Abstract
OBJECTIVE
Although the incidence of stroke after carotid endarterectomy (CEA) is low (1–3%), approximately 25% of patients experience subtle declines in postoperative neuropsychometric function. No studies have investigated the risk factors for this neurocognitive change. We sought to identify predictors of postoperative neurocognitive dysfunction.
METHODS
We enrolled 186 CEA patients, with both symptomatic and asymptomatic stenosis, to undergo a battery of neuropsychometric tests preoperatively and on postoperative Days 1 and 30. Neurocognitive dysfunction was defined as a two standard deviation decline in performance compared with a similarly aged control group of lumbar laminectomy patients. Univariate logistic regression was performed for age, sex, obesity, smoking, symptomatology, diabetes mellitus, hypertension, hypercholesterolemia, use of statin medication, previous myocardial infarction, previous CEA, operative side, duration of surgery, duration of carotid cross-clamp, and weight-adjusted doses of midazolam and fentanyl. Variables achieving univariate P < 0.10 were included in a multivariate analysis. Data is presented as (odds ratio, 95% confidence interval, P-value).
RESULTS
Eighteen and 9% of CEA patients were injured on postoperative Days 1 and 30, respectively. Advanced age predicted neurocognitive dysfunction on Days 1 and 30 (1.93 per decade, 1.15–3.25, 0.01; and 2.57 per decade, 1.01–6.51, 0.049, respectively). Additionally, diabetes independently predicted injury on Day 30 (4.26, 1.15–15.79, 0.03).
CONCLUSIONS
Advanced age and diabetes predispose to neurocognitive dysfunction after CEA. These results are consistent with risk factors for neurocognitive dysfunction after coronary bypass and major stroke after CEA, supporting an underlying ischemic pathophysiology. Further work is necessary to determine the role these neurocognitive deficits may play in appropriately selecting patients for CEA.
Keywords: Carotid endarterectomy, Cerebral ischemia, Neuropsychological tests, Risk factors
Carotid endarterectomy (CEA) reduces the risk of future stroke in patients with high-grade stenosis (5, 10, 15, 25). However, approximately 25% of CEA patients experience declines in postoperative neurocognitive function that are detected by a battery of neuropsychometric tests (NPMTs) (12, 13). Although the mechanism of post-CEA neurocognitive decline is poorly understood, it is thought to be ischemic in nature, and may be owing to hypoperfusion during carotid artery cross-clamping or the dislodgement of microemboli during vessel dissection and plaque removal.
To date, no studies have thoroughly investigated the risk factors for these neurocognitive changes after CEA. With nearly 100,000 CEAs performed annually (5, 25), many for borderline indications with small absolute benefit (11, 35), understanding the variables that predispose to subtle changes in cerebral function is crucial in appropriate patient selection. We sought to identify factors that predict postoperative neurocognitive dysfunction.
PATIENTS AND METHODS
Study Population
One hundred and eighty six consecutive patients undergoing elective CEA for both symptomatic and asymptomatic carotid artery stenosis were prospectively enrolled in this institutional review board-approved study. All CEA patients had 60% or greater stenosis of the operative carotid artery. After obtaining written informed consent, patients were evaluated with a battery of five neuropsychometric tests before surgery, 1 day after CEA, and at a follow-up examination 30 days postoperatively. As previously described (13), to account for effects of general anesthesia on NPMT performance, a control group of 67 contemporaneous patients undergoing lumbar laminectomy (LL) with a similar anesthetic regimen was included. All tests were performed more than 3 hours after administration of any analgesic or sedative medication. We excluded patients who reported pain greater than five on a zero to ten scale during testing, in both the CEA group and the LL control group, as severe pain confounds NPMT performance (14).
Anesthesia and Surgery
All patients received general anesthesia with routine hemodynamic and temperature monitoring, as previously described (13). CEA patients underwent continuous blood pressure monitoring with a radial artery catheter, and an eight-channel encephalographic monitor was used during the course of surgery (Neurotrac II; Moberg Medical, Inc., Ambler, PA). Fentanyl and midazolam were administered for preinduction sedation. General anesthesia was induced with fentanyl, midazolam, and either vecuronium or rocuronium and maintained with isoflurane. Five patients required shunt placement owing to electroencephalographic changes consistent with ischemia. All CEAs were performed by members of the neurovascular or vascular service. Surgical times averaged 153.6 ± 42 minutes. All patients were extubated in the operating room and recovered in a postoperative care or neurological intensive care unit.
