Abstract
Much research has demonstrated that events occurring in early life can have a profound influence on future biobehavioral responses to stressful and emotion provoking situations. The purpose of these studies was to determine the effects of an early environmental manipulation, handling (HAN) on cardiovascular (CV) reactivity, freezing behavior and corticosterone (CORT) responses to contextual fear conditioning in the borderline hypertensive rat (BHR), which is susceptible to environmental stressors. HAN subjects were separated from the nest for 15 min/day on post-natal days 1–14, while non-handled (NON-HAN) controls remained in the home cage. Adult subjects were exposed to the contextual fear conditioning procedure and returned to the chamber 24 h later for a 10 min test period. HAN subjects displayed significantly more freezing behavior compared to NON-HAN(92%±2.2 vs 80.7%±5.7, p < .05). Although resting MAP did not differ between groups, HAN subjects had increased MAP reactivity when re-exposed to the chamber. In addition, HAN subjects had significantly lower CORT levels at the end of the 10 min test period (174.2±9 ng/ml vs 237.2±12.9 ng/ml, p < .05). In the second experiment, CORT responses to 60 min of restraint stress and recovery following return to the home cage were assessed in separate groups of HAN and NON-HAN subjects. HAN subjects showed reduced CORT levels in response to acute restraint stress. These results indicate that neonatal handling can modulate biobehavioral responses to contextual fear conditioning in BHR and may suggest a useful model with which to study emotionality and susceptibility to CV disease.
Keywords: contextual fear conditioning, neonatal handling, mean arterial pressure, rats, corticosterone
1. Introduction
Although there are many early environmental factors that can influence the developing organism, characteristics of the mother-infant interaction, and specifically disruptions to it, have received considerable attention. In humans, several converging lines of evidence suggest that negative events early in life are capable of changing one’s vulnerability to a variety of physical and psychiatric disorders. For example, early childhood traumas such as neglect or abuse are associated with higher rats of depression, heart disease, and diabetes (1–5). In order to study the relationship between early life events and adult outcomes in a more controlled setting, the rat is commonly used to model a range of neuropsychiatric conditions (e.g., addiction, post-traumatic stress disorder, depression, etc.) as well as reactivity to stressors in large part because the dynamic interplay between dam and pup provides for not only basic survival needs (e.g., warmth, food, etc.) but also contributes to the development of biological systems, notably the hypothalamic-pituitary adrenal (HPA) axis, and central neural circuitry. The response pattern to emotion provoking stimuli over the lifespan, therefore, is the product of the ongoing interaction between environmental and genetic factors and can influence where one falls on a continuum of vulnerability. So, manipulations that impact this relationship have the potential to permanently alter biological, behavioral, and psychological processes in the offspring.
Neonatal handling is a frequently used paradigm to manipulate the early life experience of rat pups. In this procedure, each pup is removed from the nest and separated daily from the dam and littermates for a brief period (e.g., 3–15 min) for the first two weeks of life. Many studies have shown that shown that thisis intervention produces both enduring neurobiological and behavioral effects in the pups. For example, one of the most frequently documented effects of handling is a reduction in stress-induced HPA activity, often indicated by lower plasma corticosterone levels compared to controls (6–8). This finding is likely related to the observation that neonatal handling is associated with a permanent increase in hippocampal glucocorticoid receptor mRNA (9) as well as increased sensitivity to circulating glucocorticoids (7). With respect to behavioral alterations, handling generally produces a profile suggestive of reduced fear in novel environments such as increased exploration in the open field (10) and increased open-arm entries on the elevated plus maze test (11), although some strain differences have been noted (12).
The hippocampus is a critical brain structure intimately involved with learning and memory processes as well as emotionality. Moreover, this structure shows considerable postnatal developmental plasticity. The hippocampus contains large numbers of glucocorticoid receptors that are involved with HPA negative feedback. Although this receptor density increases rapidly in the post-natal period (7), evidence exists that handling may facilitate this process thereby enhancing hippocampal development, and perhaps in turn, alter learning and memory and emotional reactivity systems (13). Emotional memories that are hippocampal-dependent can be produced in rodents using contextual fear conditioning. In the simplest form of this paradigm, the animal is placed in a novel environment and allowed to explore for a period of time before receiving a brief electric footshock. Upon reintroduction to the chamber, typically 24 h later, the animal shows several characteristics indicative of a strong fear response: profound freezing, increased respiration, and elevated blood pressure and heart rate (14–18). Since early environmental manipulations such as handling (10), or naturally occurring variations in maternal care (19) can exert influence over hippocampal development, events occurring in the post natal period may have the capacity to exert a long term effect on memory systems, including those of an emotional nature.
