Summary
Interleukin-10 (IL-10) has a wide range of in vivo biological activities and is a key regulatory cytokine of immune-mediated inflammation. The authors found that murine IL-10 given 12 hours after a recombinant vaccinia virus (rVV) containing the LacZ gene significantly enhanced the treatment of mice bearing 3-day-old pulmonary metastases expressing β-galactosidase. Because IL-10 has been shown to inhibit the functions of key elements of both innate and acquired immune responses, the authors hypothesized that IL-10 might act by inhibiting clearance of the rVV, thus prolonging exposure to the experimental antigen. However, evidence that IL-10 was not acting primarily through such negative regulatory mechanisms included the following: (a) IL-10 also enhanced the therapeutic effectiveness of a recombinant fowlpox virus, which cannot replicate in mammalian cells; (b) Titers of rVV in immunized mice were lower, not higher; and (c) Although IL-10 did not alter levels of anti-vaccinia antibodies or natural killer cell activity, rVV-primed mice treated with IL-10 had enhanced vaccinia-specific cytotoxic T-lymphocyte activity. Thus, IL-10 enhanced the function of a recombinant poxvirus-based anti-cancer vaccine and may represent a potential adjuvant in the vaccination against human cancers using recombinant poxvirus-based vaccines.
Keywords: Interleukin-10, Recombinant vaccinia virus, Poxvirus, Murine tumor model
The identification of tumor-associated antigens (TAA) recognized by T lymphocytes has led to various immunization strategies that target TAA to achieve tumor rejection. One such approach uses recombinant vaccinia viruses that encode the gene for the TAA (1–3). Tumor regression in mice vaccinated with such recombinant vectors and prevention of growth of tumor lines expressing similar tumor antigens have been shown to be mediated through the generation of CD8+ cytotoxic T lymphocytes (CTL) directed against the TAA (4,5). We have previously reported the ability of different cytokines, such as interleukin-2 (IL-2) and IL-12, to augment the in vivo responsiveness of these recombinant vaccinia viruses (6,7). In initial screening studies, we included IL-10 as a control cytokine and, surprisingly, found that IL-10 enhanced, rather than suppressed, in vivo tumor regression when used as an adjuvant to vaccination.
Interleukin-10 (IL-10) is an 18-KDa cytokine that was originally identified from Th2 cells and can inhibit cytokine release from macrophages and Th1 cells (8–10). Expression of IL-10 often occurs relatively late after activation of macrophages or T cells and exhibits a wide range of immune regulatory effects, generally thought of as inhibiting cell-mediated inflammatory reactions (11,12). Recent studies suggest that IL-10 may also stimulate strong proliferative responses of murine thymocytes in the presence of IL-2 and IL-4, increase pre-cursor CTL, and significantly increase cytotoxic effector functions (13,14).
Although there have been reports that IL-10 inhibits tumor antigen presentation by Langerhans cells, prevents tumor recognition by allospecific CTL, and downregulates the expression of human leukocyte antigen class I on tumor cells and class II on macrophages, there are reports of IL-10 enhancing the in vivo treatment of tumors in animal models (15–17). The local release of IL-10 by transducing the IL-10 gene into tumor cells has been shown to prevent the growth of Chinese hamster ovary cells and a mammary adenocarcinoma cell line in mice (18,19). Systemic IL-10 was shown to elicit a specific immune response against several histologically distinct murine tumors, maintain memory responses, and synergize with IL-12 (20). Furthermore, IL-10 has been shown to inhibit the metastases of the murine M27 Lewis lung carcinoma cell line through a natural killer cell (NK)-dependent mechanism (21).
These reports suggest that IL-10 may enhance antitumor effects and augment T-cell-mediated responses under certain conditions. We now report significant augmentation of antitumor responses when IL-10 is used as an adjuvant to a recombinant vaccinia virus. The effects of IL-10 on vaccinia virus titers showed an acceleration of viral clearance in IL-10-treated mice. This increase in viral clearance was associated with increased CTL activity, without an associated increase in antibody titers. These unexpected findings suggest a more complex immunologic regulatory function of IL-10 and may support the use of IL-10 as an adjuvant to vaccination against human cancer.
MATERIALS AND METHODS
Animals and Cell Lines
Six- to 8-week-old female BALB/c (H-2d) mice were used in all animal studies (Frederick Cancer Research Center, Frederick, MD, U.S.A.), Animals were housed in the biohazard facility at the National Cancer Institute and were treated in accordance with the guidelines of the National Institutes of Health animal review committee. African green monkey kidney BSC-1 cells were grown in Dulbecco’s Modified Eagle Medium supplemented with 10% heat-inactivated fetal calf serum (FCS), 100 μg/ml streptomycin, 100 U/ml penicillin, and 50 μg/ml gentamicin. The murine colon adenocarcinoma CT26.WT and the β-gal-expressing CT26.CL25 cell lines have been previously described (22). These lines were induced in BALB/c (H-2d) mice and were grown in RPMI I640 supplemented with 10% heat-inactivated FCS, 0.03% L-glutamine, 100 μg/ml streptomycin, 100 U/ml penicillin. and 50 μg/ml gentamicin. The transfected CT26.CL25 line was supplemented with 400 μg/ml G418 (Gibco, Grand Island, NY, U.S.A.).
