Abstract
Background
Converging animal findings suggest that higher peripheral levels of inflammation are associated with activation of central inflammatory mechanisms that result in hippocampal neurodegeneration and related impairment of memory function. Consistent with animal findings, we have recently shown an inverse association between peripheral levels of interleukin-6 (IL-6), a relatively stable marker of systemic inflammation, and memory function in mid-life adults. In the current study, we extend this work to test whether systemic inflammation is associated with reduced grey matter volume of the hippocampus.
Methods
For this purpose, we used a computational structural neuroimaging method (optimized voxel-based morphometry) to evaluate the relationship between plasma IL-6 levels and hippocampal grey matter volume in a sample of 76 relatively healthy community volunteers aged 30-54.
Results
Peripheral levels of IL-6 covaried inversely with hippocampal grey matter volume, and this relationship persisted after accounting for several possible confounders, including age, sex, race, years of education, percent body fat, blood pressure, smoking, physical activity, hours of sleep, alcohol use, and total grey matter volume.
Conclusions
To our knowledge, this is the first report of a relationship between a peripheral marker of IL-6 and hippocampal grey matter volume, raising the possibility that low grade systemic inflammation could plausibly presage subclinical cognitive decline in part via structural neural pathways.
Keywords: Cognitive decline, grey matter volume, hippocampus, inflammation, interleukin-6, memory
Introduction
A growing body of evidence supports immune-to-brain communication, with peripheral immune activation being associated with behavioral, affective and cognitive disturbances. Peripheral proinflammatory cytokines, such as interleukin (IL)-6, are likely mediators of many of these effects, penetrating the blood-brain barrier directly via active transport mechanisms (1) or indirectly via activation of the afferent vagus nerve (2, 3) to stimulate the production of central proinflammatory cytokines, including IL-6 in discrete brain regions (2). Recent evidence suggests that central inflammation may adversely affect learning and memory through processes related to neurodegeneration and structural remodeling of the hippocampus (2, 4).
The hippocampus plays a key role in memory formation (5) and is particularly vulnerable to the adverse effects of IL-6. As evidence, a consistent animal literature shows peripheral IL-6 (whether the result of exogenous administration or in vivo immune challenge) to be associated with increased levels of cytokines in the hippocampus, where IL-6 and IL-6 receptors are expressed abundantly (6-8). Further, in several animal models, increased levels of hippocampal IL-6 interfere with long-term potentiation (9 - 11), neurogenesis (12), and neural plasticity (13, 14), which can all impair performance on hippocampal-dependent learning and memory tests (13, 15-17). Conversely, IL-6 receptor antagonists prevent inflammation-related disruption of hippocampal LTP and ensuing cognitive sequelae (18). Finally, IL-6 knockout mice show facilitated working memory when compared with wild type mice (19) and are refractory to peripheral endotoxin-induced impairments of spatial memory (20). Together, these animal findings suggest that higher peripheral IL-6 is associated with hippocampal inflammatory mechanisms that negatively affect cognitive processes, including memory and learning.
In parallel to animal work, studies of human dementia suggest that IL-6 is a mediator of memory decline, playing a possible pathogenic role in Alzheimer's disease (AD), vascular dementia, and age-related cognitive decline. In particular, these syndromes have been associated with high levels of central and peripheral IL-1 and IL-6 (21-26), with IL-6 levels predicting subsequent cognitive decline among the elderly (24, 26, 27). We have recently extended these findings to show an inverse association between IL-6 and memory function among relatively healthy mid-life adults, raising the possibility that IL-6 represents a novel biomarker for risk of future cognitive decline (28). Moreover, we and others have found that observed relationships between IL-6 and cognitive function are largely independent of established risk factors for subtle and clinical cognitive impairments, including age, education, hypertension, diabetes, smoking, body mass index (BMI), and subclinical atherosclerosis (e.g., 25, 28). Thus, accumulating evidence links elevated IL-6 to cognitive impairments, with animal evidence showing that hippocampal-dependent cognitive functions may be particularly vulnerable to inflammation-related processes.
If, as the animal and human clinical literatures suggest, peripheral inflammation is associated with hippocampal morphology, then mid-life adults who exhibit higher levels of IL-6, a relatively stable attribute of individual difference (29), may express a lower grey matter volume in the hippocampus, which has been previously associated with memory impairments (30), than individuals with lower levels of IL-6-related systemic inflammation. To test this possibility, we employed a computational neuroanatomical procedure, optimized voxel-based morphometry (31, 32), in a cross-sectional neuroimaging study to examine the association between plasma IL-6 levels and hippocampal grey matter volume in a subset of relatively healthy mid-life adults on whom we reported previously (28). In light of evidence that adipocytes are a primary source of circulating IL-6 (33) and that body fat covaries positively with IL-6 (34) and inversely with cognitive function (35), we also examined whether associations between IL-6 and hippocampal volume exist independently of percent body fat. Finally, we conducted exploratory analyses examining the possibility that hippocampal volume mediates any association between IL-6 and memory function.
Methods and Materials
Participants
Participants were 96 adults aged 31 to 54 from the Adult Health and Behavior (AHAB) project, a registry of behavioral and biological measurements among community volunteers. To be eligible for the neuroimaging substudy, participants had to report good general health, with no history of (1) myocardial infarction, stroke or other cerebrovascular disease, (2) neurological disorders, convulsions, or a concussion in the year prior to testing, (3) chronic kidney or liver disease, (4) cancer, (5) insulin-dependent diabetes, or (6) psychotic illness and no current DSM-IV Axis I diagnosis, as established by the Structured Clinical Interview for DSM-IV (36). Women who were pregnant or lactating were also ineligible, as were individuals taking psychotropic, glucocorticoid, hyperlipidemic, or weight-loss medications. Of the 96 participants, 10 individuals taking medications to treat immune-related diseases and 10 individuals with IL-6 levels above the maximum level reliably quantified by the current methods (10pg/ml) were dropped, resulting in a final sample of 76 subjects. No participants endorsed taking antihypertensives, anti-lipenics, nitrates, antiarrhythmics, proteases, or anti-HIV medications. Informed consent was obtained in compliance with the University of Pittsburgh Institutional Review Board.
