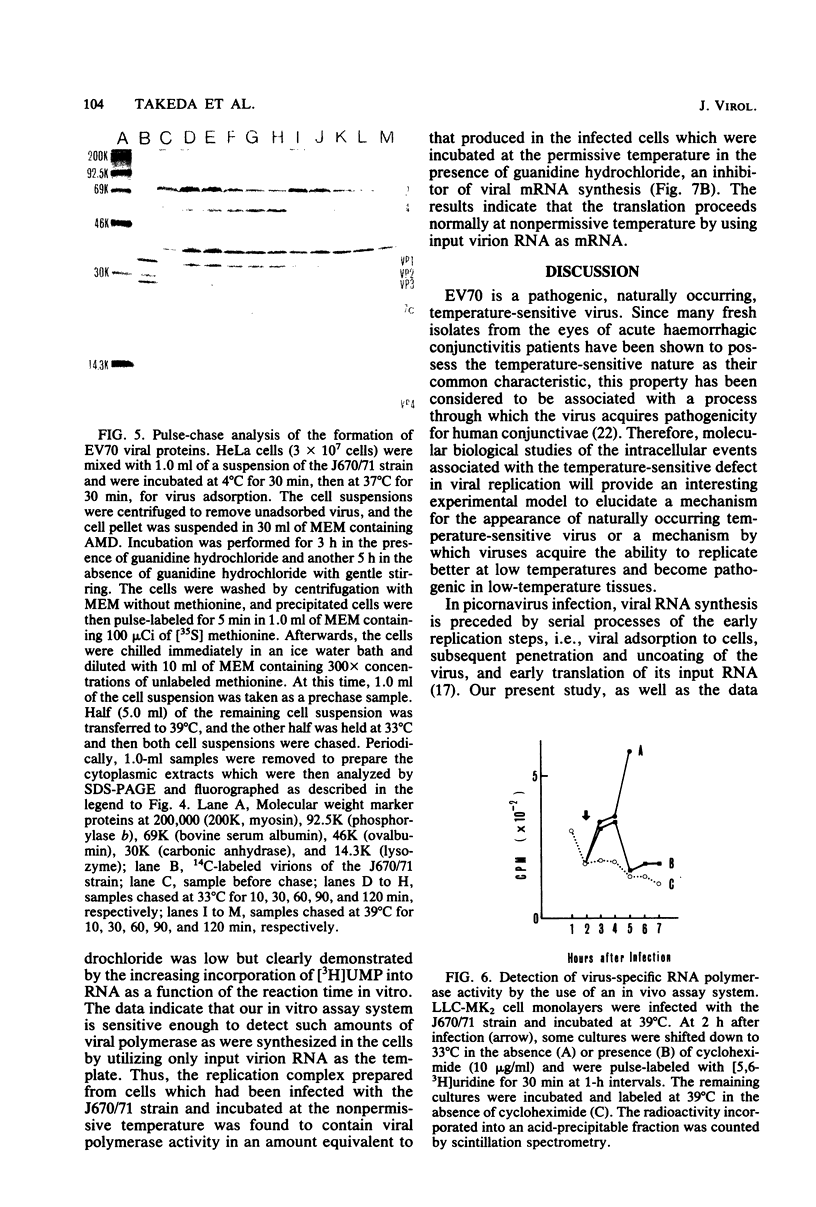
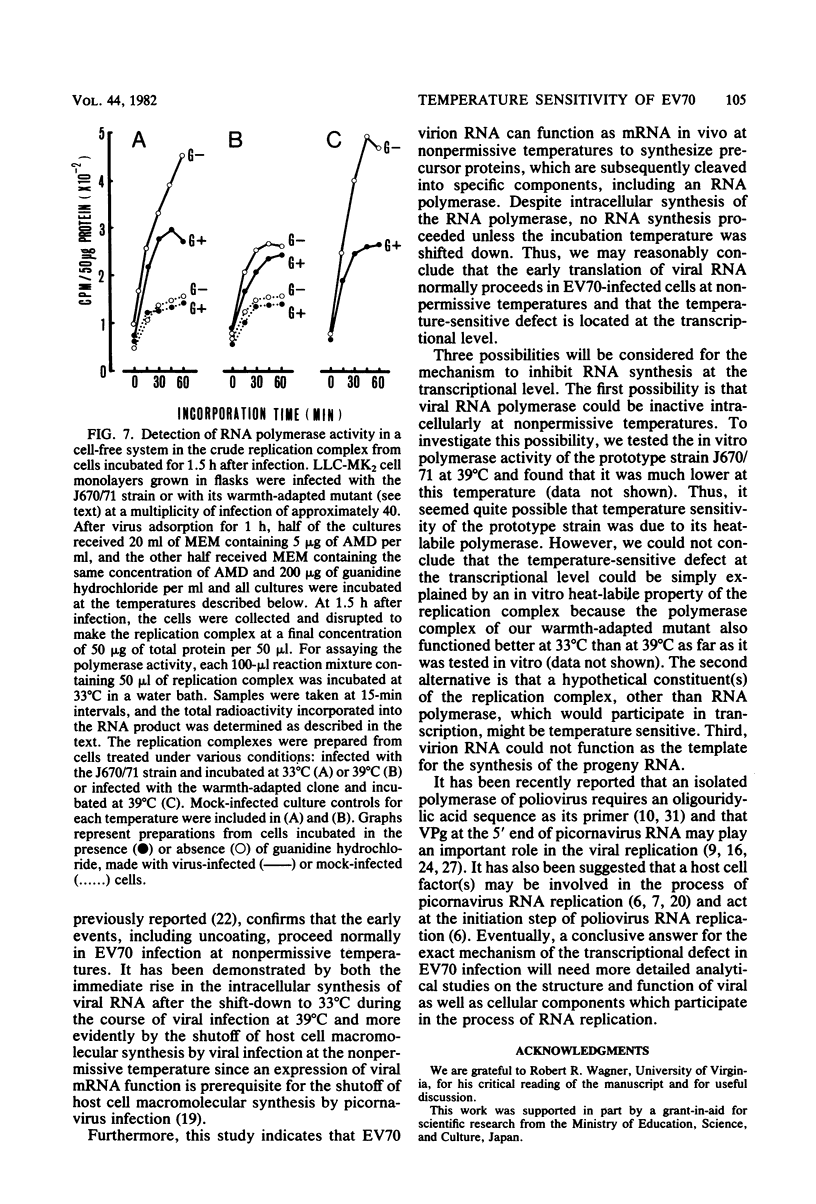

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler R., Tershak D. R. Temperature-sensitive defect of type 2 poliovirus. Virology. 1974 Mar;58(1):209–218. doi: 10.1016/0042-6822(74)90155-x. [DOI] [PubMed] [Google Scholar]
- Baltimore D., Girard M., Darnell J. E. Aspects of the synthesis of poliovirus RNA and the formation of virus particles. Virology. 1966 Jun;29(2):179–189. doi: 10.1016/0042-6822(66)90024-9. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Crawford N., Fire A., Samuels M., Sharp P. A., Baltimore D. Inhibition of transcription factor activity by poliovirus. Cell. 1981 Dec;27(3 Pt 2):555–561. doi: 10.1016/0092-8674(81)90397-4. [DOI] [PubMed] [Google Scholar]
- Cross R. K., Fields B. N. Reovirus-specific polypeptides: analysis using discontinuous gel electrophoresis. J Virol. 1976 Jul;19(1):162–173. doi: 10.1128/jvi.19.1.162-173.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta A., Zabel P., Baltimore D. Dependence of the activity of the poliovirus replicase on the host cell protein. Cell. 1980 Feb;19(2):423–429. doi: 10.1016/0092-8674(80)90516-4. [DOI] [PubMed] [Google Scholar]
- Dmitrieva T. M., Shcheglova M. V., Agol V. I. Inhibition of activity of encephalomyocarditis virus-induced RNA polymerase by antibodies against cellular components. Virology. 1979 Jan 30;92(2):271–277. doi: 10.1016/0042-6822(79)90131-4. [DOI] [PubMed] [Google Scholar]
- Ehrenfeld E. Poliovirus-induced inhibition of host-cell protein synthesis. Cell. 1982 Mar;28(3):435–436. doi: 10.1016/0092-8674(82)90195-7. [DOI] [PubMed] [Google Scholar]
- Flanegan J. B., Petterson R. F., Ambros V., Hewlett N. J., Baltimore D. Covalent linkage of a protein to a defined nucleotide sequence at the 5'-terminus of virion and replicative intermediate RNAs of poliovirus. Proc Natl Acad Sci U S A. 1977 Mar;74(3):961–965. doi: 10.1073/pnas.74.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanegan J. B., Van Dyke T. A. Isolation of a soluble and template-dependent poliovirus RNA polymerase that copies virion RNA in vitro. J Virol. 