Neuropsychometric Evaluation
Patients were assessed with a battery of five NPMTs, chosen to represent a range of cognitive domains, before surgery and on postoperative Days 1 and 30. All NPMTs were administered by one of three research assistants, each trained and supervised by a neuropsychologist. The Boston Naming Test evaluated the patients’ ability to verbally identify objects pictured on a series of cards. Halstead-Reitan Trails Parts A and B evaluated visual conceptual and visuomotor tracking by timing how long it took a subject to connect consecutively numbered circles with a single line (Part A) and then connect the same number of consecutively numbered and lettered circles by alternating between the two sequences (Part B). The Controlled Oral Word Association test assessed verbal fluency, providing information on dominant hemisphere function. Patients were asked to generate as many words as possible that begin with a certain letter within 60 seconds. Three separate trials were performed as each testing session, one each with the letters C, F, and L. The copy portion of the Rey Complex Figure test evaluated visuospatial organization, providing insight into the functioning of the non-dominant hemisphere. Patients were asked to copy the figure and a standardized scoring system was used to evaluate the presence of design-specific features and the accuracy of their locations (20).
Statistical Analysis
Each NPMT was scored individually for both the CEA patients and the LL control group. As previously described (13), the change in individual test scores from baseline to postoperative Days 1 and 30 were each converted to z-scores relative to the change within the control group, as follows:
Z-scores were converted into a point system quantifying the degree of cognitive dysfunction associated with each NPMT at Days 1 and 30, as described previously (13). For each CEA patient, these deficit points were summed to generate a total deficit score (TDS) that measures the global level of cognitive decline of each patient at Days 1 and 30. By definition, a patient is determined to have neurocognitive dysfunction when the TDS exceeds that mean total change score of the control group by two standard deviations. Using this method, neurocognitive outcome is expressed as a dichotomous variable (“injured” or “uninjured”).
Univariate logistic regression was performed for age, sex, obesity (body mass index [BMI] ≥ 30), history of smoking, symptomatology (previous stoke or transient ischemic attack), diabetes mellitus, hypertension, hypercholesterolemia, use of statin medication, previous myocardial infarction, previous contralateral CEA, operative side, duration of surgery, duration of carotid artery cross-clamp, and doses of midazolam and fentanyl adjusted by body weight. Variables with P values less than 0.10 in the univariate analysis were included in a multivariate model. Risk factors for cognitive dysfunction at Days 1 and 30 were analyzed separately. The patients were then divided into symptomatic and asymptomatic populations and evaluated for potential subgroup analysis. The logistic regression results of age are expressed as the odds ratio (OR) per each decade increase in age. Results for fentanyl and midazolam are expressed as the OR per each μg/kg or.01 mg/kg increase in weight-adjusted dose, respectively. The remaining risk factors are presented as categorical variables, with ORs that represent the risk associated with having the condition. All data is expressed as mean ± standard deviation, or (OR, 95% confidence interval, P-value), with P values less than 0.05 considered significant.
RESULTS
Cohort Characteristics
The demographic and intraoperative variables for all CEA patients are presented in Table 1. One hundred eighty-six CEA patients completed the NPMT battery on postoperative Day 1 (59% symptomatic, 41% asymptomatic), and 153 completed testing on Day 30 (59% symptomatic, 41% symptomatic). Thirty-three (18%) patients were either lost to follow-up or refused to complete the battery on Day 30. On postoperative Day 1, 33 (18%) patients experienced neurocognitive dysfunction. Fourteen patients (9%) demonstrated cognitive injury on Day 30. Mean TDS scores in the CEA and LL groups were 3.80 ± 3.95 and 2.60 ± 2.27 at Day 1, respectively, and 2.64 ± 2.93 and 2.66 ± 1.99 at Day 30.
TABLE 1.
Demographic and intraoperative parameters of carotid endarterectomy patientsa
| CEA patients (%) | |
|---|---|
| No. of patients | 186 |
| Age (yr) | 69.8 ± 8.5 |
| Male | 129 (69%) |
| BMI ≥ 30 | 39 (21%) |
| Smoker | 102 (55%) |
| Diabetes mellitus | 47 (25%) |
| Hypertensionb | 118 (63%) |
| Hypercholesterolemiac | 99 (53%) |
| Statin medication | 97 (52%) |
| Previous MI | 53 (28%) |
| Symptomatic | 77 (41%) |
| Previous contralateral CEA | 25 (13%) |
| Right operative side | 97 (52%) |
| Duration of surgery (min) | 153.6 ± 42.4 |
| Cross-clamp time (min) | 45.6 ± 18.8 |
| Shunt placement | 5 (3%) |
| Fentanyl (μg/kg) | 2.2 ± 1.2 |
| Midazolam (mg/kg) | 0.03 ± 0.01 |
CEA, carotid endarterectomy; BMI, body mass index; MI, myocardial infarction. Continuous data is presented as mean ± standard deviation.
Hypertension is defined as systolic blood pressure greater than 140 or use of antihypertensive medication.
Hypercholesterolemia is defined as blood cholesterol greater than 200 or use of anticholesterol medication.
