Abstract
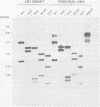
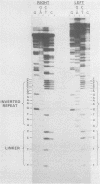
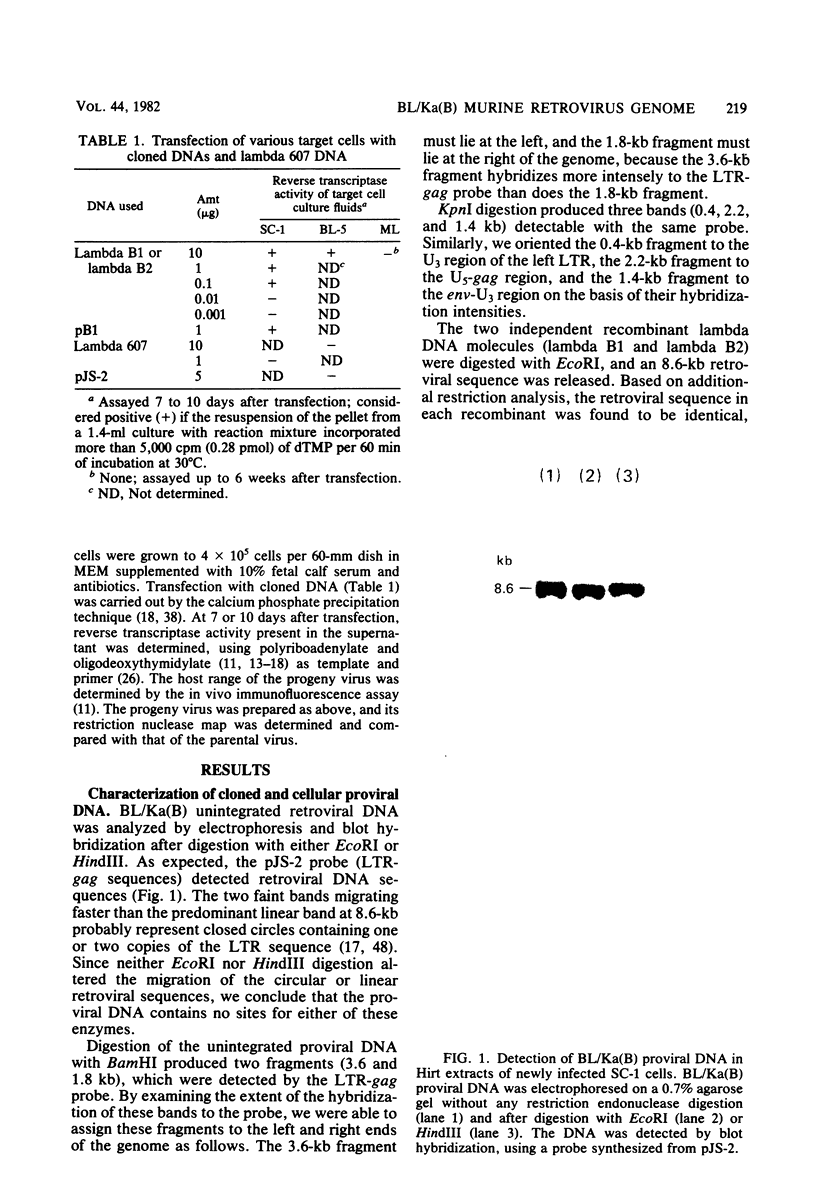
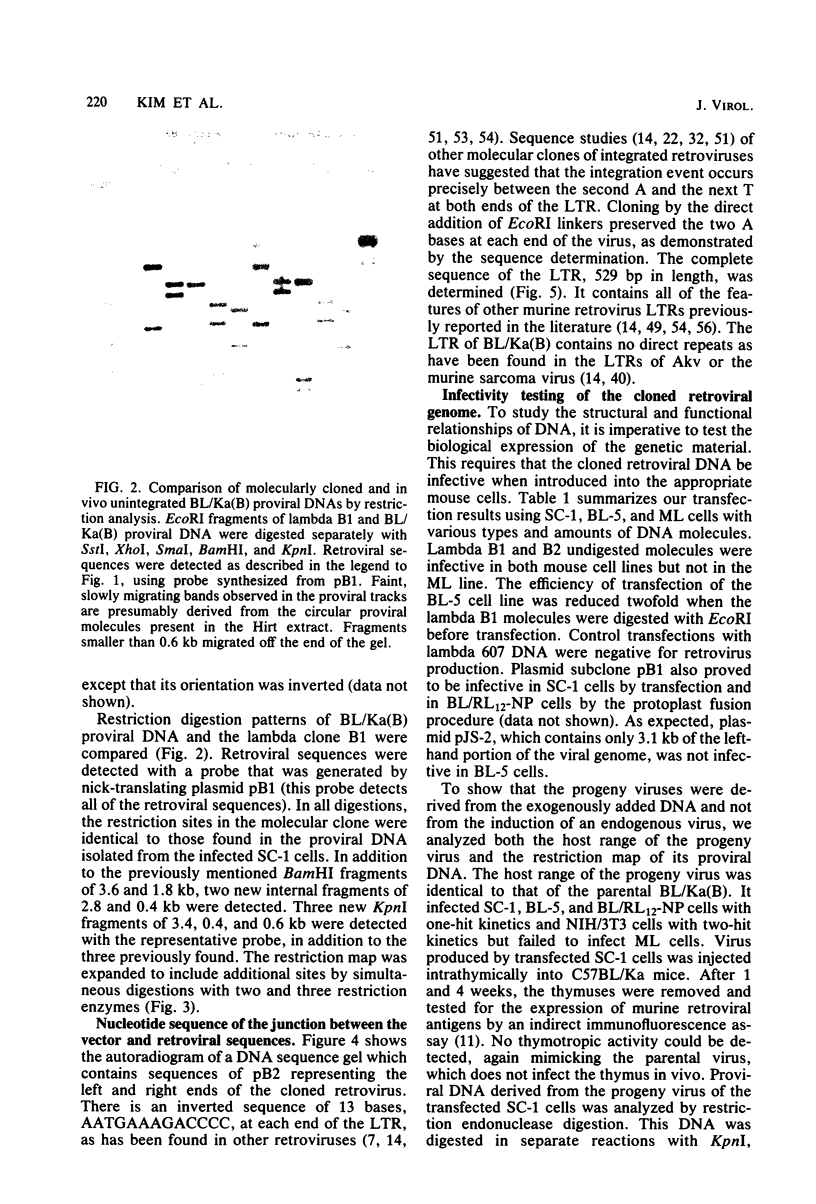
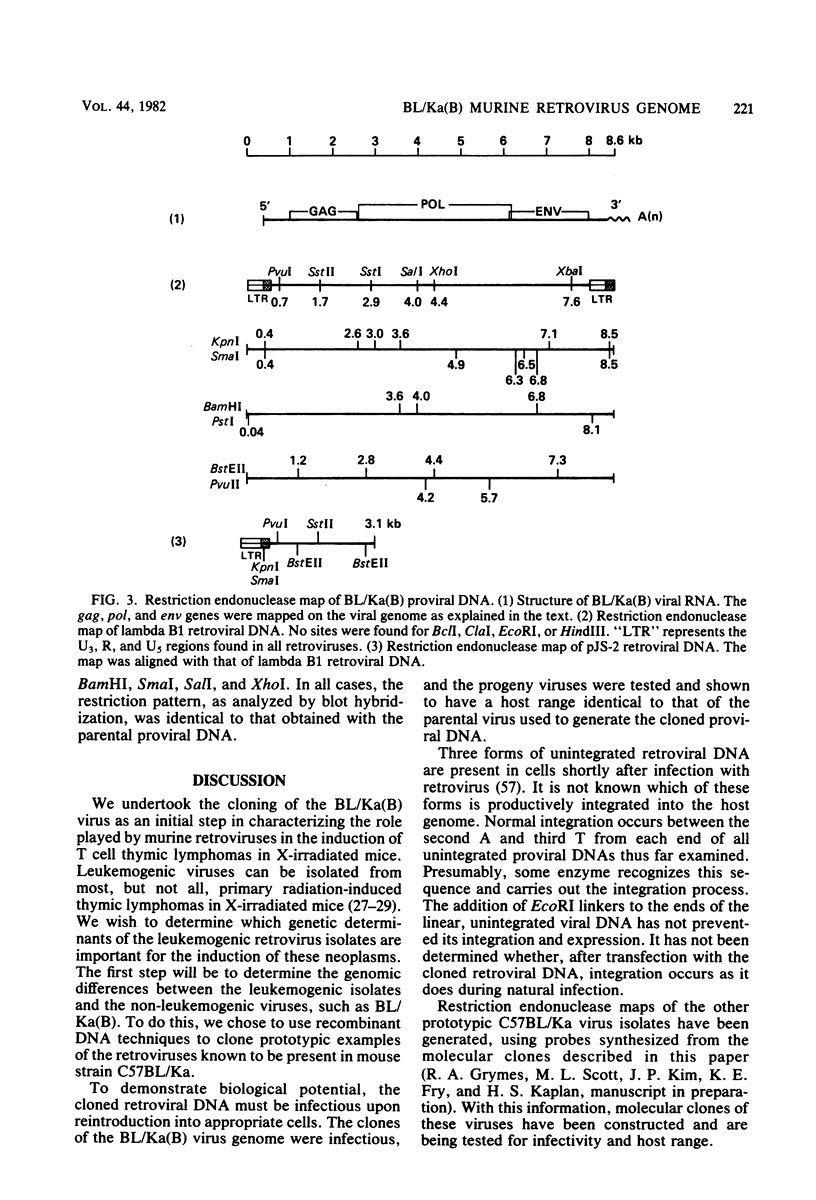
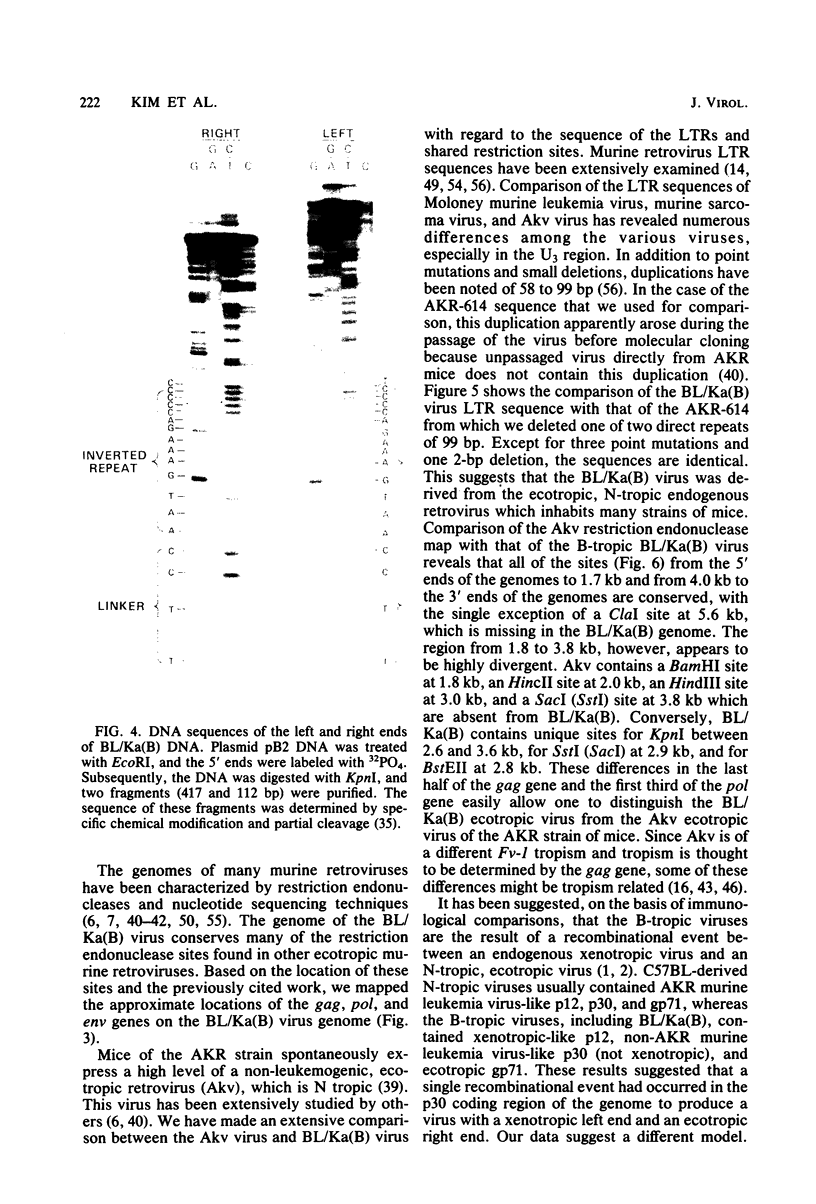
Using molecular cloning techniques, we amplified the unintegrated, linear proviral DNA of the BL/Ka(B) virus, a non-leukemogenic retrovirus of mouse strain C57BL/Ka. Two independent clones in lambda phage vector 607 and one subclone in pBR322 were infective when transfected into mouse fibroblasts. Analysis of the progeny virus revealed biological properties and a restriction map identical to those of the parental viral shock. Comparison of the restriction map with the maps of other ecotropic murine viruses reveals many similarities. Particularly interesting is the comparison of the N-tropic Akv virus and the B-tropic BL/Ka(B) virus. The long terminal repeats of the two viruses are virtually identical, as are 22 of 23 restriction sites located outside of the region which spans from 1.8 to 3.8 kilobases from the left end of the genome. Within this region, however, only three of nine sites examined are shared. This suggests that the BL/Ka(B) virus was derived from an endogenous N-tropic virus closely related to Akv by recombinational events which altered the sequence in the last half of the gag gene and the first third of the pol gene. This change is probably responsible for the observed difference in the Fv-1 tropism of the two viruses.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benade L. E., Ihle J. N., Declève A. Serological characterization of B-tropic viruses of C57BL mice: possible origin by recombination of endogenous N-tropic and xenotropic viruses. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4553–4557. doi: 10.1073/pnas.75.9.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benade L. E., Ihle J. N. Different serotypes of B-tropic murine leukemia viruses and association with endogenous ecotropic viral loci. Virology. 