1979 Oct;32(1):155–161. doi: 10.1128/jvi.32.1.155-161.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Otero G., Fernández-Tomás C., Gariglio-Vidal P. DNA-bound RNA polymerases during poliovirus infection: reduction in the number of form II enzyme molecules. Virology. 1982 Jan 30;116(2):619–628. doi: 10.1016/0042-6822(82)90153-2. [DOI] [PubMed] [Google Scholar]
- HAMBLING M. H., DAVIS P. M. SUSCEPTIBILITY OF THE LLC-MK2 LINE OF MONKEY KIDNEY CELLS TO HUMAN ENTEROVIRUSES. J Hyg (Lond) 1965 Jun;63:169–174. doi: 10.1017/s0022172400045071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura N., Semler B. L., Rothberg P. G., Larsen G. R., Adler C. J., Dorner A. J., Emini E. A., Hanecak R., Lee J. J., van der Werf S. Primary structure, gene organization and polypeptide expression of poliovirus RNA. Nature. 1981 Jun 18;291(5816):547–553. doi: 10.1038/291547a0. [DOI] [PubMed] [Google Scholar]
- Kono R., Sasagawa A., Ishii K., Sugiura S., Ochi M. Pandemic of new type of conjunctivitis. Lancet. 1972 Jun 3;1(7762):1191–1194. doi: 10.1016/s0140-6736(72)90921-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee Y. F., Nomoto A., Detjen B. M., Wimmer E. A protein covalently linked to poliovirus genome RNA. Proc Natl Acad Sci U S A. 1977 Jan;74(1):59–63. doi: 10.1073/pnas.74.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist R. E., Maizel J. V., Jr In vivo regulation of the poliovirus RNA polymerase. Virology. 1978 Sep;89(2):484–493. doi: 10.1016/0042-6822(78)90190-3. [DOI] [PubMed] [Google Scholar]
- Miyamura K., Sasagawa A., Tajiri E., Kono R. Growth characteristics of acute hemorrhagic conjunctivitis (AHC) virus in monkey kidney cells. II. Temperature sensitivity of the isolates obtained at various epidemic areas. Intervirology. 1976;7(4-5):192–200. doi: 10.1159/000149952. [DOI] [PubMed] [Google Scholar]
- Miyamura K., Yamazaki S., Tajiri E., Kono R. Growth characteristics of acute hemorrhagic conjunctivitis (AHC) virus in monkey kidney cells. I. Effect of temperature on viral growth. Intervirology. 1974;4(5):279–286. doi: 10.1159/000149860. [DOI] [PubMed] [Google Scholar]