Statistical Analysis
Sex, obesity, smoking, symptomatology, diabetes, hypertension, hypercholesterolemia, use of a statin, previous myocardial infarction, previous contralateral CEA, operative side, duration of surgery, cross-clamp time, shunt placement and weight adjusted doses of fentanyl and midazolam were not associated with neurocognitive dysfunction on Day 1. Each decade increase in patient age was associated with a 93% increase in the risk of neurocognitive decline on postoperative Day 1 (1.93, 1.15–3.25, 0.01; Table 2). Of patients younger than 65 years of age, 9.3% experienced neurocognitive dysfunction on Day 1, compared with 14.9% of 65 to 74 year-old patients, and 28.6% of patients older than 74 years (Fig. 1, analysis of variance [ANOVA] P = 0.03). Aside from age, no other variables met our P value less than 0.10 criteria for inclusion in multivariate analysis.
TABLE 2.
Risk factors for neurocognitive decline on postoperative Day 1a
| Injured (%) | Uninjured (%) | OR (95% CI) | P value | |
|---|---|---|---|---|
| No. of patients | 33 (18) | 153 (82) | — | — |
| Age (yr) | 73.2 ± 7.7 | 69.1 ± 8.5 | 1.93 (1.15, 3.25) | 0.01 |
| Male | 22 (67) | 107 (70) | — | — |
| BMI ≥ 30 | 6 (18) | 33 (22) | — | — |
| Smoker | 19 (58) | 83 (54) | — | — |
| Diabetes mellitus | 11 (33) | 36 (24) | — | — |
| Hypertensionb | 21 (64) | 97 (63) | — | — |
| Hypercholesterolemiac | 16 (48) | 83 (54) | — | — |
| Statin medication | 13 (39) | 84 (55) | — | — |
| Previous MI | 10 (30) | 43 (28) | — | — |
| Symptomatic | 16 (48) | 61 (40) | — | — |
| Previous contralateral CEA | 6 (18) | 19 (12) | — | — |
| Right operative side | 21 (64) | 76 (50) | — | — |
| Duration of surgery (min) | 153.3 ± 46.6 | 153.7 ± 18.2 | — | — |
| Cross-clamp time (min) | 47.5 ± 18.2 | 45.2 ± 19.0 | — | — |
| Shunt placement | 2 (6) | 3 (2) | — | — |
| Fentanyl (μg/kg) | 2.2 ± 1.3 | 2.2 ± 1.2 | — | — |
| Midazolam (mg/kg) | 0.03 ± 0.01 | 0.03 ± 0.01 | — | — |
OR, odds ratio; CI, confidence interval; BMI, body mass index; MI, myocardial infarction; CEA, carotid endardectomy. OR is expressed in units of decades for age. No other variables met univariate P value less than 0.10 for inclusion in multivariate analysis. Continuous data is presented as mean ± standard deviation.
Hypertension is defined as systolic blood pressure greater than 140 or use of antihypertensive medication.
Hypercholesterolemia is defined as blood cholesterol greater than 200 or use of anticholesterol medication.
FIGURE 1.
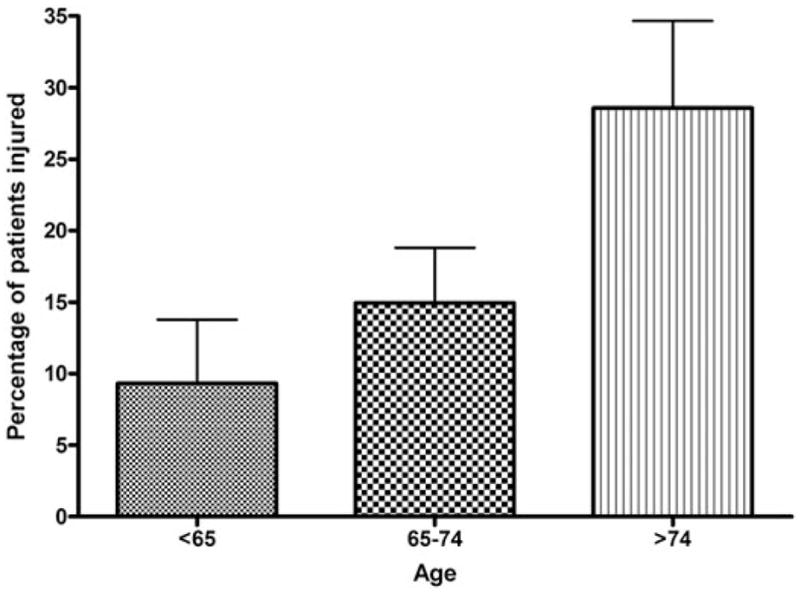
Incidence of neurocognitive decline on postoperative Day 1 by age. The rates of neurocognitive decline on Day 1 for patients younger than 65 years, 65 to 74 years, and older than 74 years of age are 9.3, 14.9, and 28.6%, respectively (Kruskal-Wallis ANOVA P = 0.03).
