Abstract
Purpose
Previous studies have shown that binocular coordination during saccadic eye movement is affected in humans with large strabismus. The purpose of this study was to examine the conjugacy of saccadic eye movements in monkeys with sensory strabismus.
Methods
The authors recorded binocular eye movements in four strabismic monkeys and one unaffected monkey. Strabismus was induced by first occluding one eye for 24 hours, switching the occluder to the fellow eye for the next 24 hours, and repeating this pattern of daily alternating monocular occlusion for the first 4 to 6 months of life. Horizontal saccades were measured during monocular viewing when the animals were 2 to 3 years of age.
Results
Horizontal saccade testing during monocular viewing showed that the amplitude of saccades in the nonviewing eye was usually different from that in the viewing eye (saccade disconjugacy). The amount of saccade disconjugacy varied among animals as a function of the degree of ocular misalignment as measured in primary gaze. Saccade disconjugacy also increased with eccentric orbital positions of the nonviewing eye. If the saccade disconjugacy was large, there was an immediate postsaccadic drift for less than 200 ms. The control animal showed none of these effects.
Conclusions
As do humans with large strabismus, strabismic monkey display disconjugate saccadic eye movements. Saccade disconjugacy varies with orbital position and increases as a function of ocular misalignment as measured in primary gaze. This type of sensory-induced strabismus serves as a useful animal model to investigate the neural or mechanical factors responsible for saccade disconjugacy observed in humans with strabismus.
Binocular alignment and binocular coordination of eye movements is important in primates, who have frontal vision and foveae to direct gaze at a particular object.1,2 Loss of sensory or motor fusion early in postnatal development leads to binocular misalignment (strabismus).3 Strabismus during the first 6 years of life occurs in 3% to 4% of all children.4 Most strabismus studies in the literature focus on issues of alignment, and, by comparison, relatively few studies have examined the state of binocular coordination in strabismic subjects. Maxwell et al.5 examined conjugacy of saccades in human subjects with one amblyopic eye and found that they were disconjugate. In addition, they showed the presence of postsaccadic drift in the amblyopic eye. In another study, Kapoula et al.6 examined saccade conjugacy in human subjects with small and large angles of strabismus but no amblyopia. They also found disconjugate saccades and postsaccadic drift in their sample of strabismic subjects and were able to show that humans with larger strabismus tended to have greater disconjugacy. In an accompanying study the same group suggested that, in humans with large strabismus, the loss of disconjugate adaptation mechanisms as a result of the loss of binocular vision could be responsible for the development of saccade disconjugacy.7 More recently, Bucci et al.8 examined saccade conjugacy in strabismic children before and after strabismus surgery and found that in addition to improvement in eye alignment as a consequence of strabismus surgery, there was also an improvement in the conjugacy of saccades.
Previously, we showed that strabismus can be induced in rhesus monkeys by rearing them with visual sensory deprivation paradigms for the first few months of life, the critical period for the development of binocularity, stereoacuity, and eye alignment (Das VE, et al. IOVS 2004;45:ARVO E-Abstract 2545).3,9-12 When infant animals were reared according to an alternate monocular occlusion method (described in Subjects and Methods), they developed large strabismus. In addition, the animals developed A/V patterns and dissociated vertical deviation (DVD), both common disorders observed in humans with strabismus. In our published study, we measured binocular eye movements in these animals and showed that static alignment patterns were reflected in their eye movements.9 In another study, we examined the efficacy of saccade adaptation using the paradigm developed by McLaughlin13 and were able to show that certain adaptive mechanisms remained conjugate even in monkeys with large strabismus.14 Thus, we were able to establish our animal model as suitable for examining various issues relating to binocular alignment and binocular coordination in the strabismic condition.
The main goal of this study was to examine the conjugacy of saccades in monkeys with alternate monocular occlusion-induced strabismus. We found that, as do humans with large strabismus, monkeys with strabismus developed disconjugate saccades and postsaccadic drift. Some of these results have appeared before in abstract form.15,16
Subjects and Methods
Subjects and Rearing Paradigms
Behavioral data were collected from four strabismic (AMO1 to AMO4) juvenile rhesus monkeys (Macaca mulatta) and 1 monkey without strabismus. Monkeys with strabismus were born and reared at the Yerkes National Primate Research Center according to an alternate monocular occlusion (AMO) method for the first few months of life designed to induce ocular misalignment but not to affect visual acuity.9,10 In the AMO rearing procedure, soon after birth (within the first 24 hours), an occluding patch (either dark opaque goggle lenses or dark opaque contact lenses) is placed in front of one eye for a period of 24 hours and then switched to the fellow eye for the next 24 hours. The patch is alternated daily for a period of 4 to 6 months. In this method, binocular vision is severely disrupted during the first few months of life, the critical period during which the monkey brain normally develops proper eye alignment, stereovision, and binocular sensitivity.3,11,12 During AMO rearing, contact lens wear was monitored every 2 hours during the day to verify compliance with the rearing protocol.
Surgical Procedures and Eye Movement Measurements
After AMO rearing, the animals were allowed to grow normally until they were approximately 2 to 3 years of age, and then behavioral experiments were begun. Sterile surgical procedures carried out under aseptic conditions with isoflurane anesthesia (1.25%-2.5%) were used to stereotaxically implant a head stabilization post. During the same surgical procedure, a scleral search coil was implanted in one eye according to the technique of Judge et al.17 Later, during second surgery, a second scleral search coil was implanted in the other eye. All procedures were performed in strict compliance with National Institutes of Health guidelines and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and the protocols were reviewed and approved by the Institutional Animal Care and Use Committee at Emory University.