1980 Oct 30;106(2):374–386. doi: 10.1016/0042-6822(80)90260-3. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattner F. R., Blechl A. E., Denniston-Thompson K., Faber H. E., Richards J. E., Slightom J. L., Tucker P. W., Smithies O. Cloning human fetal gamma globin and mouse alpha-type globin DNA: preparation and screening of shotgun collections. Science. 1978 Dec 22;202(4374):1279–1284. doi: 10.1126/science.725603. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Cloyd M. W., Linemeyer D. L., Lander M. R., Rands E., Lowy D. R. Cellular origin and role of mink cell focus-forming viruses in murine thymic lymphomas. Nature. 1982 Jan 7;295(5844):25–31. doi: 10.1038/295025a0. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lander M. R., Gupta S., Rands E., Lowy D. R. Origin of mink cytopathic focus-forming (MCF) viruses:comparison with ecotropic and xenotropic murine leukemia virus genomes. Virology. 1981 Sep;113(2):465–483. doi: 10.1016/0042-6822(81)90175-6. [DOI] [PubMed] [Google Scholar]
- Decleve A., Lieberman M., Niwa O., Kaplan H. S. Rapid in vivo assay for murine lymphatic leukaemia viruses. Nature. 1974 Nov 1;252(5478):79–81. doi: 10.1038/252079a0. [DOI] [PubMed] [Google Scholar]
- Declève A., Lieberman M., Ihle J. N., Kaplan H. S. Biological and serological characterization of radiation leukemia virus. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4675–4679. doi: 10.1073/pnas.73.12.4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Declève A., Lieberman M., Ihle J. N., Rosenthal P. N., Lung M. L., Kaplan H. S. Physicochemical, biological and serological properties of a leukemogenic virus isolated from cultured RadLV-induced lymphomas of C57BL/Ka mice. Virology. 1978 Oct 1;90(1):23–35. doi: 10.1016/0042-6822(78)90329-x. [DOI] [PubMed] [Google Scholar]
- Declève A., Lieberman M., Kaplan H. S. In vivo interaction between RNA viruses isolated from the C57BL/Ka strain of mice. Virology. 1977 Sep;81(2):270–283. doi: 10.1016/0042-6822(77)90144-1. [DOI] [PubMed] [Google Scholar]
