Summary
Smooth pursuit eye velocity to a moving target is more accurate after an initial catch-up saccade than before, an enhancement that is poorly understood. We present a novel individual differences based method for identifying mechanisms underlying a physiological response, and use it to test whether visual motion signals driving pursuit differ pre- and postsaccade. Correlating moment-to-moment measurements of pursuit over time with two psychophysical measures of speed estimation during fixation, we find two independent associations across individuals. Presaccadic pursuit acceleration is predicted by the precision of low level (motion energy based) speed estimation, and postsaccadic pursuit precision is predicted by the precision of high level (position tracking) speed estimation. These results suggest both that a low level motion signal may drive presaccadic acceleration, and that an independent high level motion signal may drive postsaccadic precision.
Keywords: postsaccadic enhancement, low level motion, high level motion, position tracking, attentive tracking, oculomotor
Introduction
Recent theories of motion perception have described both a low level system that performs an early, direct computation of motion (Nakayama, 1985), and a higher level system that first identifies some spatially localized entity (e.g. an object or salient feature), then tracks its changing position over time (Braddick, 1974; Ullman, 1979; Anstis, 1980; Cavanagh, 1992; Seiffert and Cavanagh, 1998, 1999; Lu and Sperling, 2001). The low level system has been widely modeled, takes motion energy as its direct input, and has as its likely substrate the activity of velocity sensitive neurons (Adelson and Bergen, 1985; van Santen and Sperling, 1985). While the low level system is considered to operate prior to derivation of form and position of objects, the high level position tracking system uses form and position information as its primary inputs (Anstis, 1980).
There are a number of behavioral demonstrations of the dissociability of high level and low level motion processing. For example, high level and low level motion stimuli can produce independent percepts of motion (Cavanagh, 1992) as well as independent motion aftereffects (Nishida and Sato, 1995; Culham et al., 2000). In general, stimuli with features that are too temporary or that move at too high a rate to be localized do not support position tracking, therefore provide a predominantly low level signal (Nakayama and Tyler, 1981; Heinen and Watamaniuk, 1998; Verstraten et al., 2000). Also, a drifting luminance pattern provides a predominantly low level signal when its features are masked by a more salient drifting color patter (Cavanagh, 1992). On the other hand, apparent motion stimuli that involve large steps in space or time, as well as stimuli that both lack net luminance motion and are of relatively low speed and contrast, provide a predominantly high level signal (Anstis, 1980; Baker et al., 1998; Seiffert and Cavanagh, 1998, 1999). The dissociability of low and high level motion systems allows each to be tested individually.
A distinction similar to that between low and high level motion processing has been made for the signals that drive the oculomotor tracking response. Rashbass showed that the initial presaccadic smooth pursuit response to a moving target does not take into account target position, responding instead to its position-independent motion (Rashbass, 1961). Given its position-independence, one might expect that early presaccadic pursuit is driven by a low level motion signal; several findings are consistent with this idea (Priebe et al., 2001; Lindner and Ilg, 2000; Hawken and Gegenfurtner, 2001). On the other hand, the initial catch-up saccade, when present, is largely a response to the position of the target (Rashbass, 1961; Heinen and Watamaniuk, 1998), and later stages of pursuit take into account position as well as motion information (Pola and Wyatt, 1980; Morris and Lisberger, 1987; Segraves and Goldberg, 1994). In addition, during later stages of pursuit, individuals are capable of smoothly pursuing a target whose changing position is in the direction opposite to its dominant motion energy, suggesting that a position tracking signal usable by the pursuit system exists at this time (Butzer et al., 1994; Lindner and Ilg, 2000; Hawken and Gegenfurtner, 2001). However, previous studies have not determined the degree to which a position tracking signal typically contributes to postsaccadic pursuit, nor have the relative moment-to-moment contributions of low and high level motion processing to pursuit been adequately characterized.
Suggestive evidence that a high level position tracking signal may contribute importantly to postsaccadic pursuit comes from two sources. First, smooth pursuit eye velocity to a moving target is more accurate after an initial catch-up saccade than before, and this improvement in accuracy is of a magnitude difficult to attribute to known low level mechanisms (Lisberger 1998). Second, in the presence of two moving dot targets, postsaccadic pursuit matches the velocity of whichever target was ‘chosen’ by the saccade, suggesting a motion signal closely tied to target position; on the other hand, presaccadic pursuit accelerates toward the vector average of target velocities, suggesting a low level, position independent motion signal (Gardner and Lisberger, 2001, 2002; Schoppik and Lisberger, 2006). These findings point to the saccade as an important turning point in pursuit and as a potential temporal marker for the introduction of a high level position tracking signal. However, the authors of these studies did not consider the possibility of two distinct motion signals, rather hypothesizing that a unitary motion signal is amplified postsaccade relative to presaccade.
We use a novel individual differences based method to test the hypothesis that postsaccadic pursuit is influenced by a high level position tracking signal distinct from the low level motion signal that putatively drives presaccadic pursuit. Our pursuit test is shown in Figure 1a. In separate psychophysical tests, we measure precision of speed estimation during fixation for stimuli designed respectively to isolate low and high level motion processing (see Figures 1b, 1c). We assess correlations across individuals between moment-to-moment measurements of pursuit over time and psychophysical speed estimation performance. If pursuit at a given moment in time is influenced by a given motion signal, then individuals with a greater ability to process that type of motion should exhibit higher quality pursuit at that time. Indeed, we report two such associations across our 45 participants, one between low level speed estimation and presaccadic pursuit acceleration, and a second between high level speed estimation and early postsaccadic pursuit precision. These associations provide evidence that presaccadic acceleration and postsaccadic precision are driven respectively by low and high level motion signals. These associations are temporally distinct as well as statistically independent, evidence that they reflect independent mechanisms. Our use of natural human variation to identify independent associations across the temporal course of a response represents a new method for fractionating and associating functional brain mechanisms.
Figure 1.
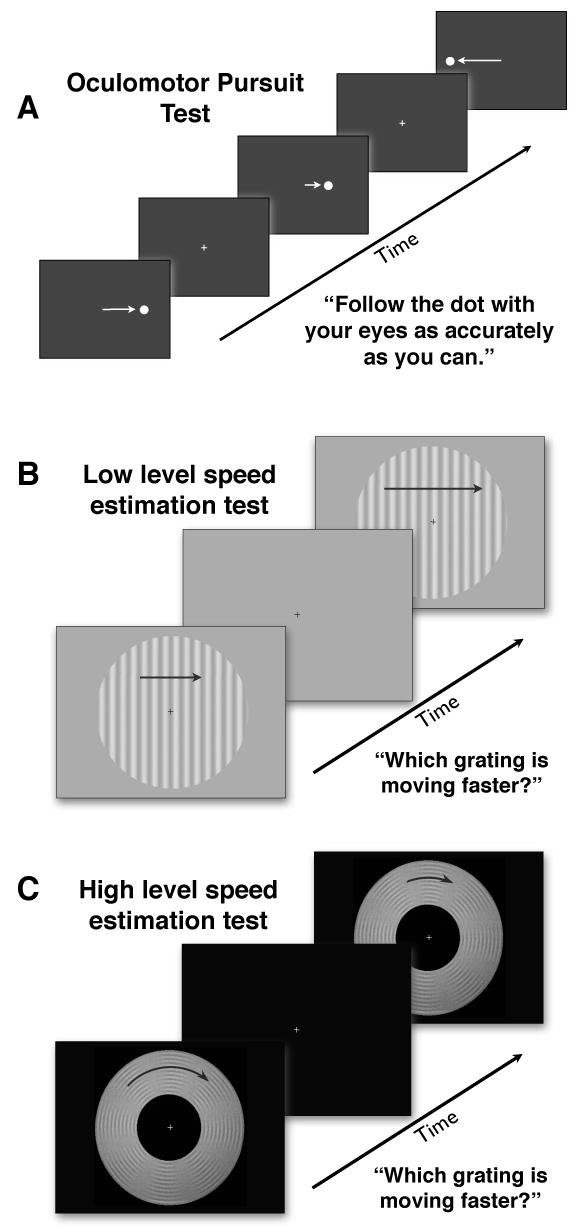
Tests. a) Oculomotor pursuit test measures presaccadic acceleration and postsaccadic precision of pursuit. b&c) Perceptual tests measure precision of speed estimation when the predominant motion signal available is (b) low level or (c) high level.
Results
Assessing associations in performance across individuals determines whether multiple processes, in this case moment-to-moment smooth pursuit and two types of motion perception, share common underlying factors. If two processes correlate across individuals, they must share common factors, and the nature of these factors can be isolated with appropriate comparisons and controls. We report two associations of interest, one between low level speed estimation and presaccadic pursuit acceleration, and a second between high level speed estimation and early postsaccadic pursuit precision (see Figure 1 for paradigms used). This pattern of associations would be expected if presaccadic acceleration and postsaccadic precision are driven respectively by low and high level visual motion signals. In several control analyses we examine the temporal characteristics of these associations, their robustness to controlling statistically for a number of factors (including measurement error), and their relation to the initial catch-up saccade. These analyses support the inference that the precision of postsaccadic pursuit is determined not by a low level motion signal that drives presaccadic acceleration, but by an independent high level motion signal.
