Abstract
Ribbon is a nuclear BTB-domain protein required for morphogenesis of the salivary gland and trachea. We recently showed that ribbon mutants exhibit decreased Crumbs and Rab11-coincident apical vesicles and increased apical Moesin activity and microvillar structure during tube elongation. To learn how these molecular and morphological changes affect the dynamics of tubulogenesis, we optimized an advanced two-photon microscope to enable high-resolution live imaging of the salivary gland and trachea. Live imaging revealed that ribbon mutant tissues exhibit slowed and incomplete lumenal morphogenesis, consistent with previously described apical defects. Since Moesin activity correlates with cortical stiffness, we hypothesize that ribbon mutants suffer from increased apical stiffness during morphogenesis. We develop this hypothesis through mechanical analysis, using the advantages of live imaging to construct computational elastic and analytical viscoelastic models of tube elongation, which suggest that ribbon mutant tubes exhibit three- to five-fold increased apical stiffness and two-fold increased effective apical viscosity.
Keywords: Apical membrane, Crumbs, live imaging, mechanics, Moesin, Ribbon, tube morphogenesis
Introduction
The Drosophila salivary gland (SG) and trachea (TR) have been excellent models for revealing the molecular and cellular basis for tube morphogenesis. Both forward and reverse genetic approaches have revealed key steps in cell type specification, invagination, branching morphogenesis, migration, elongation, fusion, lumenal maintenance and tube size control (Kerman et al., 2006). Among the genes affecting SG and TR morphogenesis identified through forward genetic screens is ribbon (rib) (Nüsslein-Volhard et al., 1984), which encodes a Broad Tramtrack Bric-a-brac (BTB)-domain containing nuclear protein that has recently been shown to partner with another BTB-domain containing protein, Lola-like, to mediate tube elongation in both the SG and the major artery of the TR, known as the dorsal trunk (DT) (Bradley and Andrew, 2001; Shim et al., 2001; Kerman et al., 2008). In both tissues, Rib is required for robust transcription of crumbs (crb), which encodes an apical membrane protein that functions in the establishment of epithelial cell polarity and in apical membrane growth. Rib also affects the phosphorylation state of Moesin (Moe), the single Drosophila member of the widespread and highly conserved Ezrin-Radixin-Moesin (ERM) family; specifically, higher levels of active, phosphorylated Moe are observed in the apical domain of rib mutant SG and TR compared with wild type (WT). Consistent with loss of rib affecting the expression and activity state of apical membrane components, rib mutants have clear defects in the apical cell domain (Kerman et al., 2008). Notably, ultrastructural and confocal analysis revealed increased microvillar structure in rib mutant SG, as well as a relative depletion of subapical Rab11-positive vesicles in rib mutant SG and TR. Since Rab11 localizes to both apical recycling endosomes (Hoekstra et al., 2004) and biosynthetic secretory vesicles (Cresawn et al., 2007; Li et al., 2007), the depletion of these vesicles in rib mutants should limit apical membrane expansion, a defect consistent with the known role of Crb in the development of embryonic epidermis and SG and adult photoreceptors (Myat and Andrew, 2002; Pellikka et al., 2002; Laprise et al., 2006). The increased microvillar structure in rib mutant SGs fits well with the known effects of loss and gain of Ezrin activity in a number of cellular contexts. Loss of Ezrin is associated with defects in mouse intestinal and retinal microvilli (Saotome et al., 2004; Bonilha et al., 2006) and expression of phosphorylated (active) Ezrin increases the length of microvilli in a mouse hepatic cell line (Lan et al., 2006).
ERM family proteins have been shown to regulate binding of membrane and cytoskeleton, with binding activity dependent upon the phosphorylation state of the ERM protein (Polesello and Payre, 2004; Saotome et al., 2004; Fievet et al., 2007). In epithelia, ERM family members predominantly localize to a complex apical network of cross-linked cytoskeletal and membrane-associated proteins, and the molecular composition and dynamics of this network are likely to affect the material properties of epithelial cells and tissues (Discher, 2000; Sheetz, 2001). Indeed, increased activity of the ERM-related protein Band 4.1 has been shown to correlate with increased membrane stiffness in vertebrate erythrocytes (Manno et al., 2005), and the degree of Moe activity was recently shown to correlate with cortical rigidity in Drosophila S2 cells (Kunda et al., 2008). In addition, since apical membrane is intimately associated with this apical protein network, membrane growth and rearrangement may also help determine cellular material properties (Sheetz et al., 2006) and, thus, the ability to deform in response to morphogenetic forces.
