Abstract
Background
Delayed renal allograft survival (DGF) after a deceased donor kidney transplant is associated with an increased risk of allograft loss. Inflammatory response and apoptosis are associated with increased risk of DGF.
Study Design
Cross Sectional Study
Setting & Participants
We first recruited 616 recipients of kidneys from 512 deceased kidney donors and the donor DNA was genotyped. These recipients who were included in a prospective cohort study of 9 transplant centers in the Delaware Valley region, had their DGF outcome obtained through medical record abstraction. Then, we identified the recipient (n=349) of the contralateral deceased kidney donor, if not part of the cohort, through the USRDS registry. The final cohort consisted of 965 recipients of deceased donor kidneys from 512 donors.
Predictors
Donor single nucleotide polymorphisms (SNPs) in genes for tumor necrosis factor α (TNF), transforming growth factor β1 (TGFB1), interleukin 10 (IL10), p53 (TP53), and heme oxygenase 1 (HMOX1).
Outcomes
DGF, defined as need for dialysis in the first week post-transplant. Secondary outcomes included acute rejection and eGFR.
Measurements
Information on DGF, acute rejection and eGFR for recipients in the Delaware Valley Cohort was obtained through medical record abstraction. For other recipients, information on DGF was obtained from UNOS forms and CMS claims in the USRDS registry.
Results
The TGFB1, IL10, TP53 and HMOX1 genes were not associated with DGF. The G allele of TNF polymorphism rs3093662 was associated with DGF in an adjusted analysis (OR= 1.85 compared to A allele, 95% C.I.=1.16–2.96, p=0.01). However this association does not achieve statistical significance after adjusting for multiple comparisons.
Limitations
Inadequate sample size for infrequent genotypes and multiple comparisons.
Conclusion
Due to the low frequency of donor SNPs of interest, a larger sample size and replication are necessary for conclusive evidence for the association of donor genotypes with DGF.
Keywords: Kidney Transplant, Deceased Donor Genotypes, Delayed Graft Function
Introduction
Delayed function of a deceased kidney allograft (DGF) defined by the need for dialysis within the first week post-transplant, portends a foreshortened allograft survival of 73% by the first year compared to 83 % for recipients who do not require dialysis. (1–10) In addition to reducing allograft survival, DGF prolongs the length of hospitalization and increases the costs of transplantation. (3) The reported incidence of DGF from various centers has previously ranged from 13.3 (8) to 52 %. (10) The increasing disparity between the number of persons on the deceased donor waiting list and the limited number of organs available for transplantation has stimulated the utilization of non-traditional donors of organs. The recent strategies to expand deceased donors who have characteristics that traditionally have precluded their use and to utilize non-heart beating persons as medically suitable donors is anticipated to increase the occurrence of DGF.
DGF on kidney allograft biopsies results from tubular epithelia damage, cellular necrosis and apoptosis, similar to that found in native kidneys with acute tubular necrosis.(11) Cytokines in the inflammatory response pathway also play an integral role in development of DGF in kidney allografts. Using inflammatory cytokines, the initial kidney injury stimulates activation of inflammatory leukocytes and this in turn, leads to formation of platelet-leukocyte plugs. These plugs along with erythrocytes, create a detrimental low-flow condition. (12–19) Production of inflammatory cytokines by the tubular cells and interstitium results in a concentration gradient that recruits inflammatory cells from the microvasculature to the matrix, and then through the injured tubular basement membrane, allowing interactions with tubular epithelial cells. The inflammatory cells and the tubular epithelial cells that have been sloughed also adhere to each other, leading to tubular obstruction and detrimentally increased intraluminal pressure. (20) Thus cytokines in the inflammatory response pathway, along with apoptosis, play an important role in occurrence of DGF.
Genes from the apoptosis and inflammatory response pathway were chosen on the basis of biological plausibility, frequency, potential role in the causal pathway of acute kidney injury and presence of known functional SNPs, at the time of genotyping.(21) Factors such as the pro-apoptotic tumor protein p53 gene (TP53) and the anti-apoptotic, antioxidant heme oxygenase 1 gene (HMOX1) play an important role in modulating apoptosis in acute kidney injury. (22, 23) Among others, the tumor necrosis factor α gene (TNF), transforming growth factor β1 gene (TGFB1), and interleukin 10 gene (IL10) play an important role in modulating inflammatory response in acute kidney injury. (22, 23) Therefore we examined the association of SNPs in TP53, HMOX1, TNF, TGFB1 and IL10 with occurrence of DGF.
Methods
Patients
We recruited a cohort consisting of pairs of kidney transplant recipients of deceased kidney donors. We first recruited recipients of kidneys from deceased kidney donors included in a prospective cohort study enrolling recipients of kidney allografts from deceased donors transplanted between 1997 and 2003 at nine transplant centers in eastern Pennsylvania within the Gift of Life Donor Program. This Delaware Valley Cohort (DVC) which was studying the impact of DNA based HLA typing, consisted of nine transplant centers including Hospital of the University of Pennsylvania, Thomas Jefferson University Hospital, Hahneman University Hospital, Albert Einstein Medical Center, Lankenau Hospital, Hershey Medical Center, Geisinger Medical Center, Temple University and Lehigh Valley Hospital. All adult transplant recipients undergoing a deceased donor transplant were eligible. Patients were consented for participation at the time of or soon after transplantation. Starting in 2000, participants were also prospectively consented for genotyping for genes associated with kidney outcomes. Participants transplanted prior to 2000, were also consented for this study. Lymph node, spleen or blood samples of deceased donors were provided by Gift of Life Donor Program. We then identified the recipient of the contralateral deceased kidney donor, if not part of the DVC, through the United States Renal Data System registry (USRDS). Outcomes of the recipient of this contralateral deceased donor kidney were accessed through the USRDS. Approval for the USRDS data was obtained from the NIH, USRDS and Center for Medicare and Medicaid Services (CMS). USRDS provides CMS claims data as well as United Network of Organ Sharing (UNOS) form data. The Institutional Review Boards at the University of Pennsylvania and Hennepin County Medical Center approved this study.
