Abstract
Although long-lasting effects of drug withdrawal are thought to play a key role in motivating continued drug use, the mechanisms mediating this type of drug-induced plasticity are unclear. As Narp is an immediate early gene product that is secreted at synaptic sites and binds to AMPA receptors, it has been implicated in mediating enduring forms of synaptic plasticity. In previous studies, we found that Narp is selectively induced by morphine withdrawal in the extended amygdala, a group of limbic nuclei that mediate aversive behavioral responses. Accordingly, in this study, we evaluated whether long-term aversive effects of morphine withdrawal are altered in Narp KO mice. We found that acute physical signs of morphine withdrawal are unaffected by Narp deletion. However, Narp KO mice acquire and sustain more aversive responses to the environment conditioned with morphine withdrawal than WT controls. Paradoxically, Narp KO mice undergo accelerated extinction of this heightened aversive response. Taken together, these studies suggest that Narp modulates both acquisition and extinction of aversive responses to morphine withdrawal and, therefore, may regulate plasticity processes underlying drug addiction.
Keywords: Immediate Early Gene, Naltrexone, Place Aversion, AMPA, Amygdala
INTRODUCTION
Although much attention has been paid to the role of reward in sustaining addiction to drugs of abuse, it has been proposed that the negative affective states associated with drug withdrawal also play an important role in motivating continued compulsive drug use (Khantzian, 1985; Koob, 1999). Several studies have focused attention on the ability of environmental cues associated with either opiate withdrawal per se (Koob and LeMoal, 2005) or with opiates given to alleviate withdrawal (Hutcheson et al., 2001) to trigger relapse in individuals who have undergone long periods of abstinence. For example, Goldberg et al. (1969) showed that presentation of previously neutral stimuli to morphine-dependent rhesus monkeys following repeated nalorphine-induced withdrawal episodes resulted in large conditioned increases in morphine self-administration. Along these lines, cues previously paired with withdrawal can precipitate a withdrawal-like state both in rats (Schulteis et al., 2000) and humans (O'Brien et al., 1977). More recently, Kenny et al., (2006) report a similar phenomenon in heroin-dependent self-administering rats. Thus, further insights into the mechanisms mediating this type of conditioning may be quite helpful in combating drug relapse.
Amongst the limbic structures thought to be key in mediating the aversive effects of morphine withdrawal are the amygdala nuclei and brain regions making up the “extended amygdala”, including the central nucleus of the amygdala (CeA), the bed nucleus of the stria terminalis (BNST) and the shell of the nucleus accumbens (NAc; Heimer et al., 1997; Koob, 1999). Recent evidence also points to a critical role for the basolateral amygdala (BLA) in mediating conditioned place aversion (CPA) following morphine withdrawal. Schulteis et al., (2000) showed that selective lesions of the BLA prevent the development of CPA in morphine-dependent rats. More recently, Frenois et al (2005) have demonstrated that following morphine withdrawal, re-exposure to the withdrawal-paired environment increases the expression of c-fos in the BLA. Hellemans et al., (2006) found that disrupting reconsolidation of conditioned withdrawal memories by injecting zif268 antisense oligonucleotides in the BLA reduces suppression of heroin seeking in rats. Similarly, other structures within the “extended amygdala” also play important roles in mediating the aversive responses to drug withdrawal (Stinus et al., 1990; Heinrichs et al., 1995; Delfs et al., 2000; Watanabe et al., 2002 a,b).
As the aversive effects of drug withdrawal can be long-lasting, it is tempting to speculate that their enduring effects may be mediated by induction of immediate early genes (IEGs). Support for this line of reasoning has come from recent studies demonstrating that morphine withdrawal is associated with heightened activation of CREB and CRE-mediated transcription in the CeA as well as other parts of the extended amygdala (Shaw-Lutchman, 2002). In addition, as mentioned above, two IEGs, c-fos and zif268, have been implicated in mediating long-term aversive responses triggered by opiate withdrawal (Frenois et al., 2005; Hellemans et al., 2006). Furthermore, in previous studies we found that Narp is induced in the extended amygdala by morphine withdrawal (Reti and Baraban, 2003).
Narp is an attractive candidate for mediating long-lasting forms of synaptic plasticity since it binds directly to AMPA receptors (O'Brien et al., 1999). Although its precise role in AMPA receptor trafficking is still under investigation, studies conducted to date have established that it is a secreted protein that is targeted to axons and released in an activity-dependent fashion (Tsui et al., 1996; O'Brien et al., 1999; Reti et al., 2002; Reti et al., 2007). Furthermore, the available evidence indicates that Narp monomers aggregate into either homomeric complexes or heteromeric complexes containing other members of the neuronal pentraxin family, NP1 and NPR (Kirkpatrick et al., 1999; Xu et al., 2003). These complexes bind to the extracellular surface of AMPA receptors and regulate their clustering at synaptic sites. Thus, Narp has emerged as an IEG that is well-positioned to regulate synaptic plasticity.
In light of these findings, we hypothesized that Narp induction in the extended amygdala (Reti and Baraban, 2003) may mediate the long-term, aversive behavioral effects induced by morphine withdrawal. We report here our evaluation of this hypothesis. Using Narp KO mice, we have assessed whether CPA acquired after morphine withdrawal is altered by Narp deletion.
MATERIALS AND METHODS
Animals
Experiments were approved by the Johns Hopkins University Institutional Animal Care and Use Committee. Both male and female mice between 3 and 5 months were used for all experiments. Mice were maintained on a 12 hour light/dark cycle and were given access to food and water ad libitum. For anatomic studies we used C57BL/6 mice (Charles River) and for behavioral experiments we used Narp KO and WT control mice on an N4 background generated by breeding Narp heterozygotes.
Generation of Narp KO mice is described elsewhere (Johnson et al., 2007). Briefly, 129 genomic DNA was prepared by screening of a mouse genomic DNA BAC library. Genomic DNA regions of the mouse Narp gene were subcloned into pBluescript vector and the targeting vector construct was based on mouse genomic DNA databases. The entire exon 2 was replaced by a neo resistant gene cassette. ES cell screening and generation of the knock out mouse line were carried out as described previously (Kim et al., 2003; Gardner et al., 2005). Deletion of exon 2 was confirmed by Southern blot and absence of Narp protein in brain was confirmed by Western blot.
