Abstract
Small GTPase Rab is a member of a large family of Ras-related proteins, highly conserved in eukaryotic cells, and thought to regulate specific type(s) and/or specific step(s) in intracellular membrane trafficking. Given our interest in synaptic transmission, we addressed the possibility that Rab27 (a close isoform of Rab3) could be involved in cytosolic synaptic vesicle mobilization. Indeed, preterminal injection of a specific antibody against squid Rab27 (anti-sqRab27 antibody) combined with confocal microscopy demonstrated that Rab27 is present on squid synaptic vesicles. Electrophysiological study of injected synapses showed that the anti-sqRab27 antibody inhibited synaptic release in a stimulation-dependent manner without affecting presynaptic action potentials or inward Ca2+ current. This result was confirmed in in vitro synaptosomes by using total internal reflection fluorescence microscopy. Thus, synaptosomal Ca2+-stimulated release of FM1-43 dye was greatly impaired by intraterminal anti-sqRab27 antibody. Ultrastructural analysis of the injected giant preterminal further showed a reduced number of docked synaptic vesicles and an increase in nondocked vesicular profiles distant from the active zone. These results, taken together, indicate that Rab27 is primarily involved in the maturation of recycled vesicles and/or their transport to the presynaptic active zone in the squid giant synapse.
Keywords: Griscelli syndrome, rabphilin, small GTPase, total internal reflection fluorescence microscopy, vesicle exocytosis
The Rab family belongs to the small GTPase Ras superfamily, and it is widely believed to be essential for the control of intracellular membrane trafficking in all eukaryotic cells (for review, see refs. 1–3). Approximately 60 distinct Rab proteins have been identified in humans (4, 5) and mice (6), and each member is thought to regulate a specific type (or a distinct step) of membrane trafficking (1–3). Among the Rab family members, Rab3 subfamily members have been proposed to control regulated secretion in a variety of cell types, including neurotransmitter release from neurons (for review, see refs. 7–9). However, because deletion of all four Rab3s (Rab3A/B/C/D) in mice leads to only a 30% reduction of probability of Ca2+-triggered neurotransmitter release (10), additional Rab isoforms must be present on synaptic vesicles and involved in the control of neurotransmitter release. Recently, considerable attention has been paid to Rab27, a close isoform of Rab3. Two Rab27 isoforms, Rab27A and Rab27B, are present in vertebrates and a single isoform in invertebrates (e.g., Caenorhabditis elegans and Drosophila) (5, 11), and mammalian Rab27A/B proteins have been shown to be involved in some forms of regulated secretion (9, 12–14). For instance, mutations in the RAB27A gene have been shown to cause human type II Griscelli syndrome (15), which is characterized by silvery hair (i.e., defect in melanosome transport in melanocytes) and immunodeficiency (i.e., defect in lytic granule exocytosis in cytotoxic T lymphocytes). Interestingly, some such patients show neurological disorders (16), suggesting an important role of Rab27 in the brain function. More recently, synaptic vesicle localization of C. elegans Rab27 has been revealed by the genetic analysis of an aex-6 mutant, which produces a neurotransmission defect and bowel movement defects (17). However, direct involvement of Rab27 protein on synaptic vesicle trafficking has not been described thus far.
In this work, we cloned squid Rab27 cDNA and generated functionally blocking antibody against squid Rab27 (referred to as anti-sqRab27 antibody below) (18). We investigated the function of Rab27 in synaptic vesicle trafficking by introduction of the anti-sqRab27 antibody into the squid giant preterminal. Electrophysiological, total internal reflection fluorescence (TIRF) microscopic, and ultrastructural analyses indicated that squid Rab27 is involved mainly in the maturation of recycled synaptic vesicles and/or their transport to the active zone. Based on our findings, we discuss the possible functions of Rab27 in synaptic vesicle trafficking.
Results
Expression of Squid Rab27 Protein in the Squid Giant Synapse.
The possible role of Rab27 in neurotransmitter release in the squid giant synapse initially required the cloning of squid Rab27 cDNA by PCR using degenerate oligonucleotides that amplify all Rab isoforms (for details, see Materials and Methods). Thirty-seven cDNA clones were sequenced, and one of them showed striking homology to the mouse Rab27A/B (Fig. 1A) rather than the mouse Rab3A/B/C/D. As judged from the phylogenetic tree of the Rab family, this Rab clone, squid Rab27 (sqRab27), should be the direct ortholog of mouse Rab27. Indeed, it fell into the small branch consisting of the Rab27 subfamily from different species, but not the Rab3 subfamily or Rab8 subfamily (black boxed in Fig. 1B). Sequence alignment of the Rab27 subfamily members indicates that a large part of the N-terminal domain of Rab27 is highly conserved among the Rab27 subfamily, whereas the C-terminal hypervariable region has no homology with each other (Fig. 1A). It should be noted that the switch II sequence, a putative recognition site by Rab27 effector molecules (19), is exactly the same in mouse Rab27A (mRab27A), mRab27B, sqRab27, and C. elegans Rab27 (ceRab27). We also identified a single isoform of squid Rab3 and Rab8 during the course of screening for sqRab27.