Clinical Data for DVC Recipients
For recipients in the DVC, clinical data were collected prospectively from patient interviews and from inpatient and outpatient medical records at 6-month intervals until July 2004 for a maximum of 36 months post-transplantation. Referring community nephrologists were contacted to obtain data on kidney allograft function for those patients who did not return to their transplant center for routine visits. The main outcome of delayed graft function was defined as need for dialysis in the first week post-transplantation. For the recipient enrolled in the DVC, this use of dialysis was determined from the medical records. Renal function was measured using the 4-variable MDRD Study equation (24) to generate an eGFR. Baseline eGFR was established between 60–120 days post transplantation. Three other kidney function outcomes were also created: persistent 25% decline in eGFR, persistent 50% decline in eGFR, and kidney allograft loss. A persistent decline in eGFR was defined as two consecutive eGFR readings one month apart demonstrating the decline. Another secondary outcome, acute rejection, was defined as a clinical rejection event requiring use of intravenous steroid and/or antibody therapy during the first year post-transplant.
Clinical Data for Recipients of Contralateral Kidney, Identified through USRDS
In order to define the DGF outcome in the USRDS, we assessed the accuracy of the CMS claims and UNOS forms using the medical record as the gold standard among the DVC recipients. Among all the 616 DVC recipients, only 263 of them have DGF outcome in CMS claims data. We utilized CMS claim data for dialysis treatment from the day after transplantation to day 7 post-transplantation. We could not utilize data from the day of transplant since the claim does not state whether the dialysis was conducted prior to or after the kidney transplant surgery. The false negative rate (FNR) and false positive rate (FPR) of claim-based DGF was 0.08 and 0.14, respectively. The FNR and FPR of UNOS form-based DGF was 0.44 and 0.05, respectively. Therefore, for the recipient of the contralateral deceased donor kidney, when not part of the DVC, dialysis use in the first week post-transplant was obtained from CMS claims and secondarily from the UNOS forms. A priori, we used simulations to compare the power using this definition of DGF outcome that enables us to include all recipients compared with an alternative strategy that eliminates recipients that only have UNOS form data. Despite the relative high false negative rate of the UNOS form-based DGF outcome, we found that using all the recipients always gave slightly better power in our simulations. Therefore, this composite is used in all of our association analysis. However, we always adjusted for the source of DGF outcome (medical record, CMS claims or UNOS form).
Genotyping
Either whole blood that remained from routine clinical testing, or lymph node or spleen specimens was used as a DNA source from donors. DNA was extracted from whole blood and tissue using Qiagen extraction kit and Puregene tissue extraction kit (both from Qiagen, Valencia, California), respectively. Given that the donor specimens used for DNA extraction were also used for clinical genotyping for HLA matching prior to transplantation, all samples provided DNA for genotyping. As a quality control measure, an A260/A280 absorbance ratio was determined for all extracted DNA to ensure adequate DNA quality for genotyping. Based on the Seattle SNP database (http://pga.gs.washington.edu), we genotyped 8, 5, and 3 SNPs in HMOX1, IL10, and TNF, respectively. Genotyping was conducted for SNPs that had a minor allele frequency of greater than 10% based on our a priori power calculations. One SNP in TNF (rs1800629) gene is in the promoter region (http://snpper.chip.org) and one SNP in IL10 (rs 3024498) is in the 3’ untranslated region and is a putative splice site variant (www.genecards.org). We also genotyped a functional (GT)n repeat in the HMOX1gene utilizing a fragment analysis method from Applied Biosystems (ABI) with Genescan™ analysis software. The IL10 SNP rs2222202 was genotyped using pyrosequencing (Biotage, Uppsala, Sweden). Due to the lack of tag SNP information for TGFB1 and TP53 in the HapMap and Seattle SNP database at the time of genotyping, we genotyped 2 functional SNPs in the TGFB1 gene (rs1800472 and rs1982073) and tag SNPs in TP53 found in the SNP500 database (http://snp500cancer.nci.nih.gov/home.cfm). We also genotyped a potentially functional SNP, rs 1042522, in TP53 by utilizing a published protocol of Storey et al(25). Patients were genotyped for remaining SNPs utilizing an ABI Taqman assay (Applied Biosystems, California). As a quality control measure, five percent of the samples were genotyped as duplicates. Tests for Hardy-Weinberg Equilibrium (HWE) were done separately in African-American and non-African American donors (since 28 SNPs were genotyped, therefore the p-value cut-off for the HWE test was <0.002 which is 0.05/28 SNPs ). (26)
Statistical Analysis of SNPs
Statistical analysis was conducted utilizing SAS v9.1 (The SAS Institute, http://www.sas.com), R (www.r-project.org) and STATA 9.0 (Stata Corporation, College Station, TX). Continuous variables were compared by t-tests and categorical variables by chi-square test and p-values were two-sided. Logistic regression with generalized estimation equations (GEE) was used to determine the association of single SNP genotypes with delayed graft function, due to two recipients being exposed to the same donor genotype. Recipients with the same donor were treated as a cluster. Some clusters have only one recipient if only a single kidney was transplanted. Exchangeable working correlation was assumed and empirical variance estimates were used to adjust for the correlation. Multivariable models were fit by adjusting for confounders such as cold ischemia time, recipient race, extended criteria donor, donor cause of death, donor race, and source of DGF information (medical record or CMS claims or UNOS forms). (27)
For participants in the DVC, separate Cox proportional hazards models were used to investigate the association of single SNP genotype on time to 25% or 50% decline in kidney function, time to graft loss, and time to acute rejection. The proportional hazards assumption was tested by graphical analysis. (28) There was no evidence that the proportional hazards assumption was violated in the unadjusted model.