IL-6 Measures
Participants were asked to fast for 8 hours and avoid exercise for 12 hours and alcohol for 24 hours before a morning blood sample was drawn for the determination of plasma IL-6 levels. Blood was collected in citrated tubes, with harvested plasma frozen at -80°C until analysis in batches. IL-6 levels were determined using a high sensitivity quantitative sandwich enzyme immunoassay kit (R & D Systems) according to manufacturer's directions. The assay standard range is 0.156 to10 pg/mL. IL-6 levels were extrapolated from a standard curve with linear regression from a log-linear curve. Samples were run in duplicate and the average coefficient of variation was 5%. Reciprocal transformation was applied to normalize raw score distributions of the IL-6 values. To aid interpretation, the signs of correlations involving reciprocally transformed measurements of IL-6 are reversed, so that positive (and negative) coefficients are interpreted as such.
Assessment of Regional Grey Matter Volume
Image acquisition
The neuroimaging substudy of the AHAB project was designed to characterize neural correlates of individual differences in risk factors for neuropsychiatric and cardiovascular disease. For this purpose, high-resolution structural brain images were acquired on a 3-Tesla Siemens Allegra scanner, equipped with a standard birdcage radiofrequency head coil. Total and regional grey matter volumes were assessed from T1-weighted 3D fast-gradient magnetization prepared rapid gradient-echo (MPRAGE) structural images (TR/TE = 1540/3.0 msec; flip angle = 8°; NEX = 1; bandwidth = 170 Hz/pixel; echo spacing = 7.7 msec), which encompassed the whole brain and consisted of 192 sagittal slices (1 mm thick; 0 mm spacing between slices; matrix size = 256 × 256 pixels; FOV = 256 mm). Prior to implementing optimized voxel-based morphometry (VBM) procedures, raw images were realigned to the axial plane of the anterior and posterior commissures.
Image processing
Optimized VBM was used to quantify regional and total grey matter volume from MPRAGE images (31,32) using statistical parametric mapping software (SPM2; Wellcome Department of Imaging Neuroscience; http://www.fil.ion.ucl.ac.uk/spm/) and MATLAB (The MathWorks, Inc., Natick, MA) scripts coauthored by John Ashburner and Christian Gaser (available at http://dbm.neuro.unijena.de/vbm.html). For further details regarding VBM processing steps, see supplemental methods (available at http://www.sobp.org/journal). In brief, we created study-specific tissue templates that were normalized to the coordinate space of the Montreal Neurological Institute (MNI). Next, individual T1-weighted MPRAGE images were segmented into grey matter, white matter, and CSF images. Grey matter images were then normalized to the study-specific grey matter template, and the Jacobian deformation parameters derived from normalization were applied to individual grey matter images on a voxel-wise basis. As a result, the relative volume change introduced by regional expansion and contraction during normalization was incorporated into each voxel value. This procedure yielded relative volumetric grey matter images conventionally referred to as optimized modulated VBM images (31, 32). Prior to analysis, modulated (volumetric) grey matter images were smoothed with a 10mm FWHM Gaussian spatial filter.
Assessment of Control Variables
Several covariates were assessed that might account in part for associations between IL-6 and hippocampal volume. These included age, sex, race, systolic and diastolic blood pressure (SBP, DBP), antihypertensive treatment, years of education, smoking status (current versus ex/non-smoker), sleep volume (hours of sleep during last 7 nights), physical activity, as measured using the Paffenbarger Physical Activity Questionnaire (37), alcohol use (average number of alcoholic drinks/week), and percentage body fat measured by bioelectrical impedance (Body Composition Analyzer, model:TBF-410, Tanita Corporation of America Inc., Illinois).
Assessment of Memory
Secondary analyses were conducted to test the association of circulating IL-6 and hippocampal volume with attention, learning and memory. For this purpose, we examined responses on the 6 primary and 3 supplemental subtests of the Wechsler Memory Scale- third edition (WMS-III;38). This battery of memory tests examines attention, working memory, and auditory and visual immediate and delayed memory (see Marsland et al (28)).
Data Reduction and Analysis
To test the hypothesis that higher plasma IL-6 is associated with lower grey matter volume in the hippocampus, we conducted a multiple regression analysis in SPM2, employing the framework of the general linear model (39), with age, sex, race, and total grey matter volume entered as covariates. After the model's regression parameters were estimated, we tested for a negative association between IL-6 and grey matter volume within the hippocampus. For this region-of-interest analysis of the hippocampus, we used a standard mask defined by the Automated Anatomical Labeling system (40) as implemented in the Wake Forest University (WFU) Pick-Atlas (41). To correct for multiple testing within the search volume of the hippocampus, we employed a statistical significance threshold of p < 0.05 using a family-wise error rate (FWE) threshold.