Fig. 1.
Identification of squid Rab27. (A) Sequence alignment of the Rab27 subfamily from mouse (GenBank accession numbers, AB232622 for mRab27A and AB232623 for mRab27B), squid (AB426126 for sqRab27), C. elegans (AB112930 for ceRab27), and Drosophila (AB112931 for dmRab27). Conserved amino acids in half of the sequences are in black background. C-terminal geranylgeranylation sites are boxed. Note that the switch II sequence, which is responsible for the recognition by Rab27 effectors (e.g., Slac2-a/melanophilin) (19), is highly conserved among the Rab27 subfamily. In contrast to other Rab27 isoforms, sqRab27 contains an additional N-terminal sequence (≈20 aa), and two potential initiation Met residues are present in this region. Because the apparent molecular mass of endogenous sqRab27 in the optic lobe was much smaller than that of recombinant sqRab27 (amino acids 1–245) (data not shown), the second Met (asterisk) is likely to function as an initiation Met of endogenous sqRab27. (B) Phylogenetic tree of the mouse Rab proteins and invertebrate Rab3/27 proteins. The phylogenetic tree was depicted with the ClustalW program (http://clustalw.ddbj.nig.ac.jp/top-e.html) as described in ref. 11. (C) Specificity of anti-sqRab3 antibody and anti-sqRab27 antibody. Two hundred nanograms of purified recombinant sqRab3 (lane 1), sqRab8 (lane 2), and sqRab27 (amino acids 11–245) (lane 3) were analyzed by 10% SDS/PAGE followed by immunoblotting with anti-sqRab3 antibody (Top; 5 μg/ml), anti-sqRab27 antibody (Middle; 5 μg/ml), and Coomassie brilliant blue (CBB) R-250 staining (Bottom). (D) Total homogenates of squid optic lobe (≈50 and 150 μg of protein was loaded in lanes 1 and 2, respectively) were analyzed by 10% SDS/PAGE followed by immunoblotting with anti-sqRab3 antibody (lane 1; 5 μg/ml) and anti-sqRab27 antibody (lane 2; 10 μg/ml). The positions of the molecular mass markers (in kDa) are shown on the left.
To determine whether sqRab27 protein is expressed in the squid giant synapse, we produced anti-sqRab27 antibody, which specifically recognized recombinant sqRab27, but not sqRab3 or sqRab8 (lanes 1–3 in Fig. 1C Middle). This antibody recognized a single ≈30-kDa band in the squid optic lobe (Fig. 1D, lane 2, arrow), which is slightly higher than the sqRab3 band (Fig. 1D, lane 1, arrowheads). Given that coincubation of the anti-sqRab27 antibody with the antigen (i.e., GST-sqRab27) caused a specific loss of a ≈30-kDa signal (data not shown), we may conclude that this band does correspond to squid Rab27 protein.
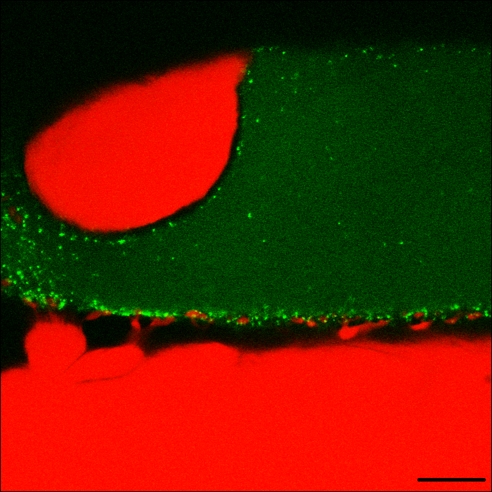
Confocal images of the squid giant preterminal injected with fluorescein-labeled anti-sqRab27 antibody showed the vesicular distribution of sqRab27 (Fig. 2, green dots), which is quite similar to that of a synaptic vesicle protein synaptotagmin I (20, 21). It should be noted that the postsynaptic dendrite-like structures (Fig. 2, red) were often surrounded by the green sqRab27 dots (20), which presumably correspond to the active zone between the pre- and postjunctional synaptic elements.
Fig. 2.
Confocal imaging of presynaptic localization of fluorescein-labeled anti-sqRab27 antibody. Fluorescein-labeled anti-sqRab27 antibody-bound vesicles are shown as a green spots by the presynaptic membrane. Note that the distribution corresponds to the size and geometrical distribution of the synaptic active zones in the terminal. Cy3 fluorescent dye was injected to postsynaptic terminal. (Scale bar, 20 μm.)
Preterminal Anti-sqRab27 Antibody Injection Reduces Neurotransmitter Release: Electrophysiological Analysis.
Preterminal injection of either the active or inactive (i.e., heat-denatured) anti-sqRab27 antibody (confirmed by fluorescence imaging of the preterminal) was followed by trains of 200-Hz electrical stimuli to the presynaptic axon (Fig. 3 B and b) until postsynaptic spikes failed occurred (Fig. 3 C, D, c, and d). This failure mostly occurs because of transmitter depletion (i.e., exhaustion of synaptic vesicles), and transmission recovery is generally observed after a 15-min rest interval (21). However, no such postsynaptic response recovery was observed in synapses injected with the active anti-sqRab27 antibody (n = 9) (Fig. 3E). By contrast, in synapses injected with the inactive anti-sqRab27 antibody transmission recovery always occurred after a 15-min rest interval (Fig. 3e) (n = 3).