Results
Baseline characteristics and DGF outcomes
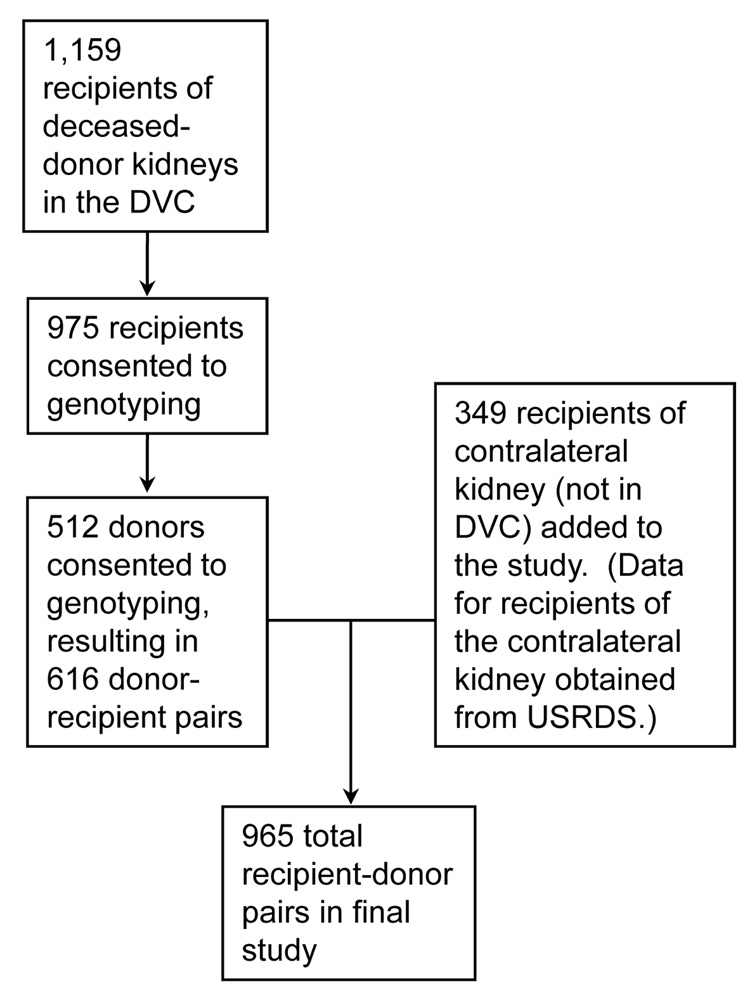
1,159 transplant recipients with deceased donors were enrolled in the DVC at 9 different centers (Figure 1). Of these, 975 recipients consented to genotyping. However the next of kin of only 512 unique donors consented to research, yielding 616 donor-recipient pairs available from the DVC. The outcomes of the remaining recipients of the contralateral deceased donor kidneys (n=349) were determined through the USRDS registry yielding a total of 965 recipients. The demographic and transplant related characteristics for the resulting 965 recipients of kidneys and their 512 deceased donors are listed in Table 1 and Table 2 respectively. Of these 965 recipients, 5 who were re-transplanted after their kidney transplant failed were included twice in our dataset. Thirty-five percent of all the recipients experienced delayed graft function (Table 3).
Figure 1.
Source of patients included in the study. Recipient-donor pairs were recruited from the Delaware Valley Cohort initially and then data for the recipient of the contra-lateral kidney of the donor was collected from the USRDS
Table 1.
Demographic Characteristics stratified by Source of Recipient Data (Number in parenthesis is %, unless otherwise noted)
| DVC (n= 616) | USRDS (n= 349) | |
|---|---|---|
| Recipient Ethnicity | ||
| African American | 188 (31 %) | 106 (30 %) |
| White | 412 (67 %) | 229 (66 %) |
| Asian | 14 (2 %) | 14 (4 %) |
| Native American | 2 (< 1%) | 0 |
| Recipient Sex | ||
| Male | 376 (61 %) | 213 (61 %) |
| Female | 240 (39 %) | 136 (39 %) |
| Recipient Mean Age | 48.6 years (±12.1) | 45.5 (±15.3) |
| Dialysis Pre-transplant | ||
| Yes | 558 (92 %) | 316 (91%) |
| No | 47 (8 %) | 33 (8 %) |
| Missing | 11 (2 %) | - |
| Cause of ESRD | ||
| Glomerulonephritis | 125 (20 %) | 92 (26 %) |
| Hypertension | 126 (20 %) | 64 (18 %) |
| Diabetes | 200 (32 %) | 85 (24 %) |
| Other kidney disease | 165 (27 %) | 108 (31 %) |
| Previous Transplant | ||
| Yes | 86 (14 %) | 51 (15 %) |
| No | 530 (84 %) | 298 (85 %) |
| Recent Panel Reactive Antigen (PRA) | ||
| >20 % | 62 (11 %) | 68 (11 %) |
| 1–20 % | 43 (8 %) | 44 (13 %) |
| < 1 % | 451 (81 %) | 258 (76 %) |
| Missing | 60 (10 %) | |
| Cold Ischemia Time | ||
| >24 hours | 79 (16 %) | 62 (21 %) |
| 12–24 hours | 338 (68 %) | 177 (60 %) |
| < 12 hours | 83 (17 %) | 56 (19 %) |
| Missing | 116 (19 %) | 54 (15 %) |
| Median (lowest – highest quartiles) distance organ traveled from procurement site to transplant center | 43.63 miles (5.63 – 80.55) | 53.96 miles (11.96 – 185.93) |
| Number of HLA mismatches | ||
| 0 | 52 (10 %) | 48 (15 %) |
| 1–2 | 94 (18 %) | 45 (14 %) |
| 3–4 | 239 (46 %) | 113 (36 %) |
| 5–6 | 134 (26 %) | 112 (35 %) |
| Missing | 97 (16 %) | 31 (9 %) |
| Medicare primary payor | 263 (43 %) | 157 (45 %) |
Table 2.
