Abstract
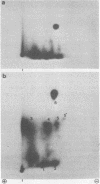
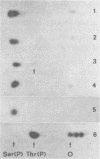
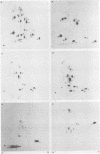
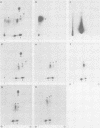
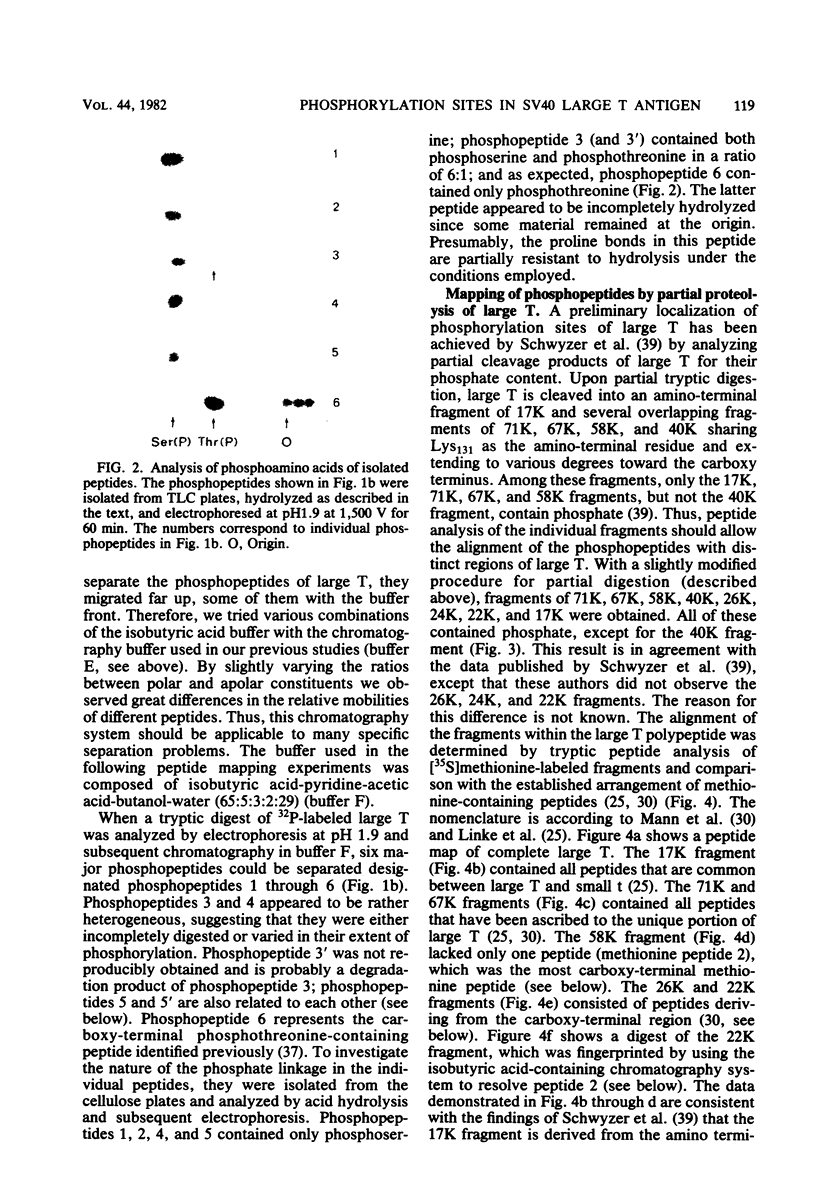
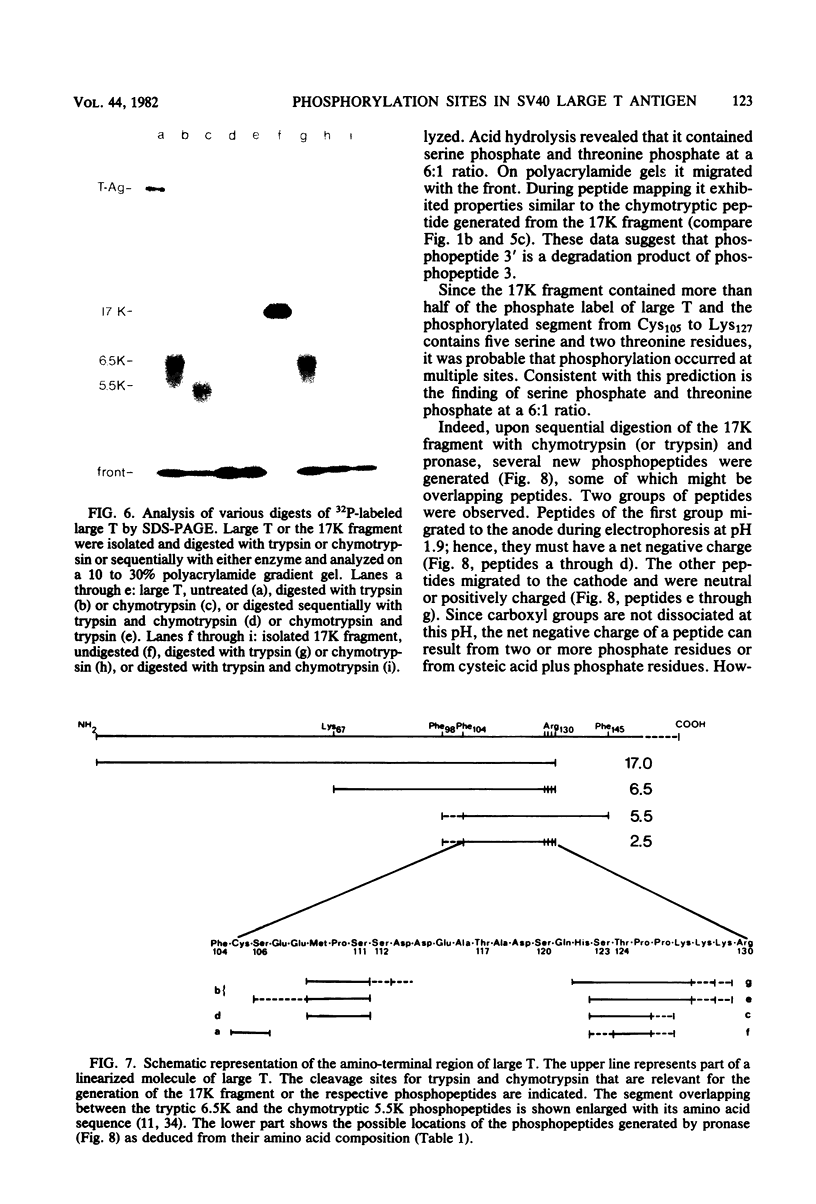
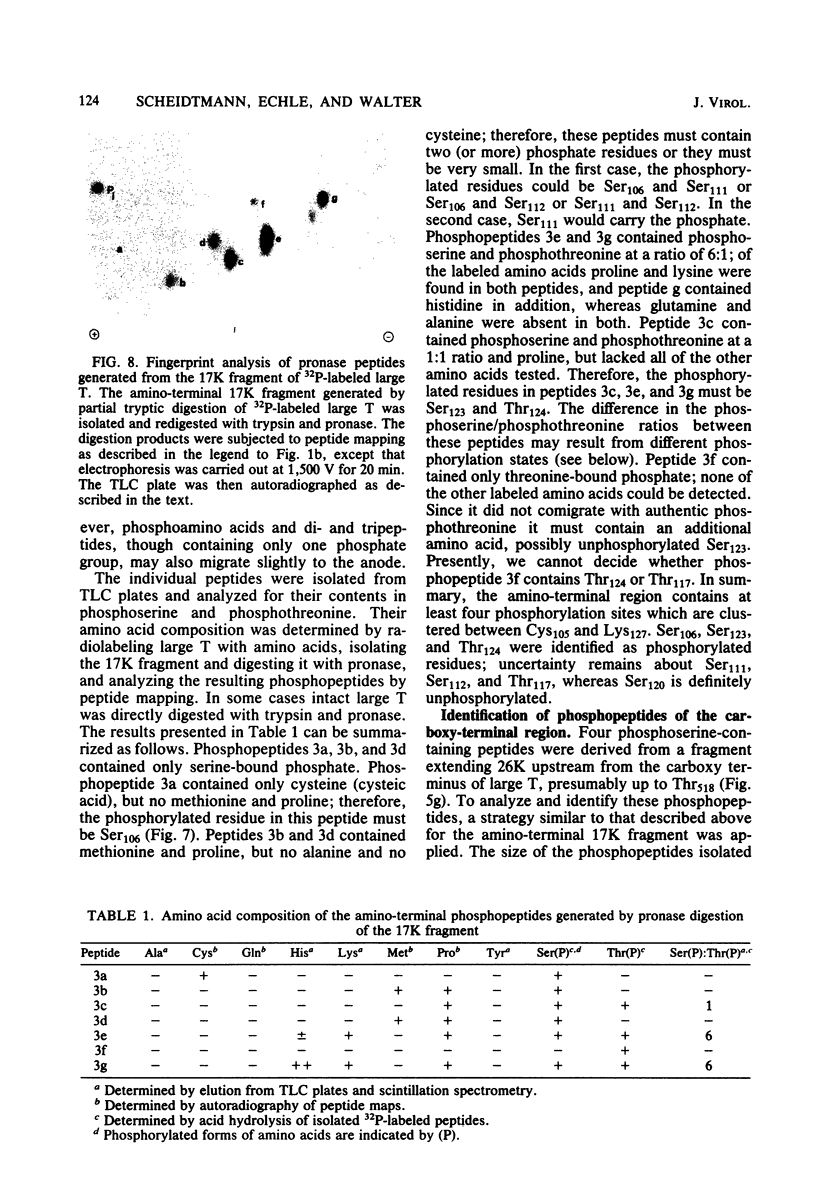
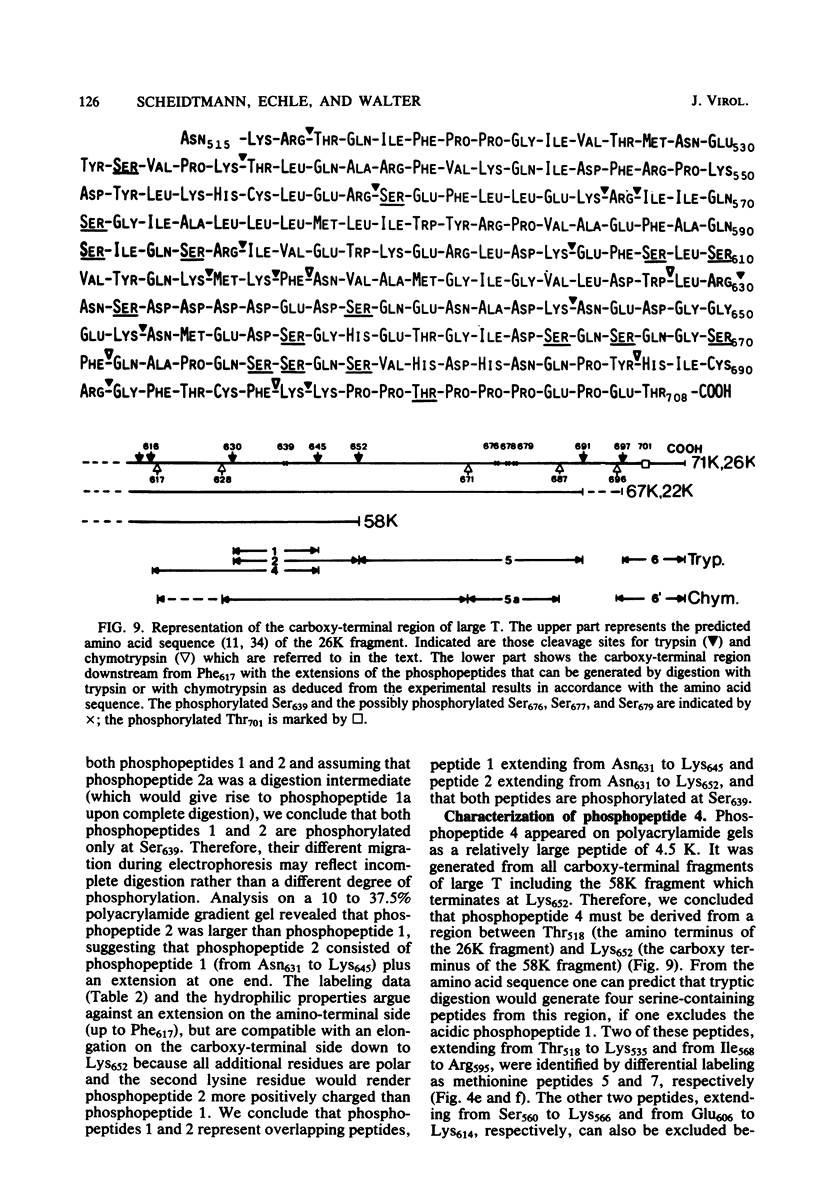
The phosphorylation sites of simian virus 40 large T antigen were determined within the primary structure of the molecule. Exhaustive digestion of 32P-labeled large T antigen with trypsin generated six major phosphopeptides which could be separated in a newly developed isobutyric acid-containing chromatography system. By partial tryptic digestion, large T antigen was cleaved into an amino-terminal fragment of 17,000 daltons and overlapping fragments from the carboxy-terminal region ranging in size between 71,000 and 13,000 daltons. The location of the phosphopeptides was then determined by fingerprint analyses of individual fragments. Their physical properties were analyzed by sizing on polyacrylamide gels and by sequential digestion and peptide mapping; their amino acid composition was determined by differential labeling with various amino acids. The amino-terminal 17,000-dalton fragment gave rise to only one phosphopeptide (phosphopeptide 3) that contained half of the phosphate label incorporated into large T antigen. It contained phosphoserine and phosphothreonine sites, all of which were clustered within a small segment between Cys105 and Lys127. This segment contained five serines and two threonines. Among these, Ser106, Ser123, and Thr124 were identified as phosphorylated residues; in addition, either one or both of Ser111 and Ser112 were phosphorylated. The neighboring residues, Ser123 and Thr124, were found in three different phosphorylation states in that either Ser123 or Thr124 or both were phosphorylated. Phosphopeptides 1, 2, 4, 5, and 6 were all derived