Binocular eye position was measured according to the magnetic search coil method (CNC Engineering, Seattle, WA).18,19 Calibration of the eye coil signal was achieved by rewarding the monkey with a small amount of juice or other incentive when the animal looked within a small region (±2° window) surrounding a 0.25° target spot that was rear projected on a tangent screen 57 cm away from the animal. The accuracy of the search coil technique itself is approximately 0.1°. In addition, eye traces were calibrated offline using data collected when the animals fixated static targets stepping from -20° to +20° (with steps of 5°) vertically and horizontally. Animals were trained for approximately 2 to 3 months before data collection. Calibration of each eye was performed independently during monocular viewing.
Experimental Paradigms, Data Acquisition, and Analysis
The primary goal of the study was to examine eye movement conjugacy during horizontal saccades. During the saccadic tasks, the target was located to the left or to the right of the vertical meridian in the visual field and stepped 5°, 10°, 15°, 20°, 25°, and 30° to the left or to the right along the horizontal meridian. All saccadic tasks were performed under monocular viewing conditions to avoid potential sensory confusion. All stimuli were under computer control.
Eye and target feedback signals were digitized to a computer with National Instruments (Austin, TX) or Cambridge Electronic Designs (CED; Cambridge, UK) hardware interface with 16-bit resolution at 1 kHz. Data analysis was performed with custom software (Matlab; Mathworks, Natick, MA). Ocular misalignment was determined from the eye-position records. During saccadic trials, we examined the misalignment at the end of the initial saccade, the postsaccadic drift that followed the initial saccade, and the final misalignment in steady state (i.e., after drift). Saccade onset and offset were determined by an acceleration criterion. Onset was defined as the time point when eye acceleration first rose to a level 3 SD higher than the average amount of acceleration within the previous 50 ms (control eye acceleration). Similarly, saccade offset was defined as the time point when eye acceleration returned to control eye acceleration values.14 Saccadic gain was defined as the ratio of target amplitude and eye amplitude. Disconjugacy was determined as the difference between the right eye and left eye positions.
Statistical Analysis
Statistical analysis was performed with one-way ANOVA. P = 0.05 was considered significant for comparisons between means for control and AMO monkeys.
Results
Static Ocular Alignment and Visual Capability
We have previously described horizontal strabismus, A/V patterns, and vertical ocular misalignment consistent with dissociated vertical dissociation (DVD) in the same AMO animals that were a part of this study.9 In our previous publication, we also showed static Hess screen charts of the animals in the present study (AMO1 here corresponds to S4 in our previous paper; AMO3 corresponds to S2; AMO4 corresponds to S3). Binocular visual acuity for the AMO animals, measured at the end of the rearing period using preferential looking techniques with sinusoidal gratings (Teller Acuity Cards), ranged from 4.1 cycles per degree (cyc/deg) to 8.3 cyc/deg around age 16 weeks, which was close to the visual acuity (5.8 cyc/deg) of animals without strabismus.20-22 We did not specifically determine the presence of amblyopia by measuring monocular acuity, but binocular visual acuity and eye preference data suggested some degree of amblyopia only in AMO3 (visual acuity. 8.3 cyc/deg). AMO animals in this study exhibited persistent ocular misalignment (strabismus) beginning at age 4 to 6 months, when the AMO rearing ceased, as noted during the visual acuity assessment. The degree of misalignment was not measured until the animals were mature enough to be behaviorally trained (2-3 years of age); therefore, we are unable to comment on whether the degree of strabismus changed in the interval. Two of the AMO monkeys (AMO1 and AMO2) were also tested for binocular stereo tracking with the use of a frame-sequential stereo display generated by Vision Research Graphic (VRG; Durham, NH). This was accomplished by having the monkey track a moving vertical stereo patch that stood out from the background approximately 20 min arc. Qualitative assessment of the tracking behavior suggested that the two AMO monkeys tested had no binocular stereo tracking, whereas the monkey without strabismus did. The rest of the study mainly focused on changes in ocular misalignment during horizontal saccades.
Characteristics of Saccades in AMO Monkeys
Figure 1 illustrates a raw eye position record for monkey AMO4 during horizontal saccades. Figure 1A illustrates eye position during monocular viewing with the left eye, and Figure 1B shows a right-eye viewing condition. The amount of ocular misalignment (disconjugacy) is also illustrated. This figure demonstrates that AMO4 had crossed ocular misalignment (esotropia) under monocular viewing conditions. The magnitude of the misalignment varied with orbital position. During left-eye viewing (Fig. 1A), misalignment increased during right gaze. During right-eye viewing, misalignment increased during left gaze (Fig. 1B). This pattern of disconjugate saccades and apparent orbital position-dependent changes in ocular misalignment was observed in all monkeys.
Figure 1.
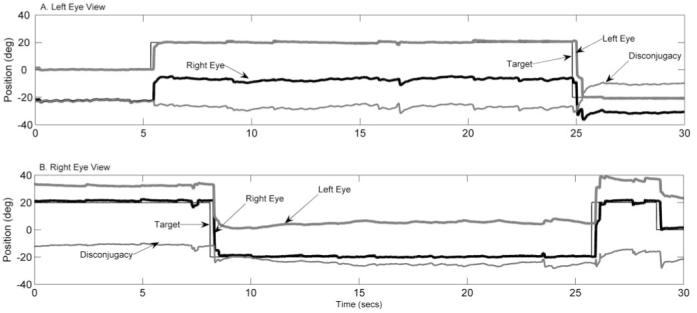
Eye position records for esotropic monkey AMO4 during monocular viewing with the left eye (A) and with the right eye (B). Traces indicate target position (black light line), right-eye position (black heavy line), left-eye position (gray heavy line), and disconjugacy (gray light line). Disconjugacy or ocular misalignment was determined by subtracting right-eye position from left-eye position. Positive values indicate rightward positions, and negative values indicate leftward positions. The figure illustrates that saccade are disconjugate and that ocular misalignment appears to vary with orbital position.