Donor Characteristics (n=512)
| Donor Mean Age in years | 40.0 (±17) |
| Donor Sex | |
| Male | 300 (59 %) |
| Donor Ethnicity | |
| African American | 58 (11 %) |
| White | 454 (89 %) |
| Donor Cause of Death | |
| Trauma | 198 (39 %) |
| Other | 314 (61%) |
| Extended Criteria Donor (ECD)1 | |
| Yes | 116 (23 %) |
| No | 396 (77 %) |
ECD defined as donor age >60; or donor age >50 with any 2 of the following donor criteria: (1) terminal serum creatinine >1.5 mg/dl, (2) hypertension, or (3) death due to CVA.
Table 3.
Outcomes post-transplantation By Source of Recipient Information
| Source of Recipient Information | Medical Chart (n=605) | CMS Claims USRDS (n= 159) | UNOS Forms USRDS (n=201) | Total (n=965) |
|---|---|---|---|---|
| Dialysis in First Week Post-transplant | ||||
| Yes | 228 (38 %) | 65 (41 %) | 49 (24 %) | 342 (35 %) |
| No | 377 (62 %) | 94 (59 %) | 152 (76 %) | 623 (65 %) |
Concordance of DGF in the 2 recipients of a single donor’s kidneys
Given that both kidneys of a deceased donor are usually utilized in two separate recipients, this study determined the concordance of DGF in the two recipients of the 512 donor kidneys in the study. The concordance rate was 57% for DGF; with 64, 194, and 195 paired recipients developing DGF, not developing DGF and being discordant on their DGF outcome. The level of concordance was statistically significantly higher than expected under chance (p=0.004) using a binomial exact test.(29)
Association of genotype with DGF
The genotypes were found to be in HWE. The frequencies of alleles were consistent with previously reported frequencies (SNP500 database and www.ensembl.org) (Table 4). Limited population frequency information was available for the TNF SNPs. The SNPs in the inflammation-related genes TGFB1 and IL10 and in the apoptosis-related genes TP53 and HMOX1 were not associated with delayed graft function (Table 4).
Table 4.
Adjusted and unadjusted association with DGF for inflammation and apoptosis related Polymorphisms [ND1]
| Polymorphism | Gene | Minor Allele [ND2] | Participants With Minor Allele (%) | Unadjusted OR (95% C.I.) | Adjusted OR (95 % C.I.) | |
|---|---|---|---|---|---|---|
| With DGF | Without DGF | |||||
| rs3024498 | IL10 | C | 107/281 (0.38) | 203/511 (0.4) | 0.93 (0.69 – 1.3) | 0.89 (0.62 – 1.3) |
| rs3024494 | IL10 | T | 3/282 (0.01) | 5/521 (0.01) | 1.1 (0.22 – 4.8) | 0.22 (0.02 – 3.3) |
| rs1878672 | IL10 | C | 157/253 (0.62) | 312/470 (0.66) | 0.83 (0.6 – 1.14) | 0.82 (0.56 – 1.2) |
| rs3024493 | IL10 | A | 56/262 (0.21) | 108/478 (0.23) | 0.93 (0.64 –1.3) | 1.1 (0.70 – 1.7) |
| rs1554286 | IL10 | A | 122/291 (0.42) | 209/514 (0.41) | 1.05 (0.79– 1.4) | 0.99 (0.70 – 1.4) |
| rs3021094 | IL10 | G | 48/260 (0.18) | 104/494 (0.21) | 0.85 (0.58– 1.2) | 0.90 (0.57 – 1.4) |
| rs2222202 | IL10 | A | 199/303 (0.66) | 378/548 (0.69) | 0.86 (0.64– 1.2) | 0.85 (0.60 – 1.2) |
| rs2071746 | HMOX1 | T | 216/294 (0.73) | 402/561 (0.72) | 1.1 (0.8– 1.5) | 0.99 (0.68 – 1.4) |
| (GT)n | HMOX1 | L | 205/326 (0.63) | 388/606 (0.64) | 0.95 (0.72– 1.3) | 1.2 (0.87 – 1.7) |
| rs2071747 | HMOX1 | C | 30/284 (0.11) | 48/522 (0.09) | 1.2 (0.71– 1.9) | 0.92 (0.53 – 1.6) |
| rs2071748 | HMOX1 | A | 191/293 (0.65) | 352/545 (0.65) | 1.0 (0.76– 1.4) | 1.09 (0.77 – 1.6) |
| rs8140669 | HMOX1 | A | 16/279 (0.06) | 26/508 (0.05) | 1.1 (0.58– 2.1) | 0.42 (0.13 – 1.3) |