Fig. 3.

Effect of presynaptic anti-sqRab27 antibody injection on synaptic transmission. (A and a) Pre- and postsynaptic response after anti-sqRab27 antibody (active form) injection and heat-denatured anti-sqRab27 antibody (inactive form) injection. (B, b, C, and c) Simultaneous presynaptic (B and b) and postsynaptic (C and c) responses after repetitive presynaptic stimulation at 200 Hz in each injection. (D and d) The stimulus train was continued until all postsynaptic spike activation failed. (E and e) After resting for 15 min, the stimulus train was partially recovered from transmitter depletion in the inactive anti-sqRab27 antibody-injected synapse (e). By contrast, the active anti-sqRab27 antibody-injected synapse showed no recovery from transmitter depletion (E).
Because modifications of presynaptic Ca2+ current (ICa) after the antibody injection can also lead to reduction of neurotransmitter release, a set of presynaptic voltage-clamp experiments was performed to test this possibility directly (n = 8). Because no reduction or delay in the presynaptic ICa accompanied the reduction in the postsynaptic potential (Fig. 4 A and B), we concluded that the anti-sqRab27 antibody had interfered most with either the vesicular trafficking (e.g., transport, docking, fusion, and/or recycling of synaptic vesicles) or a block of the release mechanism at the active zone rather than the Ca2+ entry step.
Fig. 4.

Anti-sqRab27 antibody injection reduces neurotransmitter release without modifying inward Ca2+ current amplitude. Reduction of neurotransmitter release (B) after presynaptic voltage-clamp pulse (C) at different times after anti-sqRab27 antibody (Rab27 Ab) injection. Note the lack of change in inward Ca2+ current (A).
Anti-sqRab27 Antibody Blocks Neurotransmitter Release from Synaptosomes in Vitro: TIRF Microscopic Analysis.
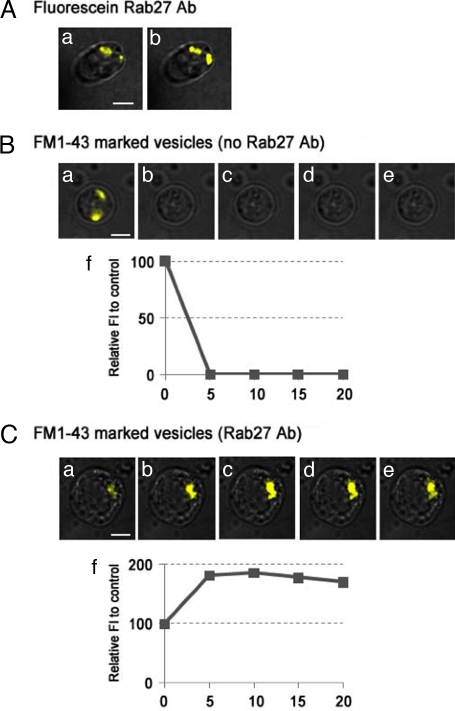
To determine which step in synaptic vesicle trafficking is blocked by the anti-sqRab27 antibody, we investigated the effect of the antibody on neurotransmitter release from synaptosomes in vitro. FM1-43 dye was introduced into synaptic vesicles of synaptosomes, and its Ca2+-dependent release was monitored by TIRF microscopy (for details, see Materials and Methods). In the control synapse (without antibody), FM1-43 fluorescence disappeared rapidly (i.e., release of FM1-43 dye from the synaptosome to the extracellular space) in response to high-KCl stimulation (Fig. 5B a–f). By contrast, however, no release of FM1-43 dye from the antibody-introduced synaptosome was observed (Fig. 5C a–f) (n = 6), indicating that sqRab27 is a critical regulator of synaptic vesicle exocytosis or of “immediately vesicular availability.”
Fig. 5.
Anti-sqRab27 antibody inhibits FM1-43 dye release from synaptosomes in vitro. Fluorescein-labeled anti-sqRab27 antibody (Rab27 Ab) is loaded during synaptosome preparation and localize on synaptic vesicle profiles (Aa). After 30-min superfusion with high KCl, fluorescence vesicular profiles remain stationary in the synaptosomal images (Ab). In the case of the FM1-43 dye-loaded synaptosome without antibody, fluorescence inside the synaptosome (Ba) rapidly disappeared after high-KCl superfusion (B b–e) (n = 7). By contrast, in the unlabeled anti-sqRab27 antibody-preloaded synaptosome, FM1-43 dye remained and accumulated after high-KCl superfusion (C a–e) (n = 6). (Scale bar, 4 μm.) Relative fluorescence intensity (percentage) to untreated control was plotted as a time-course manner (Bf and Cf).
Ultrastructural Analysis.