In this experiment we investigated the extent to which conditioned fear responses are affected by early experience in animals with a genetic predisposition to exaggerated biobehavioral reactivity to stressful stimuli. Susceptibility to emotional stimuli can be influenced by a variety of factors, not the least of which is genetic background. Borderline hypertensive rats (BHR) are the first generation offspring of spontaneously hypertensive rats (SHR) and normotensive Wistar-Kyoto rats. These animals have lifelong blood pressure of approximately 140/90 mmHg unless subjected to environmental challenges. For example, chronic exposure to either an operant-based conflict paradigm (20) or a high salt diet (21) produces elevations in arterial pressure that reach hypertensive levels. Moreover, the adult stress response in this model is sensitive to modulation by early life events. We have shown that cross-fostering BHR pups to WKY dams reduces adult cardiovascular reactivity (22), and more recently we demonstrated that maternal separation conducted during the first 14 days of life increases stress-induced Fos expression in several brain areas important for mediating the stress response (23). Thus, unlike their hypertensive parent which is genetically bred to be hypertensive and therefore largely immune to external challenges, and its normotensive parent which is largely unaffected by stress, the BHR possesses an inherent vulnerability which can be exploited for examining how a range of environmental factors can influence behavioral and biological reactivity. In the study reported herein, we have examined the effect on handling on biobehavioral responses to conditioned fear in the BHR model.
2. Materials and methods
2.1. Subjects
Subjects used in this study were male borderline hypertensive rats (BHR). These animals were the first generation offspring of female spontaneously hypertensive rats (SHR) and male Wistar-Kyoto (WKY), both of which were purchased from Taconic Farms (Germantown, NY). Following placing 2 females with 1 male for breeding, pregnant SHR were housed individually in polyprophylene cages. Animals were housed in the vivarium with a 12:12h light dark cycle (lights on at 0600h). All animals had free access to food and water throughout the study. Day of birth was defined as pups being present by 1600 h and designated at post-natal day 0. All litters were culled within 24 hours of birth to 8 pups, 4 males and 4 females where possible. No more than one male pup from each litter was used for any given measure. Subjects used in the study were housed individually following weaning at 4 weeks of age. Experiments were conducted when animals were approximately 7–9 weeks of age. All procedures were conducted in accordance with National Institutes of Health Guide for the Care and Use of Laboratory.
2. 2. Neonatal handling
The handling procedure was carried out beginning on post-natal day 1 and continued through post-natal day 14. Each dam was removed from the home cage and placed in a holding cage. Each pup was then removed and placed in an individual container for 15 min. The temperature was maintained at approximately 31–33° C. At the end of the 15 min period each pup was returned to the home cage, followed by the dam. Cage changing was suspended during this two-week period.
2.3. Surgical procedure
Arterial catheters were made from a 3 cm Micro-Renathane (.025″ OD) tip (Braintree Scientific, Braintree MA) fused to tygon tubing. Animals were anesthetized with ketamine hydrochloride+acepromazine (100 mg/kg, 2mg/kg, respectively, i.p.) and the catheter was introduced under aseptic conditions to the lower abdominal aorta via the right femoral artery. The catheter was tunneled up the back and exited dorsally at the neck. Each catheter was filled with heparinized saline (200U/ml) and capped with a stainless steel plug. The subject was fitted with a harness and swivel apparatus that permitted complete mobility in the home cage while protecting the catheter.
2.4. Contextual Fear Conditioning
Animals were allowed a 48h recovery period before being exposed to the contextual fear conditioning paradigm. On the conditioning day animals were transported individually to the lab and immediately placed the conditioning chamber which was located in ventilated sound attenuating chamber (MedAssociates, Inc., St Albans VT). The chamber was illuminated with a 40W light bulb and had background noise of 60 dB. Each animal was allowed to explore the chamber for 2 minutes prior to receiving a 2 sec, 1mA foot shock. Subjects remained in the chamber for an additional 30 sec before being immediately returned to the colony.
2.5. Testing Procedure
Prior to being placed into the chamber, baseline MAP and HR were recorded while the subject was resting quietly in the home cage. Freezing behavior, cardiovascular reactivity, and corticosterone levels were then determined in response to reintroduction to the chamber 24 h later. The subject was placed into the chamber and the swivel was attached to a ring stand. A piece of extension tubing was attached to the swivel and tunneled through a small hole in the sound attenuating box so that it could be connected to a transducer that was interfaced with PowerLab hardware and software (ADI, Grand Junction CO). Blood pressure and heart rate were recorded continuously for 10 minutes at which time a blood sample for corticosterone determinaton was collected by detaching the catheter from the transducer. A trained observer who was blind to the experimental condition of the subject recorded whether the subject was freezing every 10 seconds for the first 5 minutes via a small hole in the door of the sound attenuating box.
In a separate experiment, handled and non-handled subjects were implanted with arterial catheters as described above. Two days later resting blood samples were drawn via the catheter while the subjects were quiescent in the home cage. Subjects were then placed into a Plexiglas restraint for 60 minutes and blood samples were collected every 30 minutes. Subjects were returned to the home cage and two more samples were taken at 30 and 60 minutes following removal from the restraint stress.