Recombinant Viruses
The recombinant vaccinia virus expressing the model tumor antigen, β-gal, called VJS6, was generated by insertion of the specialized plasmid pJS6, which contains the Escherichia coli LacZ gene under the vaccinia early/late p7.5 promoter (provided by B. Moss, National Institute of Allergy and Infectious Diseases, Bethesda, MD, U.S.A.) and is inserted into the viral thymidine kinase (TK) gene by homologous recombination as previously described (23). The TK-disrupted control vaccinia virus designated V69 was constructed by generating the recombinant plasmid pGS69, which contains the influenza A/PR/8/34 nucleoprotein in flanking TK gene segments and lacks the LacZ gene (24). Viral stocks were propagated on BSC-1 cells and purified by ultracentrifugation on a 36% sucrose cushion. Recombinants were selected by expression of β-gal and for the TK− pheno-type. Virus concentration was determined by the plaque titration method using BSC-1 cells.
The fowlpox virus constructs included the wild-type strain, FPV.wt, originating from the POXVAC-TC strain (Schering Corp.. Kenilworth, NJ, U.S.A.) and the recombinant fowlpox virus, FPV.bg40k, containing the E. coli LacZ gene under the direction of the vaccinia virus 40-kDa promoter (provided by L. Gritz, Therion, Inc., Cambridge, MA, U.S.A.). The foreign sequences were inserted by homologous recombination into the BamHI J region of the fowlpox genome, as described elsewhere, and propagated on primary chick embryo dermal cultures (25).
Treatment of Mice with Established Tumors
Mice were injected intravenously (i.v.) with either the parental CT26.WT or the β-gal-expressing CT26.CL25 cell line (5 × 105) by tail vein. Three days later, they were treated with virus at the specified concentrations [105–107 plaque-forming units (PFU)] also by tail vein injection. Mice randomized to receive IL-10 were given 1 μg of murine IL-10 (PeproTech, Inc., Rocky Hill, NJ, U.S.A.) reconstituted in 1% homologous mouse serum in phosphate-buffered saline (PBS) by intraperitoneal (i.p.) injection beginning 12 hours after receiving virus. IL-10 was then administered every 24 hours for 5 days by i.p. injection. Mice were killed by CO2 asphyxiation 12 days after injection of tumor and the lungs were removed, stained with India ink, and counted in a blinded manner, as previously described (26). Statistical analysis was performed using the two-tailed Wilcoxon t test with p ≤ 0.05 used to determine significance.
Quantitation of Vaccinia Viral Titers
Two groups of mice were injected with 1 ×107 PFU of the VJS6 recombinant virus by tail vein injection. One group also received murine IL-10 (1 μg) by i.p. injection starting 12 hours after virus administration and then daily for 5 days. Two mice from each group were killed on alternating days for 8 days and the lungs, spleen, kidneys, liver, and ovaries were removed and placed in PBS. The organs were homogenized in Tris, pH 8.0, subjected to three rounds of freeze-thawing, sonicated, and diluted in minimal essential medium culture media supplemented with 2% FCS. Vaccinia titers were determined by the plaque assay method on nearly confluent BSC-1 cells, as previously described (27). All samples were run in duplicate, and titers are reported as the number of PFU per milliliter.
Direct Effect of IL-10 on Murine Tumor Cells In Vitro
To determine whether IL-10 had any direct inhibitory properties on the CT26.WT or CT26.CL25 tumor cell line in vitro, a proliferative assay was performed. Tumor cells (5 × 103) were plated into 96-well plates and incubated at 37°C for 24 hours. Murine IL-10 was added to the plates at the following concentrations in triplicate— 0, 0.008 μg, 0.04 μg, 0.2 μg, 1.0 μg, and 3.0 μg. 3H-Thymidine (1 μCi/well) was also added to each well and the plates incubated at 37°C for 5 hours. Counts were obtained on a beta counter, and the amount of 3H-thymidine release was calculated.
Enzyme-Linked Immunosorbent Assay
Three groups of mice were vaccinated with either an i.v. injection of 1 × 107 PFU of VJS6 alone, 1 ×107 PFU of VJS6 followed by 5 days of recombinant murine IL-10 administration starting 12 hours after immunization (1 μg, i.p., Q.D.), or recombinant murine IL-10 administration alone (PeproTech, Inc.). Pooled sera from two immunized mice were obtained 2, 4, 6, 14, and 21 days after treatment and were analyzed by enzyme-linked immunosorbent assay for the presence of antibodies (Abs) against β-gal protein or wild-type vaccinia virus. Briefly, microtiter plates were dried down overnight at 37°C in a nonhumidified incubator with 200 ng/well/50 μl of purified β-gal protein (Sigma Chemical Co., St. Louis, MO, U.S.A.). Alternatively, microtiter plates were coated with wild-type vaccinia virus (WT-VV) (5 × 105/well/50μl) at 4°C, overnight. The plates were incubated with 5% bovine serum albumin (BSA) in PBS on each well for 1 hour to prevent nonspecific Abs from binding. This was followed by a second 1-hour incubation with 50 μ1 of fivefold dilutions (starting at 1:50) of test sera. After washing with 1% BSA in PBS, horseradish peroxidase-conjugated sheep anti-mouse IgG F(ab′)2 fragments (1:3,000) (Amersham International, Amersham, UK) were added for 1 hour at 37°C to detect Abs immobilized on the wells. The resulting complex was detected by the chromogen, o-phenylenediazamine (Sigma Chemical Co.). Absorbance was read on a Titertek Multiskan Plus reader (Flow Laboratories, McLean, VA, U.S.A.) using a 490-nm pore filter.