from a single fragment extending 26,000 daltons upstream from the carboxy terminus of large T antigen. Phosphopeptide 6 was identical with the previously determined phosphothreonine peptide phosphorylated at Thr701. Phosphopeptides 1, 2, 4, and 5 contained only serine-bound phosphate. Phosphopeptides 1, 2, and 4 represented overlapping peptides, all of which were phosphorylated at Ser639 located next to a cluster of six acidic residues. In phosphopeptide 5, a large peptide ranging from Asn653 to Arg691, at least two of seven serines were phosphorylated. Thus, large T antigen contains at least eight phosphorylation sites. Their clustering within two separate regions might correlate with structural and functional domains of this protein.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann E. A., Hand R. Protein kinase activity associated with the D2 hybrid protein related to simian virus 40 T antigen: some characteristics of the reaction products. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3688–3692. doi: 10.1073/pnas.76.8.3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Stedman J. D. Efficient fluorography of 3H and 14C on thin layers. Anal Biochem. 1978 Aug 15;89(1):247–256. doi: 10.1016/0003-2697(78)90747-9. [DOI] [PubMed] [Google Scholar]
- Bradley M. K., Griffin J. D., Livingston D. M. Relationship of oligomerization to enzymatic and DNA-binding properties of the SV40 large T antigen. Cell. 1982 Jan;28(1):125–134. doi: 10.1016/0092-8674(82)90382-8. [DOI] [PubMed] [Google Scholar]
- Brignon G., Ribadeau Dumas B., Mercier J. C., Pelissier J. P., Das B. C. Complete amino acid sequence of bovine alphaS2-casein. FEBS Lett. 1977 Apr 15;76(2):274–279. doi: 10.1016/0014-5793(77)80167-1. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Cole C. N., Landers T., Goff S. P., Manteuil-Brutlag S., Berg P. Physical and genetic characterization of deletion mutants of simian virus 40 constructed in vitro. J Virol. 