2.6. Corticosterone Analysis
Blood samples (250–500 μl) were collected via the indwelling arterial catheter into an EDTA coated tube. Samples were centrifuged and the plasma was stored at −80°C until analysis. The corticosterone analysis was performed using a competitive immunoassay (Assay Designs, Ann Arbor MI) with donkey antibody specific to sheep IgG. The intra-assay variability was 6.6–8.0% and the inter-assay variation was 7.8–13.1%. All samples were run in duplicate.
2.7. Statistical Analyses
Resting blood pressure, heart rate, freezing behavior and corticosterone data were analyzed using the student’s t-test. Blood pressure and heart rate collected during the 10 min test period were analyzed using repeated measures ANOVA, as was the corticosterone data from the experiment using restraint stress. In all cases, a .05 level of significance was used.
2. Results
3.1. Cardiovascular Responses to Contextual Fear Conditioning
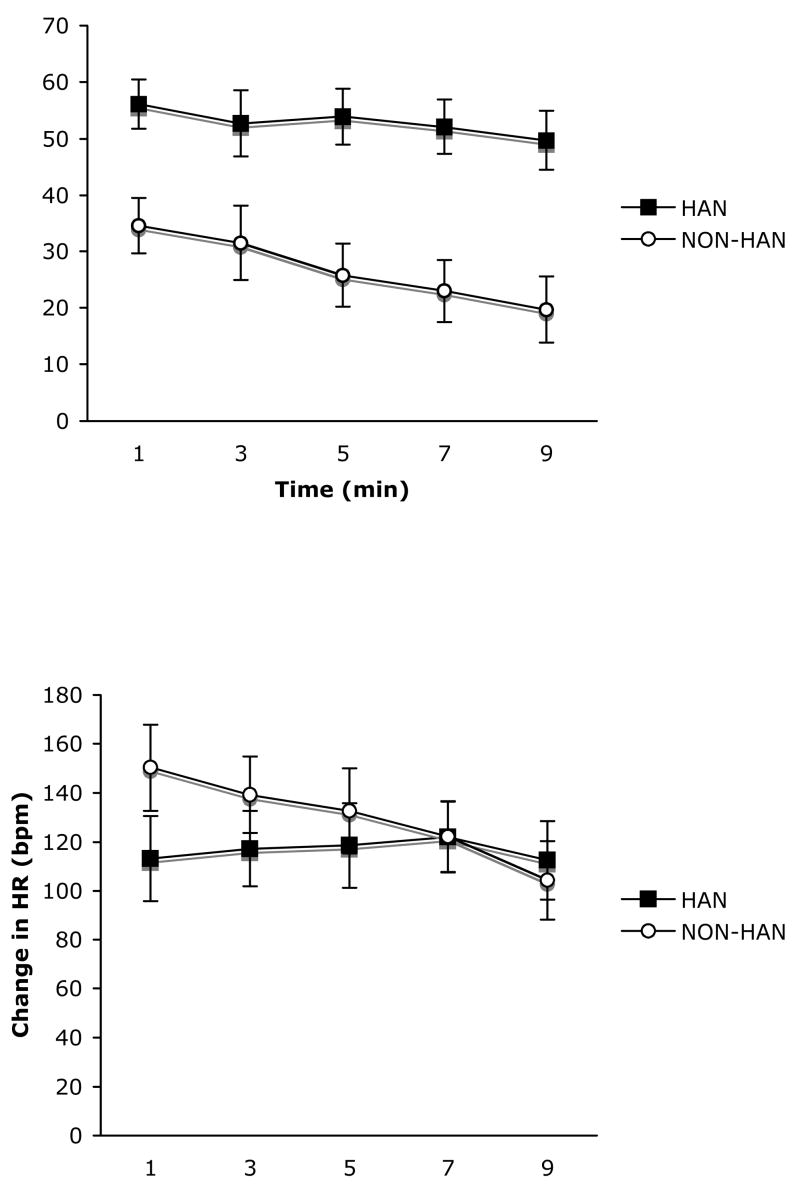
Baseline mean arterial blood pressure and heart rate were measured while the subjects were resting quietly in the home cage. There were no differences between handled and non-handled subjects with respect to MAP t(22) = .448, p = .658 or HR t(22) = .882, p = .916, (Table 1). The MAP and HR response to re-exposure to the contextual fear conditioning chamber was expressed as the difference between baseline measures and an approximately 20 second period of artifact-free recording at minutes 1, 3, 5, 7 and 9. Repeated measures ANOVA performed on MAP (Figure 1, top panel) revealed a significant main effect of time F(4,56) = 7.88, p < .001 and condition F(1,14) =12.89, p < .003. The interaction of time and condition was marginally significant F(4, 56) = 2.20, p < .081. Thus, handled subjects had significantly higher increases in MAP throughout the 10-minute test period compared to non-handled controls. A similar analysis was applied to the change in heart rate (Figure 1, bottom panel) and revealed no significant effects of time, F(4,56) = 1.89, p = .124, condition F(4,56) = 1.13, p = .305, or the interaction F(1,14) = .682, p = .607.