Thirty days after CEA, each decade increase in age elevated the risk of neurocognitive dysfunction more than 2.5 times (2.57, 1.01–6.51, 0.049) and diabetes increased the risk more than four times (4.26, 1.15–15.79, 0.03), in a multivariate analysis that also included obesity and dose of midazolam (Table 3). The neurocognitive injury rate at Day 30 was 21.1% for diabetics and 5.3% for non-diabetics (Fig. 2, P = 0.007). Sex, smoking, hypertension, hypercholesterolemia, statin medication, previous myocardial infarction, previous contralateral CEA, operative side, duration of surgery, cross-clamp time, shunt placement, and weight-adjusted dose of fentanyl all failed to reach P value less than 0.10 in univariate analysis.
TABLE 3.
Risk factors for neurocognitive decline on postoperative Day 30a
| Injured (%) | Uninjured (%) | OR (95% CI) | P value | |
|---|---|---|---|---|
| No. of patients | 14 (9) | 139 (91) | — | — |
| Age (yr) | 73.2 ± 7.6 | 69.8 ± 8.2 | 2.57 (1.01, 6.51) | <0.05 |
| Male | 12 (86) | 92 (66) | — | — |
| BMI ≥ 30 | 5 (36) | 21 (15) | 2.27 (0.61, 8.40) | NS |
| Smoker | 6 (43) | 73 (53) | — | — |
| Symptomatic | 5 (36) | 58 (42) | — | — |
| Diabetes mellitus | 8 (57) | 30 (22) | 4.26 (1.15, 15.79) | 0.03 |
| Hypertension | 7 (50) | 92 (66) | — | — |
| Hypercholesterolemia | 7 (50) | 74 (53) | — | — |
| Statin medication | 7 (50) | 73 (53) | — | — |
| Previous MI | 2 (14) | 33 (24) | — | — |
| Previous contralateral CEA | 3 (21) | 13 (9) | — | — |
| Right operative side | 7 (50) | 80 (58) | — | — |
| Duration of surgery (min) | 159.6 ± 45.8 | 155.0 ± 43.9 | — | — |
| Cross-clamp time (min) | 44.1 ± 17.2 | 45.0 ± 19.1 | — | — |
| Shunt placement | 0 (0) | 3 (2) | — | — |
| Fentanyl (μg/kg) | 1.78 ± 0.73 | 2.32 ± 1.27 | — | — |
| Midazolam (mg/kg) | 0.03 ± 0.02 | 0.03 ± 0.01 | 0.73 (0.44, 1.24) | NS |
OR, odds ratio; CI, confidence interval; BMI, body mass index; NS, not significant; MI, myocardial infarction; CEA, carotid endarterectomy. OR for age is per each decade increase. Diabetes and BMI ≥30 are categorical variables with ORs that represent the increased risk of having the condition. OR for midazolam is per 0.01mg/kg increase. Remaining variables did not meet univariate P value of less than 0.10 for inclusion in multivariate analysis. Continuous data is presented as mean ± standard deviation.
Hypertension is defined as systolic blood pressure greater than 140 or use of antihypertensive medication.
Hypercholesterolemia is defined as blood cholesterol greater than 200 or use of anticholesterol medication.
FIGURE 2.
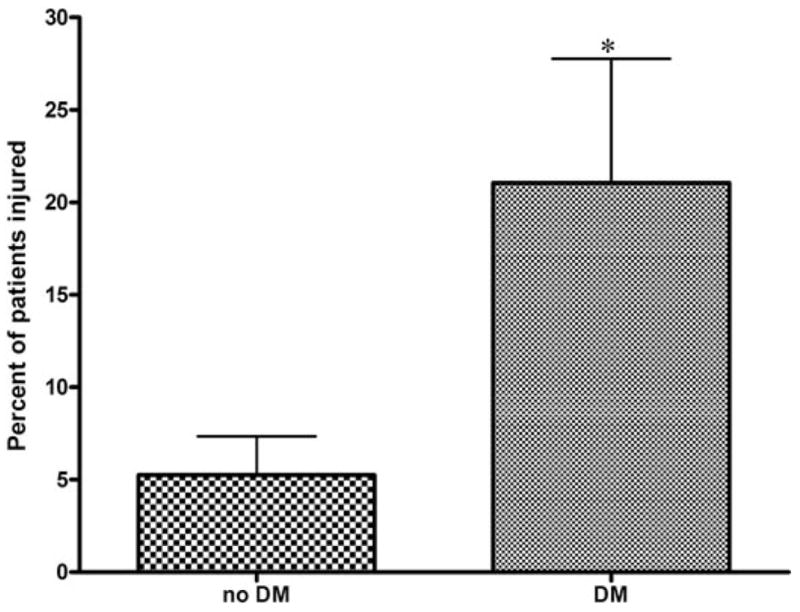
Incidence of neurocognitive decline at Day 30 by diabetes status. On Day 30, 21.1% of diabetics and 5.3% of non-diabetics experienced significant declines in NPMT performance (Fisher P = 0.007). DM, diabetes mellitus.