Morphine dependence and withdrawal
Morphine sulfate was delivered to mice via a micro-osmotic pump (Model 2001, Durect Corporation) designed to deliver morphine at a constant rate of about 4 mg/kg/hour for 7 days. The pump was placed subcutaneously on the mouse's back under light anesthesia (a solution containing ketamine 80mg/kg and xylazine 12mg/kg). Morphine withdrawal was induced in anatomic and behavioral studies by administration of naltrexone 250ug/kg via subcutaneous (sc) injection at varying time points after implantation, as described below.
Anatomical studies
Morphine dependent C57BL/6 mice were subjected to morphine withdrawal in a novel environment 3 days after pump implantation. Control mice which did not undergo morphine withdrawal were either implanted with saline filled pumps and were then administered naltrexone 250ug/kg sc, or were implanted with morphine filled pumps and then administered saline sc. Four hours after the sc injection, the mice were deeply anesthetized and then perfused transcardially with a 4% paraformaldehyde fixative. Twenty micrometer coronal brain sections were cut on a freezing microtome and tissue was processed for Narp immunohistochemistry as previously described (O'Brien et al., 1999; Reti et al., 2002b,c). Rabbit anti-Narp antibody (a gift of Dr. Paul Worley) was used at 1:1500. To control for the specificity of Narp immunostaining, we confirmed that staining is absent in brain sections from Narp KO mice.
Narp positive cell bodies were counted by a person blind to the withdrawal status of the animals. For each mouse used to monitor changes in the number of Narp-immunopositive cells, these cells were counted in 3 − 5 sections containing the structure being analyzed and the values obtained averaged. Sections counted for the BLA and CeA were cut midlevel through these nuclei at which the stria terminalis is positioned dorsomedially to the CeA. Fields counted for the BNST were cut at a level corresponding to figure 29 (Bregma +0.26mm) of Paxinos and Franklin (2001) at the branching of the anterior commissure and fields counted for the shell of the NAc were cut at a level corresponding to figure 19 (Bregma +1.42mm) of the same atlas at the anterior corpus callosum. To analyze the effects of morphine withdrawal on Narp expression compared with the two control conditions, Narp-positive cell counts were compared using a one-way analysis of variance (ANOVA). When main effects were observed, post hoc comparisons were made using the Bonferroni correction.
Somatic signs of morphine withdrawal
Narp KO and WT control mice were monitored for somatic signs of morphine withdrawal. Assessments were made in chambers located within ventilated, light- and sound-attenuating enclosures. The chambers were fitted with photobeams for monitoring locomotor activity and mouse behavior was recorded by a camera connected to a VCR. Three days after morphine pumps were implanted as described above, mice were administered a single dose of saline sc, and then placed in the chambers for 30 minutes. The following day, morphine withdrawal was induced by administration of naltrexone 250ug/kg sc followed by placement in the chambers for 30 minutes also.
Videos of the mice were analyzed by an investigator blind to their genotype and whether saline or naltrexone had been administered. We evaluated mice on several behaviors, often monitored during morphine withdrawal (Kest et al., 2001; Shaw-Luchtman et al., 2002; Georgescu et al., 2003):- Jumps, diarrhoea, locomotor activity, tremor, grooming, digging, rearing and stretching. Tremor was scored as present if the animal demonstrated either head, body and/or paw tremor. Additionally, upon evaluation of the video records we noticed, short duration repetitive front paw wipes of the nose (“nose slaps”) that were distinct from the stereotypical grooming sequences seen in rats and mice. Although we are not aware of published reports of these behaviors following morphine withdrawal they may be related to occurrence of lacrimation observed in rats following morphine withdrawal.
Conditioned place aversion
Apparatus
The conditioned cue preference apparatus was custom-built based on previous designs by Cunningham and colleagues (1992; 1995) and Vezina and Stewart (1987). It is a single-chamber design that utilizes distinct floor textures as the discriminating cue. Each place conditioning apparatus consists of an open field enclosed in a separate light-and sound-attenuating chamber (Med-Associates, St. Albans, VT). A low-level red light and constant ventilation are provided to each sound-attenuating chamber. Location in the open field is monitored by 2 methods. Firstly, there are 8 pairs of infrared photocells, located along the long axis of the chamber collecting position data which is recorded and analyzed by a Med-PC software package (Med-Associates, St. Albans, VT). Secondarily, confirmatory data on location in the apparatus is also recorded by a digital camera and subsequently analyzed by observers blind to the experimental conditions. The floor of the open field consisted of two interchangeable halves made of one of two textures: either a “grid” made from stainless-steel rods (2.3-mm stainless steel rods mounted 6.4-mm apart) or “hole” floor (16 GA stainless steel perforated with 6.4-mm diameter holes). Prior to each session the open field and floors were cleaned using a 70% ethanol solution.
Procedure
The effect of Narp deletion on CPA induced by morphine withdrawal was assessed over the following consecutive phases (see also figure 1).
Figure 1.
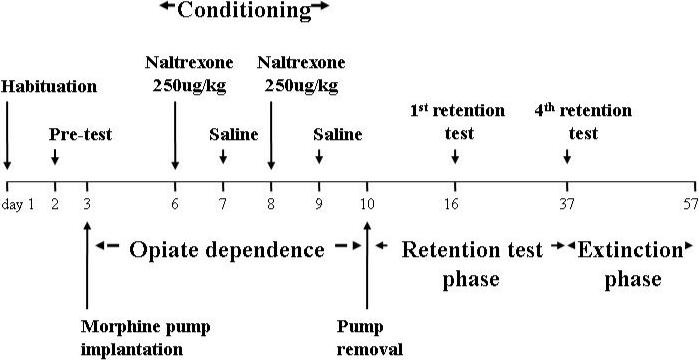
Timeline showing the conditioning, retention and extinction phases.
1. Habituation
To reduce the novelty and stress associated with handling, injection and exposure to the apparatus, mice were subjected to a one day habituation procedure. For this procedure mice were injected with saline prior to being placed on a smooth floor surface covered with white paper for 10 minutes. Importantly, mice were not exposed to the to-be-conditioned distinctive floor types to avoid latent inhibition (Cunningham et al., 1999).