Table 1.
Resting MAP and HR (Mean ± SEM).
| MAP (mmHg) | HR (bpm) | |
|---|---|---|
| HAN (n=13) | 125.71±2.8 | 343.53±9.2 |
| NON-HAN (n=11) | 127.9±4.1 | 332.57±9.8 |
Figure 1.
Change in MAP (top panel) and HR (bottom panel) in response to reexposure to the fear conditioning chamber in handled (dark squares, n=9) and non-handled (open circles, n=7) subjects. Data are expressed as mean ± SEM.
3.2. Corticosterone Responses to Contextual Fear Conditioning and Restraint Stress
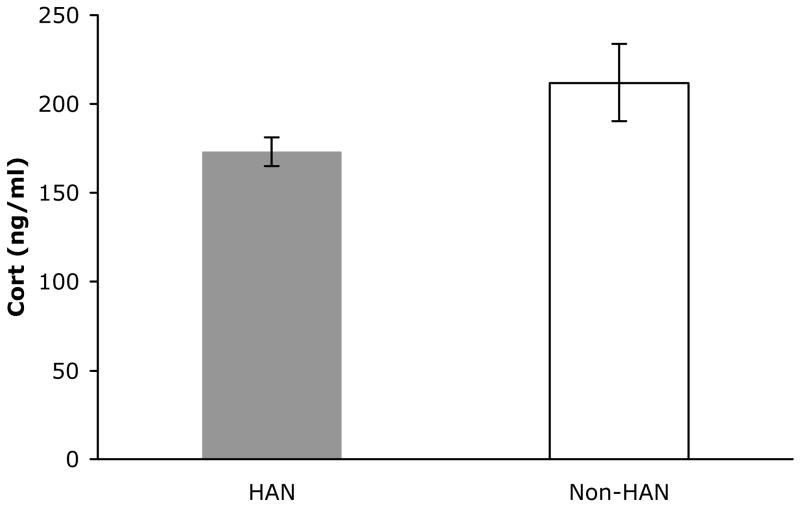
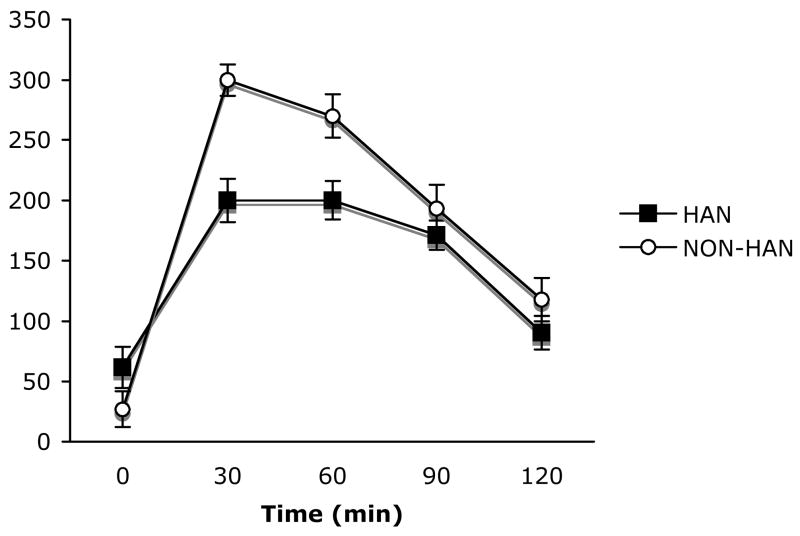
Corticosterone levels determined from samples taken at the end of the 10-minute test period were significantly lower in handled subjects compared to non-handled controls, t(10) = 4.10, p < .05 (Figure 2). In a separate group of handled and non-handled subjects, we assessed corticosterone values during baseline and at 30-minute intervals during 1 hr of restraint stress and the subsequent 1 hr post-stress period (Figure 3). Repeated measures ANOVA performed on these data revealed a significant main effect of time F(4,40) = 61.78, p < .0001, and condition F(1,10) = 4.62, p < .05, and a significant interaction of time × condition F(4,40) = 3.23, p < .02.
Figure 2.
Plamsa corticosterone levels 10 minutes following re-exposure to the fear conditioning chamber in handled (shaded bar, n = 7) and non-handled (open bar, n = 5) subjects. Data are expressed as mean ± SEM.
Figure 3.
Plasma corticosterone levels taken during a baseline period (time = 0), twice during 60 minutes of restraint stress (time = 30 and 60), and twice after return to the home cage (time = 90 and 120) in handled (dark squares, n = 7) and non-handled (open circles, n = 5) subjects. Data are expressed as mean ± SEM.
