Abstract
Between five and ten percent of women who survive a first primary breast cancer will subsequently develop a second primary cancer in the contralateral breast. The Women’s Environment Cancer and Radiation Epidemiology (WECARE) Study was designed to identify genetic and environmental determinants of contralateral breast cancer (CBC). In this study, 708 women with asynchronous CBC served as cases and 1397 women with unilateral breast cancer served as controls. ATM, a serine-threonine kinase, controls the cellular response to DNA double-strand breaks, and has been implicated in breast cancer risk. Complete mutation screening of the ATM gene in all 2105 study participants identified 240 distinct sequence variants; only 15 were observed in more than 1% of subjects. Among the rare variants, deleterious alleles resulting in loss of ATM function were associated with a non-significant increase in risk of CBC. In contrast, carriers of common variants had a statistically significant reduction in risk of CBC. Four of these 15 variants were individually associated with a significantly decreased risk of second primary breast cancer (c.1899-55T>G, RR=0.5, 95% CI=0.3–0.8; c.3161C>G, RR=0.5, 95% CI=0.3–0.9; c.5558A>T, RR=0.2, 95% CI=0.1–0.6; c.6348-54T>C RR=0.2, 95% CI=0.1–0.8). These data suggest that some alleles of ATM may exert an anti-neoplastic effect, perhaps by altering the activity of ATM as an initiator of DNA damage responses or a regulator of p53.
Introduction
ATM is a key regulator of cellular pathways protecting cells from malignant transformation that can result from exposure to genotoxic agents, such as ionizing radiation, which induce DNA double-strand breaks. Many of the proteins regulated either directly or indirectly by ATM phosphorylation, such as BRCA1, CHEK2, FANCD2 or p53, have been implicated in the etiology of various cancers, including breast cancer, raising the possibility that genetic variation in ATM might modify the activities of these downstream substrates and impact cancer risk.
Rare, severely deleterious mutations in ATM are responsible for the autosomal recessive disorder, Ataxia-Telangiectasia (A-T) (1). A-T is characterized by a progressive cerebellar ataxia, telangiectasias, oculomotor apraxia, immunodeficiency, hypersensitivity to ionizing radiation both in vitro and in vivo, and a significantly increased incidence of malignancies (2). Although A-T carriers are clinically asymptomatic for the disorder, an excess of breast cancer in mothers of A-T patients, who are obligate carriers, was first reported in the 1970s (3). Both retrospective and prospective studies of A-T families in the US, as well as independent studies from the UK, France, and Scandinavia, also based on ascertainment for A-T, have provided confirmatory results (4–9). However, case-control studies of ATM mutations in patients ascertained for breast cancer have yielded less compelling findings. To date, none of these breast cancer studies that have performed generalized screening for ATM variation have been population-based and none have included a large series of patients with CBC. Studies that have been carried out in selected populations reveal a diverse array of ATM variants in human populations. The low frequency of individual ATM variants, and, specifically, of the severely deleterious mutations observed in A-T families where breast cancer co-occurs, has made it difficult to estimate the magnitude of the role of ATM in breast cancer risk in the population. Nevertheless, there is firm evidence that infrequent ATM truncating mutations (10) and certain missense mutations (11;12) observed in A-T families and in high-risk breast cancer families, do impair ATM function and increase risk for primary breast cancer.
The population-based WECARE Study described here differs from previous studies of the role of ATM in breast cancer risk in that we restrict consideration to young women with a first primary breast cancer and then study the determinants for developing a second primary breast cancer in the contralateral breast (13). In this nested case-control study, cases were women with asynchronous CBC and controls were women diagnosed with unilateral breast cancer who were individually matched to cases by race, date of birth, registry, and date of diagnosis of first primary. Therefore, the control population represented the underlying population of breast cancer cases at risk for developing CBC, and the entire study population was enriched for genetic variants associated with breast cancer. We report here the results of screening all 2105 participants in this study for variants in the ATM gene.
Materials and Methods
Study population
The WECARE Study is a multi-center, population-based, nested case-control study including 708 cases, women with asynchronous bilateral breast cancer, and 1397 controls, women with unilateral breast cancer. All participants were identified, recruited and interviewed through five population-based cancer registries, one registry covering all of Denmark and four in the United States covering, Iowa, three counties in Southern California (Los Angeles, Orange and San Diego), and three counties in Washington state (King, Pierce and Snohomish). Blood samples were obtained from all participants at interview. The study was reviewed and approved by local Institutional Review Boards at each of these registry sites, and all biological samples and data were obtained under informed consent. The study design has been described in detail elsewhere (13).