2. Pre-conditioning test
On day 2, prior to the induction of morphine dependence, place preference/aversion was assessed during a 15 minute test session in the chambers containing one half of each of the two floor types. The time spent on each floor type was recorded to establish floor-type preference. The preferred floor-type for each mouse at pre-testing was assigned as the floor-type which in the next phase was paired with morphine withdrawal ie the conditioned stimulus (CS+); the other floor-type was assigned as CS− and paired with saline injections.
3. Conditioning
On day 3, mice were implanted with morphine pumps and allowed to recover and develop morphine dependence over 3 days. Place conditioning to morphine withdrawal was conducted on days 6 through 9 using the following protocol. Each conditioning day, the chambers were furnished with one floor type (either grid or holes). The mice were brought into the testing room and were administered either saline or naltrexone, and then confined immediately in the conditioning chamber for 30 minutes. On alternating days, mice were injected with naltrexone on the preferred floor-type and with saline on the non-prefered floor-type. Each mouse received 2 saline injections and 2 naltrexone injections on consecutive and alternating days (counterbalanced) for a total of 4 days. On the day after the last conditioning session, the pumps were removed under light, general anesthesia.
4. Post-conditioning bias tests
Mice were allowed to recover for one week after pump removal before undergoing testing for the development of a post-conditioning bias (ie conditioned place aversion) on day 16. Mice were confined for a 15 minute period to the test chambers which were each furnished with the two floor types. The time spent on the morphine withdrawal-paired floor (CS+) and the saline-paired floor (CS-) were recorded and later quantified. The time difference spent on the CS+ floor type between the pre-test and test served as an index of aversive conditioning.
5. Post-conditioning retention
CPA was subsequently assessed at 3 weekly intervals (ie at 2, 3 and 4 weeks following the last conditioning session). After retention assessment at day 37, an extinction phase was commenced.
6. Extinction
During the first 15 days of the extinction phase, 3-day extinction blocks were conducted such that on the first and second days mice received a morning and an afternoon session during which they received an injection of saline before being placed on one of the floor types (one floor type in the morning and the alternate floor in the afternoon). Then, on the third day a bias test was conducted using the same procedures as during the pre and post-conditioning bias tests. Because this extinction procedure produced no evidence of extinction in WT mice, a different procedure was used. During this phase, mice underwent repeated daily bias testing and preference for each floor was assessed throughout.
RESULTS
Narp expression in amygdala following morphine withdrawal
In our earlier studies in rats, we found induction of Narp in cell bodies in the extended amygdala following administration of low dose opiate receptor antagonists to animals that had been implanted with morphine pellets (Reti and Baraban, 2003). For the present study, we initially confirmed that Narp expression in mice parallels that seen in rats. In sections from mouse brain harvested four hours following morphine withdrawal triggered by naltrexone 250 μg/kg sc (figure 2), we found strong induction of Narp cell body staining in the lateral portion of the CeA, in the BNST and NAc. Unlike the CeA, the BLA has constitutive Narp expression. Four hours after morphine withdrawal is triggered, the number of Narp-positive cells in BLA approximately doubles compared with morphine-naïve mice treated with naltrexone or morphine-dependent mice treated with saline.
Figure 2.
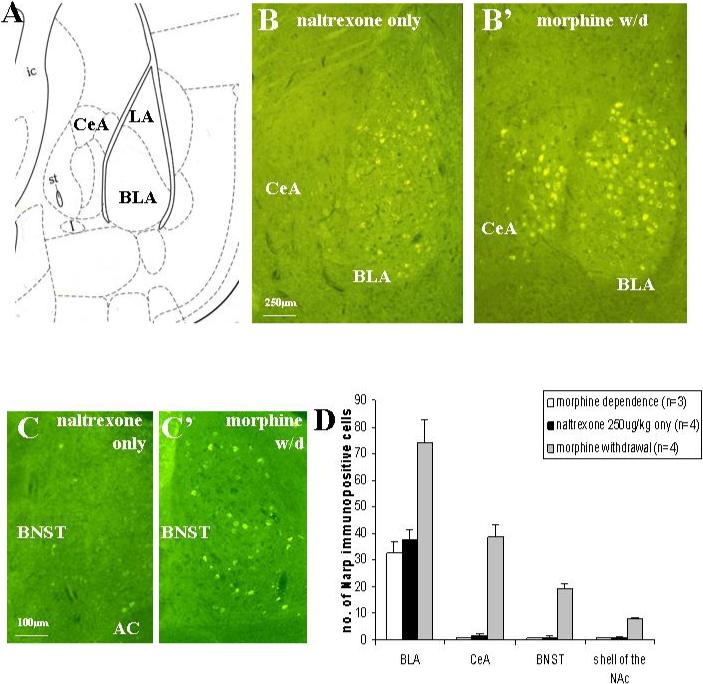
Narp immunoreactivity in the mouse amygdala is induced by morphine withdrawal. Panel (A) shows an outline of the BLA and CeA from figure 42 of the Paxinos and Franklin (2001) mouse atlas. (B) Constitutive Narp cell body staining is present in the BLA of a morphine-naive mouse treated with naltrexone 250 μg/kg sc, but Narp cell body staining is not detected in either the CeA or lateral amygdala. Four hours following administration of naltrexone 250 μg/kg sc to morphine dependent mice, Narp cell body staining is induced in both the BLA and the lateral portion of the CeA. Panel (C) shows induction of Narp staining in the BNST four hours following morphine withdrawal. (D) Narp cell body staining quantification. ANOVA comparing staining in the BLA and extended amygdala following morphine withdrawal with staining after naltrexone given to morphine-naïve animals and saline given to morphine-dependent animals. BLA: F(2,8)=12.17, p<0.005; after Bonferroni correction, number of Narp-positive cells after morphine withdrawal is significantly greater than both controls (p<0.01). CeA: F(2,8)=56.95, p<0.001; after Bonferroni correction, number of Narp-positive cells after morphine withdrawal is significantly greater than both controls (p<0.001). BNST: F(2,6)=70.22, p<0.001; after Bonferroni correction, number of Narp-positive cells after morphine withdrawal is significantly greater than both controls (p<0.001) NAc: F(2,6)=115.8, p<0.001; after Bonferroni correction, number of Narp-positive cells after morphine withdrawal is significantly greater than both controls (p<0.001). (Note: for BNST and NAc staining only 3 morphine withdrawal and 3 naltrexone only animals were examined.) Narp cell body staining levels in BLA and extended amygdala of morphine-naïve mice treated with naltrexone and morphine-dependent animals given saline is comparable to levels in naïve mice or naïve mice given saline only.