High and low level speed estimation predict different stages of pursuit
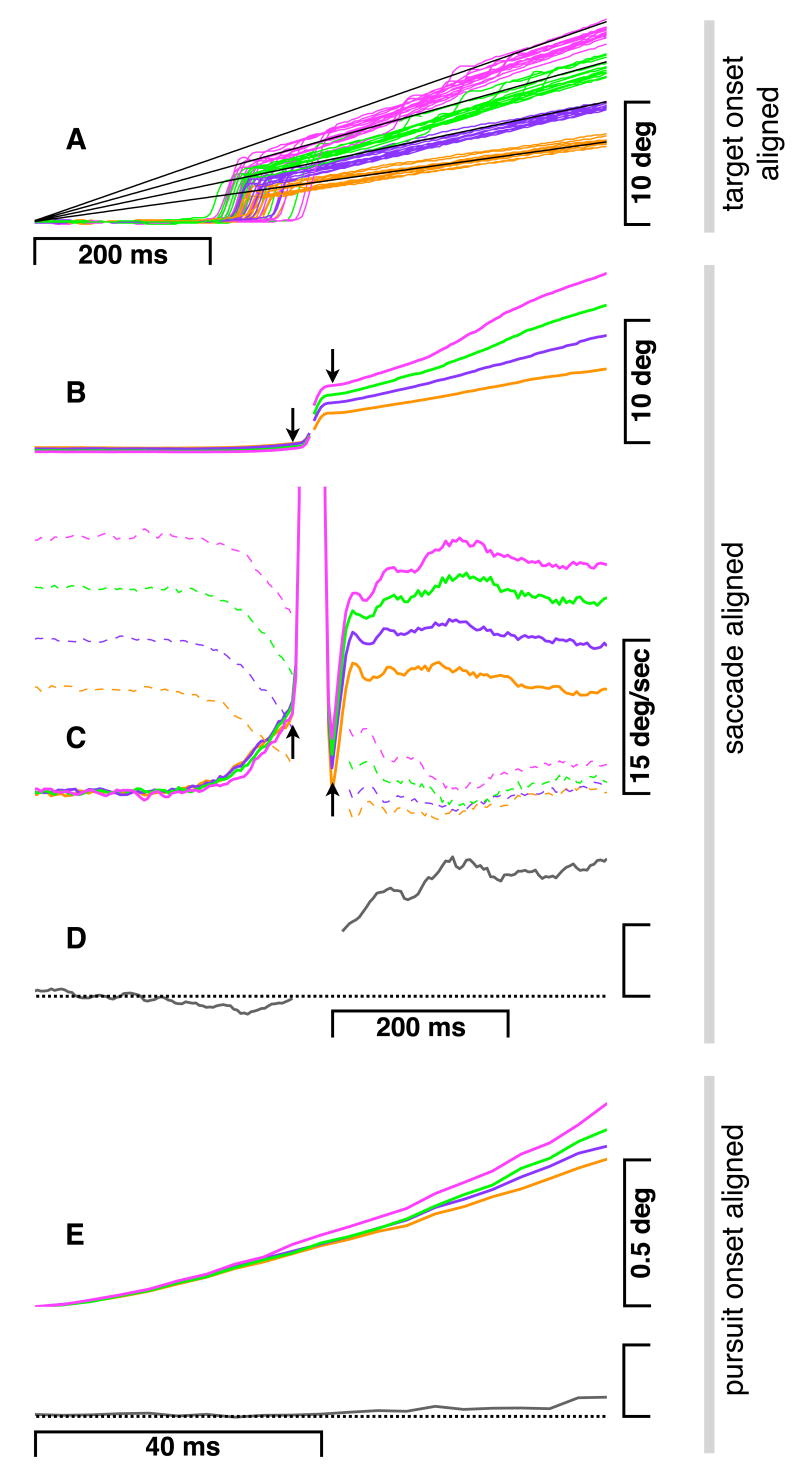
See methods for details on how we calculated pursuit performance and speed estimation, and for the stimuli used to isolate high and low level visual motion mechanisms (McKee et al., 1985; Carl and Gellman, 1987; Kowler and McKee, 1987). Figure 2 illustrates eye movements during our oculomotor pursuit test (Fig. 1a), with each color representing a different target speed. Figure 2a shows eye positions over time for a single individual (target position traces in black) starting from the appearance of the moving target. Figures 2b and 2c show eye position and velocity preceding and following the first catch-up saccade averaged across all participants. Averages were calculated separately pre- and postsaccade: presaccade with trials aligned by saccade onset, postsaccade with trials aligned by saccade offset. Saccade onset and offset are indicated by arrows. The dashed lines in Figure 2c indicate retinal slip, or the velocity of the target on the (initially static, then moving) retina. Substantially less retinal slip post- than presaccade demonstrates that pursuit matches target velocity far more closely after the first saccade than before. Therefore the retinal stabilization necessary for target examination occurs postsaccade in our task.
Figure 2.
Pursuit eye movements. a) Raw traces of eye position over time for a single individual. Black lines represent the four different target velocities, and colored lines represent eye traces for each of these target velocities. Same color scheme is used throughout Figs 2-4. b) Eye position averaged across all participants. Averages were calculated separately pre- and postsaccade: presaccade with trials aligned by saccade onset (first arrow), postsaccade with trials aligned by saccade offset (second arrow). Saccade onset and offset are plotted 46ms (average saccade duration) apart. c) Same as ‘b’ but for eye velocity. Dotted lines indicate the retinal slip of the target. d) Eye precision averaged across all participants for time period in ‘b’ and ‘c.’ Precision is the inverse of the oculomotor difference threshold, the percentage target speed increment necessary to produce a faster eye speed on 75% of trials (see methods; Kowler & McKee, 1987). Vertical bar shows one unit of precision, and horizontal line shows zero precision. e) Top trace - mean eye position across all participants for the first 80ms following initiation of eye acceleration (first saccade and all subsequent eye movements deleted). Bottom trace - for same time period, eye precision (as in ‘d’).
Figure 2d shows pursuit precision (1 / oculomotor difference threshold; see methods;Kowler & McKee, 1987) averaged across all participants for the same time periods shown in b and c. Precision, a measure of the linkage between pursuit speed and target speed (see below and methods), is enhanced dramatically at the start of the postsaccadic period relative to the presaccadic period, consistent with previous reports of postsaccadic pursuit enhancement (Lisberger, 1998; Gardner & Lisberger, 2001, 2002). An increase in precision can also be seen within the pre- and postsaccadic periods. In the postsaccadic period (Figure 2d), precision increases for another 100ms. A smaller but significant increase can also be seen in the presaccadic period. Figure 2e (note expansion in timescale) shows presaccadic eye position following pursuit initiation averaged across all participants for the four different target speeds (top traces) in relation to pursuit precision (bottom trace). Evidence for pursuit dependence on target speed (as indicated by the appropriate separation of the colored position traces) is seen only after an initial 40ms period, and this is more explicitly reflected by the small increase in pursuit precision only after this initial period (bottom trace). These results are expected as they are consistent with previous work (Tychsen & Lisberger, 1986; Krauzlis and Lisberger, 1994).
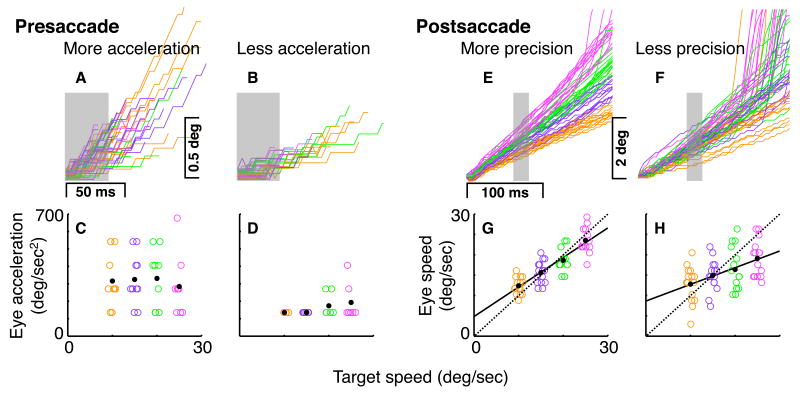
In Figure 3, we present data from four individuals illustrative of greater and lesser presaccadic acceleration (a-d) and postsaccadic precision (e-h). As can be seen, the ‘greater acceleration’ individual (Fig. 3ac) has a higher mean acceleration than the ‘lesser acceleration’ individual (Fig. 3bd), and the ‘greater precision’ individual (Fig. 3eg) comes closer than the ‘lesser precision’ individual (Fig. 3fh) to consistently modulating postsaccadic eye velocity to differing target velocities. We calculate postsaccadic pursuit precision, using the method developed by Kowler & McKee (1987), as the degree to which different stimulus velocities reliably evoke different eye velocities in a given participant (or 1 / oculomotor difference threshold; see methods; Kowler & McKee, 1987).
Figure 3.
Illustrative single cases. Data from one individual with greater (a,c) and one with lesser (b,d) presaccadic acceleration and from one with greater (e,g) and one with lesser (f,h) postsaccadic precision. Top row shows raw eye position traces, starting at time and position of acceleration initiation (presaccade, a-b) or saccade end (postsaccade, e-f). Bottom row shows eye acceleration (presaccade, c-d) or speed (postsaccade, g-h) plotted against target speed. Gray boxes represent interval over which eye acceleration or speed was calculated. Black dots indicate mean eye acceleration (c-d) or eye speed (g-h) for each target speed. Solid black and dotted lines postsaccade (g-h) indicate actual (least squares) and ideal eye speeds respectively. Because of the high magnification of the presented presaccadic results, quantization artifacts are evident (a-d). Colors correspond to target speeds as in Figure 2. To improve visibility, position traces (a,b,e,f) are jittered vertically by +/- 0.05 deg and target speeds (c,d,g,h) are jittered horizontally by +/-1 deg/sec.