3.3. Freezing Behavior Responses to Contextual Fear Conditioning
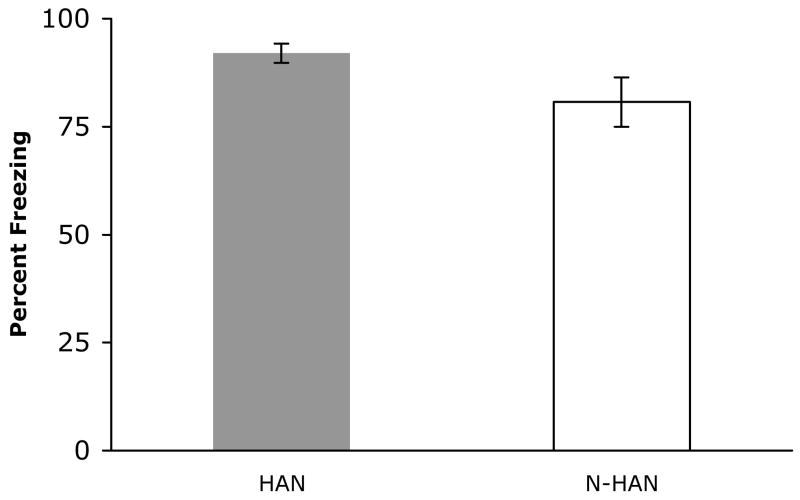
Although there was no difference in freezing between HAN and NON-HAN subjects immediately following footshock; t(27)= .762, p = .836, HAN subjects displayed significantly more freezing behavior than NON-HAN rats when tested 24 h after conditioning; t(27) = 1.97, p < .05 (Figure 4).
Figure 4.
Freezing behavior during 10 min of response to re-exposure to the fear conditioning chamber in handled (shaded bar, n = 16 ) and non-handled (open bar, n = 13) subjects. Data are mean ± SEM.
3. Discussion
The main purpose of this study was to assess the effect of neonatal handling on biobehavioral responses to conditioned fear in the BHR, a model that is sensitive to the effects of psychological stress in adulthood. Although several studies have examined how environmental challenges affect cardiovascular parameters in the adult BHR, much less is known about the role of early environmental events in altering future responses to emotional stimuli. Thus, we chose to employ the handling paradigm since it has been shown to produce a general reduction in stress-induced activation of behavioral and biological systems (6–8). Given that the BHR has an inherent vulnerability toward higher blood pressure and enhanced reactivity, we were particularly interested in cardiovascular responses provoked by fear and the extent to which handling affects these responses. In this experiment, resting MAP and HR did not differ between handled and non-handled subjects. However, upon reintroduction to the conditioning chamber, handled subjects displayed a significantly greater change from baseline in MAP (c. 60 mmHg vs 30 mmHg) compared to controls. Because our primary goal was to examine whether handling modifies cardiovascular responses in a model known to develop stress-induced hypertension as adults, we did not study normotensive subjects since they show no such tendency (24–27). However, other reports using normotensive strains have studied pressor responses to fear conditioning. In studies that condition fear to a discrete stimulus, such as a tone, typical cardiovascular responses are modest and transient, lasting just a few seconds (28, 29). However, other reports have found contextual fear conditioning produces a dramatic increase in MAP (c. 35 mmHg) upon reintroduction to the chamber (30, 31). Our observations of changes in MAP nearly twice as great as these values are particularly interesting in the light of the sensitivity of BHR to environmentally-induced (i.e., an operantly-based conflict paradigm or a high salt diet) hypertension in adulthood. Although chronic hypertension was not the endpoint of this study, the current study suggests that not only can psychological stress in the form of a negative emotional memory elicit dramatic increases in arterial pressure in a model with an inherent vulnerability for heart disease, it can do so in animals that have been subjected to neonatal handling, an intervention typically considered to blunt the biobehavioral response to stress.
Activation of the HPA axis is a hallmark of an organism’s response to stress. Regulation of glucocorticoids is achieved principally through negative feedback mechanisms to the hypothalamus and anterior pituitary gland to decrease the formation of corticotrophin releasing factor (CRF) and adrenocorticotropin hormone (ACTH), respectively. In addition, the hippocampus contains a large population of glucocorticoid receptors and has been implicated as exerting an important regulatory role of this system (7). Although plasma levels of corticosterone in the rat increase dramatically in response to a variety of stressful stimuli, several studies have shown that neonatal handling results in a significant reduction in stress-induced corticosterone levels in adulthood (6–8). This enhanced glucocorticoid negative-feedback sensitivity likely is the result of an increase in glucocorticoid receptor expression in the hippocampus, that has been associated with the increased licking and grooming behavior that is provoked by handling (32). The corticosterone responses observed in the current study are generally consistent with these observations. When reintroduced to the contextual fear conditioning chamber, handled BHR showed a lower corticosterone response compared to non-handled subjects. Although significant, the lack of a more pronounced handling effect on corticosterone may be due to the fact that the samples were taken 10 min following reintroduction which may not be long enough to see the maximum response, which is typically observed closer to 20–30 min after a stressful stimulus. However, handled BHR exposed to 60 min of restraint stress, in a separate experiment, displayed a significantly attenuated corticosterone response at 30 min compared to controls. Taken together, these data suggest that handling is capable of exerting some degree of reduction in the HPA response to emotional and stressful stimuli in BHR.