Somatic signs of morphine withdrawal are not altered by Narp deletion
Next we checked whether Narp deletion affects somatic signs of morphine withdrawal induced by injections of naltrexone given 3 or 4 days following implantation of the morphine pump. Table 1 shows the average frequency for 9 behaviors observed during a 30-min sessions following naltrexone or saline injection. Two way ANOVA revealed three patterns of results. Firstly, jumping, overall activity levels, and the occurrence of diarhoea and nose-wipes (see Methods) were significantly altered following naltrexone injection (Fs > 14.2, ps < 0.01), but these naltrexone induced changes did not vary as a function of genotype (ps > 0.78). Secondly, both grooming and digging behaviors were reduced in mice following naltrexone injection (Fs > 14.9, ps < 0.01) and this decrease did not differ between WT or KO mice. However, independent of injection, levels of grooming and digging behaviors in Narp KO mice were overall higher and lower, respectively. Thirdly, naltrexone injections failed to produce significant changes in rearing behavior or the occurrence of stretches in either WT or KO mice. Thus, statistical analysis yielded no significant effects of injection, genotype or injection x genotype interaction effects (Fs < 1.8, ps > 0.29). Additionally, the effect of naltrexone injection on the occurrence of tremors was very small and only approached significance (F(1,11) =4.3, p = 0.06) and there were no genotype or injection x genotype effects.
Table 1. Somatic signs of morphine withdrawal in Narp KO and WT mice.
Three days after morphine pump implantation, Narp KO and WT mice were administered a single dose of saline in a novel environment. The following day mice were administered a single dose of naltrexone 250ug/kg sc in the same chamber and were monitored for 30 minutes. The table shows the mean number of somatic morphine withdrawal signs for each genotype after saline and naltrexone administrations
| Genotype | Injection | Mean±SE | Effect1 | |
|---|---|---|---|---|
| Jumps equally in both WT and KO mice | WT | Naltrexone | 29.3±5.9 | Naltrexone increased |
| Saline | 0.0±0.0 | |||
| KO | Naltrexone | 32.8±8.3 | ||
| Saline | 0.0±0.0 | |||
| Diarrhoea equally in both WT and KO mice | WT | Naltrexone | 7.0±0.7 | Naltrexone increased |
| Saline | 0.0±0.0 | |||
| KO | Naltrexone | 8.1±1.2 | ||
| Saline | 0.0±0.0 | |||
| Activity equally in both WT and KO mice | WT | Naltrexone | 57.6±16.3 | Naltrexone decreased |
| Saline | 103.0±13.8 | |||
| KO | Naltrexone | 54.7±4.6 | ||
| Saline | 99.0±12.1 | |||
| Nose-wipe equally in both WT and KO mice | WT | Naltrexone | 45.3±16.3 | Naltrexone increased |
| Saline | 4.3±1.8 | |||
| KO | Naltrexone | 37.7±10.1 | ||
| Saline | 0.9±0.5 | |||
| Tremors or genotype | WT | Naltrexone | 1.7±0.5 | No effects of naltrexone |
| Saline | 0.0±0.0 | |||
| KO | Naltrexone | 3.0±2.0 | ||
| Saline | 0.0±0.0 | |||
| Grooming levels in both WT and KO mice + overall higher levels in KO mice | WT | Naltrexone | 0.0±0.0 | Naltrexone decreased |
| Saline | 8.7±2.1 | |||
| KO | Naltrexone | 1.6±0.7 | ||
| Saline | 14.4±1.6 | |||
| Digging levels in both WT and KO mice + overall lower levels in KO mice | WT | Naltrexone | 2.8±1.7 | Naltrexone decreased |
| Saline | 28.7±6.3 | |||
| KO | Naltrexone | 3.9±2 | ||
| Saline | 12.4±4.6 | |||
| Rearing or genotype | WT | Naltrexone | 21.2±2.9 | No effects of naltrexone |
| Saline | 35.5±10.1 | |||
| KO | Naltrexone | 33.3±13.3 | ||
| Saline | 26.6±4.4 | |||
| Stretches or genotype | WT | Naltrexone | 0.0±0.0 | No effects of naltrexone |
| Saline | 0.3±0.3 | |||
| KO | Naltrexone | 0.0±0.0 | ||
| Saline | 0.4±−0.4 |
Note:
Based on 2-way ANOVA that included Injection (Naltrexone versus Saline) and Genotype (WT versus KO) as within and between comparisons. Significant when p <0.05.
In summary, naltrexone significantly altered (increased or decreased) 6 of the 9 behaviors that were monitored during the 30-min test sessions and that are typically observed following morphine withdrawal. Importantly, although some overall genotype differences were seen in some behaviors (digging and grooming), no differences were evident between Narp KO and WT in naltrexone-induced somatic withdrawal symptoms.
Acquisition and retention of conditioned place aversion
Behavior testing revealed that Narp KO mice develop and retain stronger place aversion to the environment in which they underwent morphine withdrawal than their WT controls. In the pre-conditioning bias test, out of a total of 15 min, the WT mice spent 6.76 min on the CS− side and the KOs spent 5.42 min on the CS− side (CS+ versus CS− (p<0.01)). Each pair of bars in figure 4 shows the mean increase in time spent on the CS− side after conditioning compared with the pre-test evaluation. A group (KO versus WT) x session ANOVA of the increase in time spent on the CS− side revealed a significant effect of genotype (F(12,78)=5.27, p<0.0001) and session (F(1,78)=20.39, p<0.0001) as well as a genotype x session (F(12,78)=2.5, p<0.01) interaction effect, indicating the Narp KO mice develop and retain aversion more strongly than the WT controls.