We were unable to adequately assess saccade disconjugacy during binocular viewing because the animals tended to alternate the eye of fixation and did not generate saccades with a wide range of amplitudes. Therefore, saccade testing was limited to monocular viewing. Figure 2A-C shows summary data that illustrate the trajectory (mean ± 1 SD) of all saccades made to three different target steps (10°, 20°, and 30°) during right-eye viewing by the esotropic monkey (AMO4). The viewing eye (2A) landed on target with some postsaccadic drift. However, the nonviewing eye (2B) always fell short of the target. Because of this saccade disconjugacy, the degree of ocular misalignment (right eye position minus left eye position; 2C) was 12° at the beginning of the saccades and increased to 20° to 25° immediately after the saccade. Figure 2D-F shows similar summary data of the saccadic trajectory (mean ± 1 SD) for each target step in one of the exotropic monkeys (AMO3) during left-eye viewing. Saccades initially fell short of the target for the viewing eye, and the eye drifted onward to reach the target. Postsaccadic drift increased with saccade size. For each saccade, ocular misalignment decreased after the saccade.
Figure 2.
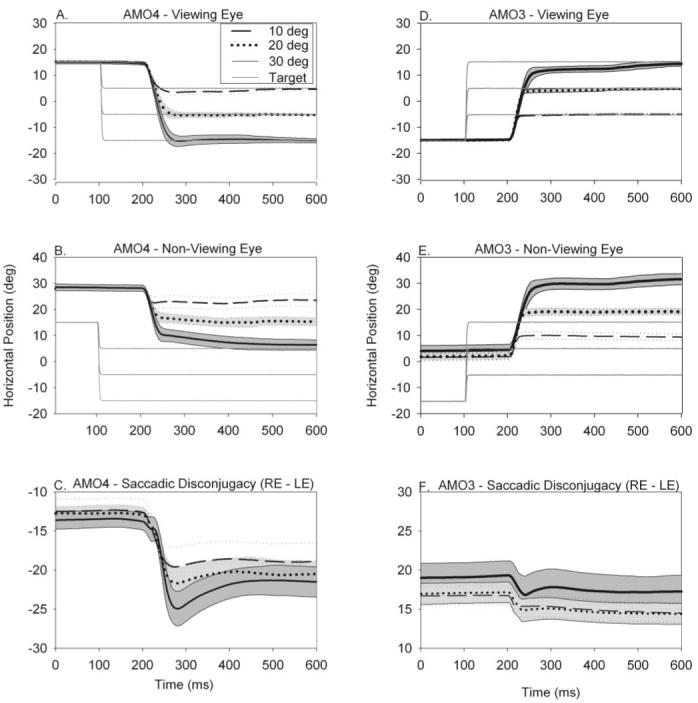
(A-C) Saccadic eye movement trajectories made to three different leftward target steps (10°, 20°, and 30°) from the same orbital eye position in esotropic monkey AMO4 when the right eye was viewing (left eye was patched). During straight-ahead binocular viewing, this animal had esotropia of 21°. Positive values indicate rightward eye position, and negative values indicate leftward eye position. (A) Trajectory of the viewing (right) eye. (B) Trajectory of the nonviewing (left) eye. (C) Degree of ocular misalignment (right-eye position - left-eye position). (D-F) Similar data from exotropic monkey AMO3 for rightward target steps during left-eye viewing. Both animals displayed disconjugate saccadic eye movements and postsaccadic drift that was orbital position dependent. Therefore, ocular misalignment before the saccade, at the end of the saccade pulse (i.e., after initial saccade), and at the end of the saccadic step (i.e., after postsaccadic drift) are all different. In all panels, trials are aligned on saccade onset.
Gain of Saccadic Pulse for the Viewing Eye and the Nonviewing Eye
Saccades generated by the viewing eye were usually able to acquire the target through a combination of an initial saccade and postsaccadic drift. To make a more precise quantitative evaluation of saccadic accuracy, we measured the gain of the initial saccade (equivalent to a measure of the saccadic pulse) in the viewing and nonviewing eyes. Figure 3 plots saccadic pulse gain (eye amplitude/target amplitude) for all the monkeys for rightward and leftward saccades in the left eye and right-eye viewing conditions (total of 14 combinations for the AMO animals). Comparison of the saccadic gain in the viewing and nonviewing eyes for the AMO animals showed significant differences (P < 0.05) in 11 of 14 conditions (only AMO2 rightward saccades left-eye viewing, AMO4 leftward saccade left-eye viewing, and AMO3 rightward saccades right-eye viewing did not show significant differences between viewing and nonviewing eyes). However, there appeared to be no consistent pattern to the pulse gain difference between viewing and nonviewing eyes.
Figure 3.