Cytotoxic T-Lymphocyte Activity
Primary CTL activity was assessed by performing a standard chromium-release assay on fresh splenocytes derived from mice treated with either VJS6 virus alone (107 PFU i.v.), VJS6 virus (107 PFU i.v.) followed by IL-10 for 5 days (1 μg i.p.), or IL-10 alone (1 μg i.p.) for 5 days. Splenocytes were harvested, filtered through wire mesh, and counted. Target cells included the CT26.WT murine adenocarcinoma cell line, a vaccinia-infected CT26.WT cell line designated CT26-VAC, and YAC-1 cells, and these were labeled with 51Cr for 1 hour and plated on 96-well plates. Splenocytes were washed in Hank’s balanced salt solution and added to the plates at various effector-to-target ratios and centrifuged. The plates were incubated for 8 hours, cells harvested, and 51Cr-release counted in a gamma counter. Percent lysis was calculated as (experimental release – spontaneous release/total release – spontaneous release) ×100%.
RESULTS
IL-10 Enhances Vaccine Treatment of Pulmonary Metastases
Systemic and local IL-10 administration has been shown to enhance immune-mediated responses against tumors (19–21). Antitumor effects of IL-10 were examined as an adjuvant to a recombinant vaccinia virus expressing a model tumor antigen. Treatment of established pulmonary metastases was tested using the recombinant vaccinia virus, VJS6, which expresses the model antigen βgalactosidase (β-gal). Pulmonary metastases developed in all mice after an intravenous injection of the murine colon adenocarcinoma cell line, CT26.WT, or the β-gal-expressing CT26.CL25 tumor cells (5 × 105), generally resulting in death of the animals within 14–20 days. Three days after establishment of pulmonary metastases, treatment with 1 × 107 PFU of the VJS6 virus intravenously resulted in a significant reduction in the number of pulmonary metastases observed on day 12. This effect was enhanced by the addition of systemic IL-10 given 12 hours after virus was injected and then daily for 5 days. No effect was observed when systemic IL-10 was used alone (Table 1). This experiment was repeated giving IL-10 just before or just after virus administration, and no effect on pulmonary metastases was observed (data not shown).
TABLE 1.
Interleukin-10 (IL-10) enhances the antitumor effect of recombinant vaccinia virus
| Mean no. of pulmonary metastases
|
|||||
|---|---|---|---|---|---|
| Experiment | Tumor used | Virus used | Dose (PFU) | −IL-10 | +IL-10 |
| 1 | CT26.CL25 | __ | _ | >250 | >250 |
| CT26.CL25 | V69 | 107 | >250 | >250 | |
| CT26.WT | VJS6 | 107 | >250 | >250 | |
| CT26.CL25 | VJS6 | 107 | 80 | 12a | |
| 2 | CT26.CL25 | — | — | >250 | >250 |
| CT26.CL25 | V69 | 106 | >250 | >250 | |
| CT26.WT | VJS6 | 106 | >250 | >250 | |
| CT26.CL25 | VJS6 | 106 | 112 | 24a | |
| 3 | CT26.CL25 | V69 | 105 | >250 | >250 |
| CT26.WT | VJS6 | 105 | >250 | >250 | |
| CT26.CL25 | VJS6 | 105 | 240 | 25a | |
BALB/c mice (five per group) were given 5 × 105 tumor cells expressing β-gal (CT26.CL25) or the non-β-gal-expressing CT26.WT. Three days later, mice were injected with intravenous vaccinia virus expressing β-gal (VJS6). a thymidine-kinase-disrupted control vaccinia virus (V69), or no virus. Viral doses were 107 plaque-forming units (PFU) (experiment 1), 106 PFU (experiment 2), or 105 PFU (experiment 3). IL-10 administration (1 μg i.p.) was started 12 hours after virus injection and continued daily for 5 days. Pulmonary metastases were counted in a blinded fashion 12 days later, and the mean for each group is shown above. IL-10 enhanced the response of VJS6 in treating the CT26.CL25 tumor at all doses.
p ≤ 0.05 (Wilcoxon t test).