Although the KOs show less preference for the CS− side during the pretest, they wind up spending more time on the CS− side than the WTs after conditioning. Therefore, the pretest bias alone cannot explain the phenotype. Nevertheless, we were concerned that the greater change among KO mice might be related to their larger unconditioned preferences between the floor types. Therefore, we also analyzed the CPA acquisition data after excluding mice with outlying pretest times, ie those mice whose pretest times lay more than 1 standard deviation outside the mean. This analysis included 8 WT's and 8 KO's with comparable mean CS− pretest times (WT: 6.39 min, KO: 6.03 min). In this subset there was still an effect of genotype on CPA acquisition and retention over four weeks. On a 2-way ANOVA: genotype (F(7,48)=3.78, p=0.0024); session (F(1,48)=6.2, p=0.0163); genotype x session (F(7,48)=1.37, p=0.24).
Extinction of conditioned place aversion
Narp KO mice display accelerated extinction of CPA following morphine withdrawal (figure 4). Whereas WT mice continue to prefer the CS− side until the 9th assessment of extinction, in contrast, Narp KO mice were spending as much time on the CS+ side by the third extinction assessment.
Figure 4.
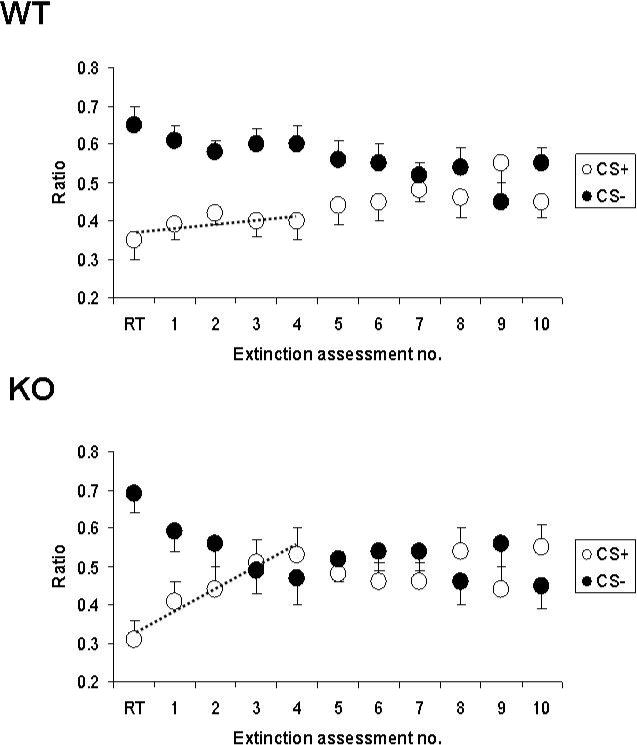
Extinction of conditioned place aversion induced by morphine withdrawal in Narp KO mice and WT controls. We present the mean proportions of time spent on the CS+ and CS− sides at each assessment of extinction for WT (top panel) and Narp KO (bottom panel) mice. The initial values are for the 4th retention trial (RT), afterwhich the extinction phase was commenced.
To determine if the rates of extinction differ by genotype, we assessed a group (KO versus WT) x session ANOVA which included the retention test and first four extinction assessments. There was a main effect of genotype x session interaction (F(12,104), p<0.01) indicating the extinction of the WT controls is significantly slower than that of the Narp KOs. There was an effect of session (F(12,104), p<0.0005) but no effect of genotype (F(1,104), p>0.05). We also used another established procedure for analyzing the rates of extinction (Crombag et al., 2000) across the first four extinction assessments. We developed a linear curve fit for each mouse and obtained slope coefficients. When analyzing across the RT and first four extinction assessments, the mean slope coefficient (S) for the Narp KO mice was significantly higher than for the WT controls (KO: S=0.053±0.013, WT: S=0.012±0.009, p<0.05, two-tailed Student's t-test). These lines are superimposed on the graphs. Thus, using two different analyses we found that the rate of extinction significantly differed as a function of genotype and that KO mice showed a faster rate of extinction during the initial phase of extinction testing compared with WT mice.
DISCUSSION
In this study, we report Narp modulates the long-lasting aversive effects of morphine withdrawal. Firstly, in Narp KO mice, the induction of morphine withdrawal in the presence of distinct environmental cues resulted in the development of more robust place aversion than observed in WT mice. This acquired place aversion was persistent and remained robust even 4 weeks following the last experience with morphine withdrawal. Secondly and most interestingly, when mice underwent extinction experience, Narp KO mice showed a markedly enhanced rate of extinction relative to WT mice. Thirdly, these effects are unlikely to be explained by differences in the physical experience of morphine withdrawal as we were unable to observe genotype specific differences in the response to naltrexone injections in morphine dependent mice. In summary, our results suggest that, in addition to playing a role in the initial acquisition of withdawal based acquired aversion, Narp plays an important role in the maintenance of this aversion under extinction conditions.
Narp increases in basolateral and extended amygdala following morphine withdrawal
As found previously in rat (Reti and Baraban, 2003), Narp is expressed in the mouse amygdala - a key region mediating CPA following morphine withdrawal (Koob, 1999; Schulteis et al., 2000). We found that following morphine withdrawal, constitutive Narp expression is increased in the BLA and is markedly induced from negligible levels in the central amygdala, and associated regions that form the extended amygdala (BNST and NAc). Recent evidence shows that these regions play a critical modulatory role in the aversive effects of morphine withdrawal and in the establishment of acquired affective responses. For instance as we discussed earlier, previous lesion and immunohistochemical studies have implicated the BLA in the establishment of CPA based on morphine withdrawal (Schulteis et al., 2000; Frenois et al 2005; Hellemans et al., (2006)) and the “extended amygdala” is increasingly considered critical (Heimer et al., 1997; Stinus et al., 1990; Heinrichs et al., 1995; Koob, 1999; Delfs et al., 2000; Watanabe et al., 2002 a,b). Therefore, we explored whether Narp deletion would alter performance in such a task, by altering morphine withdrawal experience and/or the ability of environmental cues to become endowed with aversive properties when paired with morphine withdrawal.