Women with asynchronous bilateral breast cancer were eligible to be cases if they: 1) were diagnosed between January 1, 1985 and December 31, 2000 with a primary invasive breast cancer that had not spread beyond the regional lymph nodes at diagnosis and a second primary in situ or invasive breast cancer diagnosed in the contralateral breast no earlier than one year after the first breast cancer diagnosis; 2) resided in the same study reporting area for both diagnoses; 3) had no previous or intervening cancer diagnosis; 4) were under age 55 years at the time of diagnosis of the first primary breast cancer; and 5) were alive at the time of contact, able to provide informed consent, complete the interview and provide a blood sample.
WECARE Study controls were individually matched to cases 2:1 on year of birth, year of diagnosis, registry region, and race. In addition, they met the following criteria: 1) diagnosed since January 1, 1985 with first primary invasive breast cancer while residing in one of the study reporting areas; 2) residing in the same study reporting area at the time of interview as when they were diagnosed with their breast cancer; 3) alive at the time of contact; 4) never diagnosed with a second primary breast cancer or any other cancer; 5) without prophylactic mastectomy of the contralateral breast. In addition, controls were counter-matched to cases 2:1 on whether they had received radiation therapy (13).
The analyses reported here included 693 completed triplet sets consisting of two unilateral controls matched to a single asynchronous bilateral case, 11 matched case-control pairs and 4 case-only sets. The frequency distribution of cases and controls was similar for age at reference date, race, registry, and duration of the at-risk period. Fifty-three percent of the WECARE Study population was recruited from the registries in Los Angeles and in Denmark, and the population was predominantly Caucasian.
Mutation screening
DNA for screening was prepared from blood samples by red cell lysis and phenol/chloroform extraction. All coding exons (exons 4–65) of the ATM gene along with flanking intronic sequences ranging from 50 to 100 nucleotides were screened for variation using denaturing high performance liquid chromatography (DHPLC) (14). Amplicons yielding variant results upon DHPLC analysis were evaluated by direct nucleotide sequencing. Two independent observers evaluated separately all output traces from both DHPLC and nucleotide sequencing. Discrepant readings were identified at data entry and re-tested until final resolution was obtained. Final database entries were further checked for internal consistency and cross-checked with prior reported mutations catalogued in the ATM mutation database (http://chromium.liacs.nl/LOVD2/home.php).
Screening was performed at four separate sites utilizing a standard protocol and similarly configured DHPLC devices (Transgenomic, Inc.). Most matched case-control triplets were screened on the same 96 well plate in order to minimize the effects of any variation in screening efficiency or accuracy over the course of the study. Matched samples were always screened in the same laboratory, although the laboratories were blind as to the identities of samples and any matching information. Quality control was assessed by a blinded intra-lab re-screening of 10% of samples at each site and by a second blinded inter-lab re-screening of an additional 10% by a single reference site (14). Quality control samples were distributed throughout the course of the screening. Of the 25,854 re-screening assays performed, only 103 (0.39%) yielded discrepant results. All discrepancies were subsequently resolved by nucleotide sequencing.
Statistical Analyses
To assess the association between carrier status and risk of developing second primary breast cancer, relative risks with corresponding 95% confidence intervals (CI), were estimated using conditional logistic regression. All models were adjusted for exact age and included a log weight covariate where the coefficient of this log weight was fixed at one. These computed weights account for the sampling probability of counter-matching (15), and are based upon the number of radiation exposed and unexposed subjects within the sampled risk set. In each model, the relative risk was also adjusted for other remaining ATM variants so that the rate ratios were relative to wild type. All analyses were conducted using SAS TPHREG.
A-T causing mutations were classified as those variants meeting one or more of the following criteria: 1) changes predicted to result in truncation of the ATM protein whether by direct termination or frameshift, 2) changes affecting the two highly conserved nucleotides flanking exons that direct splicing, 3) changes predicted to result in amino acid substitutions for which there is documented evidence of both a deleterious effect on ATM function and identification in diagnosed A-T patients, or 4) changes documented as A-T causing in the ATM Mutation Database.
SIFT (16) scores were calculated using a Clustal alignment of available vertebrate ATM sequences. Similar analyses were performed on the WECARE Study dataset using PolyPhen (17). The scores generated by the two programs were highly correlated and there were no significant differences in the analyses performed using either system of variant classification. For SIFT analyses, carriers whose ATM sequence differed from wild type at more than a single position were classified based on the highest scoring single variant position present.