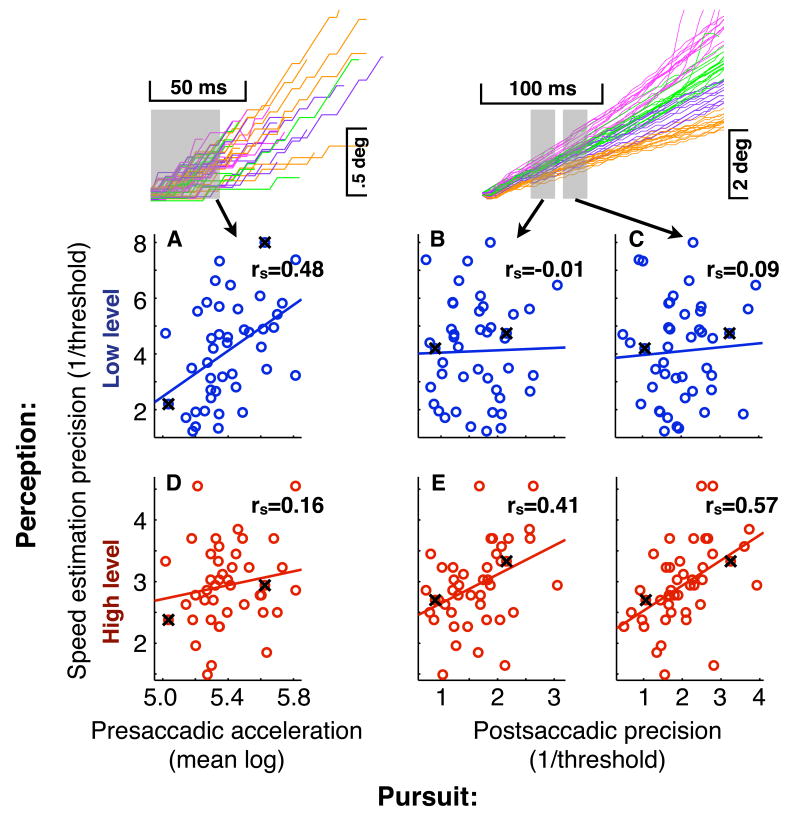
Figure 4 shows scatterplots of associations between speed estimation (low and high level) and pursuit measures (presaccadic acceleration and postsaccadic precision) for illustrative pursuit timepoints. Each dot represents performance of an individual participant. Participants from Figure 3 are marked with an ‘x’. As shown, an individual's precision of low level speed estimation predicts their eye acceleration over the first 36ms of presaccadic pursuit (Fig. 4a, rs(43)=0.48, p=0.0004, one-tailed). An individual's precision of high level speed estimation, on the other hand, does not significantly predict their eye acceleration over this period (Fig. 4d, rs(43)=0.16, p=0.15, one-tailed). The converse of the above is true for postsaccadic pursuit precision. An individual's precision of high level speed estimation predicts their postsaccadic pursuit precision for the two postsaccadic intervals shown (Fig. 4e, 40-56ms, rs(43)=0.41, p=0.004, one-tailed; Fig. 4f, 68-84ms, rs(43)=0.57, p<0.0001, one-tailed), yet an individual's precision of low level speed estimation does not significantly predict their postsaccadic pursuit precision for these intervals (Fig. 4b, 40-56ms, rs(43)=-0.01, p=0.47, one-tailed; Fig. 4c, 68-84ms, rs(43)=0.09, p=0.27, one-tailed).
Figure 4.
Associations at isolated timepoints. These scatterplots demonstrate associations between presaccadic acceleration or postsaccadic precision of pursuit (x axes) and low (blue) or high (red) level perceptual speed estimation (y axes). Each dot represents a single individual, and individuals from Figure 3 are marked with an ‘x’. Units for speed estimation precision are the inverse of the threshold percentage speed increment producing a perceptual judgement of faster speed. Units for postsaccadic precision are the inverse of the threshold percentage speed increment producing a faster eye speed. Units for presaccadic acceleration are mean log acceleration. Gray boxes represent interval over which eye acceleration or precision was calculated. Figure 3. Proportion variance explained (rs2) is listed for each association.
Temporal signature of visual motion mechanisms
Two temporal predictions can be made based on previous literature as to when low or high level visual motion processing should influence pursuit.
The first prediction follows from evidence that the low level motion system provides an early, direct computation of motion, whereas the high level motion system requires the identification of a spatially localized entity (e.g. an object or salient feature) before tracking its changing position over time (Braddick, 1974; Ullman, 1979; Anstis, 1980; Nakayama, 1985; Cavanagh, 1992; Lu and Sperling, 2001). Specifically, assuming that identifying an object or feature takes some functionally significant amount of time, the low level motion signal should be available before the high level motion signal. Indeed, as we illustrate now with Figure 5a, our low level motion test predicted earlier, presaccadic pursuit acceleration, whereas our high level motion test predicted later, postsaccadic pursuit precision.
Figure 5.
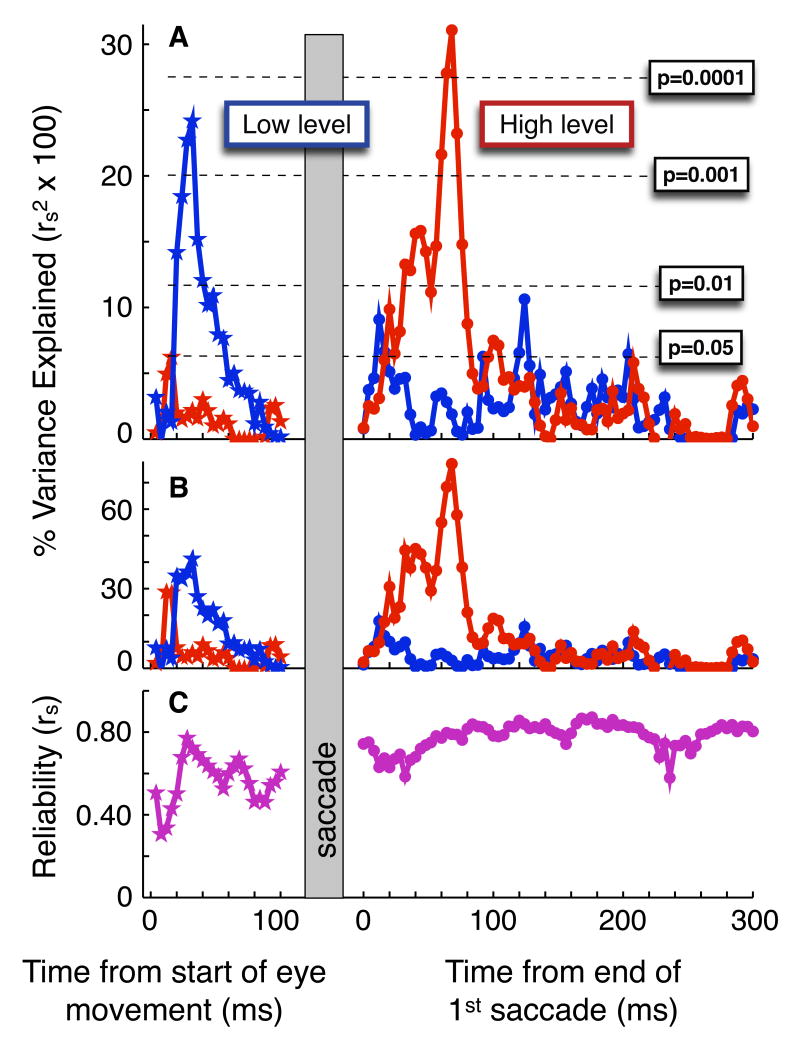
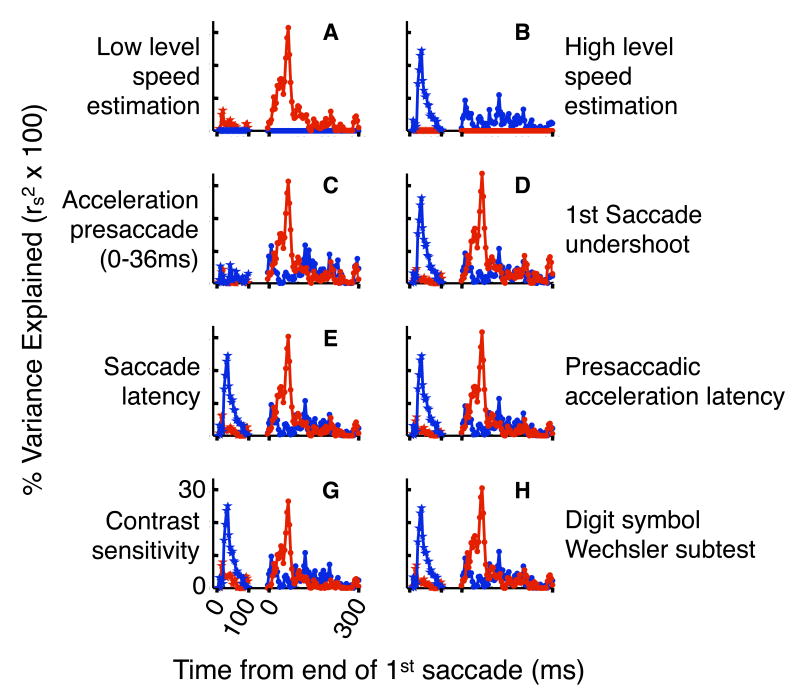
Full timecourse of associations and reliability, pre- and postsaccade. a) Percent variance explained by associations between presaccadic acceleration (stars) or postsaccadic precision (dots) of pursuit and low (blue) or high (red) level perceptual speed estimation. x-values presaccade indicate the end of the period over which acceleration was evaluated (period started at acceleration initiation), and x-values postsaccade indicate the beginning of the 16ms interval over which eye movement precision was evaluated. P-values (one-tailed) are indicated in green. b) Same as ‘a,’ except associations are corrected for attenuation due to measurement error (see results and methods). c) Reliability of presaccadic acceleration (stars) and postsaccadic precision (dots).