Next, we examined whether relationships between IL-6 and hippocampal volume were independent of demographic and health practices that could plausibly impact immune parameters or hippocampal volume. For this purpose, we extracted the unadjusted volume values from the voxel coordinates localizing the peak association between IL-6 and hippocampal grey matter volume. Grey matter volume values were then imported into Statistical Package for the Social Sciences 14.0 (SPSS, Chicago, IL), and used as dependent variables (after z-score transformation to aid in interpreting effect size) in hierarchical regression models. These hierarchical models permitted the systematic examination of the independent contribution of variables entered in each step, after taking into account the effects of variables already in the model. Finally, for completeness of reporting we executed an exploratory whole-brain analysis to examine associations between IL-6 and voxel-wise grey matter volume at puncorrected < 0.001 with a combined cluster extent threshold of 25 voxels.
Results
Associations between IL-6 and voxel-wise hippocampal grey matter volume
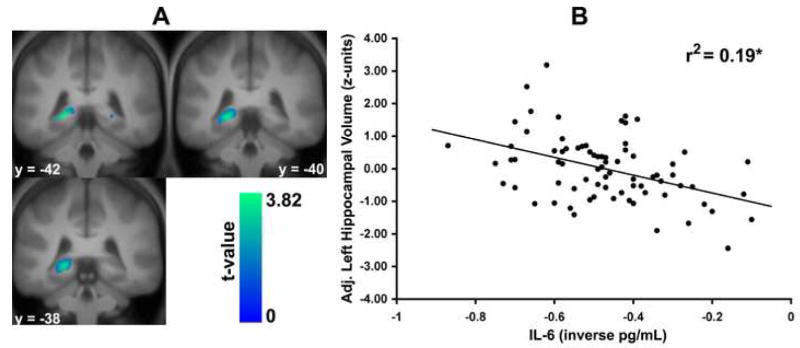
A multiple regression analysis in SPM2 with covariate control for age, sex, race, and total grey matter volume revealed an inverse association between IL-6 and grey matter volume in the left hippocampus (x, y, z MNI coordinates: -21, -42, -2; t(1, 70) = 3.82, pFWE = 0.045, cluster extent [k] = 35 voxels in mm3; Figure 1). Only at a more lenient uncorrected threshold (p < 0.05, k = 0) did we observe an inverse association between IL-6 and grey matter volume in the right hippocampus (23, -44, -2; t(1, 70) = 2.04, p = 0.02, k = 31 in mm3). As shown in Figure 1, IL-6 accounted for approximately 19% of the variance in extracted left hippocampal grey matter volume values, after controlling for age, sex, race and total grey matter volume (beta = -.44, p < .001). A similarly adjusted analysis showed that IL-6 accounted for 6% of the variance in extracted right hippocampal grey matter volume values (beta = -.25, p = .03).
Figure 1.
Higher levels of plasma interleukin-6 (IL-6) among 76 adults were associated with decreased grey matter volume in the left, but not right, hippocampus at a family-wise error rate (FWE) corrected level of statistical significance. For the left hippocampus, the x, y, & z MNI coordinates for the peak association between IL-6 and grey matter volume were -21, -42, -2; t(1, 71) = 3.82, pFWE = 0.045, k = 35. Left panel: Statistical parametric maps profiling clusters of the left and right hippocampus where higher IL-6 was associated decreased grey matter volume after controlling for age, sex, race, and total grey matter volume in a region-of-interest analysis. For display, maps are thresholded at puncorrected < 0.05. Right panel: Plotted along the y-axis are extracted left hippocampal grey matter volume values in standardized (z score) units adjusted for age, sex, race, and total grey matter volume. Plotted along the x-axis are transformed plasma IL-6 values (less negative values indicate higher IL-6 levels). *p < 0.01.
IL-6 and Hippocampal Grey Matter Volume after controlling for Covariates
Next, we tested whether several demographic and health characteristics might account for the observed relationships between circulating levels of IL-6 and hippocampal volume. Results of initial bivariate correlations are displayed in Table 1. Results of partial correlations controlling for total grey matter volume showed that lower left and right hippocampal grey matter volumes were associated with higher percent body fat (r = -.22, p = .055; r = -.30, p = .009, respectively) and a trend towards older age (r = -.23, p = .05; r = -.18, p = .12, respectively). In addition, lower left, but not right hippocampal grey matter was associated with being female (r = .28, p = .02).
Table 1.
Demographic and Health Characteristics of the Sample and their Correlations with Plasma Interleukin-6 (IL-6) and Extracted Left and Right Hippocampal Grey Matter Volumes (N=76)
| Correlation Coefficients
|
||||
|---|---|---|---|---|
| Characteristic | Mean (SD) or % | IL-6 | Left hippocampal grey matter volume | Right hippocampal grey matter volume |
| Sex1 | 42% male | -.18 | -.02 | -.03 |
| Age (years) | 45 (6.5) | -.02 | -.19 | -.17 |
| Race1 | 92% white, 8% other | .15 | -.09 | -.06 |
| Education (years) | 15.8 (2.3) | .10 | .13 | .16 |
| Percent body fat | 29 (8.7) | .23* | -.25* | -.32** |
| Current smokers1 | 7.8% | .01 | .03 | .02 |
| Physical Activity (kilocals) | 2632 (1686) | -.14 | -.01 | .08 |
| Sleep volume (hours) | 47.8 (6.6) | -.16 | .04 | -.08 |
| Alcohol (drinks/week) | 2.9 (4.1) | -.05 | -.12 | -.02 |
| Systolic blood pressure (mmHg) | 114.5 (11.5) | .29* | -.08 | .06 |
| Diastolic blood pressure (mmHg) | 77.2 (8.7) | .11 | -.07 | .01 |
| Interleukin -6 (pg/mL) | 1.58 (1.65) | -- | -.42** | -.24* |
| Total grey matter | 673.0 (61.9) | .04 | .33* | .16 |
| Left hippocampal grey matter2 | 0.32 (.05) | -.42** | -- | .65** |
| Right hippocampal grey matter2 | 0.29 (.05) | -.24* | .65** | -- |
Note. Correlations were conducted using transformed IL-6, alcohol use and physical activity variables
Point-biserial.