- Declève A., Niwa O., Kojola J., Kaplan H. S. New gene locus modifying susceptibility to certain B-tropic murine leukemia viruses. Proc Natl Acad Sci U S A. 1976 Feb;73(2):585–590. doi: 10.1073/pnas.73.2.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar R., McClements W. L., Enquist L. W., Vande Woude G. F. Nucleotide sequences of integrated Moloney sarcoma provirus long terminal repeats and their host and viral junctions. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3937–3941. doi: 10.1073/pnas.77.7.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enquist L., Sternberg N. In vitro packaging of lambda Dam vectors and their use in cloning DNA fragments. Methods Enzymol. 1979;68:281–298. doi: 10.1016/0076-6879(79)68020-5. [DOI] [PubMed] [Google Scholar]
- Gautsch J. W., Elder J. H., Schindler J., Jensen F. C., Lerner R. A. Structural markers on core protein p30 of murine leukemia virus: functional correlation with Fv-1 tropism. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4170–4174. doi: 10.1073/pnas.75.9.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa E., Goff S., Shields A., Yoshimura F., Mitra S., Baltimore D. In vitro synthesis of a 9 kbp terminally redundant DNA carrying the infectivity of Moloney murine leukemia virus. Cell. 1979 Apr;16(4):863–874. doi: 10.1016/0092-8674(79)90101-6. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Clonal cells lines from a feral mouse embryo which lack host-range restrictions for murine leukemia viruses. Virology. 1975 May;65(1):128–134. doi: 10.1016/0042-6822(75)90013-6. [DOI] [PubMed] [Google Scholar]
- Hayashi K. A cloning vehicle suitable for strand separation. Gene. 1980 Oct;11(1-2):109–115. doi: 10.1016/0378-1119(80)90091-8. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hishinuma F., DeBona P. J., Astrin S., Skalka A. M. Nucleotide sequence of acceptor site and termini of integrated avian endogenous provirus ev1: integration creates a 6 bp repeat of host DNA. Cell. 1981 Jan;23(1):155–164. doi: 10.1016/0092-8674(81)90280-4. [DOI] [PubMed] [Google Scholar]