1977 Oct;24(1):277–294. doi: 10.1128/jvi.24.1.277-294.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhart W., Hutchinson M. A., Hunter T. An activity phosphorylating tyrosine in polyoma T antigen immunoprecipitates. Cell. 1979 Dec;18(4):925–933. doi: 10.1016/0092-8674(79)90205-8. [DOI] [PubMed] [Google Scholar]
- Edwards C. A., Khoury G., Martin R. G. Phosphorylation of T-antigen and control T-antigen expression in cells transformed by wild-type and tsA mutants of simian virus 40. J Virol. 1979 Feb;29(2):753–762. doi: 10.1128/jvi.29.2.753-762.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning E., Nowak B., Burger C. Detection and characterization of multiple forms of simian virus 40 large T antigen. J Virol. 1981 Jan;37(1):92–102. doi: 10.1128/jvi.37.1.92-102.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiers W., Contreras R., Haegemann G., Rogiers R., Van de Voorde A., Van Heuverswyn H., Van Herreweghe J., Volckaert G., Ysebaert M. Complete nucleotide sequence of SV40 DNA. Nature. 1978 May 11;273(5658):113–120. doi: 10.1038/273113a0. [DOI] [PubMed] [Google Scholar]
- Friedmann T., Esty A., LaPorte P., Deininger P. The nucleotide sequence and genome organization of the polyoma early region: extensive nucleotide and amino acid homology with SV40. Cell. 1979 Jul;17(3):715–724. doi: 10.1016/0092-8674(79)90278-2. [DOI] [PubMed] [Google Scholar]
- Gibson W. Polyoma virus proteins: a description of the structural proteins of the virion based on polyacrylamide gel electrophoresis and peptide analysis. Virology. 1974 Dec;62(2):319–336. doi: 10.1016/0042-6822(74)90395-x. [DOI] [PubMed] [Google Scholar]
- Goldman N., Brown M., Khoury G. Modification of SV40 T antigen by poly ADP-ribosylation. Cell. 1981 May;24(2):567–572. doi: 10.1016/0092-8674(81)90347-0. [DOI] [PubMed] [Google Scholar]