Subgroup analysis at postoperative Day 1 demonstrated that age was the only significant predictor of neurocognitive injury in the asymptomatic population (2.84, 1.22–6.19, 0.01). Among symptomatic patients, increasing weight-adjusted doses of midazolam reduced the risk of neurocognitive dysfunction in a multivariate analysis that also included duration of surgery and prior contralateral CEA (0.53, 0.31–0.92, 0.02). Owing to the limited number of injured patients at Day 30 (symptomatic cohort, five; asymptomatic cohort, nine), a subgroup analysis of symptomatic and asymptomatic patients was underpowered to provide meaningful results.
DISCUSSION
Although CEA is considered a safe procedure, with low incidence of perioperative stroke, the prevalence of subtle neurocognitive dysfunction after CEA is being increasingly recognized. Cognitive dysfunction after CEA is detected by a battery of NPMTs designed to offer a more detailed assessment of higher cortical function than traditional neurological exam. Previously, we demonstrated that approximately 25% of CEA patients experience a decline in neurocognitive performance on a battery of NPMTs administered 1 day postoperatively, in comparison with a similarly aged population exposed to a comparable anesthetic regimen (12, 13). However, this is the first study to investigate the risk factors that predispose to this neurocognitive decline.
Although the precise mechanism of post-CEA neurocognitive dysfunction is unknown, evidence points to an ischemic etiology. Declines in cognitive function after CEA have been associated with serum elevations of protein S100b, a marker of glial cell death, indicating the occurrence of cerebral injury (3). Ischemia may be owing to transient hypoperfusion during carotid cross-clamping or the dislodgement of microemboli. The latter mechanism is supported by recent work using Doppler high-intensity transient signals analysis demonstrating that microemboli are frequently detected in the cerebral circulation during CEA (7, 36).
The association of age and diabetes with neurocognitive decline at different time points is consistent with previous studies demonstrating that these factors variably increase the risk of major stroke owing to CEA. In a retrospective review of more than 6000 CEA patients in the Ontario Carotid Endarterectomy Registry, Tu et al. (32) found that diabetes, among other factors, independently predicts stroke or death within 30 days of surgery (OR 1.28, P = 0.04). Kragsterman et al. (16) confirmed this association in a Swedish cohort (risk ratio 1.41, P = 0.02), whereas Rothwell et al. (28) demonstrated, in a meta-analysis of available literature, that age greater than 75 years confers a 36% increase in the risk of stroke or death owing to CEA. Although the concept of a high-risk CEA population remains controversial (6, 21, 26), our findings raise the possibility that post-CEA stroke and neurocognitive decline share related risk factors and etiologies.
Our findings are also consistent with studies of neurocognitive injury after coronary artery bypass surgery. Postoperative declines in NPMT performance are a well-recognized consequence of coronary artery bypass surgery, occurring in as many as 47% of patients, and are though to be ischemic in nature (2, 33). Similar to our findings, advanced age and diabetes have been identified as independent predictors of cognitive dysfunction after coronary artery bypass surgery (23, 24, 29–31).
The difference in incidence of neurocognitive injury after CEA at Day 1 (18%) and Day 30 (9%) suggests that postoperative changes in cognition vary over time, with patients gradually regaining cognitive abilities after surgery. The finding that age predicts injury at both time points, whereas diabetes becomes a significant risk factor only 1 month after surgery, suggests that cognitive decline at these time points may represent heterogenous entities with distinct underlying causes. Cognitive dysfunction on postoperative day one is age-dependent, and may be owing to age-related changes in cerebral autoregulation and oxygenation (2, 10, 27). It is likely that these changes in cognition are particular to CEA, as all scores were generated in comparison to a similarly aged control population that underwent a comparable anesthetic regimen. The CEA-specific nature of this neurocognitive decline is supported by the consistency of the mean TDS scores within the LL control group at Days 1 and 30 (2.60 ± 2.27 and 2.66 ±1.99, respectively), whereas the CEA scores improved over time (3.80 ± 3.95 and 2.64 ± 2.93, respectively). Cognitive decline on Day 30 remains age-dependent, with diabetes also predisposing to injury at this time point. This may represent an impaired ability of older and diabetic patients to adequately recover from neuropsychometric injury, and is consistent with prior studies identifying age and diabetes as predictors of poor functional recovery after stroke (17, 22, 23, 34).