Narp deletion has no effect on somatic symptoms of withdrawal
To check if Narp deletion alters the acute response to morphine withdrawal, we evaluated the physical signs of morphine withdrawal in Narp KO mice and found they did not differ from control mice. Lesion studies and intracerebral injections of opiate antagonists indicate the locus coeruleus, which contains the largest cluster of noradrenergic neurons, plays a key role in generating the physical signs of morphine withdrawal (Maldonado et al., 1992; Maldonado and Koob, 1993). We have previously demonstrated Narp expression in terminals of orexin afferents to the locus coeruleus (Reti et al., 2002b). Interestingly, Georgescu et al. (2003) recently showed that somatic signs of morphine withdrawal are attenuated in orexin KO mice. Nevertheless, we did not find Narp deletion altered the acute signs of morphine withdrawal.
Narp deletion enhances acquisition of withdrawal place aversion
Narp KO mice show increased CPA compared with wild-type controls when re-exposed to the previously conditioned environment. Moreover, the increased aversion appears to be sustained for at least 4 weeks consistent with a previous study in mice showing persistent CPA following morphine withdrawal lasting at least 6 weeks (Sakoori and Murphy, 2005). This finding suggests Narp plays a role in synaptic plasticity associated with acquisition of CPA. However, the mechanism by which this occurs is unknown. Recent studies examining the impact of neuronal pentraxins on synaptic plasticity have not detected a defect in hippocampal LTP in mice containing targeted deletions of all three neuronal pentraxins, Narp, NP1 and NPR (Bjartmar et al., 2006). However, subsequent studies indicate that these mice are deficient in AMPA receptor endocytosis and LTD (R. Cho and P. Worley, personal communication). Thus, it is tempting to speculate that a defect in AMPA receptor endocytosis may underlie the observed enhancement in acquisition of CPA found in Narp KO mice in this study.
Narp deletion enhances extinction of withdrawal place aversion
We were surprised to find that Narp KOs have accelerated extinction of CPA following morphine withdrawal. As Narp KOs conditioned by morphine withdrawal are starting the extinction phase more averse to the CS+, this makes their accelerated extinction all the more impressive. Moreover, Narp KO mice maintained CPA for 4 weeks prior to commencement of extinction, indicating their accelerated extinction does not reflect a deficit in synapse formation or stabilization in these animals. Recent work on extinction of fear conditioning suggests that a key mechanism is the establishment of inhibitory control by the prefrontal cortex (PFC) over the amygdala-based fear process (Milad and Quirk, 2002, Sotres-Bayon et al., 2004, Sotres-Bayon et al., 2006). Along these lines, it is of particular interest that the medial PFC has substantial Narp expression (Tsui et al, 1996; Lu et al., 2002). Lesion studies indicate that rats with medial PFC lesions need many more days of exposure to the CS in the absence of the unconditioned stimulus in order for the CS-elicited fear responses to be reduced to pre-training levels (Morgan et al., 1993; Morgan and LeDoux, 1995). Moreover, AMPA receptors have also been implicated in extinction learning. For example, Sutton et al., (2003) reported that extinction induced upregulation of AMPA receptors reduces cocaine-seeking behavior, and PEPA, a potentiator of AMPA facilitates extinction learning for contextual fear memory (Zushida et al., 2007). If as described in the previous paragraph, Narp acts to suppress activity by AMPA receptor mediated endocytosis, it is conceivable that in the Narp KO the medial PFC input to the amygdala is enhanced thus causing greater inhibition of the amygdala-based CPA and accelerated extinction.
As Narp KO mice show enhanced acquistion and extinction in this CPA paradigm, it will be interesting in future studies to assess whether they also display altered performance in other aversive and appetitive learning paradigms. Although behavioral characterization of these mice is still ongoing, studies conducted with food-reward learning paradigms have revealed that they are unimpaired in learning simple Pavlovian discriminations, instrumental lever pressing, and in acquisition of at least two aspects of Pavlovian incentive learning, conditioned reinforcement and Pavlovian-instrumental transfer (Johnson et al., 2007). Since Narp displays a rather heterogenous pattern of expression in the nervous system (Reti et al., 2002a; Reti et al., 2002b; Reti et al., 2002c; Reti and Baraban, 2003; Reti et al., in press), it is, perhaps, not surprising that Narp KO mice may display focal, rather than global deficits, in learning and memory. Thus, close attention to the anatomical pattern of Narp expression may provide important clues to which aspects of learning and memory are modulated by it.
In summary, we have shown that Narp deletion alters the intensity of the long-term aversive response to morphine withdrawal and accelerates extinction of that memory. As more rapid extinction of long-term behavioral responses to cues associated with drug withdrawal may be beneficial in preventing relapse and addiction, further studies aimed at understanding Narp's actions are warranted. In addition, as Narp is thought to act by regulating AMPA receptor clustering and/or trafficking, these findings implicate AMPA receptor based mechanisms of synaptic plasticity in extinction learning. Furthermore, these findings suggest that individual variations in the induction or expression of Narp may contribute to the susceptibility to drug addiction. Thus, additional studies of the role of Narp and other IEGs in long-term behavioral responses induced by drug administration may provide valuable insights into the pathophysiology of the addictive state.
Figure 3.
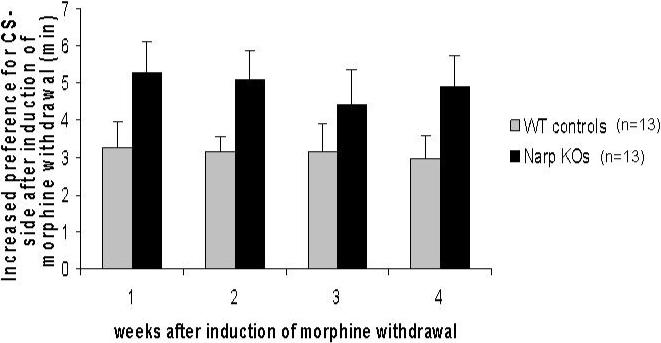
Acquisition and retention of conditioned place aversion following morphine withdrawal in Narp KO mice and WT controls. The graph shows the mean increase in time spent on the CS− side after conditioning compared with the pre-test evaluation. The retention tests are spaced weekly.
