Abstract
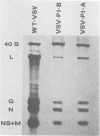
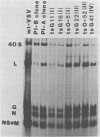
Vesicular stomatitis virus (VSV) stocks isolated from two persistently infected mouse L-cell lines (designated VSV-PI stocks) express an altered phenotype of RNA synthesis. This phenotype is different from the RNA synthesis phenotype expressed by the viruses used to initiate the persistently infected lines, wild-type VSV and VSV ts-0-23 (a group III, ts-, RNA+ mutant). At 34 and 37 degrees C in L cells productively infected with VSV-PI stocks derived from the two cell lines, transcription of virus mRNA was significantly reduced, whereas replication of the 40S genomic RNA species was enhanced compared with wild-type VSV or ts-0-23. At 34 and 37 degrees C, both VSV-PI stocks replicated with equal or greater efficiency than wild-type VSV; 37 degrees C was the temperature at which the persistently infected cultures were maintained. At 40 degrees C, both VSV-PI stocks were temperature sensitive, and clonal VSV-PI isolates from both cell lines belong to complementation group I (RNA-). Standard ts- mutants (derived by mutagenesis of wild-type VSV) belonging to RNA- complementation groups I, II, and IV do not express the VSV-PI RNA synthesis phenotype at the permissive temperature, making this phenotype distinctive to persistent infection. Since the two VSV-PI populations from persistently infected cell lines initiated with different viruses both evolved this unique phenotype of RNA synthesis, the expression of this phenotype may play an important role in the maintenance of persistence.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed R., Chakraborty P. R., Graham A. F., Ramig R. F., Fields B. N. Genetic variation during persistent reovirus infection: presence of extragenically suppressed temperature-sensitive lesions in wild-type virus isolated from persistently infected L cells. J Virol. 1980 May;34(2):383–389. doi: 10.1128/jvi.34.2.383-389.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson G. R., Matovcik L. M. Expression of murine sarcoma virus genes in uninfected rat cells subjected to anaerobic sress. Science. 1977 Sep 30;197(4311):1371–1374. doi: 10.1126/science.197602. [DOI] [PubMed] [Google Scholar]
- Bratt M. A. RNA synthesis in chick embryo cells infected with different strains of NDV. Virology. 1969 Jul;38(3):485–488. doi: 10.1016/0042-6822(69)90163-9. [DOI] [PubMed] [Google Scholar]
- Clinton G. M., Little S. P., Hagen F. S., Huang A. S. The matrix (M) protein of vesicular stomatitis virus regulates transcription. Cell. 1978 Dec;15(4):1455–1462. doi: 10.1016/0092-8674(78)90069-7. [DOI] [PubMed] [Google Scholar]
- Flamand A., Bishop D. H. In vivo synthesis of RNA by vesicular stomatitis virus and its mutants. J Mol Biol. 1974 Jul 25;87(1):31–53. doi: 10.1016/0022-2836(74)90558-0. [DOI] [PubMed] [Google Scholar]
- Holland J. J., Grabau E. A., Jones C. L., Semler B. L. Evolution of multiple genome mutations during long-term persistent infection by vesicular stomatitis virus. Cell. 1979 Mar;16(3):495–504. doi: 10.1016/0092-8674(79)90024-2. [DOI] [PubMed] [Google Scholar]
- Holland J. J., Villarreal L. P. Purification of defective interfering T particles of vesicular stomatitis and rabies viruses generated in vivo in brains of newborn mice. Virology. 1975 Oct;67(2):438–449. doi: 10.1016/0042-6822(75)90445-6. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Porter M. Translational control of protein synthesis after infection by vesicular stomatitis virus. J Virol. 1980 Dec;36(3):719–733. doi: 10.1128/jvi.36.3.719-733.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomniczi B., Meager A., Burke D. C. Virus RNA and protein synthesis in cells infected with different strains of Newcastle disease virus. J Gen Virol. 1971 Oct;13(1):111–120. doi: 10.1099/0022-1317-13-1-111. [DOI] [PubMed] [Google Scholar]
- Madansky C. H., Bratt M. A. Noncytopathic mutants of Newcastle disease virus are defective in virus-specific RNA synthesis. J Virol. 1981 Jan;37(1):317–327. doi: 10.1128/jvi.37.1.317-327.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvaldi J. L., Lucas-Lenard J., Sekellick M. J., Marcus P. I. Cell killing by viruses. IV. Cell killing and protein synthesis inhibition by vesicular stomatitis virus require the same gene functions. Virology. 1977 Jun 15;79(2):267–280. doi: 10.1016/0042-6822(77)90354-3. [DOI] [PubMed] [Google Scholar]