Finally, we investigated the number and distribution of synaptic vesicles in the antibody-injected squid giant preterminal at an electron microscopic (EM) level [see supporting information (SI) Table S1]. In the control stimulated synapse, the number of synaptic vesicles per active zone is 53.77 ± 2.83 (Fig. 6Aa), and the number of vesicles docked to the presynaptic plasma membrane is 9.24 ± 0.34 (n = 103) (Fig. 6Ac). After antibody injection, a mild reduction of vesicular density at the active zone occurred (37.21 ± 3.44; ≈70% of the control; P < 0.001, a Kruskal–Wallis test) (Fig. 6Ab) (n = 67), and the most striking defect observed was an unusually large number of synaptic vesicles distant from the active zone (referred to as “entrapped vesicles” below) in the antibody-injected terminals (Fig. 6B Lower, arrows), compared with the control synapse (Fig. 6B Upper). These entrapped vesicles presumably correspond to recycled vesicles formed after high-frequency stimulation. We also observed a reduced number of docked vesicles at the active zone in the antibody-injected synapses (4.12 ± 0.32; ≈45% of the control; P < 0.001). This finding indicates that the anti-sqRab27 antibody inhibits maturation and/or transport of recycled synaptic vesicles to the presynaptic release site. Moreover, the function of sqRab27 seems not to be limited to the synaptic vesicle availability because sqRab27 may also be involved in the recycling step of synaptic vesicles, given that the number of clathrin-coated vesicles in the antibody-injected synapse (1.94 ± 0.21) was significantly reduced compared with the control synapse (4.57 ± 0.33; P < 0.001).
Fig. 6.

Ultrastructure of anti-sqRab27 antibody (Rab27 Ab)-injected squid giant synapse. (A) Total number of synaptic vesicles was reduced (b) at the active zone compared with the control synapse (a). (c and d) Higher magnification views of the boxed area in a and b, respectively. (B) Note the large numbers of entrapped extrazonal vesicles (AZ, active zone; and arrows, entrapped vesicles away from the active zone) in the antibody-injected synapse (Lower) in contrast to the control synapse (Upper). (Scale bar, 500 nm in Aa and Ab; 100 nm in Ac and Ad; and 500 nm in B.)
Discussion
It has recently been proposed that Rab27 protein is a general regulator of secretion (22), and considerable attention has recently been focused on the function of Rab27, in addition to Rab3, in regulated secretion (9). However, direct involvement of Rab27 protein in synaptic vesicle trafficking had not been described. In the present work, we focused on the squid giant synapse to determine the function of Rab27 protein in neurotransmitter release. Electrophysiological (Fig. 3), TIRF microscopic (Fig. 5), and EM analyses (Fig. 6) of the anti-sqRab27 antibody-injected synapse indicated that sqRab27 is involved primarily in the maturation and/or transport of recycled vesicles to the presynaptic plasma membrane. In addition, a reduced number of docked vesicles and clathrin-coated vesicles at the active zone were also observed in the antibody-injected synapse, suggesting that sqRab27 may also be partly involved in the docking of synaptic vesicles to the presynaptic plasma membrane and synaptic vesicle recycling. However, such defects may all be secondary to dysfunction of synaptic vesicle maturation/transport to the release site. This can be argued because inhibition of synaptic vesicle maturation/transport would result in a reduction of docked vesicles and thus indirectly reduce the number of clathrin-coated vesicles.
The mechanism responsible for sqRab27 regulation of vesicular maturation/transport/docking is unclear at this point. Nevertheless, by analogy to the mechanism responsible in the maturation/transport/docking of Rab27-bound organelles in nonneuronal cells (23), we propose that an unidentified sqRab27 effector mediates transport/docking of recycled synaptic vesicles to the release site. This may be implemented through the simultaneous interaction of the effector with sqRab27 on the synaptic vesicle and with certain protein/lipid in the plasma membrane. Our anti-sqRab27 antibody is likely to disrupt the interaction between sqRab27 and its unidentified effector, which consequently inhibits transport/docking of synaptic vesicles to the presynaptic plasma membrane. In humans and mice, 11 Rab27 effector molecules have been identified (Slp1–5, Slac2-a–c, rabphilin, Noc2, and Munc13–4) (14), and four of them, Slp2-a (24, 25), Slp4-a/granuphilin-a (26–28), rabphilin (29, 30), and Slac2-c/MyRIP (18, 31, 32), have been shown to promote tethering or docking of Rab27-bound organelles to the plasma membrane in nonneuronal cells, in concert with Rab27 (23). Among these Rab27-binding proteins, rabphilin and Slac2-c/MyRIP have been shown to control regulated secretion from endocrine cells (11, 31, 32) and to be present in the presynapse of mouse neurons (33, 34). Both rabphilin and Slac2-c can fulfill the transport/docking function of unidentified Rab27 effector: rabphilin can interact with SNAP-25 on the presynaptic plasma membrane (29, 30), and Slac2-c can interact with actin/myosin-Va just near the presynaptic plasma membrane (11, 31, 32, 35, 36). However, we favored rabphilin as a most likely target of sqRab27. Indeed, rabphilin is the only known Rab27 effector conserved in evolution (from C. elegans to humans) (14, 17), and the Slac2 family has not been found in C. elegans or Drosophila. Second, rabphilin can also promote recruitment of hormone granules to the plasma membrane in response to Ca2+ stimulation in PC12 cells (29, 30). Third, in addition to synaptic vesicle exocytosis, rabphilin has also been shown to be involved in the endocytic pathway through interaction with Rabaptin-5 (37, 38). Fourth, microinjection of the N-terminal Rab-binding domain of bovine rabphilin into the squid giant presynapse reduced the total number of synaptic vesicles and perturbed endocytic pathway (39). The only contrary argument seems to be the lack of docking defect in neurons from rabphilin-knockout mice (40); however, this may be caused by a compensatory effect of other Rab27 effectors (e.g., Slps and Slac2s). Future identification and characterization of squid rabphilin will determine whether the sqRab27–rabphilin complex controls synaptic transmission in the squid giant synapse.