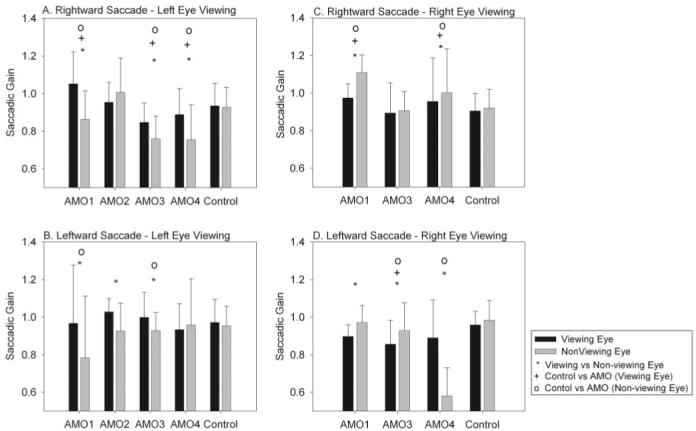
(A, B) Rightward and leftward saccadic pulse gains in all monkeys for the viewing eye and the nonviewing eye for left-eye viewing. (C, D) Rightward and leftward saccadic pulse gains in all monkeys for the viewing eye and the nonviewing eye for right-eye viewing condition. Statistically significant differences (P < 0.05) obtained from multiple comparisons (○, +, *). The main finding is that there were differences in the pulse gain between viewing and nonviewing eyes in the strabismic animals, though the differences were idiosyncratic.
Comparison of the control monkey with the AMO animals also yielded mixed results. Thus, in 9 of 14 conditions, the saccadic gain in the viewing eye of the AMO monkeys was different from the saccadic gain in the viewing eye of the control animal (one-way ANOVA with multiple comparisons at P = 0.05). In 10 of 14 conditions, the saccadic gain in the nonviewing eye of the AMO animals was different from the saccadic gain in the nonviewing eye of the control animal (one-way ANOVA with multiple comparisons at P = 0.05).
In summary, analysis of saccadic gain suggested that, even though differences were idiosyncratic, at least part of the disconjugacy in AMO animals was the result of differences in the saccadic pulse to the viewing and nonviewing eyes. Comparison with the control animal suggested that the nonviewing eye was more likely to be the affected eye though often both eyes were affected.
Main-Sequence Relationships
We also examined the amplitude-peak velocity main sequence relationship of the saccades in our sample to consider whether there might be some inherent differences in saccadic behavior between viewing and nonviewing eyes. Figure 4 plots the main-sequence relationship for the viewing and nonviewing eyes in the control animal (Fig. 4A), exotropic monkey AMO3 (Fig. 4B), and esotropic monkey AMO4 (Fig. 4C). These data suggest significant overlap in prediction bands between viewing and nonviewing eyes and also across the animals (i.e., comparing AMO3, AMO4, and the control). Our main focus for the rest of the study was analysis of the relationship between ocular misalignment and orbital position.
Figure 4.
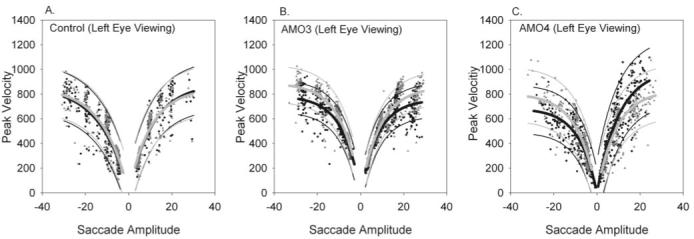
Amplitude-peak velocity relationship in viewing (gray triangles) and nonviewing (black circles) eyes in control animal (A), exotropic animal AMO3 (B), and esotropic animal AMO4 (C). Data for these figures were obtained during monocular left-eye viewing. Also plotted are exponential curve fits (heavy lines), along with 95% prediction bands (light lines). The gray lines are fits to the viewing eye data, and black lines are fits to the nonviewing eye data. There is significant overlap in the prediction intervals for the viewing and nonviewing eyes in the control animal, esotrope, and exotrope, suggesting that there was no difference in amplitude-peak velocity main-sequence relationship between viewing and nonviewing eyes. The main-sequence relationship was also similar when the strabismic animals were compared with the control animal.
Relationship between Misalignment at End of Saccadic Pulse and Orbital Position
To investigate orbital position dependence of eye misalignment, we first examined the role of disconjugacy in the saccadic pulse by measuring the magnitude of misalignment at the end of the saccadic pulse. For this data set, we analyzed all saccades of different amplitudes and directions that ended at orbital positions between left 20° to right 15°. An example of this analysis is illustrated in Figure 5A, which shows saccades made in AMO4 during left-eye viewing. For saccades ending in right orbital positions, the degree of ocular misalignment increased. Linear regression of these saccades showed a slope of - 0.14 (P < 0.001; r = 0.28). Figure 5B shows the relation between disconjugacy of saccadic pulse and orbital position for all the monkeys during left-eye viewing condition. In the exotropic monkeys (AMO1, AMO2, AMO3), decreased ocular misalignment was observed for saccades terminating in right orbital positions (slopes and goodness-of-fit shown in Table 1; estimates of slope by means of linear regression were significant at P < 0.001). Ocular misalignment increased in the esotropic monkey (AMO4). The control animal without strabismus had no ocular misalignment at different orbital positions (slope of - 0.0044; P = 0.44). In summary, the pulse of the saccades was disconjugate, and the degree of disconjugacy varied according to orbital eye position for AMO animals. We explicitly checked whether the disconjugacy was a function of saccade size by attempting linear regression between ocular misalignment and saccade amplitude (instead of orbital position). These fits yielded significantly poorer fits than those shown in Figure 5 and Table 1 and resulted in estimated slopes close to zero, indicating that there was no relationship between misalignment and saccade size.
Figure 5.