Owing to small absolute surgical benefits, the role of CEA in asymptomatic patients is particularly controversial (1, 4). This has led a number of investigators to hypothesize that symptomatic and asymptomatic carotid stenosis are distinct pathological entities (9, 18). In an effort to evaluate potential differences between symptomatic and asymptomatic patients, we attempted to perform subgroup analyses by symptomatology on Day 1 and Day 30. On postoperative Day 1, advanced age was the only significant risk factor for neurocognitive dysfunction among asymptomatic patients (2.84, 1.22–6.19, 0.01). In symptomatic patients, increasing weight-adjusted doses of midazolam reduced the risk of NPMT decline (0.53, 0.31–0.92, 0.2). Although the relevance of this finding is uncertain, previous investigations in animal stroke models have suggested a potential neuroprotective effect of midazolam (8, 37). Subgroup analysis at Day 30 was obviated by the limited number of injured patients (five in symptomatic cohort, nine in asymptomatic cohort). Currently, these findings are underpowered to draw firm conclusions regarding the nature of subtle neurocognitive decline in symptomatic versus asymptomatic patients.
We demonstrate that advanced age predisposes to neurocognitive decline after CEA on Days 1 and 30. For each decade increase in age, the risk of injury is elevated 93 and 157%, respectively. Additionally, diabetes increases the risk of neurocognitive dysfunction more than fourfold on postoperative Day 30. In the calculus of weighing the risks and benefits of CEA, the perioperative complication rate is crucial in choosing the optimal treatment. Small increases in operative risk may negate the benefits of CEA. Further work is necessary to determine the role these neurocognitive deficits may play in appropriately selecting patients for CEA.
Acknowledgments
J Mocco was supported in part by the Congress of Neurological Surgeons Wilder Penfield Clinical Research Fellowship, David A. Wilson was supported in part by the Doris Duke Clinical Research Fellowship, Ricardo J. Komotar was supported in part by a National Institutes of Health Research Training Fellowship, and Eric J. Heyer was supported in part by a grant from the National Institute on Aging (RO1 AG17604-02).
Contributor Information
J Mocco, Department of Neurological Surgery, Columbia University, New York, New York
David A. Wilson, Department of Neurological Surgery, Columbia University, New York, New York
Ricardo J. Komotar, Department of Neurological Surgery, Columbia University, New York, New York
Joseph Zurica, Department of Anesthesiology, Columbia University, New York, New York
William J. Mack, Department of Neurological Surgery, Columbia University, New York, New York
Hadi J. Halazun, Department of Anesthesiology, Columbia University, New York, New York
Raheleh Hatami, Department of Anesthesiology, Columbia University, New York, New York
Robert R. Sciacca, Department of Medicine, Columbia University, New York, New York.
E. Sander Connolly, Jr., Departments of Neurological Surgery and Neurology, Columbia University, New York, New York
Eric J. Heyer, Departments of Anesthesiology and Neurology, Columbia University, New York, New York
References
- 1.Barnett HJ. Carotid endarterectomy. Lancet. 2004;363:1486–1487. doi: 10.1016/S0140-6736(04)16182-5. [DOI] [PubMed] [Google Scholar]
- 2.Borowicz LM, Goldsborough MA, Selnes OA, McKhann GM. Neuropsychologic change after cardiac surgery: A critical review. J Cardiothorac Vasc Anesth. 1996;10:105–111. doi: 10.1016/s1053-0770(96)80185-6. [DOI] [PubMed] [Google Scholar]
- 3.Connolly ES, Jr, Winfree CJ, Rampersad A, Sharma R, Mack WJ, Mocco J, Solomon RA, Todd G, Quest DO, Stern Y, Heyer EJ. Serum S100B protein levels are correlated with subclinical neurocognitive declines after carotid endarterectomy. Neurosurgery. 2001;49:1076–1082. doi: 10.1097/00006123-200111000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dodick DW, Meissner I, Meyer FB, Cloft HJ. Evaluation and management of asymptomatic carotid artery stenosis. Mayo Clin Proc. 2004;79:937–944. doi: 10.4065/79.7.937. [DOI] [PubMed] [Google Scholar]