- Hohn B. In vitro packaging of lambda and cosmid DNA. Methods Enzymol. 1979;68:299–309. doi: 10.1016/0076-6879(79)68021-7. [DOI] [PubMed] [Google Scholar]
- KAPLAN H. S., HIRSCH B. B., BROWN M. B. Indirect induction of lymphomas in irradiated mice. IV. Genetic evidence of the origin of the tumor cells from the thymic grafts. Cancer Res. 1956 Jun;16(5):434–436. [PubMed] [Google Scholar]
- LIEBERMAN M., KAPLAN H. S. Leukemogenic activity of filtrates from radiation-induced lymphoid tumors of mice. Science. 1959 Aug 14;130(3372):387–388. doi: 10.1126/science.130.3372.387. [DOI] [PubMed] [Google Scholar]
- Lenz J., Crowther R., Straceski A., Haseltine W. Nucleotide sequence of the Akv env gene. J Virol. 1982 May;42(2):519–529. doi: 10.1128/jvi.42.2.519-529.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber M. M., Sherr C. J., Todaro G. J. S-tropic murine type-C viruses: frequency of isolation from continuous cell lines, leukemia virus preparations and normal spleens. Int J Cancer. 1974 May 15;13(5):587–598. doi: 10.1002/ijc.2910130503. [DOI] [PubMed] [Google Scholar]
- Lieberman M., Declève A., Ricciardi-Castagnoli P., Boniver J., Finn O. J., Kaplan H. S. Establishment, characterization and virus expression of cell lines derived from radiation- and virus-induced lymphomas of C57BL/Ka mice. Int J Cancer. 1979 Aug;24(2):168–177. doi: 10.1002/ijc.2910240208. [DOI] [PubMed] [Google Scholar]
- Lieberman M., Niwa O., Declève A., Kaplan H. S. Continuous propagation of radiation leukemia virus on a C57BL mouse-embryo fibroblast line, with attenuation of leukemogenic activity. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1250–1253. doi: 10.1073/pnas.70.4.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy D. R., Rands E., Chattopadhyay S. K., Garon C. F., Hager G. L. Molecular cloning of infectious integrated murine leukemia virus DNA from infected mouse cells. Proc Natl Acad Sci U S A. 1980 Jan;77(1):614–618. doi: 10.1073/pnas.77.1.