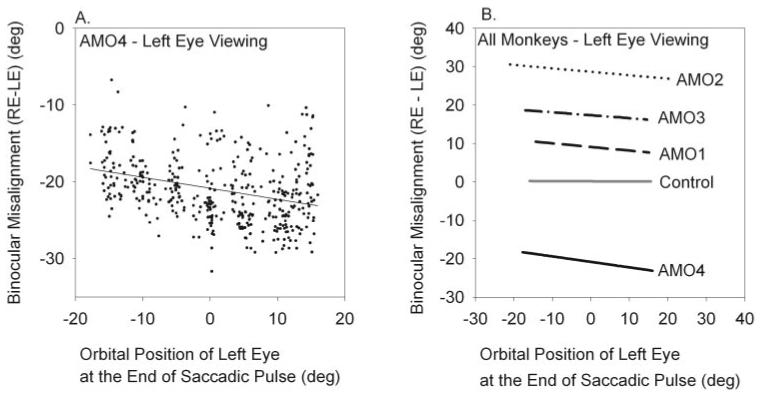
(A) Relation of binocular misalignment immediately at the end of the saccadic pulse (disconjugacy of the saccadic pulse) with orbital position in AMO4 during left-eye viewing. Black dots indicate each saccade. The black line represents linear regression to the saccade data. (B) Summary of fits for all our monkeys obtained during the left-eye viewing condition showing a linear relationship between disconjugacy of saccadic pulse and orbital position. The orbital position of the viewing (left) eye at the end of initial saccade is plotted on the x-axis. The amount of misalignment at the end of initial saccade is plotted on the y-axis. Details about each of the fits such as slopes and goodness-of-fit are provided in Table 1.
Table 1.
Summary of Slopes under Left-Eye Viewing and Right-Eye Viewing Conditions for All AMO Animals
| Saccadic Pulse Regression |
Saccadic Step Regression |
|||||
|---|---|---|---|---|---|---|
| Name | Slope | Goodness of Fit (r) | SL1 | SL2 | Inflection Point (°) | Goodness of Fit (r) |
| Left-Eye Viewing | ||||||
| AMO1 | -0.10 | 0.67 | -0.09 | 0.08 | -11 | 0.67 |
| AMO2 | -0.09 | 0.35 | -0.34 | 0.06 | 4 | 0.87 |
| AMO3 | -0.08 | 0.17 | -0.22 | 0.002 | 9 | 0.11 |
| AMO4 | -0.14 | 0.28 | -0.45 | 0.08 | -2 | 0.47 |
| Right-Eye Viewing | ||||||
| AMO1 | -0.09 | 0.47 | No change in misalignment with orbital position | |||
| AMO3 | -0.11 | 0.21 | -0.48 | 0.07 | -1 | 0.48 |
| AMO4 | 0.29 | 0.69 | 0.34 | -0.03 | -9 | 0.79 |
Regression coefficients for the control animal and for AMO1 (right-eye viewing) are not significant because there was no variation with orbital position. All other coefficients are significant at P < 0.001.
Relationship between Misalignment at End of Saccadic Step and Orbital Position
Postsaccadic drift was observed in all AMO monkeys, usually in the nonviewing eye and occasionally in the viewing eye. The effect of the postsaccadic drift was that steady state misalignment (i.e., after saccadic step) was different from the misalignment at the end of the initial saccade (i.e., after the saccadic pulse; Fig. 2C). Therefore, similar to our analysis of the relationship between ocular misalignment at end of saccade pulse and orbital position described in the previous section, we also analyzed the relationship between ocular misalignment at the end of the saccade step and orbital position. An example of this relationship in AMO4 (left-eye viewing) is shown in Figure 6A.
Figure 6.
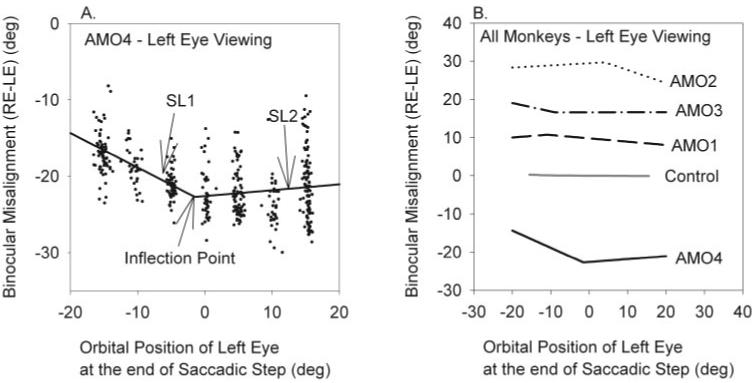
Relation of binocular misalignment at the end of the saccadic step (i.e., after postsaccadic drift) with orbital position. (A) Data collected in AMO4 during left-eye viewing. Black dots indicate each saccade. Because the data indicated that ocular misalignment was comitant for certain orbital positions after the saccadic step, we used two-segment, piecewise linear regression to fit all the saccade data. That portion of the line that varied with orbital eye position was labeled SL1. That portion of the line that varied very little with orbital eye position was labeled SL2. The inflection point is the orbital position at which the slopes changed, as determined by regression analysis. (B) Summary of two-segment fits of saccade data after saccadic step obtained during left-eye viewing. In each of the AMO animals, we were able to identify a region of relative comitancy. Details about each of the fits, such as slopes SL1, SL2, inflection point, and the goodness-of-fit are provided in Table 1.