Figure 5a presents the full timecourse of associations between speed estimation and pursuit, expressed as percentage variance explained. Low level speed estimation significantly predicts presaccadic acceleration for each acceleration value between that computed on the first 20ms of pursuit and that computed on the first 52ms of pursuit, peaking at 24% of variance explained for that computed on the first 32ms of pursuit (Fig. 5a, blue, presaccade, p<0.05, one-tailed). However, high level speed estimation predicts presaccadic acceleration significantly only for the single acceleration value computed on the first 16ms of pursuit (Fig. 5a, red, presaccade, p<0.05, one-tailed). Conversely, high level speed estimation predicts postsaccadic precision significantly over the full range of 16 postsaccadic time intervals between 20-36ms and 80-96ms postsaccade (Fig. 5a, red, postsaccade, p<0.05, one-tailed), peaking at 31% of variance explained for 68-84ms postsaccade. However, low level speed estimation predicts postsaccadic precision significantly at only five relatively scattered timepoints, including two each near the beginning and end of the period associated with high level speed estimation (Fig. 5a, blue, postsaccade, p<0.05, one-tailed). To test whether measurement error could have contributed to the temporal profile of our results, we use the classic psychometric method of attenuation correction (Schmidt and Hunter, 1996) to predict what associations would be in the absence of measurement error (Fig. 5b and see methods). Since the predicted error-less associations shown in Figure 5b have a temporal pattern qualitatively identical to the raw associations reported in Figure 5a, the temporal pattern of our results cannot be due to differential measurement reliability across time in our eye movement measures.
A second temporal prediction can be made as follows: Fast retinal slip presaccade (dashed lines in Fig. 2c) provides robust visual motion information on which to base visual estimates, both low and high level, of target speed. Since visual motion information influences pursuit after a delay of about 120ms (Tychsen and Lisberger, 1986), fast retinal slip information collected presaccade should influence early postsaccadic pursuit, whereas slow retinal slip information collected postsaccade should not influence pursuit until about 120ms postsaccade (Fig. 2c). When retinal slip is slow, much of our sense of target velocity comes from internal monitoring of eye movement commands, or efference copy (Yasui and Young, 1975; Lisberger et al., 1987). Therefore the estimate of target velocity driving pursuit after 120ms postsaccade may - relative to earlier pursuit - rely less on visual information and more on efference copy information. If such a transition happens around 120ms postsaccade, then an association between visual motion processing and pursuit quality that is present during the first 120ms postsaccade should decrease thereafter. Consistent with this prediction, we find that while high level speed estimation predicts postsaccadic precision over much of the first 120ms postsaccade, this association no longer remains significant after 120ms postsaccade (Fig. 5a). It is worth emphasizing that this association is not present late enough (>120ms postsaccade) to have been influenced by motion signals derived from slow postsaccadic retinal slips, a point that we discuss in more detail below.
The temporal signature of our results is thus consistent with previous literature suggesting that 1) a low level motion signal should be available before a high level motion signal (Braddick, 1974; Ullman, 1979; Anstis, 1980; Nakayama, 1985; Cavanagh, 1992; Lu and Sperling, 2001), and 2) pursuit should depend on visual motion signals to a lesser degree after about 120ms postsaccade (Yasui and Young, 1975; Tychsen and Lisberger, 1986; Lisberger et al., 1987). This consistency suggests that the observed associations indeed reflect low and high level visual motion processing mechanisms.
Controls for alternative mechanisms
We show above that the temporal profile of our results fits that expected of low and high level visual motion processing mechanisms. In this section we report further evidence that these associations are driven by visual motion processing mechanisms, rather than by oculomotor, other perceptual, or general performance mechanisms. To do this, we use partial correlation (see methods) to assess the contribution of alternative factors to the observed associations. To the extent that a given association remains after using partial correlation to control statistically for a given factor, that factor cannot contribute to the association. Figure 6d-h illustrates that the observed associations remain (compare to Figure 5a) after controlling for each of five factors (described below).
Figure 6.
Control analyses. Graphs indicate the associations between speed estimation and pursuit that remain after using partial correlation to statistically control for the variable listed beside each graph (compare to Fig. 5a). Colors and scales of axes same as Figure 5a.
Oculomotor factors
We consider three oculomotor factors that could have contributed to our results. First, initial saccades that fall short of their target lead to substantial eye position error immediately postsaccade. It is possible that individuals with a tendency for saccade undershoot might produce uniformly high postsaccadic pursuit speed to help the eye catch up to the target. Such uniformly high eye speed would have low precision if it did not vary according to stimulus speed. Second, the later one's first saccade, the more the time one will have presaccade to analyze the target. In theory, this extra time could allow a more precise presaccadic speed estimate, and therefore higher precision of postsaccadic pursuit. Third, earlier initiation of presaccadic pursuit could allow earlier access to efference copy signals, which might improve postsaccadic pursuit. To test these three hypotheses respectively, we controlled for: position undershoot of first saccade, latency to first saccade, and latency to presaccadic acceleration. Associations between speed estimation and pursuit were robust to controlling for each variable (Fig. 6d-f), inconsistent with the three hypotheses presented above.
Contrast sensitivity
Though our speed estimation tests were matched on physical contrast, they differed in spatial characteristics. Therefore, despite our care in choosing a contrast well within a reasonable range of visibility for both tests, it is possible that individual differences in contrast sensitivity could contribute to performance differences. In addition, at the relatively high temporal frequencies we used for the low level speed estimation test, contrast is known to serve as a cue to speed (McKee et al., 1986). Though we randomized contrast on this test, thereby reducing the reliability of this alternative cue to speed, contrast could still have provided a minor secondary cue. We thus assessed the impact on the associations between speed estimation and pursuit of controlling for contrast sensitivity (Fig. 6g). The association between low level speed estimation and presaccadic pursuit acceleration was not affected, and that between high level speed estimation and postsaccadic pursuit was affected to a small degree, suggesting that contrast sensitivity plays a minor role only in the latter association.
General intellectual ability
Do the observed associations relate to an established measure of intellectual ability? We hypothesized that the Digit Symbol subtest of the Wechsler Adult Intelligence Scale (Wechsler, 1981) might relate to perceptual and/or oculomotor skill, as it relies upon quick perceptual analysis and repeated eye movements. However, controlling for Digit Symbol performance (Fig. 6h) did not affect associations between speed estimation and pursuit, suggesting that the abilities our tests were measuring are not related to the abilities measured by the Digit Symbol test.
Statistical independence implies independent mechanisms
We have shown evidence above for two temporally distinct associations between motion processing and pursuit. In this section we ask if the observed associations are merely distinct, or in fact independent. We assess independence by using partial correlation to statistically control for each variable in each association (see methods). To the extent that associations ‘a’ and ‘b’ are independent, controlling for variables in association ‘a’ should not reduce association ‘b,’ and vice versa. On the other hand, by definition, controlling for variables in association ‘a’ will reduce association ‘a’ to zero, and controlling for variables in association ‘b’ will reduce association ‘b’ to zero. Neither of our two associations between speed estimation and pursuit was reduced by more than 1% of explained variance at any time point when controlling for the variables in the other association (at any timepoint), strong evidence that the observed associations are not only temporally distinct, but statistically independent. Three of these analyses are shown in Figure 6 (a/b - controlling for low/high level speed estimation; c - controlling for presaccadic acceleration at 0-36ms, the time of its greatest association with low level speed estimation). The statistical independence of the observed associations implies that they reflect independently varying, hence functionally independent, mechanisms.
Are associations driven by speed-tuned mechanisms?
Our psychophysical tests were selected to maximize the dissociation between low and high level motion systems (Seiffert and Cavanagh, 1998, 1999; Nakayama, 1985; Verstraten et al., 2000). Indeed, performance on our two speed estimation tests is statistically independent (rs=0.009, p=0.95), confirming a dissociation. We chose a relatively slow speed for our high level motion stimulus in order to minimize its low level motion energy and a relatively fast speed for our low level motion stimulus in order to minimize participants' ability to derive a high level position tracking signal from it. In theory, an unintended side-effect of this difference in speeds could be for our speed estimation tests to tap into distinct speed-tuned motion processing mechanisms. However, this theory is disputed by both the overlap in retinal slip speeds driving our two associations and the speeds used in our psychophysical tasks.
Since retinal slip affects pursuit after a 120ms delay (Tychsen and Lisberger, 1986), one can count back 120ms from each observed association to determine the retinal slips that drive it. The presaccadic association is driven by (fixational) retinal slips of 10, 15, 20, and 25 deg/sec in the four target speed conditions respectively (retinal slips are depicted by dashed velocity traces in Figure 2c). The postsaccadic association, at its earliest significant timepoint (Fig. 5a, red, sixth timepoint postsaccade), is driven by retinal slips averaging 6.9, 11.6, 16.8, and 22.1 deg/sec in these same four target speed conditions (specifically, these averages are taken 120ms before 36ms postsaccade, where 36ms postsaccade is the latest timepoint entering the precision calculation at the sixth timepoint postsaccade). The almost entirely overlapping retinal slips at these two times should activate similarly all but the most extreme, narrowly speed-tuned mechanisms. However, existing evidence for speed-tuned mechanisms suggests broad speed tuning (Born, 2005), and a narrowly speed-tuned mechanism would in any case be ill-suited to processing the wide range of retinal slips in our pursuit task.
Additionally, since performance on our two psychophysical tests is statistically independent, if a slow speed-tuned mechanism were to determine performance on the high level speed estimation test, it could make no contribution to the 11 deg/sec base speeds used in the faster, low level speed estimation test. Such a slow speed-tuned mechanism would inefficiently drive pursuit during our observed postsaccadic association, since retinal slips driving this period of pursuit are only consistently slower than 11 deg/sec in the slowest pursuit condition (see above). Moreover, a disproportionate contribution of the slowest pursuit condition to our results is ruled out since dropping this condition entirely from our analysis reduces the postsaccadic association only about the same as dropping the fastest condition (peak association in both cases explains 23% of variance, and number of timepoints showing statistically significant association is similar: 15 and 17 respectively, p<0.05, one-tailed).