To optimize the IL-10 effect, we administered lower doses of virus, 1 × 106–1 × 105 PFU, which resulted in decreased therapeutic responses to virus alone. However, when IL-10 was added to the treatment, a continued significant reduction in metastases was observed at both viral doses (Table 1). This effect was specific for our model antigen, β-gal, because there was no effect of virus and IL-10 together on the β-gal-negative cell line, CT26.WT, and there was no effect when the β-gal-negative virus V69 was used in combination with IL-10. All experiments were repeated with similar results. The vaccine was used intravenously in all experiments because we have previously shown that this is the most effective route of administration using this model system (22). We also examined the effect of IL-10 on survival of animals treated with the VJS6 vaccine but did not see a significant prolongation in survival. This is presumably because even a small number of remaining metastatic deposits eventually grew and killed the animals. The CT26 tumor cell line is highly aggressive and lethal within 3 weeks without any treatment.
Since IL-10 is known to inhibit inflammatory responses, we sought to determine whether a possible mechanism for IL-10 enhancement was mediated by a dampening of the host immune response against the vaccinia virus, thus resulting in prolonged exposure of the host immune system to the model antigen. To address this possibility, we explored a recombinant fowlpox virus vector that mediated the expression of β-gal, designated FPV.bg40k. Fowlpox is an avian virus that does not replicate in mammalian cells and, therefore, should not be affected by IL-10 if the enhancement effect of IL-10 is mediated by prolonging viral replication time. IL-10 enhanced the treatment of 3-day-old pulmonary metastases when administered after the recombinant FPV.bg40k virus. The IL-10 adjuvant effect became more pronounced at lower doses of virus (105–106 PFU), because at the highest dose used (107 PFU), the virus alone was very effective. No effect was seen against the β-gal-negative cell line CT26.WT or with the β-gal-negative fowlpox virus, FPV.wt, indicating an antigen-specific treatment effect (Table 2). All treatment experiments were repeated with similar results.
TABLE 2.
Interleukin-10 (IL-10) enhances the antitumor effect of recombinant fowlpox virus
| Mean no. of pulmonary metastases
|
|||||
|---|---|---|---|---|---|
| Experiment | Tumor used | Virus used | Dose (PFU) | −IL-10 | +IL-10 |
| 1 | CT26.CL25 | FPV.wt | 107 | >250 | >250 |
| CT26.WT | FPV.bg40k | 107 | >250 | >250 | |
| CT26.CL25 | FPV.bg40k | 107 | 45a | 24a | |
| 2 | CT26.CL25 | FPV.wt | 106 | >250 | >250 |
| CT26.WT | FPV.bg40k | 106 | >250 | >250 | |
| CT26.CL25 | FPV.bg40k | 106 | 72a | 4b | |
| 3 | CT26.CL25 | FPV.wt | 105 | >250 | >250 |
| CT26.WT | FPV.bg40k | 105 | >250 | >250 | |
| CT26.CL25 | FPV.bg40k | 105 | >250 | 4b | |
BALB/c mice (five per group) were treated with recombinant fowlpox virus expressing β-gal (FPV.bg40k) or wild-type fowlpox virus (FPV.wt) at a dose of 107 plaque-forming units (PFU) (experiment 1), 106 PFU (experiment 2), or 105 PFU (experiment 3) by tail vein injection 3 days after intravenous administration of 5 × 105 CT26.CL25 (β-gal-expressing) tumor cells or CT26.WT (non-β-gal-expressing). IL-10 (1 μg i.p.) was started 12 hours after virus and continued daily for 5 days. FPV.bg40k reduced the mean number of pulmonary metastases in mice bearing the CT26.CL25 tumor when administered at 107 PFU or 106 PFU (a). IL-10 did not increase the response when 107 PFU virus was used, but did reduce the number of metastases compared to FPV.bg40k alone at 106 PFU and 105 PFU (a).
p ≤ 0.05 (Wilcoxon t test).
p ≤ 0.05 (Wilcoxon t test).
IL-10 Alters the Kinetics of CTL Responses Against Vaccinia Virus
IL-10 is generally considered to be an immunosuppressive cytokine, and thus the ability to enhance the treatment of established metastases with recombinant pox viruses was unexpected (9). We sought to better understand the possible mechanisms through which IL-10 augmented the therapeutic efficacy of these viruses in vivo. To be certain that there was no direct inhibitory effect on the tumor cells, a 3H-thymidine proliferation assay was performed by exposing cells to increasing doses of murine IL-10 (0-3 μg). No direct effect on the proliferation of either the CT26.WT or the CT26.CL25 cell line was observed (data not shown), thus suggesting that the effects of IL-10 in enhancing the therapeutic efficacy of recombinant vaccinia viruses were mediated through immune mechanisms.