- Miyamura K., Yamazaki S., Tajiri E., Kono R. Growth characteristics of acute hemorrhagic conjunctivitis virus in monkey kidney cells. III. Viral RNA synthesis at permissive and nonpermissive temperatures. Intervirology. 1978;9(4):206–213. doi: 10.1159/000148938. [DOI] [PubMed] [Google Scholar]
- Nomoto A., Detjen B., Pozzatti R., Wimmer E. The location of the polio genome protein in viral RNAs and its implication for RNA synthesis. Nature. 1977 Jul 21;268(5617):208–213. doi: 10.1038/268208a0. [DOI] [PubMed] [Google Scholar]
- PENMAN S., BECKER Y., DARNELL J. E. A CYTOPLASMIC STRUCTURE INVOLVED IN THE SYNTHESIS AND ASSEMBLY OF POLIOVIRUS COMPONENTS. J Mol Biol. 1964 Apr;8:541–555. doi: 10.1016/s0022-2836(64)80010-3. [DOI] [PubMed] [Google Scholar]
- Pettersson R. F., Ambros V., Baltimore D. Identification of a protein linked to nascent poliovirus RNA and to the polyuridylic acid of negative-strand RNA. J Virol. 1978 Aug;27(2):357–365. doi: 10.1128/jvi.27.2.357-365.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasagawa A., Kono R., Konno K. Laboratory-acquired infection of the eye with AHC virus. Jpn J Med Sci Biol. 1976 Apr;29(2):95–97. doi: 10.7883/yoken1952.29.95. [DOI] [PubMed] [Google Scholar]
- Tershak D. R., Mitchell W., Garfinkle B. Effect of guanidine on the growth of LSc poliovirus. Can J Microbiol. 1972 Jun;18(6):747–755. doi: 10.1139/m72-119. [DOI] [PubMed] [Google Scholar]
- Van Dyke T. A., Flanegan J. B. Identification of poliovirus polypeptide P63 as a soluble RNA-dependent RNA polymerase. J Virol. 1980 Sep;35(3):732–740. doi: 10.1128/jvi.35.3.732-740.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S., Natori K., Kono R. Purification and biophysical properties of acute haemorrhagic conjunctivitis virus. J Virol. 1974 Dec;14(6):1357–1360. doi: 10.1128/jvi.14.6.1357-1360.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F. H., Knight E., Jr In vivo and in vitro synthesis of human rhinovirus type 2 ribonucleic acid. J Virol. 1972 Jul;10(1):93–98. doi: 10.1128/jvi.10.1.93-98.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]