- Greenspan D. S., Carroll R. B. Complex of simian virus 40 large tumor antigen and 48,000-dalton host tumor antigen. Proc Natl Acad Sci U S A. 1981 Jan;78(1):105–109. doi: 10.1073/pnas.78.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan D. S., Carroll R. B. Simian virus 40 large T antigen isoelectric focuses as multiple species with varying phosphate content. Virology. 1979 Dec;99(2):413–416. doi: 10.1016/0042-6822(79)90020-5. [DOI] [PubMed] [Google Scholar]
- Griffin J. D., Spangler G., Livingston D. M. Protein kinase activity associated with simian virus 40 T antigen. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2610–2614. doi: 10.1073/pnas.76.6.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe J., Baydoun H. Substrate specificity of the nuclear protein kinase NII from porcine liver. Studies with casein variants. Eur J Biochem. 1981 Jul;117(3):585–589. doi: 10.1111/j.1432-1033.1981.tb06378.x. [DOI] [PubMed] [Google Scholar]
- Jessel D., Landau T., Hudson J., Lalor T., Tenen D., Livingston D. M. Identification of regions of the SV40 genome which contain preferred SV40 T antigen-binding sites. Cell. 1976 Aug;8(4):535–545. doi: 10.1016/0092-8674(76)90222-1. [DOI] [PubMed] [Google Scholar]
- Krebs E. G., Beavo J. A. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. A map of temperature-sensitive mutants of simian virus 40. Virology. 1975 Jul;66(1):70–81. doi: 10.1016/0042-6822(75)90179-8. [DOI] [PubMed] [Google Scholar]
- Lanford R. E., Butel J. S. Biochemical characterization of nuclear and cytoplasmic forms of SV40 tumor antigens encoded by parental and transport-detective mutant SV40-adenovirus 7 hybrid viruses. Virology. 1980 Sep;105(2):314–327. doi: 10.1016/0042-6822(80)90033-1. [DOI] [PubMed] [Google Scholar]