Although important roles for Rab27 in synaptic transmission are evident in invertebrates (ref. 17 and the present findings), no abnormality in the brain of Rab27A/B-double knockout mice has been reported (12, 13). Such a discrepancy may relate to the different Rab-binding specificities of vertebrate and invertebrate rabphilin. Rabphilin was originally described as a specific Rab3 effector (33), but subsequent exhaustive analysis indicated that murine rabphilin functions as a dual Rab effector for Rab3 and Rab27 in hormone secretion (11, 41). By contrast, C. elegans and Drosophila rabphilin function as specific Rab27 effectors (11, 17). Moreover, whereas vertebrate rabphilin is involved in synaptic vesicle trafficking presumably through interaction with Rab3, Rab27, or both, the same as is the case for hormone secretion (42), invertebrate rabphilin regulates synaptic transmission only through interaction with Rab27 (17). Thus, the acquisition of Rab3-binding ability of vertebrate rabphilin combined with the duplication of rab3 and rab27 genes during evolution would ensure vertebrate brain function viability even if one rab3 (or rab27) gene is disrupted. In this light, analysis of mutant mice that lack both Rab3s and Rab27s will be a next important step in determining whether Rab27 is also an important regulator of synaptic vesicle trafficking in vertebrates.
In conclusion, we have investigated the effect of the anti-sqRab27 antibody on synaptic vesicle trafficking and present evidence that sqRab27 regulates maturation and/or transport of recycled vesicles to the release site.
Materials and Methods
Molecular Cloning of Squid Rab27 cDNA.
mRNA was prepared from optic lobes of squid (Loligo pealei) as described (20, 43), and optic lobe cDNA was prepared by a Marathon cDNA amplification kit (BD Biosciences) according to the manufacturer's instructions. We designed the following degenerate oligonucleotides to amplify cDNAs of all Rab isoforms: 5′-AAICCCATIGC(A/G)TC-3′ (Rab-all primer 1; antisense) and 5′-TC(C/T)TGICCIGCIGT(A/G)TCC-3′ (Rab-all primer 2; antisense). First and second PCRs were performed as described in ref. 44 by using the following sets of oligonucleotides: AP1 (BD Biosciences) and Rab-all primer 1 for the first amplification; and AP2 (BD Biosciences) and Rab-all primer 2 for the second amplification. Purified PCR products ≈0.5–1.0 kbp were directly inserted into the pGEM-T Easy vector (Promega). The cDNA inserts were then sequenced by an automated sequencer at random and identified by searching for the public database. A total of 37 clones were investigated, with one clone showing striking homology to vertebrate Rab27. We also identified the mammalian homolog of squid Rab2 (n = 3), Rab3 (n = 5), Rab5 (n = 6), Rab6 (n = 1), Rab7 (n = 1), Rab8 (n = 1), Rab10 (n = 11), Rab14 (n = 3), unknown Rabs (n = 2), Ran (n = 1), and Ras (n = 2) (numbers in parentheses indicate the number of clones we sequenced). cDNAs covering the full ORF of squid Rab8 (sqRab8) and sqRab27 were obtained by 3′-RACE as described in ref. 44 via oligonucleotides 5′-CAGCCATTGCAGCAGCAAGA-3′ (sqRab8-N1 primer; sense) and 5′-GGATCCATGGCGAAAACCTATGACTA-3′ (sqRab8-Met primer; sense; underlined, BamHI site); and 5′-TTCTCCAGGAGGAATTCCTA-3′ (sqRab27-N1 primer; sense) and 5′-CCTAGTAGGGGACTTCTTTC-3′ (sqRab27-N2 primer; sense). cDNAs encoding the full ORF of sqRab3 and sqRab27 were also amplified by PCR via oligonucleotides with a BamHI linker (underlined below) or a stop codon (bolded below): 5′-CGGATCCATGATGGCATCAGCAAATGA-3′ (sqRab3-Met primer; sense) and 5′-CTAACACTGACAGCTACCAT-3′ (sqRab3-stop primer; antisense); and 5′-GGATCCATGAAGACTGGTCTTCGGTA-3′ (sqRab27-Met primer; sense) and 5′-TTAAGCAGCCGCACCAGTGAG-3′ (sqRab27-stop primer; antisense). Purified PCR products containing the full-length sqRab cDNA were inserted directly into the pGEM-T Easy vector and sequenced by an automated sequencer as described above.