ACKNOWLEDGEMENTS
This research was funded by National Institutes of Health grants R01DA016303, RR017688 and P50DA000266-35.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at http://www.apa.org/journals/bne/
REFERENCES
- Bjartmar L, Huberman AD, Ullian EM, Rentería RC, Liu X, Xu W, Prezioso J, Susman MW, Stellwagen D, Stokes CC, Cho R, Worley P, Malenka RC, Ball S, Peachey NS, Copenhagen D, Chapman B, Nakamoto M, Barres BA, Perin MS. Neuronal pentraxins mediate synaptic refinement in the developing visual system. J Neurosci. 2006;26:6269–81. doi: 10.1523/JNEUROSCI.4212-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Badiani A, Maren S, Robinson TE. The role of contextual versus discrete drug-associated cues in promoting the induction of psychomotor sensitization to intravenous amphetamine. Behav Brain Res. 2000;116:1–22. doi: 10.1016/s0166-4328(00)00243-6. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Dickinson SD, Grahame NJ, Okorn DM, McMullin CS. Genetic differences in cocaine-induced conditioned place preference in mice depend on conditioning trial duration. Psychopharmacology (Berl) 1999;146:73–80. doi: 10.1007/s002130051090. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA. Drug-induced conditioned place preference and aversion in mice. Nat Protoc. 2006;1:1662–70. doi: 10.1038/nprot.2006.279. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, Aston-Jones G. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature. 2000;403:430–4. doi: 10.1038/35000212. [DOI] [PubMed] [Google Scholar]
- Frenois F, Stinus L, Di Blasi F, Cador M, Le Moine C. A specific limbic circuit underlies opiate withdrawal memories. J Neurosci. 2005;25:1366–74. doi: 10.1523/JNEUROSCI.3090-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner SM, Takamiya K, Xia J, Suh JG, Johnson R, Yu S, Huganir RL. Calcium-Permeable AMPA Receptor Plasticity Is Mediated by Subunit-Specific Interactions with PICK1 and NSF. Neuron. 2005;45:903–915. doi: 10.1016/j.neuron.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, Yanagisawa M, Nestler EJ, DiLeone RJ. Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J Neurosci. 2003;23:3106–11. doi: 10.1523/JNEUROSCI.23-08-03106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg SR, Woods JH, Schuster CR. Morphine: conditioned increases in self-administration in rhesus monkeys. Science. 1969;166:1306–7. doi: 10.1126/science.166.3910.1306. [DOI] [PubMed] [Google Scholar]
- Heimer L, Alheid GF, de Olmos JS, Groenewegen HJ, Haber SN, Harlan RE, Zahm DS. The accumbens: beyond the core-shell dichotomy. J Neuropsychiatry Clin Neurosci. 1997;9:354–81. doi: 10.1176/jnp.9.3.354. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Menzaghi F, Schulteis G, Koob GF, Stinus L. Suppression of corticotropin-releasing factor in the amygdala attenuates aversive consequences of morphine withdrawal. Behav Pharmacol. 1995;6:74–80. [PubMed] [Google Scholar]
- Hellemans KG, Everitt BJ, Lee JL. Disrupting reconsolidation of conditioned withdrawal memories in the basolateral amygdala reduces suppression of heroin seeking in rats. J Neurosci. 2006;26:12694–9. doi: 10.1523/JNEUROSCI.3101-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson DM, Everitt BJ, Robbins TW, Dickinson A. The role of withdrawal in heroin addiction: enhances reward or promotes avoidance? Nat Neurosci. 2001;4:943–7. doi: 10.1038/nn0901-943. [DOI] [PubMed] [Google Scholar]
- Johnson AW, Crombag HS, Takamiya K, Baraban JM, Holland PC, Huganir RL, Reti IM. Selective role of Narp in processing sensory-specific incentive learning. J Neuroscience. 2007;4:13430–5. doi: 10.1523/JNEUROSCI.4320-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Chen SA, Kitamura O, Markou A, Koob GF. Conditioned withdrawal drives heroin consumption and decreases reward sensitivity. J Neurosci. 2006;26:5894–900. doi: 10.1523/JNEUROSCI.0740-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kest B, Palmese CA, Hopkins E, Adler M, Juni A. Assessment of acute and chronic morphine dependence in male and female mice. Pharmacol Biochem Behav. 2001;70:149–56. doi: 10.1016/s0091-3057(01)00600-1. [DOI] [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of addictive disorders: focus on heroin and cocaine dependence. Am J Psychiatry. 1985;142:1259–64. doi: 10.1176/ajp.142.11.1259. [DOI] [PubMed] [Google Scholar]
- Kim JH, Lee HK, Takamiya K, Huganir RL. The role of synaptic GTPase activating protein in neuronal development and synaptic plasticity. J Neurosci. 2003;23:1119–24. doi: 10.1523/JNEUROSCI.23-04-01119.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick LL, Matzuk MM, Dodds DC, Perin MS. Biochemical interactions of the neuronal pentraxins. Neuronal pentraxin (NP) receptor binds to taipoxin and taipoxin-associated calcium-binding protein 49 via NP1 and NP2. J Biol Chem. 2000;275:17786–92. doi: 10.1074/jbc.M002254200. [DOI] [PubMed] [Google Scholar]
- Koob GF. The role of the striatopallidal and extended amygdala systems in drug addiction. Ann N Y Acad Sci. 1999;877:445–60. doi: 10.1111/j.1749-6632.1999.tb09282.x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci. 2005;8:1442–4. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Lu W, Marinelli M, Xu D, Worley PF, Wolf ME. Amphetamine and cocaine do not increase Narp expression in rat ventral tegmental area, nucleus accumbens or prefrontal cortex, but Narp may contribute to individual differences in responding to a novel environment. Eur J Neurosci. 2002;15:2027–36. doi: 10.1046/j.1460-9568.2002.02036.x. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Stinus L, Gold LH, Koob GF. Role of different brain structures in the expression of the physical morphine withdrawal syndrome. J Pharmacol Exp Ther. 1992;261:669–77. [PubMed] [Google Scholar]