| rs6518952 | HMOX1 | T | 20/297 (0.07) | 30/550 (0.05) | 1.3 (0.69– 2.2) | 0.49 (0.18 – 1.4) |
| rs2071749 | HMOX1 | G | 191/278 (0.69) | 344/506 (0.68) | 1.0 (0.76– 1.4) | 1.1 (0.74 – 1.6) |
| rs5755720 | HMOX1 | G | 146/295 (0.49) | 268/532 (0.5) | 0.97 (0.73– 1.3) | 1.0 (0.72 – 1.4) |
| rs2285112 | HMOX1 | G | 187/291 (0.64) | 332/515 (0.64) | 0.99 (0.73– 1.3) | 1.0 (0.72 –1.5) |
| rs9894946 | TP53 | T | 86/278 (0.31) | 145/519 (0.28) | 1.2 (0.84– 1.6) | 1.1 (0.75 – 1.6) |
| rs1614984 | TP53 | A | 187/277 (0.68) | 321/512 (0.63) | 1.24 (0.91– 1.69) | 1.3 (0.90 – 1.8) |
| rs17884306 | TP53 | T | 20/230 (0.09) | 35/440 (0.08) | 1.11 (0.61– 1.95) | 1.2 (0.57 – 2.4) |
| rs4968187 | TP53 | T | 4/290 (0.01) | 9/544 (0.02) | 0.85 (0.22– 2.68) | 0.67 (0.13 – 3.3) |
| rs12951053 | TP53 | C | 46/278 (0.17) | 62/507 (0.12) | 1.42 (0.94– 2.15) | 1.5 (0.90 – 2.6) |
| rs1625895 | TP53 | A | 78/278 (0.28) | 118/504 (0.23) | 1.28 (0.91– 1.78) | 1.2 (0.81 – 1.8) |
| rs1042522 | TP53 | C | 155/315 (0.49) | 260/584 (0.45) | 1.21 (0.92– 1.59) | 1.3 (0.92 – 1.8) |
| rs1800472 | TGFB1 | A | 10/292 (0.03) | 17/542 (0.03) | 1.1 (0.48– 2.42) | 0.92 (0.32– 2.6) |
| rs1982073 | TGFB1 | C | 163/256 (0.64) | 295/464 (0.64) | 1 (0.73– 1.38) | 0.91 (0.62 – 1.3) |
| rs1800629 | TNF | A | 84/294 (0.29) | 188/550 (0.34) | 0.77 (0.56– 1.05) | 0.84 (0.58 – 1.2) |
| rs3093662 | TNF | G | 53/303 (0.17) | 64/566 (0.11) | 1.66 (1.12– 2.47)* | 1.9 (1.2 – 3.0)* |
| rs3091257† | TNF | A | 43/229 (0.19) | 84/439 (0.19) | 0.98 (0.65– 1.47) | 1.5 (0.91 – 2.5) |
Adjusted for cold ischemia time, recipient race, extended criteria donor, donor cause of death, donor race, and source of DGF information (medical record or CMS claims or UNOS forms). The (GT)n SNP is categorized as short (S for ≤ 27 repeats) vs long repeats (L for >27 repeats
denotes p< 0.05
rs3091257 has been merged into rs1800628.[ND3]
TNF and DGF
The G allele of TNF SNP rs3093662 was associated with DGF (OR= 1.85 compared to A allele, 95% C.I.=1.16–2.94, p=0.009) in the model (n=965) adjusted for cold ischemia time, recipient race, extended criteria donor, donor cause of death, donor race, donor age and source of DGF information (Table 4). However, the potential association of the TNF SNP rs3093662 with DGF does not retain statistical significance after adjusting for multiple comparisons. Similar direction of association was also seen in the subset of patients in the DVC only (n=616) and in DVC plus those with claims data (n=764) but with a wider confidence interval due to small sample size (DVC only: OR= 1.29 compared to A allele, 95% C.I.= 0.68 – 2.45, p=0.4; DVC plus claims data: OR=1.83, 95% C.I. =1.07–3.14, p=0.03).
Association of genotypes with secondary outcomes in the Delaware Valley Cohort
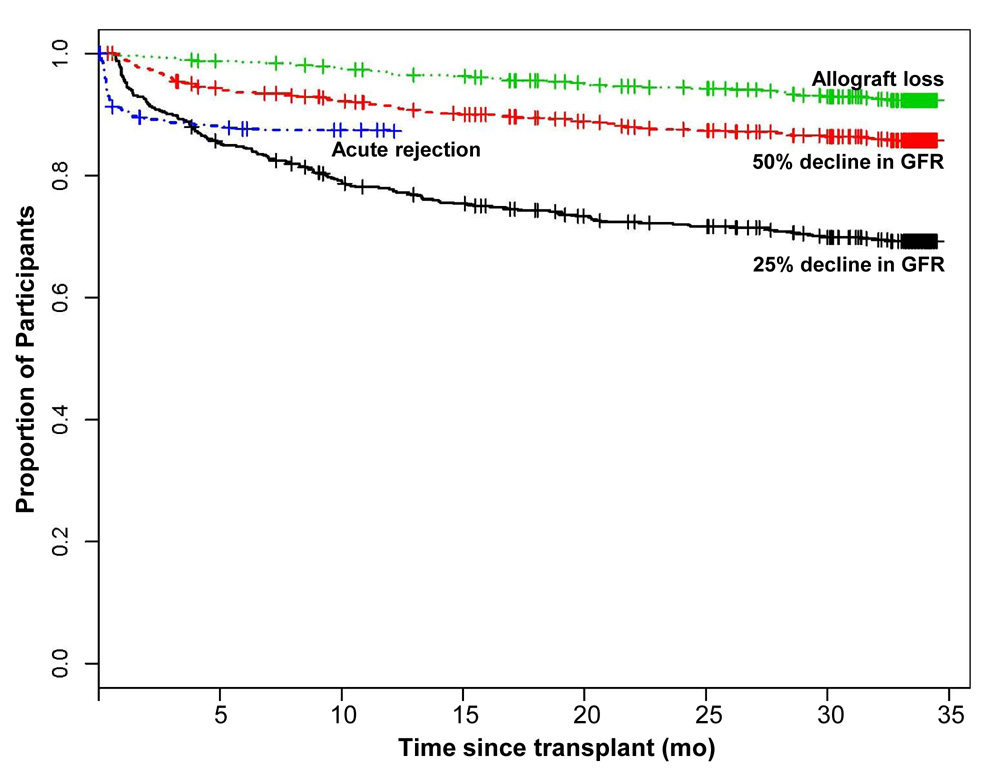
For recipients in the Delaware Valley Cohort with donor genotypes, during the median follow-up of 1010 days (range 1–1035 days) post-transplantation. The time to occurrence of 25% eGFR decline, 50% eGFR decline, kidney allograft loss, or acute rejection are shown in Figure 2. The SNPs in the inflammation-related genes TNF, TGFB1, and IL10 and apoptosis-related genes TP53 and HMOX1 were not associated with acute rejection, kidney allograft loss, or eGFR decline (Tables S1–S4; provided as online supplementary material available with this article at www.ajkd.org).