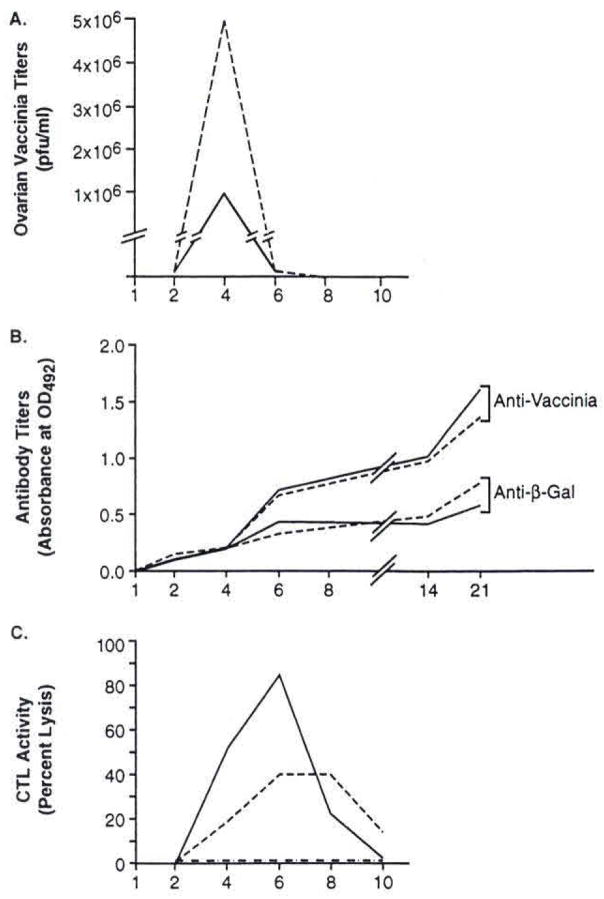
Treatment of established metastases was effective when IL-10 was given 12 hours after virus, but could not be duplicated when IL-10 was given immediately before or after virus injection (data not shown). This suggested that the timing of exposure to IL-10 was a critical determinant of the enhancement effect. To determine the effects of IL-10 on the course of vaccinia virus infection, we injected the VJS6 virus into non-tumor-bearing mice and extracted the internal organs on alternating days to determine viral liters with and without IL-10 treatment. Vaccinia tilers in the spleen, liver, kidneys, and lungs, were all less than 1,000 PFU after i.v. injection and were considered insignificant (data not shown). However, increased liters of vaccinia virus were identified in the ovaries after i.v. inoculation. A significant reduction in ovarian viral liters was seen after IL-10 administration compared to mice treated with vaccinia virus alone (Fig. 1 A). The maximum effect on viral clearance was reached 4 days after treatment, and all virus was cleared by 6 days. This finding suggested that the host immune response against vaccinia virus was actually enhanced, not suppressed, by treatment with IL-10.
FIG. 1.
Kinetics of intedeukin-10 (IL-10) exposure on the immune response against recombinant vaccinia virus is graphically illustrated here. BALB/c mice were injected intravenously with 107 plaque-forming units (PFU) of VJS6 atone (-) or VJS6 followed by IL-10 (I μg i.p.) 12 hours later and continued daily for 5 days (—). Two mice from each group were killed every other day after collection of serum for antibody titers, extraction of the visceral organs for viral titers, and harvesting of splenocytes for chromium release assays. Virus was, cleared more rapidly from the ovaries after IL-10 treatment (A) with titers less than 103 PFU in the kidneys, liver, and lungs (not shown); anti-vaccinia antibody titers, as measured by anti-vaccinia enzyme-linked immunosorbent assay, were not affected by IL-10 administration (B); anti-vaccinia cytotoxic T-cell (CTL) activity, determined by 8-hour chromium release assay against vaccinia-infected CT26.WT cells shown at an effector-to-target (E:T) ratio of 25:1, was increased after IL-10 exposure (C) with minimal natural killer cell activity, as measured in an 8-hour chromium release assay against YAC-1 cells shown at an E:T ratio of 25:1 (not shown). The peak CTL activity correlates with the rapid clearance of virus seen by the 6th day of treatment. These experiments were all repeated with nearly identical results.
To compare the effects of IL-10 administration on anti-vaccinia and anti-β-gal antibody responses after vaccination with VJS6, sera were obtained from immunized mice treated with and without IL-10. As seen in Figure 1B, both groups of mice that received VJS6 demonstrated specific antibody reactivity against wild-type vaccinia that appeared 6 days after immunization and increased slowly over a 3-week period. However, no difference in antibody titers was observed in the group of mice that received IL-10 after the immunization with VJS6 compared to the group that received VJS6 alone. Antibody titers against β-gal protein were found to increase after vaccination but did not differ between mice treated with and without IL-10 (Fig. 1B). These results did not support a role for antibody-mediated antitumor effects when IL-10 was given after a recombinant pox-virus.
Because vaccinia was cleared more rapidly from the ovaries after exposure to IL-10, we sought to determine whether IL-10 could increase CTL activity against vaccinia virus. A primary 51Cr-release assay using fresh splenocytes from mice treated with VJS6, VJS6, and IL-10, or IL-10 alone was performed. The timing of viral clearance suggested that the maximal effect of IL-10 was achieved 4 days after instituting IL-10 treatment. We thus performed a chromium release assay using splenocytes obtained every other day after vaccination. To examine CTL activity against vaccinia virus, we used a vaccinia-infected autologous tumor cell line, CT26-VAC, as a target. The noninfected CT26. WT was used as an antigen-negative target, and YAC-1 cells were included to assess NK reactivity. There was a significant increase in lysis between the 4th and 6th days after treatment with vaccinia virus and IL-10 compared to those mice treated with virus alone (Fig. 1C). There was no reactivity in mice treated with IL-10 alone (data not shown). This increased cytotoxicity correlated with the observed reduction in ovarian viral tilers seen on the 4th day of treatment with IL-10. Primary CTL against β-gal was attempted, but could not be obtained, consistent with previous results (6). Minimal amounts of NK activity were observed in the mice treated with IL-10 (data not shown).