- Maldonado R, Koob GF. Destruction of the locus coeruleus decreases physical signs of opiate withdrawal. Brain Res. 1993;605:128–38. doi: 10.1016/0006-8993(93)91364-x. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–4. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: contribution of medial prefrontal cortex. Neurosci Lett. 1993;163:109–13. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- Morgan MA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci. 1995;109:681–8. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- O'Brien CP, Testa T, O'Brien TJ, Brady JP, Wells B. Conditioned narcotic withdrawal in humans. Science. 1977;195:1000–2. doi: 10.1126/science.841320. [DOI] [PubMed] [Google Scholar]
- O'Brien RJ, Xu D, Petralia RS, Steward O, Huganir RL, Worley P. Synaptic clustering of AMPA receptors by the extracellular immediate early gene product Narp. Neuron. 1999;23:309–323. doi: 10.1016/s0896-6273(00)80782-5. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 2nd Edn. Academic Press; New York: 2001. [Google Scholar]
- Reti IM, Reddy R, Worley PF, Baraban JM. Prominent Narp expression in projection pathways and terminal fields. Journal of Neurochemistry. 2002a;82:935–44. doi: 10.1046/j.1471-4159.2002.01051.x. [DOI] [PubMed] [Google Scholar]
- Reti IM, Reddy R, Worley PF, Baraban JM. Selective expression of Narp, a secreted neuronal pentraxin, in orexin neurons. Journal of Neurochemistry. 2002b;82:1561–5. doi: 10.1046/j.1471-4159.2002.01141.x. [DOI] [PubMed] [Google Scholar]
- Reti IM, Minor LB, Baraban JM. Selective expression of Narp in central vestibular pathways: dependence on sensory input. European Journal of Neuroscience. 2002c;16:1949–58. doi: 10.1046/j.1460-9568.2002.02284.x. [DOI] [PubMed] [Google Scholar]
- Reti IM, Baraban JM. Opiate withdrawal induces Narp in the extended amygdale. Neuropsychopharmacology. 2003;28:1606–13. doi: 10.1038/sj.npp.1300205. [DOI] [PubMed] [Google Scholar]
- Reti IM, Miskimon M, Dickson M, Petralia RS, Takamiya K, Bland R, Saini J, During MJ, Huganir RL, Baraban JM. Activity-dependent release of Narp from vasopressin neurons into the systemic circulation. Neuroscience. 2007 doi: 10.1016/j.neuroscience.2007.10.040. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakoori K, Murphy NP. Maintenance of conditioned place preferences and aversion in C57BL6 mice: effects of repeated and drug state testing. Epub 2004 Dec 29. Behav Brain Res. 2005;160:34–43. doi: 10.1016/j.bbr.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Ahmed SH, Morse AC, Koob GF, Everitt BJ. Conditioning and opiate withdrawal. Nature. 2000;405:1013–4. doi: 10.1038/35016630. [DOI] [PubMed] [Google Scholar]
- Shaw-Lutchman TZ, Barrot M, Wallace T, Gilden L, Zachariou V, Impey S, Duman RS, Storm D, Nestler EJ. Regional and cellular mapping of cAMP response element-mediated transcription during naltrexone-precipitated morphine withdrawal. J Neurosci. 2002;22:3663–72. doi: 10.1523/JNEUROSCI.22-09-03663.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush DE, LeDoux JE. Emotional perseveration: an update on prefrontal-amygdala interactions in fear extinction. Learn Mem. 2004;11:525–35. doi: 10.1101/lm.79504. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Cain CK, LeDoux JE. Brain mechanisms of fear extinction: historical perspectives on the contribution of prefrontal cortex. Biol Psychiatry. 2006;60:329–36. doi: 10.1016/j.biopsych.2005.10.012. Erratum in: Biol Psychiatry 60, 666. [DOI] [PubMed] [Google Scholar]
- Stinus L, LeMoal M, Koob GF. Nucleus accumbens and amygdala are possible substrates for the aversive stimulus effects of opiate withdrawal. Neurosci. 1990;37:767–773. doi: 10.1016/0306-4522(90)90106-e. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Schmidt EF, Choi KH, Schad CA, Whisler K, Simmons D, Karanian DA, Monteggia LM, Neve RL, Self DW. Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. Nature. 2003;421:70–5. doi: 10.1038/nature01249. [DOI] [PubMed] [Google Scholar]
- Tsui CC, Copeland NG, Gilbert DJ, Jenkins NA, Barnes C, Worley P. Narp, a novel member of the pentraxin family, promotes neurie outgrowth and is dynamically regulated by neuronal activity. J Neurosci. 1996;16:2463–2478. doi: 10.1523/JNEUROSCI.16-08-02463.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P, Stewart J. Conditioned locomotion and place preference elicited by tactile cues paired exclusively with morphine in an open field. Psychopharmacology (Berl) 1987;91:375–80. doi: 10.1007/BF00518195. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Nakagawa T, Yamamoto R, Maeda A, Minami M, Satoh M. Involvement of glutamate receptors within the central nucleus of the amygdala in naloxone-precipitated morphine withdrawal-induced conditioned place aversion in rats. Jpn J Pharmacol. 2002a;88:399–406. doi: 10.1254/jjp.88.399. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Yamamoto R, Maeda A, Nakagawa T, Minami M, Satoh M. Effects of excitotoxic lesions of the central or basolateral nucleus of the amygdala on naloxone-precipitated withdrawal-induced conditioned place aversion in morphine-dependent rats. Brain Res. 2002b;958:423–8. doi: 10.1016/s0006-8993(02)03468-6. [DOI] [PubMed] [Google Scholar]
- Xu D, Hopf C, Reddy R, Cho RW, Guo L, Lanahan A, Petralia RS, Wenthold RJ, O'Brien RJ, Worley P. Narp and NP1 form heterocomplexes that function in developmental and activity-dependent synaptic plasticity. Neuron. 2003;39:513–28. doi: 10.1016/s0896-6273(03)00463-x. [DOI] [PubMed] [Google Scholar]
- Zushida K, Sakurai M, Wada K, Sekiguchi M. Facilitation of extinction learning for contextual fear memory by PEPA: a potentiator of AMPA receptors. J Neurosci. 2007;27:158–66. doi: 10.1523/JNEUROSCI.3842-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]