The importance of the saccade
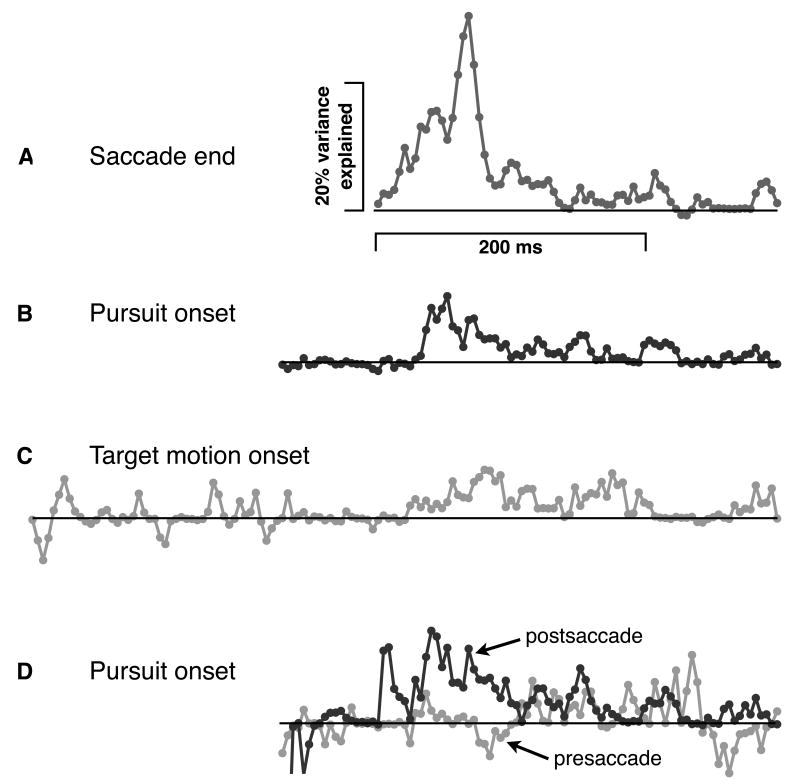
Is the postsaccadic association we observe functionally tied to the saccade, or is it instead tied to another event such as pursuit onset or target motion onset? To answer this question, we assess the degree of temporal linkage between the association and each of these events, reasoning that the largest association should be timelocked to the most functionally important event. We compute pursuit precision from saccade-removed traces aligned with either pursuit onset or target motion onset, then assess the associations of these new precision measures with high level speed estimation, comparing the results with our original saccade-aligned analysis. Results, shown in Figure 7, suggest that the observed association is linked with the saccade, and is spread out and watered down when traces are aligned with pursuit onset or target motion onset. In Figure 7a, where traces are aligned by the saccade, the association peaks at 32 percent of variance explained. In Figure 7b, where traces are aligned by pursuit onset, relative to which saccade timing is spread out with a standard deviation of 42ms, the peak association reduces to 11 percent variance explained. Finally, in Figure 7c, with traces aligned by target motion onset, relative to which saccade timing is spread out with a standard deviation of 49ms, the peak association reduces even further to 8 percent variance explained. The greater the spread of the saccade relative to the alignment of eye traces the lower the association, clearly supporting the idea that the observed postsaccadic association is functionally tied to the saccade. We rule out measurement error as an explanation for these differences with the same attenuation correction approach shown in Figure 5b (Supplementary Fig. 1 and see Methods).
Figure 7.
The importance of the saccade. Percent variance explained by associations between high level speed estimation and precision of pursuit, with precision calculated separately for traces aligned with a) end of first saccade (same as Fig. 5a), b) pursuit onset, and c) target motion onset. Initial timepoints are shifted horizontally by mean time of a) saccade end (260ms), b) pursuit onset (188ms), and c) target motion onset (0ms). All saccades were removed from velocity traces before calculating precision. d) Same as b, except that a distinction is made between whether a saccade has happened or not in order to understand whether the saccade has a functional role in the associations seen above. The association at each timepoint in ‘d’ was calculated separately for pursuit precision based on eye traces that, at that timepoint, were before (‘presaccade,’ light) and after (‘postsaccade,’ dark) the first catch-up saccade. As in all figures unless noted otherwise, horizontal and vertical scales are the same for a-d.
The trial to trial variation of the timing of pursuit onset and the saccade provides a good opportunity to assess the importance of the saccade itself. At any given instant relative to pursuit onset, pursuit will sometimes be presaccade and sometimes postsaccade depending on the exact timing of the saccade for that trial. While pursuit just after initiation is typically presaccade and pursuit much later in time is typically postsaccade, the natural variability in saccade timing relative pursuit onset allows us to separately assess associations confined to either pre- or postsaccadic pursuit for intermediate time points. If the saccade is critical, there should be a strong association between high level speed estimation performance and precision postsaccade but this should be diminished significantly for presaccadic associations. The results, shown in Figure 7d, show a clear difference. While presaccadic precision (light) shows essentially no association with high level speed estimation, postsaccadic precision (dark) shows a strong association, stronger even than that seen for the whole data set (as shown in Figure 7b). This is consistent with the idea that the association in Figure 7b is reduced by trials where the pursuit is presaccade. In Figure 7d, we have a purer measure of the postsaccadic association. Thus, high level speed estimation does not relate to precision per se, but specifically to postsaccadic precision.
A separate study conducted with 12 additional participants confirms the importance of the saccade (results presented in Suppl. Fig. 2). First, this study replicates our main finding of a strong saccade-aligned association which is reduced when traces are aligned with pursuit onset or target motion onset. Second, this study demonstrates that in the same participants, when position error is minimized such that saccades rarely occur (using a so-called Rashbass step-ramp paradigm), this association disappears.
Together, these results demonstrate that high level speed estimation relates only to pursuit precision after, and timelocked to, the saccade, evidence for a tight functional link between the saccade and high level motion influenced pursuit. An important question for future research will be whether the saccade marks a full transition in the motion signal relied upon, from low to high level, or whether a high level motion signal introduced postsaccade instead supplements a low level motion signal (e.g., conceivably, a low level motion signal may continue to drive pursuit acceleration postsaccade).
Discussion
We use a novel individual differences based method to test the hypothesis that postsaccadic pursuit is influenced by a high level, position tracking signal distinct from the low level motion signal that may drive presaccadic pursuit. We find two independent associations across individuals between speed estimation during fixation and moment-to-moment measurements of pursuit. Presaccadic pursuit acceleration is predicted by the precision of low level (motion energy based) speed estimation, and postsaccadic pursuit precision is predicted by the precision of high level (position tracking) speed estimation. These results support our hypothesis, providing evidence that presaccadic acceleration and postsaccadic precision are influenced respectively by independent low and high level motion signals. While previous reports of enhanced pursuit postsaccade have hypothesized that the saccade allows an existing low level motion signal to be used differently (Lisberger, 1998; Gardner and Lisberger, 2001, 2002), our results highlight a plausible alternative mechanism for this enhancement: the introduction of a high level motion signal.
We provide four lines of evidence that the observed associations indeed reflect independent contributions of low and high level visual motion processing to pursuit. First, the temporal profiles of these associations fit two predictions laid out in results: a) that a low level motion signal should be available to the pursuit system earlier than a high level motion signal, and b) that after 120ms postsaccade, the influence of visual motion signals on pursuit should decrease. Importantly, these temporal profiles are not an artifact of differing measurement reliability over the temporal course of the pursuit response (Fig. 5b). Second, explicit controls show that the observed associations cannot be explained by individual differences in contrast sensitivity, a measure of general intellectual ability, or three aspects of oculomotor control (Fig. 6). Third, the finding that our two reported associations are statistically independent suggests that low and high level motion processing make functionally independent contributions to pursuit. Fourth, the broadly overlapping retinal slip speeds driving these associations, and the statistical independence of our psychophysical tasks, suggests that these associations are due to low and high level, rather than fast and slow speed-tuned, motion processing mechanisms.
What is the importance of the saccade to the effects we observe? We report three lines of evidence that the saccade, at minimum, shares a substrate with high level motion influenced pursuit, and may even be necessary for such pursuit. First, we show that the observed postsaccadic association is temporally linked to the saccade, rather than to pursuit onset or target motion onset. Second, we show that at the same time relative to pursuit onset, high level motion processing relates to pursuit precision calculated from postsaccadic, but not presaccadic, pursuit. Third, we report evidence from a small experiment using a saccade-less “Rashbass” paradigm suggesting that pursuit without a catch-up saccade may lack an influence of high level motion processing on pursuit.
Similar inputs could be necessary for both saccade targeting and high level position tracking. Both processes clearly rely upon the derivation of target position, and both may also require an active attentional focus on that position signal (Cavanagh, 1992). Trial-by-trial variation in the temporal dynamics of one or more such shared processes could be responsible for a tight functional link between the saccade and high level motion processing influenced pursuit.
Our study joins others in suggesting that the visual motion signals influencing pursuit differ over time. Previous studies using bars and other elongated stimuli demonstrated that the visual motion signal driving both perception and pursuit is initially based on local image velocity, but shifts over 150-250ms to represent more global object velocity (Lorenceau et al., 1993; Pack and Born, 2001; Masson and Stone, 2002). Our results suggest an additional temporal distinction for pursuit: between low level motion driven acceleration presaccade and high level motion driven precision postsaccade. It may be that the low level motion system provides a quick but rough speed estimate used to get the eye within an operating range under which a more refined position and/or object based high level speed estimate facilitates precise retinal stabilization of the pursued target.