Figure 2.
Percentage of Recipients in the Delaware Valley Cohort experiencing 25% decline in eGFR, 50% decline in eGFR, acute rejection and graft loss.
Discussion
We showed that the TNF SNP rs3093662 in donors was associated with increased risk of DGF. The G allele was associated with 1.8 fold increased odds of DGF compared to the A allele in the study population (Table 4). However this association does not retain statistical significance after adjusting for multiple comparisons. The association of the donor TNF SNP with DGF has a similar direction of association among the subset of study participants enrolled in the Delaware Valley Transplant Cohort. The study did find a statistically significant concordance rate for DGF of 57% among the two recipients of a deceased donor kidney.
Our study is the first to study the association of DGF with the tag and functional SNPs in IL10, TP53, TGFB1, and TNF in deceased donors, in a multi-center cohort study of kidney transplant recipients (Table 4). This study also utilized a unique study design by utilizing both recipients of a deceased donor utilizing USRDS registry data.
The donor TNF SNP rs3093662 with a trend for association with DGF is located in the first intron of the TNF.(30, 31) Prior studies have genotyped another TNF SNP, namely the promoter SNP rs1800629 in kidney transplant recipients, but not in donors, and found it to be associated with decreased kidney allograft survival.(32) The TNF SNP may contribute to DGF through several potential mechanisms. TNF-α, expressed in donor kidney tissue (33, 34), is a proinflammatory cytokine which upregulates cell adhesion molecules.(35) TNF-α also contributes to kidney injury, since neutralizing antibodies to TNF-α decrease neutrophil infiltration and kidney injury in mice.(33, 34) Higher levels of TNF-α expression occur in kidney allografts experiencing DGF than those without DGF. (36) Therefore it is biologically plausible that a donor polymorphism that increases TNF-α activity could make a recipient more susceptible to DGF.
Our study did not find associations with TP53, HMOX1, TGFB1 and IL10 donor gene polymorphisms and DGF. The literature supports the role of growth factors such as TGF-β1 expressed by tubular and interstitial cells in response to acute kidney injury.(37–39) The donor TP53 and HMOX1 gene products, probably expressed in the kidney tubules (40), play a role in tubular apoptosis seen in ischemia reperfusion injury in human kidney allografts.(41) Similarly, IL-10, expressed in donor kidney tissue, plays a role in ischemia-reperfusion injury.(34) Thus, SNPs in genes beyond the ones studied here that upregulate apoptosis and increase inflammatory response, may increase the risk of DGF. Other studies in kidney transplantation have not used the tag SNP approach used in our study. One study found association of HMOX1 (GT)n repeat with kidney allograft survival but not with DGF.(42) It is possible that the association of SNPs in these genes was not seen in our study due to the small effect sizes of these SNPs and the limitation of our sample size. The limited sample size could possibly explain why the TNF SNP rs3093662, with a trend for association with DGF, was not associated with decreased kidney function and rejection in the subset of study patients in the DVC.
Our study has several limitations. First, DGF was defined as need for dialysis in the first week post-transplant. DGF was not determined by utilizing creatinine clearance to determine kidney clearance. However, registry and patient-level studies usually define DGF as need for dialysis, as we did in our study. Also utilizing creatinine clearance is problematic in participants that are dialyzed. Second, not all recipient data was obtained from review of medical records. The study utilized USRDS data instead of medical record abstraction for the recipient of contrateral kidneys from donors providing organs to DVC participants. However, it was not practical to obtain consent from the recipient of the contralateral kidney to review medical records because this recipient could be in another part of the country given the national sharing system for kidneys. This study adjusted for the source of DGF data in the multivariate analysis (Table 4). The potential association of the TNF SNP rs3093662 with DGF was in the same direction in the subset of DVC patients with medical records data only. Third, a larger cohort of deceased donors with longer follow-up, is needed to determine the impact of the TNF polymorphism on DGF and long-term kidney allograft outcomes. The trend for association of the TNF SNP rs3093662 with DGF in this study, does not achieve statistical significance after adjusting for multiple comparisons. A larger cohort is also needed because the SNPs of interest had a low frequency thus require a larger sample size for proper assessment. Lastly, a larger cohort may allow for both donor and recipients SNPs to be studied together since both recipient and donor SNPs could play a role in DGF.
In conclusion, the donor TNF SNP rs3093662 needs to be further studied for its trend for association with DGF. This study highlights the need to study donor genotypes in determining the impact of donors on kidney allograft outcomes. If the association of TNF SNPs with DGF or kidney allograft outcomes is validated in independent studies, drugs that modulate TNF function can be studied to reduce the incidence of DGF.
Supplementary Material
Table S1: Adjusted association with 25% decline in eGFR for subjects in the Delaware Valley Cohort.
Table S2: Adjusted association with 50% decline in eGFR for subjects in the Delaware Valley Cohort.
Table S3: Adjusted association with allograft loss for subjects in the Delaware Valley Cohort.
Table S4: Adjusted association with acute rejection for subjects in the Delaware Valley Cohort.
Note: The supplementary material accompanying this article (doi:_____) is available at www.ajkd.org.
Acknowledgements
We would like to thank the staff and patients at the nine participating transplant centers, Kyle Walker and Amy Walker of the Molecular Epidemiology Laboratory at the University of Pennsylvania and Grace Wilson and Jenny Vigliaturo at Hennepin County Medical Center for their assistance. Presented in part as an oral presentation at the 2007 American Society of Nephrology meeting, San Francisco and poster at American Transplant Congress in May 2006.