Results
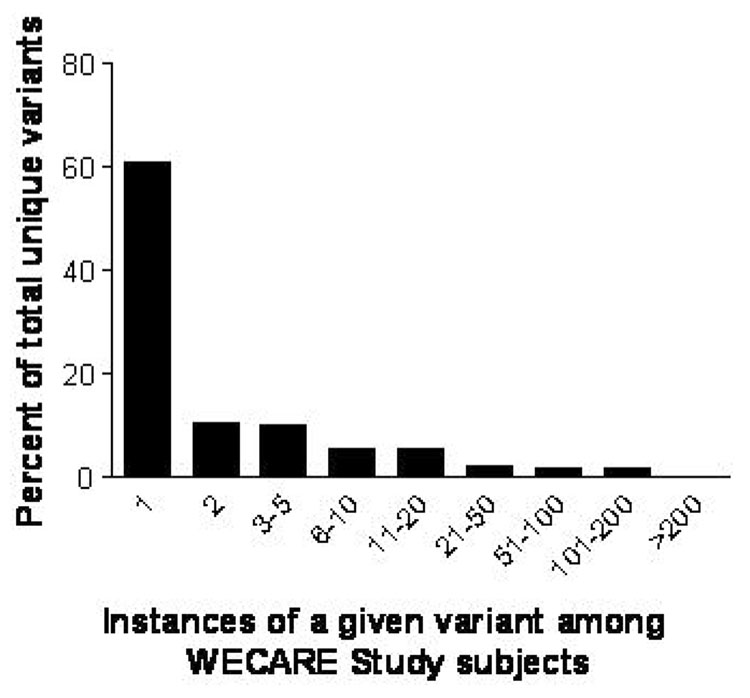
All 2105 WECARE Study participants were screened for variants occurring in any of the 62 coding exons and flanking intronic sequences of ATM. A total of 2153 variant sequences were identified, corresponding to 240 unique variants. The distribution was strongly skewed towards rare variants; fewer than half of the variants had more than a single occurrence in the study population (Figure 1).
Figure 1.
Distribution of ATM variants (N = 240) in the WECARE Study population.
Consideration of the reported associations of breast cancer with obligate carriers ascertained from A-T families suggests that ATM alleles that increase risk for breast cancer would likely be 1) rare in the population, given the low population incidence of A-T; and 2) highly deleterious, given the absence of detectable ATM function in most A-T cell lines. However, consideration of the role of ATM in regulating the products of other genes implicated in cancer risk such as BRCA1, CHEK2, FANCD2 or TP53, makes no prediction as to the frequency of alleles of interest or the direction of their effect. Therefore, in analyzing the data derived from ATM screening in WECARE Study subjects, the effects of common variants, i.e., those with minor allele frequencies greater than 1%, and rare variants, were considered separately. Because of the large size of the WECARE Study population screened, in contrast to past studies, it was possible to compare the distribution of individual or groups of variants to that for the reference wild-type sequence, allowing for either positive or negative effects on risk to be discerned.
Overall, compared to controls, cases (CBC) were less likely to be carriers of an ATM variant, although this difference was not statistically significant (Table 1). The observed difference was largely attributable to the effects of the 15 common variants, for which, as a group, there were significantly fewer carriers among cases as compared to controls (RR=0.8, 10 95% CI= 0.6–0.9). The proportions of carriers of rare variants did not differ between case and control populations.
Table 1.
Risk of developing second primary breast cancer associated with ATM gene carrier status.
| ATM variants classification | Cases (N) | Controls (N) | Rate Ratio* | 95% CI† |
|---|---|---|---|---|
| Overall | ||||
| Wildtype | 271 | 480 | 1.0 | |
| Carrier of any ATM variant | 437 | 917 | 0.8 | 0.7–1.0 |
| Common‡ | ||||
| Wildtype | 271 | 480 | 1.0 | |
| Carrier of any common ATM variant | 355 | 778 | 0.8 | 0.6–0.9 |
| Rare‡ | ||||
| Wildtype | 271 | 480 | 1.0 | |
| Carrier of any rare ATM variant | 148 | 264 | 1.0 | 0.8–1.4 |
Adjusted for exact age at diagnosis of the first primary and counter-matching weight. Common and Rare models also adjusted for carriers of other remaining ATM variants.
95% CI is 95% confidence interval.
Common variants are defined as those carried by greater than or equal to 1% of the WECARE Study participants. Rare variants are those carried by fewer than 1% of the participants.