DISCUSSION
Identification of T-cell restricted tumor antigen epitopes has renewed interest in using recombinant vaccines for the treatment of cancer. Expression of foreign antigens by poxvirus vectors has been well established, and several recombinant viruses containing different antigens have been generated as potential cancer vaccines (28). Enhancement of poxvirus vaccines with other cytokines has been previously reported, including enhanced anti-tumor responses with IL-2 and IL-12 added to a vaccinia virus vaccine (6,7). We now report that IL-10 also enhances the effects of a recombinant vaccinia virus directed against a model tumor antigen in a murine pulmonary metastases model. This effect was not related to the immunosuppressive effects of IL-10 because we observed similar treatment effects with the nonreplicating fowlpox virus vaccine, and vaccinia virus was cleared more rapidly from IL-10-treated animals.
We observed enhanced therapeutic responses when IL-10 was administered 12 hours after vaccine was given, but did not see any treatment augmentation when the cytokine was given immediately before or just after vaccination. This suggests that the timing of exposure between virus and IL-10 was a critical factor in determining the physiological effect of IL-10 in this system. Because little is known about the interactions of IL-10 with vaccinia virus, we sought to understand the mechanism of IL-10 enhancement by studying the interaction of IL-10 with vaccinia virus in a non-tumor-bearing animal. Our results support the function of IL-10 as an agent that increases CTL activity and promotes viral clearance when administered in vivo 12 hours after immunization. Vaccinia virus is known to induce potent CD8+ CTL responses, and it is possible that IL-10 influences activated (i.e., vaccinia primed) T cells differently from its effects on resting (i.e., naive) T cells. This would explain why we saw no treatment effect when IL-10 was given before or just after vaccination, because T cells would not have been adequately activated by vaccinia at these time points. Yang et al. have shown that IL-10 can prime in vitro tumor-reactive CTL against the mouse mastocytoma cell line P815 using naive splenocytes (29). The in vitro priming was enhanced by costimulation with B7 but was also observed in B7-negative P815 cells when IL-10 and IL-2 were used together. Furthermore, they were able to adoptively transfer IL-10-primed CTL into mice bearing P815 ascites tumor and observed antitumor responses. This is consistent with our findings suggesting that IL-10 played a role in the induction of a tumor-specific CTL response when given 12 hours after vaccination with virus.
Although we tried to elicit primary CTL against β-gal in our model, we could not observe any evidence of specific CTL responses. This is consistent with previous reports that primary CTL could not be generated after vaccination with a β-gal vaccine (6). We have been able to generate only secondary CTL after a course of in vitro stimulation with β-gal peptide. Because secondary CTL responses may not correlate with in vivo tumor responses, we did not attempt to perform such an assay (30). However, we can infer specificity of the CTL response because we saw no therapeutic effects against the (β-gal-negative tumor, CT26. It is possible that other xenogeneic antigens are present on the vaccinia virus because it may capture portions of the cell membrane used to culture virus stocks. This is unlikely to represent a significant mechanism of tumor rejection because we observed no effect when vaccinia virus and IL-10 were used for treatment of the CT26 colon carcinoma line.
We found a minimal contribution from NK activity after IL-10, although there is a previous report of NK-mediated tumor regression using IL-10 in several different murine tumor models (21). Our model differs from this previous report in that we used a different tumor cell line, a different model antigen, and a different strain of mouse, all of which could influence the induction and degree of antitumor immunity. Also, we used IL-10 as an adjuvant to recombinant vaccinia virus, not as a sole treatment agent, and this may have influenced the immune response. Other cytokines, such as tumor necrosis factor, have been reported to have multiple and often contradictory effects under different physiological conditions. IL-10 has evolved as a key regulatory cytokine and mediates multiple functions, which may explain our finding that the timing of exposure was critical for the antitumor effects observed. Murine IL-10 has been reported to enhance CD8+ T-cell cultures in other settings, such as after ConA and anti-CD3 activation (14). Increased CTL activity of in vivo vaccinia-activated T cells after IL-10 treatment is thus consistent with other reports.
Another possible explanation for our results was that IL-10 influenced an unidentified, non-T-cell population of cells. A likely candidate would be the macrophage, known to express IL-10 receptors, and murine macrophages have been shown to be highly sensitive to IL-10 exposure (31–33). Other investigators have reported a reduction of in vivo macrophage infiltration into tumor sites of mice treated with local IL-10, implicating a decrease in macrophage activation after IL-10 treatment (18). This study used tumor cells transduced with IL-10 to simulate local IL-10 release. The route of administration and dose of IL-10 could play an important role in the net effect on CTL production. Although we have not formally tested this possibility yet, it remains interesting that both local IL-10 release and systemic adjuvant therapy to recombinant vaccinia virus vaccines give similar antitumor efficacy data.