An unexpected observation shown in Figure 6A and observed in all the animals was that ocular misalignment at the end of the saccade step (i.e., in the steady state) did not vary linearly with orbital position for the entire range of orbital positions tested. This result was attributed to postsaccadic drift also being orbital position dependent, as can be observed in Figure 2A. The data suggested that the postsaccadic drift appeared to have an effect of producing a comitant misalignment for certain orbital positions. To quantify this relationship, we used two-segment, piecewise linear regression for all the saccade data in steady state (i.e., after postsaccadic drift). The choice of a two-segment regression rather than some other fitting function was arbitrary and driven by the observation that in the steady state, orbital misalignment appeared comitant for a range of orbital positions and incomitant elsewhere (as shown in Fig. 6A). Linear regression consisted of fitting two intersecting lines of different slopes and intercepts to the entire data set such that global error was minimized. No a priori assumptions were made on the intersection point. Parameters calculated from the fit were the slopes and intercepts of the two lines and the intersection point. Figure 6A shows a linear relationship between ocular misalignment and orbital position from -15° to -5° (slope of 0.34, SL1). The degree of misalignment remained almost the same from 0° to 15° (slope of - 0.03, SL2). Figure 6B plots steady state ocular misalignment of the saccadic step as a function of ending orbital position of saccades in all monkeys. For each monkey, orbital positions were observed in which the misalignment was more constant (SL2). These positions can be referred to as comitant orbital positions. Other orbital positions, in which the misalignment changed linearly (SL1), can be referred to as incomitant orbital positions. Given that the choice of the two-segment regression was arbitrary, we also attempted simple linear regression using the same data. In each case, the linear regression resulted in poorer fits (lower goodness-of-fit measure) than the two-segment fit.
Slopes of the comitant and incomitant positions and inflection points for each monkey are shown in Table 1. For the incomitant orbital positions, postsaccadic drift reduced the amount of ocular misalignment at some orbital positions for all AMO monkeys except AMO3, who showed a decreased saccadic pulse for the viewing eye. Because of the scatter in the data, some of the r signifying goodness-of-fit were poor, but all coefficients reported in the table were significant at P < 0.001.
Discussion
The main finding in our study is that the ocular misalignment in monkeys reared with AMO is not comitant but varies according to orbital position. Monkeys with XT had decreased abduction in the nonfixating eye. The monkey with ET had decreased adduction in the nonfixating eye. The degree of incomitance increased in monkeys with the largest degree of strabismus in primary gaze.
How do these incomitant patterns of static ocular misalignment measured during the steady state relate to saccadic eye movements? There are three possibilities. First, saccades could be conjugate, followed by disconjugate drifts that establish the new steady state magnitude of misalignment. Second, saccades could be disconjugate by an amount that establishes the new steady state. Third, the steady state misalignment at each orbital position could evolve over time from a combination of disconjugate saccades, followed by disconjugate drifts. In other words, there is a problem with the pulse (saccade gain is abnormal) and the step (postsaccadic drift varies for different orbital positions, resulting in different steady state misalignment). This third possibility applies to our animals. In our study, the saccadic pulse was disconjugate because the saccadic gain of the nonviewing eye was generally different from that of the viewing eye. The degree of disconjugacy of the saccadic pulse depended on orbital position. Disconjugacy increased for more eccentric eye positions. We also found substantial disconjugate postsaccadic drift at the end of the initial saccades. The nonviewing eye of each AMO monkey drifted after each saccade to reach the target. Postsaccadic drift was disconjugate in such a way that it affected final ocular alignment. At certain orbital positions, the ocular misalignment was nearly concomitant, i.e., it changed little or not at all (more apparent in AMO2, AMO3, and AMO4). Why drift should produce a region of comitancy is unclear. The lack of stereoacuity or the lack of comitancy in a large part of the oculomotor range appears to argue against a condition producing a visual benefit such as anomalous retinal correspondence (ARC). Our studies do not rule out all forms of binocular vision because we did not test for summation from each eye or coarse stereopsis.23
Comparison of our data with human studies showed that our monkeys with strabismus made disconjugate saccades that were similar to those of humans with infantile strabismus.6 However, the amount of disconjugacy in our monkeys was significantly more than that reported in human subjects. Thus, Kapoula et al.6 showed that in human subjects with ET of 2° to 18°, accurate saccades are observed in the viewing eye but saccades of decreased amplitude are observed in the nonviewing eye. Saccades in the nonviewing eye were followed by postsaccadic eye drift. The amount of saccade disconjugacy and direction of eye drift in the nonviewing eye was a function of the amount of misalignment. Other than relative magnitude, our results agree with the human data. In an accompanying paper, Bucci et al.7 suggested that impairment of binocular control of saccades may be attributed to the deficiency of disconjugate adaptive mechanisms needed to compensate for natural asymmetries or developmental changes of the oculomotor plants.24 However, conjugate adaptive mechanisms such as the saccade adaptation produced by the backward gain adaptation paradigm are intact in our animals.14 Therefore, our results support the suggestion that binocular vision is important in maintaining binocular oculomotor coordination, but disconjugacy may not be attributed to a generalized loss of the adaptive process. In another publication, Bucci et al.8 suggested that conjugacy of saccades improves after strabismus surgery because of central adaptive mechanisms. Although this hypothesis may be true and is supported by our previous saccade adaptation study, another possibility is that strabismus surgery has simply placed the nonfixating eye in orbital positions that result in smaller disconjugacy.