Visual motion processing is known to provide a signal that determines the acceleration of the pursuit system (Krauzlis and Lisberger, 1994, Lisberger et al., 1987). Given that performance on our low level motion test relates specifically to presaccadic pursuit acceleration, we have hypothesized that a low level visual motion signal drives presaccadic pursuit acceleration. One question remains: Why does precision of low level speed estimation predict magnitude of presaccadic acceleration? This question is beyond the scope of our inquiry but we suggest two possibilities: First, a noisy visual motion signal could be attenuated in magnitude by some kind of gain control, leading to weaker pursuit acceleration (Stocker and Simoncelli, 2006). Second, our low level motion test (relatively high in temporal frequency at 11hz) could rely upon a high temporal frequency mechanism important for robust pursuit acceleration (Mandler and Makous, 1984).
Recent functional magnetic resonance imaging, electrophysiological, and lesion studies suggest that human area IPL (inferior parietal lobe), monkey areas LIP (lateral intraparietal area) and 7a, and other parietal areas may play a role in high level motion processing (Battelli et al., 2001; Claeys et al., 2003; Williams et al., 2003; Merchant et al., 2005). The middle temporal complex (MT/V5+), on the other hand, is believed to play a crucial role in low level motion processing (Born, 2005). We suggest that future studies of motion processing in these identified areas could probe for an evident change, timelocked with the initial catch-up saccade to a moving target, that supports the introduction of a high level visual motion signal to the pursuit system.
The individual differences based method we demonstrate here represents a novel technique for identifying, with fine-grained temporal and functional specificity, the mechanisms underlying a physiological response. Though not necessary to the use of this method, independent associations such as those we report support a strong inference that the mechanisms assessed contribute independently to that physiological response. Our method is part of a trend toward studying variation between individuals or responses to fractionate and associate functional brain mechanisms (Peterzell and Teller, 2000; Kosslyn et al., 2002; Osborne et al., 2005). Such covariance based methods provide a complement to more common methods that focus on the average individual or response, while also establishing reliability and validity of measures, an essential prerequisite for genetic and clinical investigations.
Experimental procedures
Subjects
Forty five college students with normal or corrected to normal vision participated in this study for course credit. Participants gave informed written consent before taking part in this study, which was approved by the Faculty of Arts and Sciences Human Subjects Committee at Harvard University. During a 1.5-2 hour testing session, each participant completed a battery of perceptual tests, an oculomotor test, and a measure of general intellectual ability. All vision tests were self-paced, and participants were encouraged to pause for rest as needed. For each perceptual test, participants were given at least 20 practice trials, more if needed, to ensure a good understanding of the task.
Apparatus
All perceptual and oculomotor testing was conducted in a darkened room. Perceptual tests were run on a Power Macintosh 7100 with a 12 inch Apple© High-Resolution Monochrome monitor, calibrated for linearity. The oculomotor test was run on a G3 Macintosh with a 17 inch color monitor. Both monitors provided a 67hz, 480×640 pixel display. An ISR Video Attenuator provided accurate control of contrast for the psychophysical tests. Viewing distance was 57 and 50 cm for the perceptual and oculomotor tests respectively. Eye movements for the oculomotor test were recorded at 250hz from the right eye using an Eyelink I infrared eye tracker (www.eyelinkinfo.com) and chin and cheek rests minimized head movement. All tests were programmed in C using routines created by Raynald Comtois (www.kagi.com/visionshell/), and analyses were conducted in Matlab (www.mathworks.com).
Statistical analysis
To demonstrate that all results represent robust trends in the data unaffected by extreme individuals or datapoints, all reported correlations are Spearman (rs) rank order correlations. All conclusions remain the same when using Pearson correlations stripped of statistical outliers (those datapoints more than 1.5 interquartile distance outside the interquartile range). As an additional precaution against extreme values, all measures of central tendency were calculated after removing statistically outlying datapoints.
Any measurement tool has random error, which biases associations downward. For example, if the true association between two processes accounts for 50% of their combined variance, and the processes are measured in a way that captures 30% of their true variance, the average measured association will explain only 50% × 30% = 15% of variance. Conversely, given a measured association (say 15% variance explained) and an estimate of the true variance captured by the measures (the product of their reliabilities provides an upper-bound estimate of the latter, say 30%), one can estimate the true association between the processes being tapped as 15% / 30% = 50% of variance, or the percentage of reliable (non-error) variance accounted for. This simple technique of estimating true explained variance is a backbone of psychometric theory and a basic property of tools like structural equation modeling (Schmidt and Hunter, 1996). As this estimate statistically equates reliability across measures or over the timecourse of a single measure, it provides a powerful tool for detecting when different sized associations are merely due to differential measurement reliability. We use this so-called attenuation correction technique for analyses reported in Figure 5b. See individual tests below for reliability calculations.
Partial correlation measures the size of an association after controlling for a third variable. Formally, partial correlation regresses the controlled for variable on each of the two variables in the original association, then computes the association between the residuals of these two regressions. We use partial correlation for analyses reported in Figure 6. All conclusions remain the same when our partial correlation analyses are corrected for measurement error using an attenuation correction procedure (Schmidt and Hunter, 1996).
Perceptual tests
Low level speed estimation
Our low level speed estimation test (Fig. 1b) assessed participants' ability to estimate speed when the only robust motion signal was low level. The task was to decide which of two sequentially presented stimuli moved faster. The stimulus was a circular window subtending 15 degrees of visual angle, containing a drifting luminance defined sinusoidal grating of spatial frequency 1 cycle/degree. This stimulus drifted at a temporal frequency (>=11hz), too high to allow position tracking, which requires temporal frequencies of 7hz or less (Verstraten et al., 2000). Therefore, while this stimulus provided a robust low level motion signal (Nakayama, 1985), it did not provide a usable high level motion signal.
A two interval forced choice 3-down-1-up staircase procedure determined the smallest speed difference that each individual could reliably discriminate. The slower speed was fixed at 11 hz (11 deg/sec). The faster speed began at 13 hz (13 deg/sec). The faster speed decreased by 30% of the difference between the two speeds after three consecutive correct responses, and increased by 30% of this difference after each incorrect response. Each stimulus was presented for 195ms, with 500ms between. Direction of movement, right or left, was the same for the two stimuli in a trial but randomly determined for each trial to avoid adaptation effects. Contrast, which appears lower for fast stimuli, was randomized between values 10%, 12.5%, 15%, 17.5%, and 20% so that contrast would not provide a reliable alternative cue to stimulus speed (McKee et al, 1986). The staircase procedure ended after 12 reversals, and the mean speed difference at reversals was divided by the slower 11 hz speed to determine the percentage speed difference detectable 79.4% of the time. We took the inverse of this percentage as the threshold value because it rendered the data more normally distributed and made larger values correspond to higher precision. The mean of three thresholds per participant was used in subsequent analyses, and the reliability of this value was calculated across subjects (Cronbach's alpha=0.81).
High level speed estimation
Our high level speed estimation test (Fig. 1c) assessed participants' ability to estimate speed when the only robust motion signal was high level. The task was to decide which of two sequentially presented stimuli moved faster. The stimulus was an annulus containing a circularly drifting second order “contrast modulated rings” pattern developed by Seiffert and Cavanagh (1998, 1999). This stimulus evokes little to no low level motion percept. Therefore, a robust percept of motion results only from position tracking, and in the absence of active position tracking these stimuli appear to slow down substantially.
The annulus, extending from 3 to 7.5 degrees of visual angle surrounding fixation, was composed of 25 concentric rings of equal 0.18 degree thickness alternating between ‘dark’ and ‘light.’ Each ‘dark’ ring varied sinusoidally from low luminance to middle luminance, and each ‘light’ ring varied sinusoidally from high luminance to middle luminance, in eight full cycles around its 360 degrees of space. The low and high luminance portions of the rings were aligned, making average luminance identical over any given radial cross section of the stimulus. Thus, rotating this stimulus provided a motion signal with no net luminance motion. We defined contrast for this stimulus as the contrast between rings at maximal luminance difference. We set this contrast to 16%, 10 times the least sensitive participant's detection threshold in Seiffert and Cavanagh (1998, 1999). This value is high enough for easy visibility, even for a participant with rather poor contrast sensitivity, yet low enough to provide a predominantly high level motion signal (Seiffert and Cavanagh, 1998, 1999). The annulus was used because it takes the motion signal away from fixation, discouraging eye movements during the relatively long presentation times necessary to engage position tracking. Subjects were instructed to keep their eyes on the fixation cross in the center of the annulus.
A two interval forced choice staircase procedure determined the smallest speed difference that each individual could reliably discriminate. The slower speed was fixed at 1 hz, or 45 degrees of rotation per second (deg/sec). This corresponded to a speed of 5.89 deg/sec at the outer edge of the grating, and 2.36 deg/sec at its inner edge. The faster speed began at 1.39 hz, decreasing by 0.03 hz for each correct identification of the faster stimulus and increasing by 0.09 hz for each incorrect answer. Direction of movement, clockwise or counterclockwise, was the same for the two stimuli in a trial but randomly determined for each trial to avoid adaptation effects. Presentation time randomly varied between one and two seconds so total stimulus rotation would not provide a reliable alternative cue to stimulus speed (McKee et al., 1986). Time between stimuli was 500 ms. The staircase procedure ended after eight reversals, and mean speed difference at reversals was divided by the slower 1 hz speed to determine the percentage speed difference detectible 75% of the time. We took the inverse of this percentage as the threshold value because it rendered the data more normally distributed and made larger values correspond to higher precision. The mean of three thresholds per participant was used in subsequent analyses, and the reliability of this value was calculated across subjects (Cronbach's alpha=0.51).