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majors J. E., Varmus H. E. Nucleotide sequences at host-proviral junctions for mouse mammary tumour virus. Nature. 1981 Jan 22;289(5795):253–258. doi: 10.1038/289253a0. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Manteuil-Brutlag S., Liu S. L., Kaplan H. S. Radiation leukemia virus contains two distinct viral RNAs. Cell. 1980 Mar;19(3):643–652. doi: 10.1016/s0092-8674(80)80041-9. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClements W. L., Enquist L. W., Oskarsson M., Sullivan M., Vande Woude G. F. Frequent site-specific deletion of coliphage lambda murine sarcoma virus recombinants and its use in the identification of a retrovirus integration site. J Virol. 1980 Aug;35(2):488–497. doi: 10.1128/jvi.35.2.488-497.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray N. E., Brammar W. J., Murray K. Lambdoid phages that simplify the recovery of in vitro recombinants. Mol Gen Genet. 1977 Jan 7;150(1):53–61. doi: 10.1007/BF02425325. [DOI] [PubMed] [Google Scholar]
- Parker B. A., Stark G. R. Regulation of simian virus 40 transcription: sensitive analysis of the RNA species present early in infections by virus or viral DNA. J Virol. 1979 Aug;31(2):360–369. doi: 10.1128/jvi.31.2.360-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rands E., Lowy D. R., Lander M. R., Chattopadhyay S. K. Restriction endonuclease mapping of ecotropic murine leukemia viral DNAs: size and sequence heterogeneity of the long terminal repeat. Virology. 1981 Jan 30;108(2):445–452. doi: 10.1016/0042-6822(81)90451-7. [DOI] [PubMed] [Google Scholar]
- Rassart E., Jolicoeur P. Restriction endonuclease mapping of unintegrated viral DNA of B- and N-tropic BALB/c murine leukemia virus. J Virol. 1980 Sep;35(3):812–823. doi: 10.1128/jvi.35.3.812-823.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy E. P., Smith M. J., Canaani E., Robbins K. C., Tronick S. R., Zain S., Aaronson S. A. Nucleotide sequence analysis of the transforming region and large terminal redundancies of Moloney murine sarcoma virus. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5234–5238. doi: 10.1073/pnas.77.9.5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelaere J., Donis-Keller H., Hopkins N. RNA sequencing provides evidence for allelism of determinants of the N-, B- or NB-tropism of murine leukemia viruses. Cell. 1979 Jan;16(1):43–50. doi: 10.1016/0092-8674(79)90186-7. [DOI] [PubMed] [Google Scholar]
- Rothenberg E., Donoghue D. J., Baltimore D. Analysis of a 5' leader sequence on murine leukemia virus 21S RNA: heteroduplex mapping with long reverse transcriptase products. Cell. 1978 Mar;13(3):435–451. doi: 10.1016/0092-8674(78)90318-5. [DOI] [PubMed] [Google Scholar]
- Roychoudhury R., Jay E., Wu R. Terminal labeling and addition of homopolymer tracts to duplex DNA fragments by terminal deoxynucleotidyl transferase. Nucleic Acids Res. 1976 Apr;3(4):863–877. doi: 10.1093/nar/3.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler J., Gautsch J. W., Lerner R. A., Hopkins N. Biochemical analysis of the p30's of N-, B-, and B leads to NB-tropic murine leukemia viruses of BALB/c origin. J Virol. 1981 Sep;39(3):703–712. doi: 10.1128/jvi.39.3.703-712.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M. L., McKereghan K., Kaplan H. S., Fry K. E. Molecular cloning and partial characterization of unintegrated linear DNA from gibbon ape leukemia virus. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4213–4217. doi: 10.1073/pnas.78.7.4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank P. R., Hughes S. H., Kung H. J., Majors J. E., Quintrell N., Guntaka R. V., Bishop J. M., Varmus H. E. Mapping unintegrated avian sarcoma virus DNA: termini of linear DNA bear 300 nucleotides present once or twice in two species of circular DNA. Cell. 1978 Dec;15(4):1383–1395. doi: 10.1016/0092-8674(78)90063-6. [DOI] [PubMed] [Google Scholar]