Thus, a potential mechanism consistent with known effects of IL-10 is the downregulation of a macrophage population. Theoretically, the presence of a suppressor macrophage that can be selected by activation with vaccinia virus and that is sensitive to inhibition by systemic IL-10 therapy would result in an enhanced CD8+ CTL response. This would lead to more rapid clearance of virus, increased vaccinia-specific CTL activity, and enhanced antitumor effectiveness if CTL were also generated against the inserted antigen, findings consistent with our results. Our laboratory has identified such a suppressor macrophage population by treating mice with a vaccinia virus expressing β-gal and IL-2. A macrophage population was induced and characterized by surface expression of Mac-1+ and Gr-1+, and was shown to functionally mediate the suppression of CD8+ T-cell responses against β-gal (Bronte V. et al, unpublished observations). We have preliminary evidence that mice treated with vaccinia virus followed by IL-10 exhibit increased apoptosis of fresh splenocyte preparations, and we are currently trying to identify the cell lineage of the apoptotic cells after IL-10 exposure. It is possible that IL-10 inhibits this suppressor population after being activated by vaccinia virus, and secondarily promotes enhanced CTL responses against the foreign immunogen.
We have demonstrated that IL-10 administered 12 hours after a recombinant vaccinia virus expressing a model tumor antigen can enhance the treatment of 3-day-old pulmonary metastases. Therapeutic responses correlated with increased vaccinia-specific CTL and a more rapid clearance of virus from murine ovaries. IL-10 may act to enhance in vivo activated T-cell responses when given after poxvirus vaccinations. Further studies on the exact mechanisms of how IL-10 enhances pox virus-based vaccines are necessary to better understand the potential pleiotropic actions of IL-10 when used in vivo. These insights into the biology of IL-10 should allow a more rational approach to the design of clinical trials using recombinant vaccines in the treatment of human cancers.
Acknowledgments
The authors thank Dr. Scott Abrams for critical review of the manuscript, Arnold Mixon for his assistance with FACS scanning, and Paul Spiess for expert technical support.
References
- 1.Kaufman H, Kantor J, Schlom J. A recombinant vaccinia virus expressing human carcinoemhryonic antigen (CEA) Int J Cancer. 1991;48:900–7. doi: 10.1002/ijc.2910480618. [DOI] [PubMed] [Google Scholar]
- 2.Hareuveni M, Wreschner DH, Kieny MP, et al. Vaccinia recombinants expressing secreted and transmembrane forms of breast cancer-associated epithelial tumor antigen (ETA) Vaccine. 1991;9:618–26. doi: 10.1016/0264-410x(91)90185-9. [DOI] [PubMed] [Google Scholar]
- 3.Hodge JW, Schlom J, Donohue SJ, et al. A recombinant vaccinia virus expressing human prostate-specific antigen (PSA): safety and immunogenicity in a non-human primate. Int J Cancer. 1995;63:231–37. doi: 10.1002/ijc.2910630215. [DOI] [PubMed] [Google Scholar]
- 4.Kantor J, Irvine K, Abrams S, Kaufman H, DiPietro J, Schlom J. Anti-tumor activity and immune responses induced by a recombinant vaccinia-carcinoembryonic antigen (CEA) vaccine. J Natl Cancer Inst. 1992;84:1084–91. doi: 10.1093/jnci/84.14.1084. [DOI] [PubMed] [Google Scholar]
- 5.Abrams SI, Hodge JW, McLaughtin JP, Steinberg SM, Kantor JA, Schlom J. Adoptive immunotherapy as an in vivo model to explore antitumor mechanisms induced by a recombinant anticancer vaccine. J Immunother. 1997;20:48–59. doi: 10.1097/00002371-199701000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Bronte V, Tsung K, Rao JB, et al. IL-2 enhances the function of recombinant poxvirus-based vaccines in the treatment of established pulmonary metastases. J Immunol. 1995;154:5282–92. [PMC free article] [PubMed] [Google Scholar]
- 7.Rao JB, Chamberlain RS, Bronte V, et al. IL-12 is an effective adjuvant to recombinant vaccinia virus-based tumor vaccines: enhancement by simultaneous B7-1 expression. J Immunol. 1996;156:3357–65. [PMC free article] [PubMed] [Google Scholar]
- 8.de Waal Malefyt R, Yssel H, Roncarolo M, Spits H, de Vries JE. Interleukin-10. Curr Opin Immunol. 1992;4:314–20. doi: 10.1016/0952-7915(92)90082-p. [DOI] [PubMed] [Google Scholar]
- 9.Florentine DF, Zlotnik A, Mossman TR, Howard M, O’Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–22. [PubMed] [Google Scholar]
- 10.Fiorentino DF, Bond MW, Mossman TR. Two types of mouse helper T cell IV Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–95. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Waal Malefyt R, Abrams J, Bennett B, Figdor C, de Vries J. IL-10 inhibits cytokine synthesis by human monocytes: an auto-regulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–20. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arai T, Hiromatsu K, Kobayashi N, et al. IL-10 is involved in the protective effect of dibutyryl cyclic adenosine monophosphate on endotoxin-induced inflammatory liver injury. J Immunol. 1995;155:5743–19. [PubMed] [Google Scholar]