We further examined the rare variant category, which would be expected to include all of the A-T causative alleles in the WECARE Study population. Considering only confirmed A-T-causing mutations in this category resulted in a modest, but non-significant increase in the rate of CBC in comparison to participants with wild type alleles (Table 2). These known A-T causing variants are primarily rare frameshift or nonsense variants, whereas missense variants constitute the largest proportion of the rare variants identified in the WECARE Study. Since few ATM missense variants have been the subject of functional studies, it is reasonable to assume that additional A-T-causing missense mutations may exist. To address this possibility, we utilized the software program SIFT (16) to classify the rare ATM missense variants into those likely to be deleterious or tolerated. In this analysis, having a deleterious variant was non-significantly associated with an increased rate ratio (Table 2).
Table 2.
Risk of developing second primary breast cancer associated with rare ATM variants.
| ATM variants classification | Cases (N) | Controls (N) | Rate Ratio* | 95% CI† |
|---|---|---|---|---|
| A-T causing mutations | ||||
| Wildtype | 271 | 480 | 1.0 | |
| A-T causing‡ | 14 | 13 | 1.4 | 0.6–3.4 |
| Variants of unknown effect classified by SIFT§ | ||||
| Wildtype | 271 | 480 | 1.0 | |
| Deleterious | 39 | 56 | 1.3 | 0.8–2.2 |
| Tolerated | 36 | 72 | 0.9 | 0.6–1.4 |
Adjusted for exact age at diagnosis of the first primary, countermatching weight and for carriers of the other remaining ATM variants.
95% CI is 95% confidence interval.
Meeting one or more of the following criteria: (1) changes predicted to result in truncation of the ATM protein whether by direct termination or frameshifting, (2) changes affecting the two highly conserved nucleotides flanking exons that direct splicing, (3) changes predicted to result in amino acid substitutions for which there is documented evidence of both a deleterious effect on ATM function and identification in diagnosed A-T patients, or (4) changes documented as AT causing in the ATM Mutation Database.
Defined as by SIFT: Variants with normalized probabilities less than 0.05 are predicted to be deleterious, while those greater than or equal to 0.05 are predicted to be tolerated.. Results for missense variants are adjusted for other variants.
For the common ATM variants there were sufficient observations in the WECARE Study population to allow their individual assessment for association with CBC (Table 3). Four of these individual variants were associated with a significantly decreased risk of CBC and none were associated with a significantly increased risk (Table 3). Two of these variants, c.3161C>G (p.Pro1054Arg) and c.5558A>T (p.Asp1853Val), predict amino acid substitutions that would have deleterious effects on protein structure based on either SIFT (Score = 0.00 for each variant) or PolyPhen (PSIC = 2.025, “probably damaging” for each variant) (16;17). Several of the negatively associated alleles were in linkage disequilibrium, suggesting that they may not have independent effects but no common haplotype containing all of these alleles could be defined. These common variants displayed no significant interaction with other risk factors such as age at diagnosis, family history or treatment modality although power to evaluate interaction effects was only modest given the frequencies of these variants.
Table 3.
Risk of developing second primary breast cancer associated with common ATM variants*
| Variant† | Effect | dbSNP‡ | CasesN (%) | Controls N (%) | Rate Ratio§ | 95% CI║ |
|---|---|---|---|---|---|---|
| c. 378T>A | p.Asp126Glu | rs2234997 | 8 (1.1) | 17 (1.1) | 0.7 | 0.2–2.0 |
| c.735C>T | Silent | rs3218674 | 21 3.0) | 40 (2.8) | 1.0 | 0.6–1.9 |
| c.1899-55T>G | Silent | rs4987943 | 34 (4.8) | 121 (9.5) | 0.5 | 0.3–0.8 |
| c.2119T>C | p.Ser707Pro | rs4986761 | 20 (2.8) | 30 (3.0) | 1.0 | 0.5–1.9 |
| c.2572T>C | p.Phe858Leu | rs1800056 | 14 (2.0) | 42 (2.7) | 0.5 | 0.2–1.0 |
| c.3161C>G | p.Pro1054Arg | rs1800057 | 23 (3.2) | 64 (4.7) | 0.5 | 0.3–0.9 |
| c.3285-10delT | Silent | 8 (1.1) | 15 (1.1) | 0.8 | 0.3–2.0 | |
| c.4258C>T | p.Leu1420Phe | rs1800058 | 24 (3.4) | 47 (3.4) | 0.8 | 0.4–1.4 |
| c.4578C>T | Silent | rs1800889 | 52 (7.3) | 121 (9.0) | 0.7 | 0.5–1.1 |
| c.5497-8T>C | Silent | rs3092829 | 37 (5.2) | 69 (4.9) | 0.9 | 0.5–1.4 |
| c.5557G>A | p.Asp1853Gln | rs1801516 | 173 (24.4) | 339 (24.5) | 0.9 | 0.7–1.1 |
| c.5558A>T | p.Asp1853Val | rs1801673 | 4 (.06) | 30 (2.6) | 0.2 | 0.1–0.6 |
| c.5762+27G>A | Silent | rs3218673 | 8 (1.1) | 22 (1.5) | 0.6 | 0.2–1.6 |
| c.6348-54T>C | Silent | 3 (0.4) | 19 (1.5) | 0.2 | 0.1–0.8 | |
| c.8786+8A>C | Silent | 39 (5.5) | 99 (6.3) | 0.7 | 0.4–1.1 |
Variants carried by more than 1% of the WECARE Study subjects.