- 5.Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995;273:1421–1428. [PubMed] [Google Scholar]
- 6.Gasparis AP, Ricotta L, Cuadra SA, Char DJ, Purtill WA, Van Bemmelen PS, Hines GL, Giron F, Ricotta JJ. High-risk carotid endarterectomy: Fact or fiction. J Vasc Surg. 2003;37:40–46. doi: 10.1067/mva.2003.56. [DOI] [PubMed] [Google Scholar]
- 7.Gaunt ME, Martin PJ, Smith JL, Rimmer T, Cherryman G, Ratliff DA, Bell PR, Naylor AR. Clinical relevance of intraoperative embolization detected by transcranial Doppler ultrasonography during carotid endarterectomy: A prospective study of 100 patients. Br J Surg. 1994;81:1435–1439. doi: 10.1002/bjs.1800811009. [DOI] [PubMed] [Google Scholar]
- 8.Gilby KL, Sydserff SG, Robertson HA. Differential neuroprotective effects for three GABA-potentiating compounds in a model of hypoxiaischemia. Brain Res. 2005;1035:196–205. doi: 10.1016/j.brainres.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 9.Golledge J, Greenhalgh RM, Davies AH. The symptomatic carotid plaque. Stroke. 2000;31:774–781. doi: 10.1161/01.str.31.3.774. [DOI] [PubMed] [Google Scholar]
- 10.Halliday A, Mansfield A, Marro J, Peto C, Peto R, Potter J, Thomas D. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: Randomised controlled trial. Lancet. 2004;363:1491–1502. doi: 10.1016/S0140-6736(04)16146-1. [DOI] [PubMed] [Google Scholar]
- 11.Halm EA, Chassin MR, Tuhrim S, Hollier LH, Popp AJ, Ascher E, Dardik H, Faust G, Riles TS. Revisiting the appropriateness of carotid endarterectomy. Stroke. 2003;34:1464–1471. doi: 10.1161/01.STR.0000072514.79745.7D. [DOI] [PubMed] [Google Scholar]
- 12.Heyer EJ, Adams DC, Solomon RA, Todd GJ, Quest DO, McMahon DJ, Steneck SD, Choudhri TF, Connolly ES., Jr Neuropsychometric changes in patients after carotid endarterectomy. Stroke. 1998;29:1110–1115. doi: 10.1161/01.str.29.6.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heyer EJ, Sharma R, Rampersad A, Winfree CJ, Mack WJ, Solomon RA, Todd GJ, McCormick PC, McMurtry JG, Quest DO, Stern Y, Lazar RM, Connolly ES., Jr A controlled prospective study of neuropsychological dysfunction following carotid endarterectomy. Arch Neurol. 2002;59:217–222. doi: 10.1001/archneur.59.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heyer EJ, Sharma R, Winfree CJ, Mocco J, McMahon DJ, McCormick PA, Quest DO, McMurtry JG, Riedel CJ, Lazar RM, Stern Y, Connolly ES., Jr Severe pain confounds neuropsychological test performance. J Clin Exp Neuropsychol. 2000;22:633–639. doi: 10.1076/1380-3395(200010)22:5;1-9;FT633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hobson RW, 2nd, Weiss DG, Fields WS, Goldstone J, Moore WS, Towne JB, Wright CB. Efficacy of carotid endarterectomy for asymptomatic carotid stenosis. The Veterans Affairs Cooperative Study Group. N Engl J Med. 1993;328:221–227. doi: 10.1056/NEJM199301283280401. [DOI] [PubMed] [Google Scholar]
- 16.Kragsterman B, Logason K, Ahari A, Troeng T, Parsson H, Bergqvist D. Risk factors for complications after carotid endarterectomy: A population-based study. Eur J Vasc Endovasc Surg. 2004;28:98–103. doi: 10.1016/j.ejvs.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 17.Leys D, Bandu L, Henon H, Lucas C, Mounier-Vehier F, Rondepierre P, Godefroy O. Clinical outcome in 287 consecutive young adults (15 to 45 years) with ischemic stroke. Neurology. 2002;59:26–33. doi: 10.1212/wnl.59.1.26. [DOI] [PubMed] [Google Scholar]
- 18.Liapis CD, Kakisis JD, Kostakis AG. Carotid stenosis: Factors affecting symptomatology. Stroke. 2001;32:2782–2786. doi: 10.1161/hs1201.099797. [DOI] [PubMed] [Google Scholar]
- 19.Lu PH, Boone KB, Cozolino L, Mitchell C. Effectiveness of the ReyOsterrieth Complex Figure Test and the Meyers and Meyers recognition trial in the detection of suspect effort. Clin Neuropsychol. 2003;17:426–440. doi: 10.1076/clin.17.3.426.18083. [DOI] [PubMed] [Google Scholar]
- 20.Mehagnoul-Schipper DJ, Vloet LC, Colier WN, Hoefnagels WH, Jansen RW. Cerebral oxygenation declines in healthy elderly subjects in response to assuming the upright position. Stroke. 2000;31:1615–1620. doi: 10.1161/01.str.31.7.1615. [DOI] [PubMed] [Google Scholar]