- 13.MacNeil IA, Suda T, Moore KW, Mossman TR, Zlotnick A. IL-10: a novel growth cofactor for mature and immature T cells. J Immunol. 1990;145:4167–73. [PubMed] [Google Scholar]
- 14.Chen W, Zlotnik A. IL-10: a novel cytotoxic T celi differentiation factor. J Immunol. 1991;147:528–34. [PubMed] [Google Scholar]
- 15.Beisserl S, Hosoi J, Grabbe S, Asahina A, Granstein RD. IL-10 inhibits tumor antigen presentation by epidermal antigen-presenting cells. J Immunol. 1995;154:1280–6. [PubMed] [Google Scholar]
- 16.Matsuda M, Salazar F, Petersson M, et al. Interleukin 10 pretreatment protects target cells from tumor- and allo-specific cytotoxic T cells and downregulated HLA class I expression. J Exp Med. 1994;180:2371–6. doi: 10.1084/jem.180.6.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Waal Malefyt R, Haanen J, Spits H, et al. Interleukin-10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II MHC expression. J Exp Med. 1991;174:915–24. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richter G, Kruger-Krasagakes S, Hein G, et al. Interleukin 10 iransfected into Chinese hamster ovary cells prevents tumor growth and macrophage infiltration. Cancer Res. 1993;53:4134–7. [PubMed] [Google Scholar]
- 19.Giovarelli M, Musiani P, Modesti A, et al. Local release of IL-10 by transfected mouse mammary adenocarcinoma cells does not suppress but enhances antiturnor reaction and elicits a strong cytotoxic lymphocyte and antibody-dependent immune memory. J Immunol. 1995;155:3112–23. [PubMed] [Google Scholar]
- 20.Berman RM, Suzuki T, Tahara H, Robbins PD, Narula SK, Lotze MT. Systemic administration of cellular IL-10 induces an effecttive, specific, and long-lived immune response against established tumors in mice. J Immunol. 1996;157:231–8. [PubMed] [Google Scholar]
- 21.Zheng LM, Ojcius DM, Garaud F, et al. Interleukin-10 inhibits tumor metastasis through an NK cell-dependent mechanism. J Exp Med. 1996;184:579–84. doi: 10.1084/jem.184.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang M, Bronte V, Chen PW, et al. Active immunotherapy of cancer with a nonreplicating recombinant fowlpox virus encoding a model tumor-associated antigen. J Immunol. 1995;154:4685–92. [PMC free article] [PubMed] [Google Scholar]
- 23.Davison AJ, Moss B. New vaccinia virus recombination plasmids incorporating a synthetic late promoter for high level expression of foreign proteins. Nucleic Acids Res. 1990;18:4285–6. doi: 10.1093/nar/18.14.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith GL, Levin JZ, Palese P, Moss B. Synthesis and cellular location of the influenza polypeptides individually expressed by recombinant vaccinia viruses. Virology. 1987;160:336–45. doi: 10.1016/0042-6822(87)90004-3. [DOI] [PubMed] [Google Scholar]
- 25.Jenkins S, Gritz L, Fedor CH, O’Neill EM, Cohen LK, Panicali DL. Formation of lentivirus panicles by mammalian cells infected with recombinant fowlpox virus. AIDS Res Hum Retroviruses. 1991;7:991–8. doi: 10.1089/aid.1991.7.991. [DOI] [PubMed] [Google Scholar]
- 26.Restifo NP, Spiess PJ, Karp SE, Mule JJ, Rosenberg SA. A non-immunogenic sarcoma transduced with the cDNA for interferon gamma elicits CDS+ T cells against the wild-type tumor: correlation with antigen presentation capability. J Exp Med. 1992;175:1423–31. doi: 10.1084/jem.175.6.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mackett M, Smith GL, Moss B. General method for production and selection of infectious vaccinia virus recombinants expressing foreign genes. J Virol. 1984;49:857–64. doi: 10.1128/jvi.49.3.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moss B. Genetically engineered poxviruses for recombinant gene expression, vaccination, and safety. Proc Natl Acad Sci USA. 1996;93:11341–48. doi: 10.1073/pnas.93.21.11341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang G, Hellstrom KE, Mizuno MT, Chen L. In vitro priming of tumor-reactive cytolytic T lymphocytes by combining IL-10 with B7-CD28 costimulation. J Immunol. 1995;155:3897–903. [PubMed] [Google Scholar]
- 30.Rosenberg SA, Yang JC, Schwartzentruber DJ. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nature Med. 1998;4:321–6. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bogdan C, Vodovotz Y, Nathan C. Macrophage deactivation by interleukin 10. J Exp Med. 1991;174:1549–55. doi: 10.1084/jem.174.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fiorentino DF, Zlotnik A, Vieira P, et al. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–51. [PubMed] [Google Scholar]
- 33.Ding L, Linsley PS, Huang L, Germain RN, Shevach EM. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993;151:1224–34. [PubMed] [Google Scholar]