Derived from extracting the unadjusted volume value from the voxel coordinates localizing the peak association between IL-6 and hippocampal grey matter volume.
p < .05,
p < .005.
After controlling for age, sex, race, and total grey matter volume, a 2-step hierarchical regression analysis confirmed a relationship between IL-6 and left hippocampal volume (beta = -.37, t(1,70) = -3.82, p < .001), with IL-6 accounting for 13% of the variance in left hippocampal grey matter volume above-and-beyond the covariates (delta R2 = .13, F(1,70) = 14.62, p < .001). A similarly adjusted regression analyses showed that IL-6 also predicted variance in right hippocampal grey matter volume (beta = -.24, t (1,70) = -2.04, p = .045), accounting for 5% of independent variance (delta R2 = .053, F(1,70)=4.18, p = .045). The correlations between IL-6 and extracted grey matter volume values were not significantly different between the left and right hippocampus (r's = -.42 and -.24, respectively; z = 1.23, p = 0.22), suggesting the absence of IL-6 and volumetric laterality effects in this sample.
The Role of Body Fat
Consistent with existing evidence, our initial bivariate correlations revealed that IL-6 and bilateral hippocampal grey matter volumes covaried with percent body fat (see Table 1). Thus, we next explored whether IL-6 predicts hippocampal volume independently of body fat. In a 3-step hierarchical regression analysis, with demographic and total gray matter covariates entered in step 1, and body fat in step 2, IL-6 continued to predict left hippocampal grey matter volume (beta = -.34, t (1,69 = -3.07, p = .003), accounting for 8.5% of the variance over-and-above the contribution of demographic characteristics and body fat (delta R2 = .085, F(1,69) = 9.43, p = .003). Thus, IL-6 was inversely associated with left hippocampal grey matter volume independently of body fat. However, when IL-6 was entered before body fat in step 2 of the model, there was no significant independent effect of body fat (beta = -.15, p = .23), suggesting that the association between body fat and left hippocampal grey matter volume is largely related to variance in IL-6. On examination of right hippocampal grey matter volume, a similar regression analysis revealed no independent effect of IL-6 with the demographic covariates, total grey matter volume and body fat in the model (beta = -.12, p = .33).
The Role of Hypertension
Initial bivariate analyses revealed associations of higher blood pressure with higher IL-6 and a tendency towards lower left hippocampal grey matter volume. However, entering resting SBP and DBP into the second step of the regression equation with the standard demographic controls and total gray matter volume already in the model revealed no independent effect of blood pressure on left hippocampal gray matter volume. In contrast, IL-6 remained an independent predictor of left hippocampal gray matter volume (beta = -.42, p < .001).
Exploratory Whole-Brain Analysis
A whole-brain regression analysis in SPM2 with covariate control for age, sex, race, and total grey matter volume showed that higher IL-6 was associated with decreased grey matter volume in the left hippocampus (-21, -44, -2; t(1,70) = 3.85, p < 0.001, k = 350), Brodmann area (BA) 9 of the medial prefrontal cortex (-7, 60, 37; t(1, 70) = 3.83, p < 0.001, k = 632), and the posterior cerebellum (8, -75, -42, t(1, 70) = 3.49, p < 0.001, k = 541; see Supplemental Figure 1, available at http://www.sobp.org/journal).
Supplementary Analyses
In a prior study, we found an inverse association between IL-6 and performance on tests of auditory recognition memory, attention/working memory, and executive function in a larger group of 500 individuals taken from the same parent project (28). The current findings raise the possibility that hippocampal volume is one of several potential mediators of these effects. In support of this possibility, correlational analyses revealed positive associations between left and right hippocampal grey matter volume and performance on the immediate (r = .23, p = .04; r = .25, p = .03, respectively) and general memory (r = .22, p = .06; r = .20, p = .08) indices of the WMS-III (38), independent of age, sex, race and total grey matter volume. However, we did not find a significant association between IL-6 and any of the Wechsler memory indices in the current smaller sample. Thus, it was not possible to conduct formal mediational analyses. It is possible that our failure to replicate this relationship is due to the smaller current sample size and relative lack of power to detect effects. Indeed, based on the effect size from our earlier study, our power to detect associations between IL-6 and memory in the present smaller sample was 0.15-0.28 and well below conventional limits of acceptable power (e.g., 0.80 (42)).
Discussion
This study provides initial evidence for an inverse association between peripheral levels of the inflammatory cytokine IL-6 and hippocampal grey matter volume among a community sample of relatively healthy adults aged 30-54 years. Consistent with animal literature supporting an association between peripheral inflammation and activation of central inflammatory mechanisms that result in hippocampal remodeling, particularly neurodegeneration (e.g., 12), we found that higher plasma IL-6 was associated with lower hippocampal grey matter volume. This relationship withstood adjustment for multiple demographic and health factors, including age, sex, race, years of education, percent body fat, blood pressure, smoking, physical activity, hours of sleep, alcohol use, and total grey matter volume. To our knowledge, this is the first report of a relationship between IL-6 and hippocampal grey matter volume, raising the possibility that peripheral low grade systemic inflammation could plausibly relate to subclinical cognitive decline via hippocampal pathways.