Support: This research was supported in part by National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant K23-DK062829 to Dr Israni, the Norman S. Coplon Grant from Satellite Healthcare to Dr Israni, an NIH individual postdoctoral fellowship to Dr Cizman, and NIH NIDDK grant K24-DK002651 and NIH grant R01-AI043295 to Dr Feldman. Dr. Israni was the recipient of a Beginning Grant-in-Aid (Pennsylvania/Delaware) and Dr. Feldman was an Established Investigator of the American Heart Association during the conduct of part of this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Descriptive Text for Online Delivery
Hyperlink: Supplementary Table S1 (PDF)
About: Adjusted association with 25% decline in eGFR for subjects in the Delaware Valley Cohort.
Hyperlink: Supplementary Table S2 (PDF)
About: Adjusted association with 50% decline in eGFR for subjects in the Delaware Valley Cohort.
Hyperlink: Supplementary Table S3 (PDF)
About : Adjusted association with allograft loss for subjects in the Delaware Valley Cohort.
Hyperlink: Supplementary Table S4 (PDF)
About: Adjusted association with acute rejection for subjects in the Delaware Valley Cohort.
References
- 1.Cecka JM. The OPTN/UNOS renal transplant registry. Clin Transplant. 2005:1–16. [PubMed] [Google Scholar]
- 2.Daly PJ, Power RE, Healy DA, Hickey DP, Fitzpatrick JM, Watson RW. Delayed graft function: A dilemma in renal transplantation. BJU Int. 2005;96(4):498–501. doi: 10.1111/j.1464-410X.2005.05673.x. [DOI] [PubMed] [Google Scholar]
- 3.Rosenthal J, Danovitch G, Wilkinson A, Ettenger R. The high cost of delayed graft function in cadaveric renal transplantation. Transplantation. 1991;51(5):1115–1118. [PubMed] [Google Scholar]
- 4.Cecka J, Cho Y, Terasaki P. Analyses of the UNOS Scientific Renal Transplant Registry at three years - early events affecting transplant success. Transplantation. 1992;53(1):59–64. doi: 10.1097/00007890-199201000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Cacciarelli T, Sumrani N, Delaney V, et al. The influence of delayed graft function on long-term outcome in the cylcosporine era. Clin Nephrol. 1993;39(6):335–339. [PubMed] [Google Scholar]
- 6.Pallardo L, Garcia J, Sanchez J, Gorriz J, Orero E, Calabuig F. Posttransplant renal allograft dysfunction as a prognostic factor in triple therapy-treated patients. Transplant Proc. 1992;24(1):113–114. [PubMed] [Google Scholar]
- 7.Kasiske BL. Clinical correlates to chronic renal allograft rejection. Kidney Int Suppl. 1997;63:S71–S74. [PubMed] [Google Scholar]
- 8.Franco A, Gas J, Gasso M, Prados M, Jimenez L, Olivares J. Prevention measures for severe acute tubular necrosis in cadaveric kidney transplants. Transplant Proc. 1992;24(1):48–49. [PubMed] [Google Scholar]
- 9.Isa W, Robles J, Rosell D, et al. Cadaveric renal transplantation: multifactorial analysis of possible causes affecting graft outcome in recipients treated with and without cyclosporine. Transplant Proc. 1992;24(1):115–117. [PubMed] [Google Scholar]
- 10.Feldman H, Gayner R, Berlin JA, et al. Delayed function reduces renal allograft survival independent of acute rejection. Nephrol Dial Transplant. 1996;11:1306–1313. [PubMed] [Google Scholar]
- 11.Solez K, Racusen L, Marcussen N, et al. Morphology of ischemic acute renal failure, normal function, and cyclosporine toxicity in cyclosporine-treated renal allograft recipients. Kidney Int. 1993;43:1058–1067. doi: 10.1038/ki.1993.148. [DOI] [PubMed] [Google Scholar]
- 12.Rabb H, Daniels F, O'Donnell M, et al. Pathophysiologic role of T lymphocytes in renal ischemia-reperfusion injury in mice. Am J Physiol Renal Physiol. 2000;279:F525–F531. doi: 10.1152/ajprenal.2000.279.3.F525. [DOI] [PubMed] [Google Scholar]
- 13.Vadiei K, Brunner L, Luke D. Effects of pentoxifylline in experimental acute renal failure. Kidney Int. 1989;36:466–470. doi: 10.1038/ki.1989.218. [DOI] [PubMed] [Google Scholar]
- 14.Mason J, Welsch J, Torhorst J. The contribution of vascular obstruction to the functional defect that follows renal ischemia. Kidney Int. 1987;31:65–71. doi: 10.1038/ki.1987.10. [DOI] [PubMed] [Google Scholar]
- 15.Hellberg P, Kallskog T. Neutrophil-mediated postischemic tubular leakage in the rat kidney. Kidney Int. 1989;36:555–561. doi: 10.1038/ki.1989.230. [DOI] [PubMed] [Google Scholar]
- 16.Klausner J, Paterson I, Goldman G, et al. Postischemic renal injury is mediated by neutrophils and leukotrienes. AM J Physiol. 1989;1989:F794–F802. doi: 10.1152/ajprenal.1989.256.5.F794. [DOI] [PubMed] [Google Scholar]
- 17.Paller M. Effect of neutrophil depletion on ischemic renal injury in the rat. J Lab Clin Med. 1989;113:379–386. [PubMed] [Google Scholar]
- 18.Takada M, Chandraker A, Nadeau K, et al. The role of the B7 costimulatory pathway in experimental cold ischemia-reperfusion injury. J Clin Invest. 1997;100:1199–1203. doi: 10.1172/JCI119632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly K, Williams W, Colvin R, Bonventre J. Antibody to intercellular adhesion molecule-1 protects the kidney against ischemic injury. Proc Natl Acad Sci. 1994;91:812–816. doi: 10.1073/pnas.91.2.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rabb H, Star R. Inflammatory response and its consequences in acute renal failure. In: Molitoris B, Finn W, editors. Acute renal failure: A companion to Brenner & Rector's The Kidney. Philadelphia: W.B. Saunders; 2001. pp. 89–100. [Google Scholar]