Variants indicated relative to the reference sequence for the ATM Mutation Database (http://chromium.liacs.nl/lovd/refseq/ATM_codingDNA.html). Nomenclature as recommended by the Human Variome Project.
rs numbers are provided for those SNPs currently listed in dbSNP.
Adjusted for exact age at diagnosis of the first primary, countermatching weight and for carriers of the other remaining ATM variants so that the rate ratio is relative to those for wildtype for ATM variants.
95% CI is 95% confidence interval.
Discussion
Our findings suggest a model in which genetic variation in ATM has a more complex relationship with breast cancer risk than previously anticipated, which might explain some of the persistent difficulties in defining its role. In our studies, ATM alleles known to cause A-T, as well as other predicted deleterious missense alleles, which may also be A-T causative, were associated with a modestly increased risk of CBC. These classes of alleles have been previously demonstrated to be highly penetrant for first primary breast cancer (10;12). Their rarity, however, undermines the importance of their contribution to population risk. More important from a population perspective is the novel finding we report here, that some ATM alleles appear to confer a protective effect, at least against CBC.
The current study differs from past studies of CBC in its population-based design which allowed us to ascertain large numbers of women with CBC and potentially extrapolate our findings to the general population. The WECARE Study is limited to women who survived their breast cancer; results may have differed if women who were deceased but otherwise eligible could have been included. However, the source population for the WECARE Study consists of women with early stage breast cancer. Since the preponderance of the women in this population are cured of their cancer, they would be less susceptible to biased sampling based on breast cancer survival. Further strengths of our study include the comprehensive nature of ATM screening and the size the population screened. While these features allowed us to detect the main effects of several putatively protective alleles at ATM, we had only limited power to evaluate their statistical interactions with other risk factors. In particular, we were unable to incorporate information on treatment into our evaluation of the effects of these alleles due to instability of the risk estimates resulting from small numbers of observations.
A significant protective effect for CBC associated with ATM variants has not been previously reported and there are no current studies with comparable designs to WECARE that would allow for immediate replication testing of our findings. However, we note that in several smaller previously published studies of ATM and breast cancer (18–20), a similar trend is present for at least one of these same variant alleles, although not remarked upon. For most of the variants, the minor allele is too infrequent to observe any significant effects, but for one of the more common of these variants, c.3161C>G, there are relevant data from several studies. Broeks et al., in a study of unmatched CBC cases and UBC controls, reported a higher frequency of carriers among controls (OR = 0.47, 95% C.I. = 0.19–1.2) (21). Although not statistically significant, this finding is consistent with our observations in the WECARE Study population. Two other studies have examined the incidence of this variant in primary breast cancer cases as compared to unaffected controls. Bretsky et al. (18) observed an increased number of carriers among control individuals (OR = 0.61, 95% C.I. = 0.25–1.5) while Angele et al. (19) observed no significant difference (OR = 1.07, 95% C.I.= 0.57–2.00). Finally, Einarsdottir et al. reported a reduced hazard ratio for c.3161C>G carriers (HR = 0.62, 95% CI = 0.16–2.46) (22). While none of these published studies are large enough to draw statistically significant conclusions regarding the role of this variant in breast cancer risk, the trends are consistent with our findings in the WECARE Study and raise the possibility that the reduced risk associated with this variant may apply to primary breast cancers as well as to CBC.
Given the prominent role of ATM in the mammalian cellular response to DNA damage, its role as a regulator of the tumor suppressor p53 as well as other proteins specifically involved in breast cancer risk such as BRCA1 or CHEK2, and the large number of ATM variants present in human populations, the observation of a range of effects, both positive and negative, on breast cancer risk at this single locus should not be entirely unexpected. However, it raises the important question of how the presence of specific alleles at ATM might reduce the risk of second primary breast cancer.