Contrast sensitivity
Our contrast sensitivity test determined the lowest contrast value at which participants could discriminate orientations. A two interval forced choice 3-down-1-up staircase procedure determined the lowest contrast at which participants could reliably identify which of two sequentially presented gratings was non-vertical. The stimulus was a circular window subtending 15 degrees of visual angle that contained a 0.5 cycle per degree, static, luminance defined sinusoidal grating. The non-vertical grating was tilted 4 degrees clockwise. Stimulus contrast started at 1.5% contrast, a level visible to all participants. It was decreased by 30% after three consecutive correct responses, and increased by 30% after each incorrect response. The two stimuli were presented for 300ms each, with 500ms in between. The staircase procedure ended after 12 reversals. Mean contrast across reversals was taken as the 79.4% detection threshold for non-vertical orientation, and its reciprocal as contrast sensitivity. The mean of three contrast sensitivity values per participant was used in subsequent analyses, and the reliability of this value was calculated across subjects (Cronbach's alpha=0.66).
Oculomotor pursuit test
Our oculomotor pursuit test (Fig. 1a) assessed the ability to accelerate to and precisely follow a moving target with the eyes. The target was a dot that appeared at fixation and immediately began to move in one of the four cardinal directions at one of four constant velocities (10, 15, 20, or 25 deg/sec), continuing until it disappeared off the edge of the screen. Participants were instructed to “follow each dot with your eyes, as accurately as you can,” and their eye movements were tracked. We calculated eye acceleration and precision, latency to presaccadic acceleration and to the first saccade, and the degree to which the first saccade undershot its target (overshoots were rare).
To calculate split-half reliability for each measure, we first randomly split the 16 trials for each stimulus condition for each participant into two bins of 8 trials each, leaving two half-datasets for each participant. Second, we calculated each measure twice for each participant, once for each half-dataset. Third, we calculated a Spearman correlation coefficient across participants between the two half-dataset measures. We performed steps 1-3 iteratively 500 times, and estimated the split-half reliability of each measure as the mean of the 500 resulting correlations. As split-half reliability estimates the reliability of a measure computed on half of the dataset, we used the Spearman-Brown formula to estimate the reliability of the full dataset. We report this estimate in Figure 5c for presaccadic acceleration and postsaccadic precision, and below for saccade undershoot, acceleration latency and saccade latency.
Each participant performed 128 trials, 8 trials at each of the four target directions and speeds. For each trial, the participant fixated a cross at the center of the screen, then pressed a button when ready. The fixation cross disappeared after 500ms, and after a delay of either 250 or 750ms an off-white (2.55 cd/m2), 1 degree in diameter circle appeared in its place and immediately began to move across a dark background (0.05 cd/m2). The variable delay, direction, and speed minimized the potential for stimulus prediction. As the quality of eye movement measurement with our setup is better horizontally than vertically, we focus our analysis on the 64 horizontal trials per individual. As is typical in tasks involving saccades (Fischer and Ramsperger, 1984), we observed a bimodal distribution of first saccade latencies, with a minority of 9% of first saccades occurring at short latencies. In order to focus on saccades within the typical range of latencies, we excluded both the short latency mode of the distribution (latencies <128ms, 9% of trials) and the 2.5% of trials with no saccade within the first 350ms of target movement. We defined saccades as any timepoint within 12ms of an eye velocity above 45 deg/sec or below -45 deg/sec. Eye velocity was calculated by subtracting each eye position from that 12ms later, dividing by 0.012 sec, then attaching this value to the time point 4ms hence. We defined latency of smooth acceleration as the latest timepoint before the initial saccade (or, for saccade-less trials, before 350ms from target motion onset) at which the eye had made no net forward movement over the preceding 40ms. Individually viewed position and velocity traces of each trial confirmed the robustness of our saccade and acceleration detection algorithms.
Presaccadic acceleration
In Fig. 3a-d, we present the data from two extreme cases, individuals chosen as illustrative of greater (Fig. 3a,c) and lesser (Fig. 3b,d) acceleration. As demonstrated both by eye traces (Fig. 3a,b) and by plots of eye acceleration vs. target speed (Fig. 3c,d), the greater acceleration participant produces higher average eye accelerations.
To calculate acceleration, we first lined up all traces for a given individual by the initiation of acceleration, as in Figures 3a and 3b, with first saccade and all subsequent eye movements deleted. In 4ms increments between acceleration initiation and 100ms hence, we calculated the constant acceleration needed to produce each trial's change in position since acceleration initiation (Carl and Gellman, 1987). We found each individual's mean log acceleration for each increment (taking logs rendered acceleration values normally distributed). Nearly identical results were obtained when z scores were calculated for each target speed condition before computing the mean (thereby ensuring equal weighting of each condition). The reliability of this measure is reported in Figure 5c.
Postsaccadic precision
In Fig. 3e-h, we present the data from two extreme cases, individuals chosen as illustrative of high (Fig. 3e,g) and low (Fig. 3f,h) precision. As demonstrated both by eye traces (Fig. 3e,f) and by plots of eye speed vs. target speed (Fig. 3g,h), the high precision participant more clearly and consistently modulates postsaccadic eye velocity to the velocity of the stimulus. The high precision participant has both a greater difference in eye speed between target speed conditions, indicated by a steeper slope of the (solid) least squares regression line, and lower variation in eye speed within target speed conditions.
To calculate pursuit precision from this data, we first determined the ‘oculomotor difference threshold’ (Kowler and McKee, 1987). That is, the change in stimulus speed that would be necessary to predict an increase in eye speed 75% of the time, given the slope of the eye speed (y-axis) vs. stimulus speed (x-axis) regression line and the standard deviation of residual eye speed values around that line. An increase in eye speed of 1.349 standard deviations is required to reach this 75% threshold. The oculomotor difference thresholds for the data shown are 5.81 deg/sec (high precision) and 16.79 deg/sec (low precision). We divided the oculomotor difference threshold by the average stimulus velocity (17.5 deg/sec) to obtain a percentage speed difference. We define pursuit precision as the inverse of this value. This renders the data more normally distributed and makes larger values correspond to higher precision. For the data shown, this value was 3.01 (high precision participant) and 1.04 (low precision participant). We calculated pursuit precision in 16ms blocks, beginning every 4ms from 0-300ms postsaccade, from 0-400ms after pursuit onset, and from 0-600ms after target motion onset. In the latter two cases we included trials whether or not a saccade was made, but removed all saccades prior to calculating precision. The reliability of the postsaccadic measure is reported in Figure 5c.
Other pursuit measures
We calculated three additional global pursuit measurements for each individual in order to control for them statistically (Fig. 5): saccade latency, presaccadic acceleration latency, and first saccade undershoot. We computed each individual's mean value for each measure. Results stayed the same when z scores were calculated for each target speed condition before computing means (thereby ensuring equal weighting for each condition). Derivation of latencies is described above. First saccade undershoot was defined as the difference, at the end of the first saccade, between eye position and target position, divided by the deviation of target position from initial fixation. The reliability of these measures are: mean saccade latency (rs=0.91), mean presaccadic acceleration latency (rs=0.92), and mean first saccade undershoot (rs=0.91).
General intellectual ability test
Participants completed a focal measure of intellectual ability, the digit symbol subtest of the Wechsler Adult Intelligence Scale - Revised (Wechsler, 1981). This subtest is useful in research: it can be quickly administered (90 seconds) and correlates 0.58 with Full Scale IQ, 0.55 with Verbal IQ, and 0.50 with Performance IQ in 18- and 19-year-olds (Wechsler, 1981).