Which neural circuit, if any, might be disrupted, leading to the generation of disconjugate saccades? One possibility is the cerebellum because it is involved in binocular control of eye movements. Humans with cerebellar degeneration have ocular misalignment in the horizontal plane that is noncomitant and that generates disconjugate saccades.25 These same defects occur in mature monkeys with lesions of the dorsal oculomotor vermis.26 The dorsal oculomotor vermis, along with the underlying fastigial oculomotor region (FOR) and the pontine nuclei (dorsolateral pontine nucleus and nucleus reticularis tegmenti pontis), may be part of a circuit involved in vergence and binocular eye movement control.27,28 Takagi et al.28 postulate that this circuit controls the static and the dynamic aspects of maintaining eye alignment. To ensure normal concomitancy between both eyes, static alignment may be set by linearizing vergence tone as a function of the position of the eyes in the orbit. To ensure normal saccade conjugacy, this circuit may also control the saccadic pulse to each eye and the vergence tone during a saccade. Although disruption of this circuit could partially have been responsible for the inability to maintain binocular control of eye movements in our animals, there are some differences between the previously published monkey lesion data28 and data from our strabismic animals and from strabismic humans6 that suggest a more pervasive problem. First, the angle of strabismus produced by cerebellar vermal lesions is small (less than 2°) compared with approximately 20° in the strabismic monkeys in or humans. Second, the amount of saccade disconjugacy after vermal lesions is on the order of 1° to 2° for 20° to 30° saccades, whereas disconjugacy in strabismic monkeys and humans is at least an order higher. Therefore, one hypothesis could be that static and dynamic defects in binocular control of eye movements occurred in our monkeys as a result of loss of sensory binocular fusion during development, which in turn led to a cascade of oculomotor problems including a pulse-step mismatch and an inability of the ponto-cerebellar circuits to maintain binocular control of eye movements.
Mechanical factors could also have contributed to saccade disconjugacy in our animals. Recent work has suggested that the oculomotor plant is a more complex structure than previously thought.29 Extraocular muscle pulleys have been shown to play an important role in ensuring normal ocular motility including pulse-step matching.30,31 In our animals, the nonviewing eye is both exotropic and hypertropic. The eccentric location of the nonviewing eye could, therefore, result in different mechanical loads in the two eyes, orbital position-dependent disconjugacy, and pulse-step mismatches. The lack of difference in main-sequence relationships between viewing and nonviewing eyes could suggest that mechanical factors are not relevant. Caution must be exercised with this argument, however, because the disconjugacies are sometimes small, and corresponding differences in the main-sequence relationship between viewing and nonviewing eyes may not be easily discernible. One way to measure the contribution of mechanical factors would be to offset the target vertically during the horizontal saccadic testing. Vertical offsets would alter the degree of exotropia/hypertropia (because of the presence of A/V patterns and DVD) and presumably mechanical loads on the viewing/nonviewing eyes and possibly change the relationships between ocular misalignment and orbital position that we have reported. Another way to test the relative contributions of neural and mechanical factors in influencing saccade disconjugacy would be to record burst neuron activity during saccade behavior. If saccade disconjugacy is caused solely by mechanical factors, the prediction would be that identical neural burst signals are delivered to each eye.
Acknowledgments
The authors thank Tracey Brozyna and Riva Feldman for expert technical assistance.
Supported by National Institutes of Health Grants EY015312 (VED), EY06069 (MJM), and RR00165 (Yerkes base grant).
Footnotes
Disclosure: L. Fu, None; R.J. Tusa, None; M.J. Mustari, None; V.E. Das, None
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be marked “advertisement” in accordance with 18 U.S.C. §1734 solely to indicate this fact.
References
- 1.von Noorden GK. Binocular Vision and Ocular Motility: Theory and Management of Strabismus. 5th ed. CV Mosby; St. Louis: 1996. [Google Scholar]
- 2.Leigh RJ, Zee DS. The Neurology of Eye Movements. 3rd ed. Oxford University Press; New York: 1999. Contemporary Neurology Series. [Google Scholar]
- 3.Boothe RG, Dobson V, Teller DY. Postnatal development of vision in human and nonhuman primates. Ann Rev Neurosci. 1985;8:495–545. doi: 10.1146/annurev.ne.08.030185.002431. [DOI] [PubMed] [Google Scholar]
- 4.Lorenz B. Genetics of isolated and syndromic strabismus: facts and perspectives. Strabismus. 2002;10:147–156. doi: 10.1076/stra.10.2.147.8133. [DOI] [PubMed] [Google Scholar]
- 5.Maxwell GF, Lemij HG, Collewijn H. Conjugacy of saccades in deep amblyopia. Invest Ophthalmol Vis Sci. 1995;36:2514–2522. [PubMed] [Google Scholar]