The presence of DNA double-strand breaks activates ATM, a process characterized by rapid dissociation of inactive ATM dimers and phosphorylation of the resulting monomers in trans (23). Active ATM has a number of anti-neoplastic effects, including the stabilization and accumulation of p53 (24–27), activation of cell cycle checkpoints and induction of apoptotic programs (28). ATM can also be activated in the absence of DNA damage by agents, such as chloroquine, that relax chromatin (23). In such cases, ATM phosphorylates and stabilizes p53, leading to its accumulation, without activating additional biochemical pathways that are dependent on the recruitment of ATM to sites of DNA damage. Mice carrying supernumerary copies of p53 have been shown to resist chemical induction of tumors while aging normally (29) and pretreatment of mice with chloroquine, activating ATM, has been shown to protect against chemically induced mammary carcinomas (30). Thus, allelic products that display increased sensitivity to activation or a higher basal level of activated ATM could reduce the risk of malignant transformation or the subsequent proliferation of transformed cells by increasing the endogenous levels of the tumor suppressor p53. The alleles described here might achieve this effect by increasing the total cellular amount of ATM, lowering its threshold for activation or increasing its kinase activity. Functional studies of ATM activity in cells from carriers of these variant alleles should help to resolve their effects.
Acknowledgments
The study was supported by the National Cancer Institute, awards CA097397, CA098438, and CA112450.
The WECARE Study Collaborative Group
P.I.: Jonine L. Bernstein, Ph.D.; Co-investigators named on grant: Hoda Anton-Culver, Ph.D., Colin Begg., Ph.D., Leslie Bernstein, Ph.D., John Boice, Jr., Ph.D., Anne-Lise Børresen-Dale, Ph.D., Marinela Capanu, Ph.D., Patrick Concannon, Ph.D., Richard A. Gatti, Ph.D., Robert W. Haile, Dr.P.H., Ph.D., Bryan M. Langholz, Ph.D., Charles F. Lynch, M.D., Ph.D., Kathleen E. Malone, Ph.D., Jørgen H. Olsen, M.D., DMSc., Barry Rosenstein, Ph.D., Roy E. Shore, Ph.D., Dr.P.H., Marilyn Stovall, Ph.D., Duncan C. Thomas, Ph.D., W. Douglas Thompson, Ph.D.
Coordinating Center: Memorial Sloan-Kettering Cancer Center (New York, NY) Jonine L. Bernstein, Ph.D. (WECARE Study P.I.), Xiaolin Liang, M.D., M.S. (Informatics Specialist), Abigail Wolitzer, M.S.P.H. (Project Director); National Cancer Institute (Bethesda, MD) Daniela Seminara, Ph.D., M.P.H. (Program Officer).
Laboratories: Benaroya Research Institute at Virginia Mason (Seattle, WA) Patrick Concannon, Ph.D. (P.I.), Sharon Teraoka, Ph.D. (Laboratory Director), Eric R. Olson (Laboratory Manager), Kia Kham-Lee; University of Southern California (Los Angeles, CA) Robert W. Haile, Dr.P.H. (P.I.), Anh T. Diep (Laboratory Director), Nianmin Zhou, M.D. (Laboratory Manager), Yong Liu, M.D. (Director of Blood Processing), Evgenia Ter-Karapetova (Supervisor of Biospecimen Processing), Andre Hernandez; Rikshospitalet-Radiumhospitalet Medical Centre (Oslo, Norway) Anne-Lise Børresen-Dale, Ph.D. (P.I.), Laila Jansen (Laboratory Manager); Mount Sinai School of Medicine (New York, NY) Barry S. Rosenstein, Ph.D. (P.I.), David P. Atencio, Ph.D. (Laboratory Manager); University of California at Los Angeles (Los Angeles, CA) Richard A. Gatti, Ph.D. (Consultant); Memorial Sloan-Kettering Cancer Center (New York, NY) Irene Orlow, Ph.D. (Laboratory Director, Biorepository).
Data Collection Centers: University of Southern California (Los Angeles, CA) Leslie Bernstein, Ph.D. (P.I.), Laura Donnelly-Allen (Project Manager); Danish Cancer Society (Copenhagen, Denmark) Jørgen H. Olsen, M.D., DMSc. (P.I.), Lene Mellemkjær, Ph.D., MSc. (Project Manager); University of Iowa (Iowa City, IA) Charles F. Lynch, M.D., Ph.D. (P.I.), Jeanne DeWall, M.A. (Project Manager); Fred Hutchinson Cancer Research Center (Seattle, WA) Kathleen E. Malone, Ph.D. (P.I.), Noemi Epstein (Project Manager); University of California at Irvine (Irvine, CA) Hoda Anton-Culver, Ph.D. (P.I.), Joan Largent, Ph.D., M.P.H. (Project Manager).