Antibody Production.
cDNA of sqRab27, sqRab8, or sqRab3 was subcloned into the pGEX-4T-3 vector (Amersham Biosciences). Recombinant sqRab proteins were expressed as GST in Escherichia coli JM109. Purification of GST-sqRab proteins and thrombin digestion were implemented by using standard protocols. New Zealand White rabbits were immunized with the purified GST-sqRab27 (or GST-sqRab3), and a specific antibody was affinity-purified by exposure to antigen-bound Affi-Gel 10 beads (Bio-Rad) as described in ref. 45. The specificity of the anti-sqRab27 (or anti-sqRab3) antibody was checked by immunoblotting with purified recombinant sqRab3, sqRab8, and sqRab27 (see Fig. 1C). Conjugation of IgG with 5-carboxyfluorescein (Invitrogen; catalog no. C-2210) was performed according to the manufacturer's instructions (20).
Synaptosomal Preparation and FM Dye Loading.
Squid optic lobe synaptosomes were prepared as described in ref. 46 with minor modifications. Briefly, six optic lobes were homogenized in 6 ml of ice-cold homogenization buffer [0.7 M sucrose, 20 mM Tris·HCl (pH 7.4)]. The suspension was centrifuged at 3,000 × g for 11 min at 4°C, and the supernatant was recentrifuged at 14,000 × g for 30 min at 4°C. The floating cohesive layer at the top was transferred to a small dish and resuspended with a fire-polished glass pipette in 2–3 ml of homogenization buffer. In the case of the antibody-loaded experiment, we mixed 2 μg of antibody with homogenization buffer in this step. The loading of antibody into synaptosomes was confirmed by the fluorescence of fluorescein-labeled anti-sqRab27 antibody (Fig. 5A). After resuspension, synaptosomes were plated in glass-bottom dishes (Willco Wells) and incubated for 0.5–1 h at 18°C. For FM dye staining, 4 μM FM1-43 dye in 50 mM K+/10 mM Ca2+ seawater was added to synaptosomes, which were washed with Ca2+-free artificial seawater. After dye loading, synaptosomes were washed with Ca2+-free artificial seawater at least three times. All of the experiments were performed at room temperature.
TIRF Microscopy.
Images were acquired on an Olympus IX71 microscope equipped with a ×60, 1.45 N.A., oil-immersion objective and an argon ion laser. Optical images were obtained with an ORCA digital CCD Camera (C4742-95-12; Hamamatsu Photonics). By using this system, differential interference contrast or fluorescence images were obtained at 5-min intervals. The image data were analyzed and displayed with an in-house program written under the LabVIEW programming environment (National Instruments). The analysis of vesicle release with the FM1-43 dye was implemented, and the poststimulus decreased rate was calculated from fluorescence intensity determination at the region of interest after stimulation.
Miscellaneous Procedures.
The isolation of the squid stellate ganglion, presynaptic injection of the anti-sqRab27 antibody, electrophysiological techniques and protocol, and the composition of the continuously superfused artificial seawater were the same as in our previous experiments (20, 47). Confocal microscopy and electron microscopy were also performed as described in ref. 21.
Supplementary Material
Acknowledgments.
We thank Teresa P. Maglia for the excellent EM technical support. We acknowledge Dr. Thomas Reese for use of his EM laboratory facilities at MBL. This work was supported in part by Ministry of Education, Culture, Sports, and Technology of Japan Grants 18207015, 19044003, 20022004, and 20054001 and by the Brain Science Foundation, the Naito Foundation, the Takeda Science Foundation, the Gushinkai Foundation, the Uehara Memorial Foundation (M.F.), Cooperação Interinstitucional de Apoio à Pesquisa sobre o Cérebro Program Project 05-56447-7 FAPESP, Brasil (to J.E.M.), and by National Institutes of Health Grant NS 13742–30 (to R.L. and M.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AB426126).
This article contains supporting information online at www.pnas.org/cgi/content/full/0804825105/DCSupplemental.