Freezing behavior is a commonly used measure of fear in rodents (16). Animals returned to an environment in which something aversive occurred, such as footshock, display a predictable absence of motor behavior, and variations in the degree to which this occurs represents the robustness of the memory for the prior negative event. Several lines of research suggest that the hippocampus is critical for mediating the memory of the fearful event that provokes freezing behavior. For example, electrolytic lesions of the dorsal hippocampus reduce freezing behavior when the animal is reintroduced to the chamber where tone-footshock pairings were received; no such reduction in freezing occurs to the tone (33, 34). In addition, other research suggests that the level of circulating corticosterone can affect hippocampal-dependent memory processes. For example, performance in the Morris water maze, an assay for spatial memory, is impaired following adrenalectomy (35) and administration of the Type II glucocorticoid receptor antagonist disrupts contextual fear conditioning (36). Thus, changes in the structure and/or functioning of the hippocampus as well as alterations in the level of circulating corticosterone may have the ability to alter memory function. Of interest to the current study is the finding of an association between dams that display naturally high licking/grooming behavioral phenotype and an increased expression hippocampal glucocorticoid receptor of mRNA and glucocorticoid sensitiviy in the offspring (19). Thus, although not measured in this study, the increased licking/grooming behavior that is reliably provoked by neonatal handling may promote hippocampal changes that mediate the enhanced memory processes and glucocorticoid sensitivity as suggested by increased freezing behavior and corticosterone responses observed in the present study.
The changes that handling produces in glucocorticoid receptor density in the hippocampus and the implication of this for emotional memory certainly provide important context for understanding the results of the current study. However, an alternative explanation also deserves consideration. The reduction in biobehavioral responses to stress that handling produces have been characterized almost exclusively in normal, healthy animals (Long-Evans, Sprague-Dawley, etc). The current study, however, utiltized BHR subjects that are known to be stress-sensitive. It is possible that the handling procedure represents a significant source of stress for these neonates that results in exaggerated cardiovascular reactivity in adulthood. Removal from the nest, even for a brief period, may result in transient hypothermia or nutrition deficit if a nursing bout is interrupted, as well as the usual increase in licking and grooming that the dam displays upon return of the pups. All of these events, as well as others, may be more stressful for a neonate that is already predisposed to heightened reactivity, especially of the cardiovascular system, to noxious stimuli. Thus, even brief periods of separation may result in biobehavioral hypereactivity that is typically seen in other strains subjected to longer periods of separation (37, 38). In support of the contention that early life events have the potential to impact later cardiovascular functioning is the observation that both brief (15 min) and long (3 h) periods of separation results in a reduction of preautonomic neurons in the central nucleus of the amygdala and bed nucleus of the stria terminalis, both of which are involved in processing emotionally relevant stimuli (39).
In summary, the current study reports that neonatal handling enhances the arterial pressure response to contextual fear conditioning in BHR, while producing the typical increase in freezing behavior and reduction in stress-induced plasma corticosterone. These results extend previous work examining how other early environments such as cross-fostering and maternal separation may modulate cardiovascular responses to future stressful events. In addition, since this is the first study that has employed a memory-based emotional stressor in the BHR, these results may suggest a potentially useful strategy for exploring the relationship between early experience, emotionality, and biobehavioral responses. For example, this model may lend itself to the study of clinical disorders such as post-traumatic stress disorder which can be influenced by early adversity (40) and has a cardiovascular component. Ongoing research is aimed at further exploring this relationship.