Biostatistics Core: University of Southern California (Los Angeles, CA) Bryan M. Langholz, Ph.D., Duncan C. Thomas, Ph.D.; Memorial Sloan-Kettering Cancer Center (New York, NY) Colin Begg., Ph.D., Marinela Capanu, Ph.D.; University of Southern Maine (Portland, ME) W. Douglas Thompson, Ph.D. (P.I.).
External Advisors: Stanford University (Palo Alto, CA) Alice Whittemore, Ph.D.
References
- 1.Savitsky K, Bar-Shira A, Gilad S, Rotman G, Ziv Y, Vanagaite L, et al. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science. 1995 Jun 23;268(5218):1749–1753. doi: 10.1126/science.7792600. [DOI] [PubMed] [Google Scholar]
- 2.Boder E. Ataxia-telangiectasia: an overview. In: Gatti RA, Swift M, editors. Ataxia-Telangiectasia: Genetics, Neuropathology, and Immunology of a Degenerative Disease of Childhood. New York, NY: Alan R. Liss, Inc.; 1985. pp. 1–63. [Google Scholar]
- 3.Swift M, Sholman L, Perry M, Chase C. Malignant neoplasms in the families of patients with ataxia-telangiectasia. Cancer Res. 1976 Jan;36(1):209–215. [PubMed] [Google Scholar]
- 4.Swift M, Reitnauer PJ, Morrell D, Chase CL. Breast and other cancers in families with ataxia-telangiectasia. N Engl J Med. 1987;316:1289–1294. doi: 10.1056/NEJM198705213162101. [DOI] [PubMed] [Google Scholar]
- 5.Swift M, Morrell D, Massey RB, Chase CL. Incidence of cancer in 161 families affected by ataxia-telangiectasia. N Engl J Med. 1991;325:1831–1836. doi: 10.1056/NEJM199112263252602. [DOI] [PubMed] [Google Scholar]
- 6.Pippard EC, Hall AJ, Barker DJP, Bridges B. Cancer in homozygotes and heterozygotes of ataxia-telangiectasia and xeroderma pigmentosum in Britain. Cancer Res. 1988;48:2929–2932. [PubMed] [Google Scholar]
- 7.Janin N, Andrieu N, Ossian K, Lauge A, Croquette MF, Griscelli C, et al. Breast cancer risk in ataxia telangiectasia (AT) heterozygotes: haplotype study in French AT families. Br J Cancer. 1999 Jun;80(7):1042–1045. doi: 10.1038/sj.bjc.6690460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olsen JH, Hahnemann JM, Borresen-Dale AL, Brondum-Nielsen K, Hammarstrom L, Kleinerman R, et al. Cancer in patients with ataxia-telangiectasia and in their relatives in the nordic countries. J Natl Cancer Inst. 2001 Jan;93(2):121–127. doi: 10.1093/jnci/93.2.121. [DOI] [PubMed] [Google Scholar]
- 9.Borresen A-L, Anderson TI, Tretli S, Heiberg A, Moller P. Breast and other cancers in Norwegian families with ataxia-telangiectasia. Genes Chromosomes Cancer. 1990;2:339–340. doi: 10.1002/gcc.2870020412. [DOI] [PubMed] [Google Scholar]
- 10.Renwick A, Thompson D, Seal S, Kelly P, Chagtai T, Ahmed M, et al. ATM mutations that cause ataxia-telangiectasia are breast cancer susceptibility alleles. Nat Genet. 2006 Aug;38(8):873–875. doi: 10.1038/ng1837. [DOI] [PubMed] [Google Scholar]
- 11.Stankovic T, Kidd AM, Sutcliffe A, McGuire GM, Robinson P, Weber P, et al. ATM mutations and phenotypes in ataxia-telangiectasia families in the British Isles: expression of mutant ATM and the risk of leukemia, lymphoma, and breast cancer. Am J Hum Genet. 1998 Feb;62(2):334–345. doi: 10.1086/301706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernstein JL, Teraoka S, Southey MC, Jenkins MA, Andrulis IL, Knight JA, et al. Population-based estimates of breast cancer risks associated with ATM gene variants c.7271T>G and c.1066-6T>G (IVS10-6T>G) from the Breast Cancer Family Registry. Hum Mutat. 2006 Nov;27(11):1122–1128. doi: 10.1002/humu.20415. [DOI] [PubMed] [Google Scholar]
- 13.Bernstein JL, Langholz B, Haile RW, Bernstein L, Thomas DC, Stovall M, et al. Study design: evaluating gene-environment interactions in the etiology of breast cancer - the WECARE study. Breast Cancer Res. 2004;6(3):R199–R214. doi: 10.1186/bcr771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernstein JL, Teraoka S, Haile RW, Borresen-Dale AL, Rosenstein BS, Gatti RA, et al. Designing and implementing quality control for multi-center screening of mutations in the ATM gene among women with breast cancer. Hum Mutat. 2003 May;21(5):542–550. doi: 10.1002/humu.10206. [DOI] [PubMed] [Google Scholar]