Based on the present findings, the mechanisms by which peripheral IL-6 relates to hippocampal grey matter volume remain unclear. Animal models show that peripheral inflammation stimulates the production of IL-6 by activated mononuclear and glial cells in the hippocampus (e.g., 8), which, in turn, inhibits neurogenesis, decreases synaptic plasticity, and disrupts learning and memory (12, 14, 15, 18, 20, 43). It is feasible that this pathway accounts for the observed anatomically-specific relationship between higher peripheral IL-6 and lower hippocampal grey matter volume, which may reflect a possible structural neural correlate of inflammation-related cognitive decline. In this regard, human evidence shows an inverse association between circulating IL-6 and mild cognitive decline in well-functioning mid-life and older persons (24, 26, 28). Furthermore, polymorphisms of the IL-6 gene that predict lower levels of plasma IL-6 are associated with decreased risk of developing dementia (44). However, we note that the optimized VBM procedures employed here do not reveal whether IL-6 may relate to such endpoints via neural changes in cell size, dendritic branching complexity, cell proliferation and degeneration, or even cell packing and dendritic spine density. Further, it is possible that IL-6 could relate to other structural neural changes in the integrity of white matter connective tracts that link distributed cortical and subcortical nodes of cognitive processing networks. In this regard, an important future direction will be to apply diffusion tensor imaging methods in examining the impact of inflammatory processes on brain networks implicated in cognitive decline. Finally, there is recent evidence that acute peripheral inflammation, as induced by typhoid vaccination, slows reaction times to cognitive processing tasks and affects corresponding changes in functional neural activation (45). These findings further highlight the potential influence of peripheral inflammation not only on structural neural pathways, but also on functional neural pathways supporting neurocognitive functions.
Decreased hippocampal volume is frequently, but not always, associated with poorer performance on tests of hippocampal-dependent cognitive function (30). Here, we found a positive association between bilateral hippocampal grey matter volume and performance on clusters of tests assessing attention/working and general memory, independently of age, sex, race and total grey matter volume. However, in contrast to our prior findings (28), we did not find a significant association between IL-6 and learning and memory function in the present smaller sample. Our failure to detect significant effects is not surprising given the relatively small size of the effect observed in the larger sample of 500 individuals (28) and lack of power (0.15-.28) in the current sample of only 76 individuals. Hence, larger sample sizes are needed to formally test whether hippocampal remodeling partially mediates the association of IL-6 with cognitive function. Nonetheless, the current findings do suggest that IL-6 is not the only factor contributing to the relationship between hippocampal grey matter volume and memory function.
Low grade systemic inflammation, as defined by 2- to 3-fold increases in circulating levels of proinflammatory cytokines, increases with age (46) and is associated with a range of chronic inflammatory conditions, including atherosclerosis, type 2 diabetes, hypertension, and cardiovascular and autoimmune diseases (47-51). Although adults recruited for the current study were relatively healthy, their levels of plasma IL-6 (mean = 1.56; SD = 1.65, range = .16 to 9.47 pg/ml) indicate the presence of subclinical inflammatory conditions, with 18.4% of the sample having IL-6 greater than 2 pg/ml. Adipose tissue is a potent source of peripheral IL-6 thought to account for approximately 30% of circulating levels (33). Further, greater BMI in middle and later life is associated with poorer cognitive function independently of age (52) and predicts temporal lobe and global brain atrophy, cognitive decline, and the incidence of dementia (35, 52-56). Animal studies also show that obesity is associated with impaired hippocampal LTP (57) and deficits in hippocampal-dependent learning and memory (58). Consistent with the findings of others (34, 59), we found that percent body fat was associated with higher plasma levels of IL-6 (r = .23, p < .05) and lower right and left hippocampal grey matter volume (r = -.32, p = .009; r = -.25, p = .055, respectively). However, the relationship between IL-6 and hippocampal grey matter volume held after controlling for body fat, making it unlikely that adipose tissue is the sole source of the variability in IL-6 levels associated with hippocampal grey matter volume. In contrast, the relationship between body fat and left hippocampal grey matter volume was largely related to variance in IL-6. These findings could suggest that inverse relationships between BMI and cognitive function may be secondary to inflammation-related changes in hippocampal grey matter.
In addition to relationships with hippocampal grey matter volume, our exploratory whole brain analyses revealed an inverse association between peripheral IL-6 and grey matter volume of BA9 in the medial prefrontal cortex and of the posterior lobe of the right cerebellum. It is noteworthy that existing evidence shows that IL-6 receptors are in fact concentrated in the prefrontal cortex (6-8), although their role remains unclear. Further investigation of inflammation in these brain regions is warranted.
The current findings have potential implications for the early detection and treatment of systemic inflammation. Of interest here, treatment with nonsteroidal anti-inflammatory drugs has been shown to ameliorate the progression of memory loss in patients with dementia (60, 61), decrease risk of developing Alzheimer's disease (22) and restore hippocampal neurogenesis in rats after systemic endotoxin-induced inflammation and cranial irradiation (12). Thus, longitudinal studies should explore whether mid-life IL-6 predicts future hippocampal atrophy and memory pathology and to examine the potential benefit of pre-emptive anti-inflammatory therapy.
The present novel findings should be interpreted in context of a number of study limitations. First, our cross-sectional study design precludes causal interpretations. Indeed, there is much debate about whether peripheral cytokine levels are the cause or consequence of brain inflammation (62) and it is possible that raised circulating IL-6 reflects spillover from the central nervous system and is thus a marker of subclinical neuroinflammatory conditions (63). It is also possible that the relationship between peripheral IL-6 levels and hippocampal grey matter volume are independently accounted for by a third, possibly genetic, factor. Another limitation of the current study is the single assessment of IL-6. Although evidence suggests IL-6 is relatively stable over extended periods (e.g., 29), a more reliable indicator of chronic interindividual variability would be derived from multiple assessments over time. In the future, larger, longitudinal investigations are warranted beginning in early adulthood and employing serial assessments of low grade systemic inflammation and structural brain images. In this work, it will be important to determine whether variation in IL-6 and associated hippocampal grey matter volume in mid-life adults predict cognitive decline and the onset of dementia.