- 6.Kapoula Z, Bucci MP, Eggert T, Garraud L. Impairment of the binocular coordination of saccades in strabismus. Vis Res. 1997;37:2757–2766. doi: 10.1016/s0042-6989(97)00064-3. [DOI] [PubMed] [Google Scholar]
- 7.Bucci MP, Kapoula Z, Eggert T, Garraud L. Deficiency of adaptive control of the binocular coordination of saccades in strabismus. Vision Res. 1997;37:2767–2777. doi: 10.1016/s0042-6989(97)00093-x. [DOI] [PubMed] [Google Scholar]
- 8.Bucci MP, Kapoula Z, Yang Q, Roussat B, Bremond-Gignac D. Binocular coordination of saccades in children with strabismus before and after surgery. Invest Ophthalmol Vis Sci. 2002;43:1040–1047. [PubMed] [Google Scholar]
- 9.Das VE, Fu LN, Mustari MJ, Tusa RJ. Incomitance in monkeys with strabismus. Strabismus. 2005;13:33–41. doi: 10.1080/09273970590910298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tusa RJ, Mustari MJ, Das VE, Boothe RG. Animal models for visual deprivation-induced strabismus and nystagmus. Ann NY Acad Sci. 2002;956:346–360. doi: 10.1111/j.1749-6632.2002.tb02833.x. [DOI] [PubMed] [Google Scholar]
- 11.Harwerth RS, Smith EL, 3rd, Crawford ML, von Noorden GK. Behavioral studies of the sensitive periods of development of visual functions in monkeys. Behav Brain Res. 1990;41:179–198. doi: 10.1016/0166-4328(90)90107-p. [DOI] [PubMed] [Google Scholar]
- 12.O’Dell C, Boothe RG. The development of stereoacuity in infant rhesus monkeys. Vis Res. 1997;37:2675–2684. doi: 10.1016/s0042-6989(97)00080-1. [DOI] [PubMed] [Google Scholar]
- 13.Mclaughlin SC. Parametric adjustment in saccadic eye movements. Percept Psychophys. 1967;2:359–362. [Google Scholar]
- 14.Das VE, Ono S, Tusa RJ, Mustari MJ. Conjugate adaptation of saccadic gain in non-human primates with strabismus. J Neurophysiol. 2004;91:1078–1084. doi: 10.1152/jn.00205.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Das VE, Fu LN, Ono S, Tusa RJ, Mustari MJ. Saccade disconjugacy and adaptation in strabismic monkeys. Ann NY Acad Sci. 2003;1004:381–384. [Google Scholar]
- 16.Fu LN, Das VE, Mustari MJ, Tusa RJ. Saccadic disconjugacy in monkeys with strabismus (Abstract) Soc Neurosci. 2001;27:71.42. [Google Scholar]
- 17.Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vis Res. 1980;20:535–538. doi: 10.1016/0042-6989(80)90128-5. [DOI] [PubMed] [Google Scholar]
- 18.Fuchs AF, Robinson DA. A method for measuring horizontal and vertical eye movement chronically in the monkey. J Appl Physiol. 1966;21:1068–1070. doi: 10.1152/jappl.1966.21.3.1068. [DOI] [PubMed] [Google Scholar]
- 19.Hess BJ, Van Opstal AJ, Straumann D, Hepp K. Calibration of three-dimensional eye position using search coil signals in the rhesus monkey. Vis Res. 1992;32:1647–1654. doi: 10.1016/0042-6989(92)90157-e. [DOI] [PubMed] [Google Scholar]
- 20.Teller DY, Boothe R. Development of vision in infant primates. Trans Ophthalmol Soc UK. 1979;99:333–337. [PubMed] [Google Scholar]
- 21.Tusa RJ, Repka MX, Smith CB, Herdman SJ. Early visual deprivation results in persistent strabismus and nystagmus in monkeys. Invest Ophthalmol Vis Sci. 1991;32:134–141. [PubMed] [Google Scholar]
- 22.Boothe RG, Louden TM, Lambert SR. Acuity and contrast sensitivity in monkeys after neonatal intraocular lens implantation with and without part-time occlusion of the fellow eye. Invest Ophthalmol Vis Sci. 1996;37:1520–1531. [PubMed] [Google Scholar]
- 23.Crawford ML, Harwerth RS, Chino YM, Smith EL., 3rd. Binocularity in prism-reared monkeys. Eye. 1996;10(pt 2):161–166. doi: 10.1038/eye.1996.41. [DOI] [PubMed] [Google Scholar]
- 24.Bucci MP, Kapoula Z, Eggert T, Garraud L. Deficiency of adaptive control of the binocular coordination of saccades in strabismus. Vis Res. 1997;37:2767–2677. doi: 10.1016/s0042-6989(97)00093-x. [DOI] [PubMed] [Google Scholar]
- 25.Versino M, Hurko O, Zee DS. Disorders of binocular control of eye movements in patients with cerebellar dysfunction. Brain. 1996;119:1933–1950. doi: 10.1093/brain/119.6.1933. [DOI] [PubMed] [Google Scholar]
- 26.Takagi M, Zee DS, Tamargo RJ. Effects of lesions of the oculomotor vermis on eye movements in primate: saccades. J Neurophysiol. 1998;80:1911–1931. doi: 10.1152/jn.1998.80.4.1911. [DOI] [PubMed] [Google Scholar]
- 27.Gamlin PD, Yoon K, Zhang H. The role of cerebro-ponto-cerebellar pathways in the control of vergence eye movements. Eye. 1996;10:167–171. doi: 10.1038/eye.1996.42. [DOI] [PubMed] [Google Scholar]
- 28.Takagi M, Tamargo R, Zee DS. Effects of lesions of the cerebellar oculomotor vermis on eye movements in primate: binocular control. Prog Brain Res. 2003;142:19–33. doi: 10.1016/S0079-6123(03)42004-9. [DOI] [PubMed] [Google Scholar]
- 29.Miller J. No oculomotor plant, no final common path. Strabismus. 2003;11:205–211. doi: 10.1076/stra.11.4.205.24308. [DOI] [PubMed] [Google Scholar]
- 30.Demer JL. Current concepts of mechanical and neural factors in ocular motility. Curr Opin Neurol. 2006;19:4–13. doi: 10.1097/01.wco.0000198100.87670.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quaia C, Optican LM. Commutative saccadic generator is sufficient to control a 3-D ocular plant with pulleys. J Neurophysiol. 1998;79:3197–3215. doi: 10.1152/jn.1998.79.6.3197. [DOI] [PubMed] [Google Scholar]