- Linke H. K., Hunter T., Walter G. Structural relationship between the 100,000- and 17,000- molecular-weight T antigens of simian virus 40 (SV40) as deduced by comparison with the SV40-specific proteins coded by the nondefective adenovirus type 2-SV40 hybrid viruses. J Virol. 1979 Jan;29(1):390–394. doi: 10.1128/jvi.29.1.390-394.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamrack M. D., Olson M. O., Busch H. Amino acid sequence and sites of phosphorylation in a highly acidic region of nucleolar nonhistone protein C23. Biochemistry. 1979 Jul 24;18(15):3381–3386. doi: 10.1021/bi00582a026. [DOI] [PubMed] [Google Scholar]
- Mann K., Hunter T. Phosphorylation of SV40 large T antigen in SV40 nucleoprotein complexes. Virology. 1980 Dec;107(2):526–532. doi: 10.1016/0042-6822(80)90320-7. [DOI] [PubMed] [Google Scholar]
- Mann K., Hunter T., Walter G., Linke H. Evidence for simian virus 40 (SV40) coding of SV40 T-antigen and the SV40-specific proteins in HeLa cells infected with nondefective adenovirus type 2-SV40 hybrid viruses. J Virol. 1977 Oct;24(1):151–169. doi: 10.1128/jvi.24.1.151-169.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montenarh M., Henning R. Simian virus 40 T-antigen phosphorylation is variable. FEBS Lett. 1980 May 19;114(1):107–110. doi: 10.1016/0014-5793(80)80870-2. [DOI] [PubMed] [Google Scholar]
- Oren M., Winocour E., Prives C. Differential affinities of simian virus 40 large tumor antigen for DNA. Proc Natl Acad Sci U S A. 1980 Jan;77(1):220–224. doi: 10.1073/pnas.77.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintel D., Bouck N., di Mayorca G., Thimmappaya B., Swerdlow B., Shenk T. SV40 mutant tsA1499 is heat-sensitive for lytic growth but generates cold-sensitive rat-cell transformants. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):305–309. doi: 10.1101/sqb.1980.044.01.035. [DOI] [PubMed] [Google Scholar]
- Reddy V. B., Thimmappaya B., Dhar R., Subramanian K. N., Zain B. S., Pan J., Ghosh P. K., Celma M. L., Weissman S. M. The genome of simian virus 40. Science. 1978 May 5;200(4341):494–502. doi: 10.1126/science.205947. [DOI] [PubMed] [Google Scholar]