Supplementary Material
Acknowledgments
This study was supported by grants P01HL40962 from the National Heart Lung and Blood Institute (SBM), NR008237 from the National Institute of Nursing Research (ALM), R01 MH072837 from the National Institute of Mental Health (ARH), and the John D. and Catherine T. MacArthur Foundation Network of Socioeconomic Status and Health and the NARSAD Bowman Family Investigator fund (ARH). The expert technical assistance of Ramasri Saathanoori, MS, Karen Petersen, Ph.D., and Cyndi Kravitz is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
Drs. Marsland, Gianaros, Brown, Manuck, and Hariri reported no biomedical financial interests or potential conflicts of interest.
References
- 1.Banks WA, Kastin AJ. Blood to brain transport of interleukin links the immune and central nervous systems. Life Sci. 1991;48:PL117–121. doi: 10.1016/0024-3205(91)90385-o. [DOI] [PubMed] [Google Scholar]
- 2.Maier SF, Watkins LR. Cytokines for psychologists: implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol Rev. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- 3.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 4.Van Wagoner NJ, Benveniste EN. Interleukin-6 expression and regulation in astrocytes. J Neuroimmunol. 1999;100:124–139. doi: 10.1016/s0165-5728(99)00187-3. [DOI] [PubMed] [Google Scholar]
- 5.Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- 6.Gadient RA, Otten U. Expression of interleukin-6 (IL-6) and interleukin-6 receptor (IL-6R) mRNAs in rat brain during postnatal development. Brain Res. 1994;637:10–14. doi: 10.1016/0006-8993(94)91211-4. [DOI] [PubMed] [Google Scholar]
- 7.Schobitz B, De Kloet ER, Holsboer F. Gene expression and function of interleukin 1, interleukin 6 and tumor necrosis factor in the brain. Prog Neurobiol. 1994;44:397–432. doi: 10.1016/0301-0082(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 8.Vitkovic L, Konsman JP, Bockaert J, Dantzer R, Homburger V, Jacque C. Cytokine signals propagate through the brain. Mol Psychiatry. 2000;5:604–615. doi: 10.1038/sj.mp.4000813. [erratum appears in Mol Psychiatry 2001Mar;6(2):249] [DOI] [PubMed] [Google Scholar]
- 9.Bellinger FP, Madamba S, Siggins GR. Interleukin 1 beta inhibits synaptic strength and long-term potentiation in the rat CA1 hippocampus. Brain Res. 1993;628:227–234. doi: 10.1016/0006-8993(93)90959-q. [DOI] [PubMed] [Google Scholar]
- 10.Bellinger FP, Madamba SG, Campbell IL, Siggins GR. Reduced long-term potentiation in the dentate gyrus of transgenic mice with cerebral overexpression of interleukin-6. Neurosci Lett. 1995;198:95–98. doi: 10.1016/0304-3940(95)11976-4. [DOI] [PubMed] [Google Scholar]
- 11.Tancredi V, D'Antuono M, Cafe C, Giovedi S, Bue MC, D'Arcangelo G, et al. The inhibitory effects of interleukin-6 on synaptic plasticity in the rat hippocampus are associated with an inhibition of mitogen-activated protein kinase ERK. J Neurochem. 2000;75:634–643. doi: 10.1046/j.1471-4159.2000.0750634.x. [DOI] [PubMed] [Google Scholar]
- 12.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 13.Heyser CJ, Masliah E, Samimi A, Campbell IL, Gold LH. Progressive decline in avoidance learning paralleled by inflammatory neurodegeneration in transgenic mice expressing interleukin 6 in the brain. Proc Natl Acad Sci USA. 1997;94:1500–1505. doi: 10.1073/pnas.94.4.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poluektova L, Meyer V, Walters L, Paez X, Gendelman HE. Macrophage-induced inflammation affects hippocampal plasticity and neuronal development in a murine model of HIV-1 encephalitis. Glia. 2005;52:344–353. doi: 10.1002/glia.20253. [DOI] [PubMed] [Google Scholar]
- 15.Gibertini M, Newton C, Friedman H, Klein TW. Spatial learning impairment in mice infected with Legionella pneumophila or administered exogenous interleukin-1-beta. Brain Behav Immun. 1995;9:113–128. doi: 10.1006/brbi.1995.1012. [DOI] [PubMed] [Google Scholar]
- 16.Pugh CR, Kumagawa K, Fleshner M, Watkins LR, Maier SF, Rudy JW. Selective effects of peripheral lipopolysaccharide administration on contextual and auditory-cue fear conditioning. Brain Behav Immun. 1998;12:212–229. doi: 10.1006/brbi.1998.0524. [DOI] [PubMed] [Google Scholar]
- 17.Arai K, Matsuki N, Ikegaya Y, Nishiyama N. Deterioration of spatial learning performances in lipopolysaccharide-treated mice. J Pharmacol. 2001;87:195–201. doi: 10.1254/jjp.87.195. [DOI] [PubMed] [Google Scholar]
- 18.Balschun D, Wetzel W, Del Rey A, Pitossi F, Schneider H, Zuschratter W, et al. Interleukin-6: a cytokine to forget. FASEB J. 2004;18:1788–1790. doi: 10.1096/fj.04-1625fje. [DOI] [PubMed] [Google Scholar]