- 15.Langholz B, Goldstein L. Risk set sampling in epidemiologic cohort studies. Statistical Science. 1996;11:35–53. [Google Scholar]
- 16.Ng PC, Henikoff S. Predicting deleterious amino acid substitutions. Genome Res. 2001 May;11(5):863–874. doi: 10.1101/gr.176601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sunyaev S, Ramensky V, Koch I, Lathe W, III, Kondrashov AS. Bork P. Prediction of deleterious human alleles. Hum Mol Genet. 2001 Mar 15;10(6):591–597. doi: 10.1093/hmg/10.6.591. [DOI] [PubMed] [Google Scholar]
- 18.Bretsky P, Haiman CA, Gilad S, Yahalom J, Grossman A, Paglin S, et al. The relationship between twenty missense ATM variants and breast cancer risk: the Multiethnic Cohort. Cancer Epidemiol Biomarkers Prev. 2003 Aug;12(8):733–738. [PubMed] [Google Scholar]
- 19.Angele S, Romestaing P, Moullan N, Vuillaume M, Chapot B, Friesen M, et al. ATM haplotypes and cellular response to DNA damage: association with breast cancer risk and clinical radiosensitivity. Cancer Res. 2003 Dec 15;63(24):8717–8725. [PubMed] [Google Scholar]
- 20.Teraoka SN, Malone KE, Doody DR, Suter NM, Ostrander EA, Daling JR, et al. Increased frequency of ATM mutations in breast carcinoma patients with early onset disease and positive family history. Cancer. 2001 Aug 1;92(3):479–487. doi: 10.1002/1097-0142(20010801)92:3<479::aid-cncr1346>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 21.Broeks A, Braaf LM, Huseinovic A, Schmidt MK, Russell NS, van Leeuwen FE, et al. The spectrum of ATM missense variants and their contribution to contralateral breast cancer. Breast Cancer Res Treat. 2008 Jan;107(2):243–248. doi: 10.1007/s10549-007-9543-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Einarsdottir K, Rosenberg LU, Humphreys K, Bonnard C, Palmgren J, Li Y, et al. Comprehensive analysis of the ATM, CHEK2 and ERBB2 genes in relation to breast tumour characteristics and survival: a population-based case-control and follow-up study. Breast Cancer Res. 2006;8(6):R67. doi: 10.1186/bcr1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003 Jan 30;421(6922):499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- 24.Kastan MB, Zhan Q, el-Deiry WS, Carrier F, Jacks T, Walsh WV, et al. A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992;71:587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- 25.Khanna KK, Keating KE, Kozlov S, Scott S, Gatei M, Hobson K, et al. ATM associates with and phosphorylates p53: mapping the region of interaction. Nat Genet. 1998 Dec;20(4):398–400. doi: 10.1038/3882. [DOI] [PubMed] [Google Scholar]
- 26.Canman CE, Lim DS, Cimprich KA, Taya Y, Tamai K, Sakaguchi K, et al. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science. 1998 Sep 11;281(5383):1677–1679. doi: 10.1126/science.281.5383.1677. [DOI] [PubMed] [Google Scholar]
- 27.Banin S, Moyal L, Shieh S, Taya Y, Anderson CW, Chessa L, et al. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science. 1998 Sep 11;281(5383):1674–1677. doi: 10.1126/science.281.5383.1674. [DOI] [PubMed] [Google Scholar]
- 28.Lukas J, Lukas C, Bartek J. Mammalian cell cycle checkpoints: signalling pathways and their organization in space and time. DNA Repair (Amst) 2004 Aug;3(8–9):997–1007. doi: 10.1016/j.dnarep.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Cao I, Garcia-Cao M, Martin-Caballero J, Criado LM, Klatt P, Flores JM, et al. "Super p53" mice exhibit enhanced DNA damage response, are tumor resistant and age normally. EMBO J. 2002 Nov 15;21(22):6225–6235. doi: 10.1093/emboj/cdf595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loehberg CR, Thompson T, Kastan MB, Maclean KH, Edwards DG, Kittrell FS, et al. Ataxia telangiectasia-mutated and p53 are potential mediators of chloroquine-induced resistance to mammary carcinogenesis. Cancer Res. 2007 Dec 15;67(24):12026–12033. doi: 10.1158/0008-5472.CAN-07-3058. [DOI] [PubMed] [Google Scholar]