- Rio D., Robbins A., Myers R., Tjian R. Regulation of simian virus 40 early transcription in vitro by a purified tumor antigen. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5706–5710. doi: 10.1073/pnas.77.10.5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundell K., Collins J. K., Tegtmeyer P., Ozer H. L., Lai C. J., Nathans D. Identification of simian virus 40 protein A. J Virol. 1977 Feb;21(2):636–646. doi: 10.1128/jvi.21.2.636-646.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidtmann K. H., Kaiser A., Carbone A., Walter G. Phosphorylation of threonine in the proline-rich carboxy-terminal region of simian virus 40 large T antigen. J Virol. 1981 Apr;38(1):59–69. doi: 10.1128/jvi.38.1.59-69.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwyzer M., Weil R., Frank G., Zuber H. Amino acid sequence analysis of fragments generated by partial proteolysis from large simian virus 40 tumor antigen. J Biol Chem. 1980 Jun 25;255(12):5627–5634. [PubMed] [Google Scholar]
- Shalloway D., Kleinberger T., Livingston D. M. Mapping of SV40 DNA replication origin region binding sites for the SV40 T antigen by protection against exonuclease III digestion. Cell. 1980 Jun;20(2):411–422. doi: 10.1016/0092-8674(80)90627-3. [DOI] [PubMed] [Google Scholar]
- Shaw S. B., Tegtmeyer P. Binding of dephosphorylated A protein to SV40 DNA. Virology. 1981 Nov;115(1):88–96. doi: 10.1016/0042-6822(81)90091-x. [DOI] [PubMed] [Google Scholar]
- Shortle D. R., Margolskee R. F., Nathans D. Mutational analysis of the simian virus 40 replicon: pseudorevertants of mutants with a defective replication origin. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6128–6131. doi: 10.1073/pnas.76.12.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small D., Chou P. Y., Fasman G. D. Occurrence of phosphorylated residues in predicted beta-turns: implications for beta-turn participation in control mechanisms. Biochem Biophys Res Commun. 1977 Nov 7;79(1):341–346. doi: 10.1016/0006-291x(77)90101-2. [DOI] [PubMed] [Google Scholar]