- 19.Braida D, Sacerdote P, Panerai AE, Bianchi M, Aloisi AM, Losue S, et al. Cognitive function in young and adult IL (interleukin)-6 deficient mice. Behav Brain Res. 2004;153:423–429. doi: 10.1016/j.bbr.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 20.Sparkman NL, Buchanan JB, Heyen JR, Chen J, Beverly JL, Johnson RW. Interleukin-6 facilitates lipopolysaccharide-induced disruption in working memory and expression of other proinflammatory cytokines in hippocampal neuronal cell layers. J Neurosci. 2006;26:10709–10716. doi: 10.1523/JNEUROSCI.3376-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griffin WS, Sheng JG, Roberts GW, Mrak RE. Interleukin-1 expression in different plaque types in Alzheimer's disease: significance in plaque evolution. J Neuropathol Exp Neurol. 1995;54:276–281. doi: 10.1097/00005072-199503000-00014. [DOI] [PubMed] [Google Scholar]
- 22.McGeer PL, McGeer EG. The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Research - Brain Research Reviews. 1995;21:195–218. doi: 10.1016/0165-0173(95)00011-9. [DOI] [PubMed] [Google Scholar]
- 23.Singh VK, Guthikonda P. Circulating cytokines in Alzheimer's disease. J Psychiatr Res. 1997;31:657–660. doi: 10.1016/s0022-3956(97)00023-x. [DOI] [PubMed] [Google Scholar]
- 24.Weaver JD, Huang MH, Albert M, Harris T, Rowe JW, Seeman TE. Interleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology. 2002;59:371–378. doi: 10.1212/wnl.59.3.371. [DOI] [PubMed] [Google Scholar]
- 25.Wright CB, Sacco RL, Rundek TR, Delman JB, Rabbani LE, Elkind MSV. Interleukin-6 is associated with cognitive function: the Northern Manhattan Study. J Stroke and Cerebrovascular Diseases. 2006;15:34–38. doi: 10.1016/j.jstrokecerebrovasdis.2005.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yaffe K, Lindquist K, Penninx BW, Simonsick EM, Pahor M, Kritchevsky S, et al. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology. 2003;61:76–80. doi: 10.1212/01.wnl.0000073620.42047.d7. [DOI] [PubMed] [Google Scholar]
- 27.Luterman JD, Haroutunian V, Yemul S, Ho L, Purohit D, Aisen PS, et al. Cytokine gene expression as a function of the clinical progression of Alzheimer disease dementia. Arch Neurol. 2000;57:1153–1160. doi: 10.1001/archneur.57.8.1153. [DOI] [PubMed] [Google Scholar]
- 28.Marsland AL, Petersen KL, Sathanoori R, Muldoon MF, Neumann SA, Ryan C, et al. Interleukin-6 covaries inversely with cognitive performance among middle-aged community volunteers. Psychosom Med. 2006;68:895–903. doi: 10.1097/01.psy.0000238451.22174.92. [DOI] [PubMed] [Google Scholar]
- 29.Rao KM, Pieper CS, Currie MS, Cohen HJ. Variability of plasma IL-6 and crosslinked fibrin dimers over time in community dwelling elderly subjects. Am J Clin Pathol. 1994;102:802–805. doi: 10.1093/ajcp/102.6.802. [DOI] [PubMed] [Google Scholar]
- 30.Van Petten C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: review and meta-analysis. Neuropsychologia. 2004;42:1394–1413. doi: 10.1016/j.neuropsychologia.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 32.Good CD, Wade AM, Hayward RD, Phipps KP, Michalski AJ, Harkness WF, et al. Surveillance neuroimaging in childhood intracranial ependymoma: how effective, how often, and for how long? J Neurosurg. 2001;94:27–32. doi: 10.3171/jns.2001.94.1.0027. [DOI] [PubMed] [Google Scholar]
- 33.Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82:4196–4200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- 34.Khaodhiar L, Ling PR, Blackburn GL, Bistrian BR. Serum levels of interleukin-6 and C-reactive protein correlate with body mass index across the broad range of obesity. J Parenter Enteral Nutr. 2004;28:410–415. doi: 10.1177/0148607104028006410. [DOI] [PubMed] [Google Scholar]
- 35.Elias MF, Elias PK, Sullivan LM, Wolf PA, D'Agostino RB. Lower cognitive function in the presence of obesity and hypertension: the Framingham heart study. International Journal of Obesity & Related Metabolic Disorders: Journal of the International Association for the Study of Obesity. 2003;27:260–268. doi: 10.1038/sj.ijo.802225. [DOI] [PubMed] [Google Scholar]
- 36.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, research version, non-patient edition. New York: New York State Psychiatric Institute, Biometrics Research Department; 1996. [Google Scholar]
- 37.Paffenbarger RS, Jr, Blair SN, Lee I, Hyde RT. Measurement of physical activity to assess health effects in free-living populations. Med Sci Sport Exer. 1993;25:60–70. doi: 10.1249/00005768-199301000-00010. [DOI] [PubMed] [Google Scholar]
- 38.Wechsler D. The WMS-III administration and scoring manual. Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 39.Friston KJ, Frith CD, Frackowiak RS, Turner R. Characterizing dynamic brain responses with fMRI: a multivariate approach. Neuroimage. 1995;2:166–172. doi: 10.1006/nimg.1995.1019. [DOI] [PubMed] [Google Scholar]
- 40.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 41.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 42.Cohen J. Statistical power analysis for the behavioral sciences. 2nd. Hillsdale, NJ: L. Erlbaum Associates; 1988. [Google Scholar]