- Shimotohno K., Mizutani S., Temin H. M. Sequence of retrovirus provirus resembles that of bacterial transposable elements. Nature. 1980 Jun 19;285(5766):550–554. doi: 10.1038/285550a0. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Shoemaker C., Goff S., Gilboa E., Paskind M., Mitra S. W., Baltimore D. Structure of a cloned circular Moloney murine leukemia virus DNA molecule containing an inverted segment: implications for retrovirus integration. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3932–3936. doi: 10.1073/pnas.77.7.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G., Shinnick T. M., Verma I. M., Lerner R. A. Nucleotide sequence of Moloney leukemia virus: 3' end reveals details of replications, analogy to bacterial transposons, and an unexpected gene. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3302–3306. doi: 10.1073/pnas.77.6.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beveren C., Goddard J. G., Berns A., Verma I. M. Structure of Moloney murine leukemia viral DNA: nucleotide sequence of the 5' long terminal repeat and adjacent cellular sequences. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3307–3311. doi: 10.1073/pnas.77.6.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beveren C., Rands E., Chattopadhyay S. K., Lowy D. R., Verma I. M. Long terminal repeat of murine retroviral DNAs: sequence analysis, host-proviral junctions, and preintegration site. J Virol. 1982 Feb;41(2):542–556. doi: 10.1128/jvi.41.2.542-556.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beveren C., van Straaten F., Galleshaw J. A., Verma I. M. Nucleotide sequence of the genome of a murine sarcoma virus. Cell. 1981 Nov;27(1 Pt 2):97–108. doi: 10.1016/0092-8674(81)90364-0. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Heasley S., Kung H. J., Oppermann H., Smith V. C., Bishop J. M., Shank P. R. Kinetics of synthesis, structure and purification of avian sarcoma virus-specific DNA made in the cytoplasm of acutely infected cells. J Mol Biol. 1978 Mar 25;120(1):55–82. doi: 10.1016/0022-2836(78)90295-4. [DOI] [PubMed] [Google Scholar]