- Soeda E., Arrand J. R., Smolar N., Griffin B. E. Sequence from early region of polyoma virus DNA containing viral replication origin and encoding small, middle and (part of) large T antigens. Cell. 1979 Jun;17(2):357–370. doi: 10.1016/0092-8674(79)90162-4. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P., Andersen B., Shaw S. B., Wilson V. G. Alternative interactions of the SV40 A protein with DNA. Virology. 1981 Nov;115(1):75–87. doi: 10.1016/0042-6822(81)90090-8. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P., Rundell K., Collins J. K. Modification of simian virus 40 protein A. J Virol. 1977 Feb;21(2):647–657. doi: 10.1128/jvi.21.2.647-657.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R., Robbins A., Clark R. Catalytic properties of the SV40 large T antigen. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):103–111. doi: 10.1101/sqb.1980.044.01.012. [DOI] [PubMed] [Google Scholar]
- Tjian R. The binding site on SV40 DNA for a T antigen-related protein. Cell. 1978 Jan;13(1):165–179. doi: 10.1016/0092-8674(78)90147-2. [DOI] [PubMed] [Google Scholar]
- Van Heuverswyn H., Cole C., Berg P., Fiers W. Nucleotide sequence analysis of two simian virus 40 mutants with deletions in the region coding for the carboxyl terminus of the T antigen. J Virol. 1979 Jun;30(3):936–941. doi: 10.1128/jvi.30.3.936-941.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Roy F., Fransen L., Fiers W. Phosphorylation patterns of tumour antigens in cells lytically infected or transformed by simian virus 40. J Virol. 1981 Oct;40(1):28–44. doi: 10.1128/jvi.40.1.28-44.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter G., Flory P. J., Jr Phosphorylation of SV40 large T antigen. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):165–169. doi: 10.1101/sqb.1980.044.01.019. [DOI] [PubMed] [Google Scholar]