References
- 1.Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 2.Segev N. Ypt and Rab GTPases: Insight into functions through novel interactions. Curr Opin Cell Biol. 2001;13:500–511. doi: 10.1016/s0955-0674(00)00242-8. [DOI] [PubMed] [Google Scholar]
- 3.Pfeffer SR. Rab GTPases: Specifying and deciphering organelle identity and function. Trends Cell Biol. 2001;11:487–491. doi: 10.1016/s0962-8924(01)02147-x. [DOI] [PubMed] [Google Scholar]
- 4.Bock JB, et al. A genomic perspective on membrane compartment organization. Nature. 2001;409:839–841. doi: 10.1038/35057024. [DOI] [PubMed] [Google Scholar]
- 5.Pereira-Leal JB, Seabra MC. Evolution of the Rab family of small GTP-binding proteins. J Mol Biol. 2001;313:889–901. doi: 10.1006/jmbi.2001.5072. [DOI] [PubMed] [Google Scholar]
- 6.Itoh T, et al. Screening for target Rabs of TBC (Tre-2/Bub2/Cdc16) domain-containing proteins based on their Rab-binding activity. Genes Cells. 2006;11:1023–1037. doi: 10.1111/j.1365-2443.2006.00997.x. [DOI] [PubMed] [Google Scholar]
- 7.Takai Y, et al. Rab3A small GTP-binding protein in Ca2+-dependent exocytosis. Genes Cells. 1996;1:615–632. doi: 10.1046/j.1365-2443.1996.00257.x. [DOI] [PubMed] [Google Scholar]
- 8.Geppert M, Südhof TC. RAB3 and synaptotagmin: The yin and yang of synaptic membrane fusion. Annu Rev Neurosci. 1998;21:75–95. doi: 10.1146/annurev.neuro.21.1.75. [DOI] [PubMed] [Google Scholar]
- 9.Fukuda M. Regulation of secretory vesicle traffic by Rab small GTPases. Cell Mol Life Sci. 2008;65:2801–2813. doi: 10.1007/s00018-008-8351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schlüter OM, et al. A complete genetic analysis of neuronal Rab3 function. J Neurosci. 2004;24:6629–6637. doi: 10.1523/JNEUROSCI.1610-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukuda M, et al. Rabphilin and Noc2 are recruited to dense-core vesicles through specific interaction with Rab27A in PC12 cells. J Biol Chem. 2004;279:13065–13075. doi: 10.1074/jbc.M306812200. [DOI] [PubMed] [Google Scholar]
- 12.Tolmachova T, et al. Rab27b regulates number and secretion of platelet-dense granules. Proc Natl Acad Sci USA. 2007;104:5872–5877. doi: 10.1073/pnas.0609879104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomi H, et al. Rab27b is expressed in a wide range of exocytic cells and involved in the delivery of secretory granules near the plasma membrane. Mol Biol Cell. 2007;18:4377–4386. doi: 10.1091/mbc.E07-05-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukuda M. Versatile role of Rab27 in membrane trafficking: Focus on the Rab27 effector families. J Biochem. 2005;137:9–16. doi: 10.1093/jb/mvi002. [DOI] [PubMed] [Google Scholar]
- 15.Ménasché G, et al. Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nat Genet. 2000;25:173–176. doi: 10.1038/76024. [DOI] [PubMed] [Google Scholar]
- 16.Anikster Y, et al. Evidence that Griscelli syndrome with neurological involvement is caused by mutations in RAB27A, not MYO5A. Am J Hum Genet. 2002;71:407–414. doi: 10.1086/341606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahoney TR, et al. Regulation of synaptic transmission by RAB-3 and RAB-27 in Caenorhabditis elegans. Mol Biol Cell. 2006;17:2617–2625. doi: 10.1091/mbc.E05-12-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imai A, et al. The small GTPase Rab27B regulates amylase release from rat parotid acinar cells. J Cell Sci. 2004;117:1945–1953. doi: 10.1242/jcs.01048. [DOI] [PubMed] [Google Scholar]
- 19.Fukuda M. Synaptotagmin-like protein (Slp) homology domain 1 of Slac2-a/melanophilin is a critical determinant of GTP-dependent specific binding to Rab27A. J Biol Chem. 2002;277:40118–40124. doi: 10.1074/jbc.M205765200. [DOI] [PubMed] [Google Scholar]
- 20.Mikoshiba K, et al. Role of the C2A domain of synaptotagmin in transmitter release as determined by specific antibody injection into the squid giant synapse preterminal. Proc Natl Acad Sci USA. 1995;92:10703–10707. doi: 10.1073/pnas.92.23.10703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Llinás RR, et al. Vesicular reuptake inhibition by a synaptotagmin I C2B domain antibody at the squid giant synapse. Proc Natl Acad Sci USA. 2004;101:17855–17860. doi: 10.1073/pnas.0408200101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tolmachova T, et al. A general role for Rab27a in secretory cells. Mol Biol Cell. 2004;15:332–344. doi: 10.1091/mbc.E03-07-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukuda M. Rab27 and its effectors in secretory granule exocytosis: A novel docking machinery composed of a Rab27·effector complex. Biochem Soc Trans. 2006;34:691–695. doi: 10.1042/BST0340691. [DOI] [PubMed] [Google Scholar]
- 24.Saegusa C, et al. Decreased basal mucus secretion by Slp2-a-deficient gastric surface mucous cells. Genes Cells. 2006;11:623–631. doi: 10.1111/j.1365-2443.2006.00964.x. [DOI] [PubMed] [Google Scholar]