- 21.Khoury J, Beaty T, Cohen B. Fundamentals in genetic epidemiology. New York: Oxford University Press; 1993. Study of genetic factors in disease; pp. 124–163. [Google Scholar]
- 22.Kanakiriya S, Nath K. Heme Oxygenase and acute renal injury. In: Molitrois B, Finn W, editors. Acute Renal Failure. Philadelphia: WB Saunders; 2001. pp. 78–88. [Google Scholar]
- 23.Ueda N, Kaushal G, Shah S. Apoptotic mechanisms in acute renal failure. Am J Med. 2000;108:403–415. doi: 10.1016/s0002-9343(00)00311-9. [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate.[see comment] Annals of Internal Medicine. 2006;145(4):247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 25.Storey A, Thomas M, Kalita A, et al. Role of p53 polymorphism in the development of human papillomavirus-associated cancer. Nature. 1998;393:229–234. doi: 10.1038/30400. [DOI] [PubMed] [Google Scholar]
- 26.Emigh T. Comparison of tests for Hardy-Weinberg Equilibrium. Biometrics. 1980;36:627–642. [PubMed] [Google Scholar]
- 27.Cox D. Regression models and life-tables. Journal of the Royal Statistics Society. 1972;34:187–220. [Google Scholar]
- 28.Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 29.Rosner B. Fundamentals of biostatistics. 5th edition ed. Pacific Grove, California: Duxbury Thomson Learning; 2000. Two-sample test for binomial proportions; pp. 361–371. [Google Scholar]
- 30.2008 http://www.ncbi.nlm.nih.gov.
- 31.2008 www.genecards.org.
- 32.Mytilineos J, Laux G, Opelz G. Relevance of IL10, TGFbeta1, TNFa, and IL4Ra gene polymorphisms in kidney transplantation: A collaborative transplant study report. American Journal of Transplantation. 2004;4(10):1684–1690. doi: 10.1111/j.1600-6143.2004.00561.x. [DOI] [PubMed] [Google Scholar]
- 33.Donnahoo K, Meng X, Ayala A, et al. Early kidney TNF-alpha expression mediates neutrophil infiltration and injury after renal ischemia-reperfusion. Am J Physiol. 1999;277:R922–R929. doi: 10.1152/ajpregu.1999.277.3.R922. [DOI] [PubMed] [Google Scholar]
- 34.Daemen M, Van de Ven M, Heineman E, Buurman W. Involvement of endogenous interleukin-10 and tumor necrosis factor-alpha in renal ischemia-reperfusion injury. Transplantation. 1999;67:792–800. doi: 10.1097/00007890-199903270-00003. [DOI] [PubMed] [Google Scholar]
- 35.Toback F. Regeneration after acute tubular necrosis. Kidney Int. 1992;41:226. doi: 10.1038/ki.1992.32. [DOI] [PubMed] [Google Scholar]
- 36.Wiggins MC, Bracher M, Mall A, Hickman R, Robson SC, Kahn D. Tumour necrosis factor levels during acute rejection and acute tubular necrosis in renal transplant recipients. Transplant Immunology. 2000;8(3):211–215. doi: 10.1016/s0966-3274(00)00027-7. [DOI] [PubMed] [Google Scholar]
- 37.Basile D, Martin D, Hammerman M. Extracellular matrix-related genes in kidney after ischemic injury: potential role for TGF-beta in repair. Am J Physiol. 1998;275:F894–F903. doi: 10.1152/ajprenal.1998.275.6.F894. [DOI] [PubMed] [Google Scholar]
- 38.Basile D, Rovak J, Martin D, et al. Increased TGF-beta expression in regenerating rate renal tubules following ischemic injury. Am J Physiol(Renal Fluid Electrolyte Physiol) 1996;39:F500. doi: 10.1152/ajprenal.1996.270.3.F500. [DOI] [PubMed] [Google Scholar]
- 39.Ando T, Okuda S, Tamaki K, et al. Localization of transforming growth factor-beta and latent transforming growth factor beta binding protein in rat kidney. Kidney Int. 1995;47:733–739. doi: 10.1038/ki.1995.112. [DOI] [PubMed] [Google Scholar]
- 40.Agarwal A, Kim Y, Matas AJ, et al. Gas-generating systems in acute renal allograft rejection in the rat: co-induction of heme oxygenase and nitric oxide synthase. Transplantation. 1996;61:93. doi: 10.1097/00007890-199601150-00019. [DOI] [PubMed] [Google Scholar]
- 41.Burns A, Davies D, Mclaren A, Cerundolo L, Morris P, Fuggle S. Apoptosis in ischemia/reperfusion injury of human renal allografts. Transplantation. 1998;66(7):872–876. doi: 10.1097/00007890-199810150-00010. [DOI] [PubMed] [Google Scholar]
- 42.Baan C, Peeters A, Lernos F, Uitterlinden A, Doxiadis I, Claas F, et al. Fundamental role for HO-1 in the self-protection of renal allografts. American Journal of Transplantation. 2004;4:811–818. doi: 10.1111/j.1600-6143.2004.00420.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Adjusted association with 25% decline in eGFR for subjects in the Delaware Valley Cohort.
Table S2: Adjusted association with 50% decline in eGFR for subjects in the Delaware Valley Cohort.
Table S3: Adjusted association with allograft loss for subjects in the Delaware Valley Cohort.
Table S4: Adjusted association with acute rejection for subjects in the Delaware Valley Cohort.
Note: The supplementary material accompanying this article (doi:_____) is available at www.ajkd.org.