Supplementary Material
Acknowledgments
We thank Patrick Cavanagh, Richard Born, Anne Dwyer Wilmer, and David Schoppik and other members of Stephen Lisberger's lab for comments on the manuscript, Anne Grossetete for statistical consultation, Jeff Wetherhold for programming assistance, and members of the Harvard Vision Lab for helpful discussions. We also thank three anonymous reviewers for their constructive input. This work was supported by an NSF Graduate Research Fellowship to JBW and NSF grant IIS-0433226 to KN.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adelson EH, Bergen JR. Spatiotemporal energy models for the perception of motion. J Opt Soc Am A Opt Image Sci Vis. 1985;2:284–299. doi: 10.1364/josaa.2.000284. [DOI] [PubMed] [Google Scholar]
- Anstis SM. The perception of apparent motion. Philos Trans R Soc Lond B Biol Sci. 1980;290:153–168. doi: 10.1098/rstb.1980.0088. [DOI] [PubMed] [Google Scholar]
- Baker CL, Boulton JC, Mullen KT. A nonlinear chromatic motion mechanism. Vision Res. 1998;38:291–302. doi: 10.1016/s0042-6989(97)00069-2. [DOI] [PubMed] [Google Scholar]
- Battelli L, Cavanagh P, Intriligator J, Tramo MJ, Henaff MA, Michel F, Barton JJ. Unilateral right parietal damage leads to bilateral deficit for high-level motion. Neuron. 2001;32:985–995. doi: 10.1016/s0896-6273(01)00536-0. [DOI] [PubMed] [Google Scholar]
- Born RT. Structure and function of visual area MT. Annu Rev Neurosci. 2005;28:157–189. doi: 10.1146/annurev.neuro.26.041002.131052. [DOI] [PubMed] [Google Scholar]
- Braddick O. Short-range process in apparent motion. Vision Res. 1974;14:519–527. doi: 10.1016/0042-6989(74)90041-8. [DOI] [PubMed] [Google Scholar]
- Butzer F, Ilg UJ, Zanker JM. Smooth-pursuit eye movements elicited by first-order and second-order motion. Exp Brain Res. 1994;115:61–70. doi: 10.1007/pl00005686. [DOI] [PubMed] [Google Scholar]
- Carl JR, Gellman RS. Human smooth pursuit - stimulus-dependent responses. J Neurophysiol. 1987;57:1446–1463. doi: 10.1152/jn.1987.57.5.1446. [DOI] [PubMed] [Google Scholar]
- Cavanagh P. Attention-based motion perception. Science. 1992;257:1563–1565. doi: 10.1126/science.1523411. [DOI] [PubMed] [Google Scholar]
- Claeys KG, Lindsey DT, De Schutter E, Orban GA. A higher order motion region in human inferior parietal lobule: evidence from fMRI. Neuron. 2003;40:631–642. doi: 10.1016/s0896-6273(03)00590-7. [DOI] [PubMed] [Google Scholar]
- Culham JC, Verstraten FAJ, Ashida H, Cavanagh P. Independent aftereffects of attention and motion. Neuron. 2000;28:607–615. doi: 10.1016/s0896-6273(00)00137-9. [DOI] [PubMed] [Google Scholar]
- Fischer B, Ramsperger E. Human express saccades - extremely short reaction-times of goal directed eye-movements. Exp Brain Res. 1984;57:191–195. doi: 10.1007/BF00231145. [DOI] [PubMed] [Google Scholar]
- Gardner JL, Lisberger SG. Linked target selection for saccadic and smooth pursuit eye movements. J Neurosci. 2001;21:2075–2084. doi: 10.1523/JNEUROSCI.21-06-02075.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner JL, Lisberger SG. Serial linkage of target selection for orienting and tracking eye movements. Nat Neurosci. 2002;5:892–899. doi: 10.1038/nn897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawken MJ, Gegenfurtner KR. Pursuit eye movements to second-order motion targets. J Opt Soc Am A Opt Image Sci Vis. 2001;18:2282–2296. doi: 10.1364/josaa.18.002282. [DOI] [PubMed] [Google Scholar]
- Heinen SJ, Watamaniuk SNJ. Spatial integration in human smooth pursuit. Vision Res. 1998;38:3785–3794. doi: 10.1016/s0042-6989(97)00422-7. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Cacioppo JT, Davidson RJ, Hugdahl K, Lovallo WR, Spiegel D, Rose R. Bridging psychology and biology: the analysis of individuals in groups. Am Psychol. 2002;57:341–51. [PubMed] [Google Scholar]
- Kowler E, McKee SP. Sensitivity of smooth eye-movement to small differences in target velocity. Vision Res. 1987;27:993–1015. doi: 10.1016/0042-6989(87)90014-9. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Lisberger SG. Temporal properties of visual-motion signals for the initiation of smooth-pursuit eye-movements in monkeys. J Neurophysiol. 1994;72:150–162. doi: 10.1152/jn.1994.72.1.150. [DOI] [PubMed] [Google Scholar]
- Lindner A, Ilg UJ. Initiation of smooth-pursuit eye movements to first-order and second-order motion stimuli. Exp Brain Res. 2000;133:450–456. doi: 10.1007/s002210000459. [DOI] [PubMed] [Google Scholar]
- Lisberger SG. Postsaccadic enhancement of initiation of smooth pursuit eye movements in monkeys. J Neurophysiol. 1998;79:1918–1930. doi: 10.1152/jn.1998.79.4.1918. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Morris EJ, Tychsen L. Visual-motion processing and sensory-motor integration for smooth pursuit eye-movements. Annu Rev Neurosci. 1987;10:97–129. doi: 10.1146/annurev.ne.10.030187.000525. [DOI] [PubMed] [Google Scholar]
- Lorenceau J, Shiffrar M, Wells N, Castet E. Different motion sensitive units are involved in recovering the direction of moving lines. Vision Res. 1993;33:1207–1217. doi: 10.1016/0042-6989(93)90209-f. [DOI] [PubMed] [Google Scholar]
- Lu ZL, Sperling G. Three-systems theory of human visual motion perception: review and update. J Opt Soc Am A Opt Image Sci Vis. 2001;18:2331–2370. doi: 10.1364/josaa.18.002331. [DOI] [PubMed] [Google Scholar]
- Mandler MB, Makous W. A 3 channel model of temporal frequency perception. Vision Res. 1984;24:1881–1887. doi: 10.1016/0042-6989(84)90021-x. [DOI] [PubMed] [Google Scholar]
- Masson GS, Stone LS. From following edges to pursuing objects. J Neurophysiol. 2002;88:2869–2873. doi: 10.1152/jn.00987.2001. [DOI] [PubMed] [Google Scholar]
- McKee SP, Silverman GH, Nakayama K. Precise velocity discrimination despite random variations in temporal frequency and contrast. Vision Res. 1986;26:609–619. doi: 10.1016/0042-6989(86)90009-x. [DOI] [PubMed] [Google Scholar]
- Merchant H, Battaglia-Mayer A, Georgopoulos AP. Decoding of path-guided apparent motion from neural ensembles in posterior parietal cortex. Exp Brain Res. 2005;161:532–540. doi: 10.1007/s00221-004-2100-1. [DOI] [PubMed] [Google Scholar]
- Morris EJ, Lisberger SJ. Different responses to small visual errors during initiation and maintenance of smooth-pursuit eye-movements in monkeys. J Neurophysiol. 1987;58:1351–1369. doi: 10.1152/jn.1987.58.6.1351. [DOI] [PubMed] [Google Scholar]
- Nakayama K. Biological image motion processing - a review. Vision Res. 1985;25:625–660. doi: 10.1016/0042-6989(85)90171-3. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Tyler CW. Psychophysical isolation of movement sensitivity by removal of familiar position cues. Vision Res. 1981;21:427–433. doi: 10.1016/0042-6989(81)90089-4. [DOI] [PubMed] [Google Scholar]
- Nishida S, Sato T. Motion aftereffect with flickering test patterns reveals higher stages of motion processing. Vision Res. 1995;35:477–490. doi: 10.1016/0042-6989(94)00144-b. [DOI] [PubMed] [Google Scholar]
- Osborne LC, Lisberger SG, Bialek W. A sensory source for motor variation. Nature. 2005;437:412–416. doi: 10.1038/nature03961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pack CC, Born RT. Temporal dynamics of a neural solution to the aperture problem in visual area MT of macaque brain. Nature. 2001;409:1040–1042. doi: 10.1038/35059085. [DOI] [PubMed] [Google Scholar]
- Peterzell DH, Teller DY. Spatial frequency tuned covariance channels for red-green and luminance modulated gratings: psychophysical data from human adults. Vision Res. 2000;40:417–30. doi: 10.1016/s0042-6989(99)00187-x. [DOI] [PubMed] [Google Scholar]
- Pola J, Wyatt HJ. Target position and velocity - the stimuli for smooth pursuit eye-movements. Vision Res. 1980;20:523–534. doi: 10.1016/0042-6989(80)90127-3. [DOI] [PubMed] [Google Scholar]
- Priebe NJ, Churchland MM, Lisberger SG. Reconstruction of target speed for the guidance of pursuit eye movements. J Neurosci. 2001;21:3196–3206. doi: 10.1523/JNEUROSCI.21-09-03196.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashbass C. Relationship between saccadic and smooth tracking eye movements. J Physiol. 1961;159:326–338. doi: 10.1113/jphysiol.1961.sp006811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt FL, Hunter JE. Measurement in psychological research: lessons from 26 research scenarios. Psychol Methods. 1996;1:119–223. [Google Scholar]
- Schoppik D, Lisberger SG. Saccades exert spatial control of motion processing for smooth pursuit eye movements. J Neurosci. 2006;26:7607–7618. doi: 10.1523/JNEUROSCI.1719-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segraves MA, Goldberg ME. Effect of stimulus position and velocity upon the maintenance of smooth-pursuit eye velocity. Vision Res. 1994;34:2477–2482. doi: 10.1016/0042-6989(94)90291-7. [DOI] [PubMed] [Google Scholar]
- Seiffert AE, Cavanagh P. Position displacement, not velocity, is the cue to motion detection of second-order stimuli. Vision Res. 1998;38:3569–3582. doi: 10.1016/s0042-6989(98)00035-2. [DOI] [PubMed] [Google Scholar]
- Seiffert AE, Cavanagh P. Position-based motion perception for color and texture stimuli: effects of contrast and speed. Vision Res. 1999;39:4172–4185. doi: 10.1016/s0042-6989(99)00129-7. [DOI] [PubMed] [Google Scholar]
- Stocker AA, Simoncelli EP. Noise characteristics and prior expectations in human visual speed perception. Nat Neurosci. 2006;9:578–585. doi: 10.1038/nn1669. [DOI] [PubMed] [Google Scholar]
- Tychsen L, Lisberger SG. Visual-motion processing for the initiation of smooth-pursuit eye-movements in humans. J Neurophysiol. 1986;56:953–968. doi: 10.1152/jn.1986.56.4.953. [DOI] [PubMed] [Google Scholar]
- Ullman S. The interpretation of visual motion. Cambridge, Massachusetts, USA: MIT Press; 1979. [Google Scholar]
- Van Santen JPH, Sperling G. Elaborated reichardt detectors. J Opt Soc Am A Opt Image Sci Vis. 1985;2:300–321. doi: 10.1364/josaa.2.000300. [DOI] [PubMed] [Google Scholar]
- Verstraten FAJ, Cavanagh P, Labianca AT. Limits of attentive tracking reveal temporal properties of attention. Vision Res. 2000;40:3651–3664. doi: 10.1016/s0042-6989(00)00213-3. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale - revised (WAIS-R) manual. New York: Psychological Corporation; 1981. [Google Scholar]
- Williams ZM, Elfar JC, Eskandar EN, Toth LJ, Assad JA. Parietal activity and the perceived direction of ambiguous apparent motion. Nat Neurosci. 2003;6:616–623. doi: 10.1038/nn1055. [DOI] [PubMed] [Google Scholar]
- Yasui S, Young LR. Perceived visual motion as effective stimulus to pursuit eye movement system. Science. 1975;190:906–908. doi: 10.1126/science.1188373. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.