- 25.Kuroda TS, Fukuda M. Rab27A-binding protein Slp2-a is required for peripheral melanosome distribution and elongated cell shape in melanocytes. Nat Cell Biol. 2004;6:1195–1203. doi: 10.1038/ncb1197. [DOI] [PubMed] [Google Scholar]
- 26.Gomi H, et al. Granuphilin molecularly docks insulin granules to the fusion machinery. J Cell Biol. 2005;171:99–109. doi: 10.1083/jcb.200505179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsuboi T, Fukuda M. The Slp4-a linker domain controls exocytosis through interaction with Munc18-1·syntaxin-1a complex. Mol Biol Cell. 2006;17:2101–2112. doi: 10.1091/mbc.E05-11-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomas A, et al. Munc 18-1 and granuphilin collaborate during insulin granule exocytosis. Traffic. 2008;9:813–832. doi: 10.1111/j.1600-0854.2008.00709.x. [DOI] [PubMed] [Google Scholar]
- 29.Tsuboi T, Fukuda M. The C2B domain of rabphilin directly interacts with SNAP-25 and regulates the docking step of dense core vesicle exocytosis in PC12 cells. J Biol Chem. 2005;280:39253–39259. doi: 10.1074/jbc.M507173200. [DOI] [PubMed] [Google Scholar]
- 30.Tsuboi T, et al. The polybasic sequence in the C2B domain of rabphilin is required for the vesicle docking step in PC12 cells. J Neurochem. 2007;100:770–779. doi: 10.1111/j.1471-4159.2006.04266.x. [DOI] [PubMed] [Google Scholar]
- 31.Desnos C, et al. Rab27A and its effector MyRIP link secretory granules to F-actin and control their motion toward release sites. J Cell Biol. 2003;163:559–570. doi: 10.1083/jcb.200302157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waselle L, et al. Involvement of the Rab27-binding protein Slac2c/MyRIP in insulin exocytosis. Mol Biol Cell. 2003;14:4103–4113. doi: 10.1091/mbc.E03-01-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shirataki H, et al. Rabphilin-3A, a putative target protein for smg p25A/rab3A p25 small GTP-binding protein related to synaptotagmin. Mol Cell Biol. 1993;13:2061–2068. doi: 10.1128/mcb.13.4.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El-Amraoui A, et al. MyRIP, a novel Rab effector, enables myosin VIIa recruitment to retinal melanosomes. EMBO Rep. 2002;3:463–470. doi: 10.1093/embo-reports/kvf090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ivarsson R, et al. Myosin 5a controls insulin granule recruitment during late-phase secretion. Traffic. 2005;6:1027–1035. doi: 10.1111/j.1600-0854.2005.00342.x. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe M, et al. Myosin-Va regulates exocytosis through the submicromolar Ca2+-dependent binding of syntaxin-1A. Mol Biol Cell. 2005;16:4519–4530. doi: 10.1091/mbc.E05-03-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohya T, et al. Involvement of Rabphilin3 in endocytosis through interaction with Rabaptin5. J Biol Chem. 1998;273:613–617. doi: 10.1074/jbc.273.1.613. [DOI] [PubMed] [Google Scholar]
- 38.Coppola T, et al. Rabphilin dissociated from Rab3 promotes endocytosis through interaction with Rabaptin-5. J Cell Sci. 2001;114:1757–1764. doi: 10.1242/jcs.114.9.1757. [DOI] [PubMed] [Google Scholar]
- 39.Burns ME, et al. Rabphilin-3A: A multifunctional regulator of synaptic vesicle traffic. J Gen Physiol. 1998;111:243–255. doi: 10.1085/jgp.111.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deák F, et al. Rabphilin regulates SNARE-dependent repriming of synaptic vesicles for fusion. EMBO J. 2006;25:2856–2866. doi: 10.1038/sj.emboj.7601165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fukuda M. Distinct Rab binding specificity of Rim1, Rim2, rabphilin, and Noc2: Identification of a critical determinant of Rab3A/Rab27A recognition by Rim2. J Biol Chem. 2003;278:15373–15380. doi: 10.1074/jbc.M212341200. [DOI] [PubMed] [Google Scholar]
- 42.Tsuboi T, Fukuda M. Rab3A and Rab27A cooperatively regulate the docking step of dense-core vesicle exocytosis in PC12 cells. J Cell Sci. 2006;119:2196–2203. doi: 10.1242/jcs.02962. [DOI] [PubMed] [Google Scholar]
- 43.Fukuda M, et al. Inositol 1,3,4,5-tetrakisphosphate binding to C2B domain of IP4BP/synaptotagmin II. J Biol Chem. 1994;269:29206–29211. [PubMed] [Google Scholar]
- 44.Fukuda M, et al. Conserved N-terminal cysteine motif is essential for homo- and heterodimer formation of synaptotagmins III, V, VI, and X. J Biol Chem. 1999;274:31421–31427. doi: 10.1074/jbc.274.44.31421. [DOI] [PubMed] [Google Scholar]
- 45.Fukuda M, Mikoshiba K. A novel alternatively spliced variant of synaptotagmin VI lacking a transmembrane domain: Implications for distinct functions of the two isoforms. J Biol Chem. 1999;274:31428–31434. doi: 10.1074/jbc.274.44.31428. [DOI] [PubMed] [Google Scholar]
- 46.Crispino M, et al. Neurofilament proteins are synthesized in nerve endings from squid brain. J Neurochem. 1993;61:1144–1146. doi: 10.1111/j.1471-4159.1993.tb03632.x. [DOI] [PubMed] [Google Scholar]
- 47.Llinás R. The Squid Giant Synapse. New York: Oxford Univ Press; 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.