Acknowledgments
This work was supported by NIH grant HL-073894.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bifulco A, Brown GW, Adler Z. Early sexual abuse and clinical depression in adult life. Br J Psychiatry. 1991;159:115–122. doi: 10.1192/bjp.159.1.115. [DOI] [PubMed] [Google Scholar]
- 2.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 3.Holmes SJ, Robins LN. The role of parental disciplinary practices in the development of depression and alcoholism. Psychiatry. 1988;51:24–36. doi: 10.1080/00332747.1988.11024377. [DOI] [PubMed] [Google Scholar]
- 4.Taylor SE, Seeman TE. Psychosocial resources and the SES-health relationship. Ann NY Acad Sci. 1999;896:210–215. doi: 10.1111/j.1749-6632.1999.tb08117.x. [DOI] [PubMed] [Google Scholar]
- 5.Trickett PK, McBride-Chang C. The developmental impact of different forms of child abuse and neglect. Dev Res. 1995;15:311–337. [Google Scholar]
- 6.Levine S, Haltmeyer GC, Karas G, Denenberg VH. Physiological and behavioral effects of infantile stimulation. Physiol Beh. 1967;2:55–63. [Google Scholar]
- 7.Meaney MJ, Aitken DH, Sharma S, Viau V, Sarrieau A. Postnatal handling increases hippocampal type II glucocorticoid receptors and enhances adrenocortical negative feedback in the rat. J Neuroendocrinol. 1989;5:597–604. doi: 10.1159/000125287. [DOI] [PubMed] [Google Scholar]
- 8.Viau V, Sharma S, Plotsky PM, Meaney MJ. The hypothamic-pituitary-adrenal response to stress in handled and nonhandled rats: Differences in stress-induced plasma ACTH secretion are not dependent upon increased corticosterone levels. J Neurosci. 1993;13:1097–1105. doi: 10.1523/JNEUROSCI.13-03-01097.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meaney MJ, Diorio J, Widdowson J, Laplante P, Caldji C, Seckl JR, Plotsky PM. Early environmental regulation of forebrain glucocorticoid receptor gene expression: Implications for adrenocortical responses to stress. Dev Neurosci. 1996;18:49–72. doi: 10.1159/000111395. [DOI] [PubMed] [Google Scholar]
- 10.Caldji C, Diorio J, Meaney MJ. Variations in maternal care in infancy regulate the development of stress reactivity. Biol Psychiatry. 2000;48:1164–1174. doi: 10.1016/s0006-3223(00)01084-2. [DOI] [PubMed] [Google Scholar]
- 11.McIntosh J, Anisman H, Merali Z. Short- and long-periods of neonatal maternal separation differentially affect anxiety and feeding in adult rats: gender-dependent effects. Dev Brain Res. 1999;113:97–106. doi: 10.1016/s0165-3806(99)00005-x. [DOI] [PubMed] [Google Scholar]
- 12.Boccia ML, Pedersen CA. Brief vs long maternal separations in infancy: contrasting relationships with adult maternal behavior and lactation levels of aggression and anxiety. Pyschoneuroendocrinology. 2001;26:657–672. doi: 10.1016/s0306-4530(01)00019-1. [DOI] [PubMed] [Google Scholar]
- 13.Beane ML, Cole MA, Spencer RL, Rudy JW. Neonatal handling enhances contextual fear conditioning and alters corticosterone stress responses in young rats. Horm Beh. 2002;41:33–40. doi: 10.1006/hbeh.2001.1725. [DOI] [PubMed] [Google Scholar]
- 14.Carrive P. Dual activation of cardiac sympathetic and parasympathetic components during conditioned fear to context in the rat. Clin Exp Pharm Physiol. 2006;33:1251–1254. doi: 10.1111/j.1440-1681.2006.04519.x. [DOI] [PubMed] [Google Scholar]
- 15.Antoniadis EA, McDonald RJ. Discriminative fear conditioning to context expressed by multiple measures of fear in the rat. Behav Brain Res. 1999;101:1–13. doi: 10.1016/s0166-4328(98)00056-4. [DOI] [PubMed] [Google Scholar]
- 16.Blanchard RJ, Blanchard DC. Crouching as an index of fear. J Comp Physiol Psychol. 1969;67:370–375. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- 17.Furlong T, Carrive P. Neurotoxic lesions centered on the perifornical hypothalamus abolish the cardiovascular and behavioral responses of conditioned fear to context but not of restraint. Brain Res. 2007;1128:107–119. doi: 10.1016/j.brainres.2006.10.058. [DOI] [PubMed] [Google Scholar]
- 18.Vianna DM, Carrive P. Changes in cutaneous and body temperature during and after conditioned fear to context in the rat. Eur J Neurosci. 2005;21:2505–2512. doi: 10.1111/j.1460-9568.2005.04073.x. [DOI] [PubMed] [Google Scholar]
- 19.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 20.Sanders BJ, Lawler JE. The borderline hypertensive rat (BHR) as a model for environmentally-induced hypertension: a review and update. Neurosci Biobehav Rev. 1992;16:207–217. doi: 10.1016/s0149-7634(05)80181-2. [DOI] [PubMed] [Google Scholar]