- 43.Guan Z, Fang J. Peripheral immune activation by lipopolysaccharide decreases neurotrophins in the cortex and hippocampus in rats. Brain Behav Immun. 2006;20:64–71. doi: 10.1016/j.bbi.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 44.Papassotiropoulos A, Bagli M, Jessen F, Bayer TA, Maier W, Rao ML, et al. A genetic variation of the inflammatory cytokine interleukin-6 delays the initial onset and reduces the risk for sporadic Alzheimer's disease. Ann Neurol. 1999;45:666–668. doi: 10.1002/1531-8249(199905)45:5<666::aid-ana18>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 45.Brydon L, Harrison NA, Walker C, Steptoe A, Critchley HD. Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biol Psychiatr. doi: 10.1016/j.biopsych.2007.12.007. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol. 2004;39:687–699. doi: 10.1016/j.exger.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 47.Duncan BB, Schmidt MI, Pankow JS, Ballantyne CM, Couper D, Vigo A, et al. Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes. 2003;52:1799–1805. doi: 10.2337/diabetes.52.7.1799. [DOI] [PubMed] [Google Scholar]
- 48.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 49.Kozora E, Thompson LL, West SG, Kotzin BL. Analysis of cognitive and psychological deficits in systemic lupus erythematosus patients without overt central nervous system disease. Arthritis Rheum. 1996;39:2035–2045. doi: 10.1002/art.1780391213. [see comment] [DOI] [PubMed] [Google Scholar]
- 50.Kozora E, Laudenslager M, Lemieux A, West SG. Inflammatory and hormonal measures predict neuropsychological functioning in systemic lupus erythematosus and rheumatoid arthritis patients. J Int Neuropsychol Soc. 2001;7:745–754. doi: 10.1017/s1355617701766106. [DOI] [PubMed] [Google Scholar]
- 51.Strachan MW, Gough K, McKnight JA, Padfield PL. Ambulatory blood pressure monitoring: is it necessary for the routine assessment of hypertension in people with diabetes? Diabet Med. 2002;19:787–789. doi: 10.1046/j.1464-5491.2002.00771.x. [see comment] [DOI] [PubMed] [Google Scholar]
- 52.Cournot M, Marquie JC, Ansiau D, Martinaud C, Fonds H, Ferrieres J, et al. Relation between body mass index and cognitive function in healthy middle-aged men and women. Neurology. 2006;67:1208–1214. doi: 10.1212/01.wnl.0000238082.13860.50. [DOI] [PubMed] [Google Scholar]
- 53.Gunstad J, Paul RH, Cohen RA, Tate DF, Gordon E. Obesity is associated with memory deficits in young and middle-aged adults. Eating & Weight Disorder. 2006;11:e15–19. doi: 10.1007/BF03327747. [DOI] [PubMed] [Google Scholar]
- 54.Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch Intern Med. 2003;163:1524–1528. doi: 10.1001/archinte.163.13.1524. [DOI] [PubMed] [Google Scholar]
- 55.Gustafson D, Lissner L, Bengtsson C, Bjorkelund C, Skoog I. A 24-year follow-up of body mass index and cerebral atrophy. Neurology. 2004;63:1876–1881. doi: 10.1212/01.wnl.0000141850.47773.5f. [DOI] [PubMed] [Google Scholar]
- 56.Ward MA, Carlsson CM, Trivedi MA, Sager MA, Johnson SC. The effect of body mass index on global brain volume in middle-aged adults: a cross sectional study. BMC Neurology. 2005;5:23. doi: 10.1186/1471-2377-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gerges NZ, Aleisa AM, Alkadhi KA. Impaired long-term potentiation in obese zucker rats: possible involvement of presynaptic mechanism. Neuroscience. 2003;120:535–539. doi: 10.1016/s0306-4522(03)00297-5. [DOI] [PubMed] [Google Scholar]
- 58.Winocur G, Greenwood CE, Piroli GG, Grillo CA, Reznikov LR, Reagan LP, et al. Memory impairment in obese Zucker rats: an investigation of cognitive function in an animal model of insulin resistance and obesity. Behav Neurosci. 2005;119:1389–1395. doi: 10.1037/0735-7044.119.5.1389. [DOI] [PubMed] [Google Scholar]
- 59.Bermudez EA, Rifai N, Buring J, Manson JE, Ridker PM. Interrelationships among circulating interleukin-6, C-reactive protein, and traditional cardiovascular risk factors in women. Arterioscler Thromb Vasc Biol. 2002;22:1668–1673. doi: 10.1161/01.atv.0000029781.31325.66. [see comment] [DOI] [PubMed] [Google Scholar]
- 60.Rogers J, Kirby LC, Hempelman SR, Berry DL, McGeer PL, Kaszniak AW, et al. Clinical trial of indomethacin in Alzheimer's disease. Neurology. 1993;43:1609–1611. doi: 10.1212/wnl.43.8.1609. [DOI] [PubMed] [Google Scholar]
- 61.in t' Veld BA, Ruitenberg A, Hofman A, Launer LJ, van Duijn CM, Stijnen T, et al. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer's disease. N Engl J Med. 2001;345:1515–1521. doi: 10.1056/NEJMoa010178. [DOI] [PubMed] [Google Scholar]
- 62.Licastro F, Pedrini S, Caputo L, Annoni G, Davis LJ, Ferri C, et al. Increased plasma levels of interleukin-1, interleukin-6 and alpha-1-antichymotrypsin in patients with Alzheimer's disease: peripheral inflammation or signals from the brain? J Neuroimmunol. 2000;103:97–102. doi: 10.1016/s0165-5728(99)00226-x. [DOI] [PubMed] [Google Scholar]
- 63.Meyer JS, Rauch G, Rauch RA, Haque A. Risk factors for cerebral hypoperfusion, mild cognitive impairment, and dementia. Neurobiol Aging. 2000;21:161–169. doi: 10.1016/s0197-4580(00)00136-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.