- McAllister P. E., Wagner R. R. Differential inhibition of host protein synthesis in L cells infected with RNA - temperature-sensitive mutants of vesicular stomatitis virus. J Virol. 1976 May;18(2):550–558. doi: 10.1128/jvi.18.2.550-558.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan J. J., Emerson S. U., Wagner R. R. The plus-strand leader RNA of VSV inhibits DNA-dependent transcription of adenovirus and SV40 genes in a soluble whole-cell extract. Cell. 1982 Feb;28(2):325–333. doi: 10.1016/0092-8674(82)90350-6. [DOI] [PubMed] [Google Scholar]
- Nishiyama Y., Ito Y., Shimokata K. Properties of the viruses selected during persistent infection of L cells with VSV. J Gen Virol. 1978 Aug;40(2):481–484. doi: 10.1099/0022-1317-40-2-481. [DOI] [PubMed] [Google Scholar]
- Nishiyama Y. Studies of L cells persistently infected with VSV: factors involved in the regulation of persistent infection. J Gen Virol. 1977 May;35(2):265–279. doi: 10.1099/0022-1317-35-2-265. [DOI] [PubMed] [Google Scholar]
- Perlman S. M., Huang A. S. RNA synthesis of vesicular stomatitis virus. V. Interactions between transcription and replication. J Virol. 1973 Dec;12(6):1395–1400. doi: 10.1128/jvi.12.6.1395-1400.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preble O. T., Youngner J. S. Temperature-sensitive defect of mutants isolated from L cells persistently infected with Newcastle disease virus. J Virol. 1973 Sep;12(3):472–480. doi: 10.1128/jvi.12.3.472-480.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramseur J. M., Friedman R. M. Prolonged infection of L cells with vesicular stomatitis virus. Defective interfering forms and temperature-sensitive mutants as factors in the infection. Virology. 1978 Mar;85(1):253–261. doi: 10.1016/0042-6822(78)90429-4. [DOI] [PubMed] [Google Scholar]
- Rowlands K., Grabau E., Spindler K., Jones C., Semler B., Holland J. Virus protein changes and RNA termini alterations evolving during persistent infection. Cell. 1980 Apr;19(4):871–880. doi: 10.1016/0092-8674(80)90078-1. [DOI] [PubMed] [Google Scholar]
- Simonsen C. C., Batt-Humphries S., Summers D. F. RNA synthesis of vesicular stomatitis virus-infected cells: in vivo regulation of replication. J Virol. 1979 Jul;31(1):124–132. doi: 10.1128/jvi.31.1.124-132.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria M., Little S. P., Huang A. S. Characterization of vesicular stomatitis virus nucleocapsids. I. Complementary 40 S RNA molecules in nucleocapsids. Virology. 1974 Sep;61(1):270–280. doi: 10.1016/0042-6822(74)90261-x. [DOI] [PubMed] [Google Scholar]
- Stamminger G. M., Lazzarini R. A. RNA synthesis in standard and autointerfered vesicular stomatitis virus infections. Virology. 1977 Mar;77(1):202–211. doi: 10.1016/0042-6822(77)90418-4. [DOI] [PubMed] [Google Scholar]
- Weck P. K., Carroll A. R., Shattuck D. M., Wagner R. R. Use of UV irradiation to identify the genetic information of vesicular stomatitis virus responsible for shutting off cellular RNA synthesis. J Virol. 1979 Jun;30(3):746–753. doi: 10.1128/jvi.30.3.746-753.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B., Rosenthal R., Schlesinger S. Establishment and maintenance of persistent infection by Sindbis virus in BHK cells. J Virol. 1980 Jan;33(1):463–474. doi: 10.1128/jvi.33.1.463-474.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz G. W. Isolation of possible replicative intermediate structures from vesicular stomatitis virus-infected cells. Virology. 1978 Mar;85(1):271–285. doi: 10.1016/0042-6822(78)90431-2. [DOI] [PubMed] [Google Scholar]
- Youngner J. S., Dubovi E. J., Quagliana D. O., Kelly M., Preble O. T. Role of temperature-sensitive mutants in persistent infections initiated with vesicular stomatitis virus. J Virol. 1976 Jul;19(1):90–101. doi: 10.1128/jvi.19.1.90-101.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngner J. S., Jones E. V., Kelly M., Frielle D. W. Generation and amplification of temperature-sensitive mutants during serial undiluted passages of vesicular stomatitis virus. Virology. 1981 Jan 15;108(1):87–97. doi: 10.1016/0042-6822(81)90529-8. [DOI] [PubMed] [Google Scholar]
- Youngner J. S., Preble O. T., Jones E. V. Persistent infection of L cells with vesicular stomatitis virus: evolution of virus populations. J Virol. 1978 Oct;28(1):6–12. doi: 10.1128/jvi.28.1.6-13.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngner J. S., Quagliana D. O. Temperature-sensitive mutants of vesicular stomatitis virus are conditionally defective particles that interfere with and are rescued by wild-type virus. J Virol. 1976 Jul;19(1):102–107. doi: 10.1128/jvi.19.1.102-107.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]