- 21.Lawler JE, Sanders BJ, Chen YF, Nagahama S, Oparil S. Hypertension produced by a high sodium diet in the borderline hypertensive rat (BHR) Clin Exp Hypertens A. 1987;9:1713–1731. doi: 10.3109/10641968709158968. [DOI] [PubMed] [Google Scholar]
- 22.Sanders BJ, Gray MJ. Early environmental influences can attenuate the blood pressure response to acute stress in borderline hypertensive rats. Physiol Behav. 1997;61:749–754. doi: 10.1016/s0031-9384(96)00530-6. [DOI] [PubMed] [Google Scholar]
- 23.Sanders BJ, Anticevic A. Maternal separation enhances neuronal activation and cardiovascular responses to acute stress in borderline hypertensive rats. Behav Brain Res. 2007;183:25–30. doi: 10.1016/j.bbr.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrap SB, Louis WJ, Doyle AE. Failure of psychosocial stress to induce hypertension in the rat. J Hyperten. 1984;2:653–662. doi: 10.1097/00004872-198412000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Herd AJ, Morse WH, Kelleher RT. Arterial hypertension in the squirrel monkey during behavioral experiments. Am J Physiol. 1969;217:424–429. doi: 10.1152/ajplegacy.1969.217.1.24. [DOI] [PubMed] [Google Scholar]
- 26.Smookler HH, Buckley JP. Relationships between brain catecholamine synthesis, pituitary adrenal function and the production of hypertension during prolonged exposure to environmental stress. Int J Neuropharmacol. 1969;8:33–41. doi: 10.1016/0028-3908(69)90032-x. [DOI] [PubMed] [Google Scholar]
- 27.Shapiro AP, Melhado J. Observations on blood pressure and other physiologic and biochemical mechanisms in rats with behavioral disturbances. J Psychosom Med. 1958;20:303–317. doi: 10.1097/00006842-195807000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Helmstetter FJ, Tershner SA. Lesions of the periaqueductal gray and rostral ventromedial medulla disrupt antinociceptive but not cardiovascular aversive conditioned responses. J Neurosci. 1994;14:7099–7108. doi: 10.1523/JNEUROSCI.14-11-07099.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwata J, LeDoux JE. Dissociation of associative and non associative concomitants of classical conditioning in the freely behaving rat. Behav Neurosci. 1988;102:66–76. doi: 10.1037//0735-7044.102.1.66. [DOI] [PubMed] [Google Scholar]
- 30.Carrive P. Conditioned fear to environmental context: cardiovascular and behavioral components in the rat. Brain Res Inter. 2000;858:440–445. doi: 10.1016/s0006-8993(00)02029-1. [DOI] [PubMed] [Google Scholar]
- 31.Carrive P. Dual activation of cardiac sympathetic and parasympathetic components during conditioned fear to context in the rat. Clin Exp Pharm Phys. 2006;33:1251–1254. doi: 10.1111/j.1440-1681.2006.04519.x. [DOI] [PubMed] [Google Scholar]
- 32.Lee M, Williams D. Changes in licking behavior of rat mother following handling of young. Anim Beh. 1974;22:679–681. [Google Scholar]
- 33.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 34.Kim JJ, Rison RA, Fanselow MS. Effects of amygdala, hippocampus and periaqueductal gray lesions on short- and long-term contextual fear. Behav Neurosci. 1993;107:1093–1098. doi: 10.1037//0735-7044.107.6.1093. [DOI] [PubMed] [Google Scholar]
- 35.Oitzl MS, de Kloet RE. Selective corticosterone antagonists modulate specific aspects of spatial orientation learning. Behav Neurosci. 1992;106:62–71. doi: 10.1037//0735-7044.106.1.62. [DOI] [PubMed] [Google Scholar]
- 36.Pugh CR, Fleshner M, Rudy JW. Type II glucocorticoid receptor antagonists impair contextual but not auditory-cue fear conditioning in juvenile rats. Neurobiol Learning Memory. 1997;67:75–79. doi: 10.1006/nlme.1996.3741. [DOI] [PubMed] [Google Scholar]
- 37.Lehmann J, Pryce CR, Bettschen D, Feldon J. The maternal separation paradigm and adult emotionality and cognition in male and female Wistar rats. Pharmacol Biochem Behav. 1999;64:705–715. doi: 10.1016/s0091-3057(99)00150-1. [DOI] [PubMed] [Google Scholar]
- 38.Paulus MP, Bakshi VP, Geyer MA. Isolation rearing affects sequential organization of motor behavior in post-pubertal but not pre-pubertal Lister and Sprague-Dawley rats. Behav Brain Res. 1998;94:271–280. doi: 10.1016/s0166-4328(97)00158-7. [DOI] [PubMed] [Google Scholar]
- 39.Card RP, Levitt P, Gluhovsky M, Rinaman L. Early experience modifies the postnatal assembly of autonomic emotional motor neurons in rats. J Neurosci. 2005;25:9102–9111. doi: 10.1523/JNEUROSCI.2345-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bremner JD, Southwick SM, Johnson DR, Yehuda R, Charney DS. Childhood physical abuse and combat related post traumatic stress disorder in Vietnam veterans. Am J Psychiatry. 1993;150:235–239. doi: 10.1176/ajp.150.2.235. [DOI] [PubMed] [Google Scholar]