- 21.Mozes G, Sullivan TM, Torres-Russotto DR, Bower TC, Hoskin TL, Sampaio SM, Gloviczki P, Panneton JM, Noel AA, Cherry KJ., Jr Carotid endarterectomy in SAPPHIRE-eligible high-risk patients: Implications for selecting patients for carotid angioplasty and stenting. J Vasc Surg. 2004;39:958–965. doi: 10.1016/j.jvs.2003.12.037. [DOI] [PubMed] [Google Scholar]
- 22.Naess H, Nyland HI, Thomassen L, Aarseth J, Myhr KM. Long-term outcome of cerebral infarction in young adults. Acta Neurol Scand. 2004;110:107–112. doi: 10.1111/j.1600-0404.2004.00273.x. [DOI] [PubMed] [Google Scholar]
- 23.Newman MF, Croughwell ND, Blumenthal JA, Lowry E, White WD, Spillane W, Davis RD, Jr, Glower DD, Smith LR, Mahanna EP, Reves JG. Predictors of cognitive decline after cardiac operation. Ann Thorac Surg. 1995;59:1326–1330. doi: 10.1016/0003-4975(95)00076-w. [DOI] [PubMed] [Google Scholar]
- 24.Newman MF, Croughwell ND, Blumenthal JA, White WD, Lewis JB, Smith LR, Frasco P, Towner EA, Schell RM, Hurwitz BJ, Reves JG. Effect of aging on cerebral autoregulation during cardiopulmonary bypass: Association with postoperative cognitive dysfunction. Circulation. 1994;90(Suppl 2):243–249. [PubMed] [Google Scholar]
- 25.North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 26.Ouriel K. Regarding Carotid endarterectomy in SAPPHIRE-eligible high-risk patients: Implications for selecting patients for carotid angioplasty and stenting. J Vasc Surg. 2004;40:595–596. doi: 10.1016/j.jvs.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 27.Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev. 1990;2:161–192. [PubMed] [Google Scholar]
- 28.Rothwell PM, Slattery J, Warlow CP. Clinical and angiographic predictors of stroke and death from carotid endarterectomy: Systematic review. BMJ. 1997;315:1571–1577. doi: 10.1136/bmj.315.7122.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selnes OA, Goldsborough MA, Borowicz LM, Jr, Enger C, Quaskey SA, McKhann GM. Determinants of cognitive change after coronary artery bypass surgery: A multifactorial problem. Ann Thorac Surg. 1999;67:1669–1676. doi: 10.1016/s0003-4975(99)00258-1. [DOI] [PubMed] [Google Scholar]
- 30.Shaw PJ, Bates D, Cartlidge NE, French JM, Heaviside D, Julian DG, Shaw DA. An analysis of factors predisposing to neurological injury in patients undergoing coronary bypass operations. Q J Med. 1989;72:633–646. [PubMed] [Google Scholar]
- 31.Townes BD, Bashein G, Hornbein TF, Coppel DB, Goldstein DE, Davis KB, Nessly ML, Bledsoe SW, Veith RC, Ivey TD. Neurobehavioral outcomes in cardiac operations. A prospective controlled study. J Thorac Cardiovasc Surg. 1989;98:774–782. [PubMed] [Google Scholar]
- 32.Tu JV, Wang H, Bowyer B, Green L, Fang J, Kucey D. Risk factors for death or stroke after carotid endarterectomy: Observations from the Ontario Carotid Endarterectomy Registry. Stroke. 2003;34:2568–2573. doi: 10.1161/01.STR.0000092491.45227.0F. [DOI] [PubMed] [Google Scholar]
- 33.van Dijk D, Keizer AM, Diephuis JC, Durand C, Vos LJ, Hijman R. Neurocognitive dysfunction after coronary artery bypass surgery: A systematic review. J Thorac Cardiovasc Surg. 2000;120:632–639. doi: 10.1067/mtc.2000.108901. [DOI] [PubMed] [Google Scholar]
- 34.Weimar C, Ziegler A, Konig IR, Diener HC. Predicting functional outcome and survival after acute ischemic stroke. J Neurol. 2002;249:888–895. doi: 10.1007/s00415-002-0755-8. [DOI] [PubMed] [Google Scholar]
- 35.Wennberg DE, Lucas FL, Birkmeyer JD, Bredenberg CE, Fisher ES. Variation in carotid endarterectomy mortality in the Medicare population: Trial hospitals, volume, and patient characteristics. JAMA. 1998;279:1278–1281. doi: 10.1001/jama.279.16.1278. [DOI] [PubMed] [Google Scholar]
- 36.Wolf O, Heider P, Heinz M, Poppert H, Sander D, Greil O, Weiss W, Hanke M, Eckstein HH. Microembolic signals detected by transcranial Doppler sonography during carotid endarterectomy and correlation with serial diffusion-weighted imaging. Stroke. 2004;35:373–375. doi: 10.1161/01.STR.0000143184.69343.ec. [DOI] [PubMed] [Google Scholar]
- 37.Zhang PB, Liu Y, Li J, Chen XL, Tian YF, Sun JJ, Liu JX. Effects of ketaminemidazolam anesthesia on focal cerebral ischemic injury in rats. Di Yi Jun Yi Da Xue Xue Bao. 2004;24:1337–1341. [in Chinese] [PubMed] [Google Scholar]