To date, analysis of the rib mutant phenotype has been limited to observations on fixed samples. To learn more about how the observed molecular and morphological changes result in perturbed tube morphogenesis, we optimized a highly sensitive two-photon microscope to enable high-resolution live imaging of deep tissue morphogenesis in the Drosophila embryo. This imaging allows us to conclude that rib SG and TR fail in tube elongation due to slowed and incomplete lumenal morphogenesis, in agreement with the apically-localized molecular and morphological defects in rib mutants shown previously (Kerman et al., 2008). We hypothesize, based on studies linking membrane and ERM family members to cellular material properties (Manno et al., 2005; Kunda et al., 2008), that the apically-localized defects observed in rib mutants lead to increased resistance to deformation in the apical region. Since high-resolution live imaging enables accurate measurements of cell dimensions and measurement of the rate of tube elongation, we incorporate this new data into mechanical models to test the hypothesis of increased apical resistance and to gain further insight into the mechanics of tube elongation. We conclude that the molecular, morphological, and kinetic defects observed in rib mutant tissues are consistent with increased apical resistance to deformation during tube morphogenesis, and we propose that remodeling of the apical region to decrease resistance during epithelial morphogenesis could be integral to tissue development.
Results
SG tube morphogenesis begins during embryonic stage 11, when cells in a dorsal posterior portion of the primordia undergo apical constriction and internalize to form a rudimentary tube (Myat and Andrew, 2000). As additional cells change shape and invaginate, the tube elongates along a dorsal-posterior trajectory until the distal portion of the gland contacts the visceral mesoderm (VM) during embryonic stage 12. Once in contact with the VM, the SG tube initiates posterior migration (Fig. 1A; Bradley et al., 2003), moving directly on the VM through stages 13 and early 14 to become realigned with its long axis parallel to the anterior-posterior axis of the embryo (Fig. 1C; Vining et al., 2005). In rib mutants, the early stages of SG morphogenesis appear relatively normal (data not shown). It is only when the distal portion of the SG contacts the VM that defects become obvious. Specifically, the rib SG appears to stall at this stage, failing to turn and initiate posterior migration (Fig. 1B, D; Bradley and Andrew, 2001). Since rib is expressed and presumably required in many of the tissues used as landmarks for staging embryos, it is unclear whether the early stages of SG morphogenesis in rib mutants occur in the same time frame as in WT. Similarly, although changes in apical proteins and apical cell morphology are evident in rib mutant tissues (Kerman et al., 2008), the impact of these changes on cell dynamics is unknown. Thus, we turned to live imaging to directly compare SG morphogenesis in WT and rib mutants.
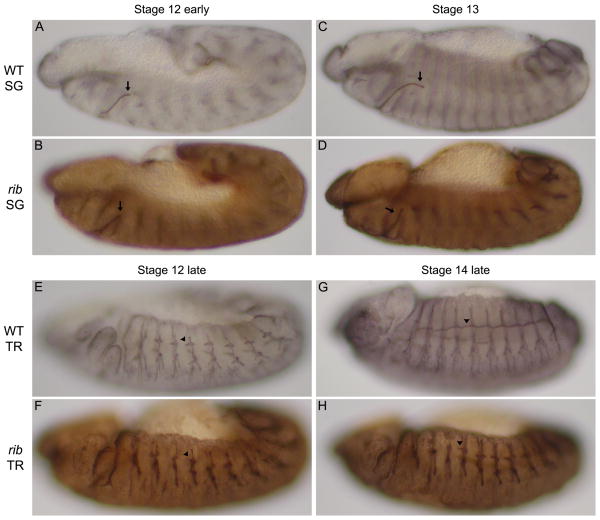
Fig. 1.
Ribbon is required for tube elongation during SG and TR morphogenesis. (A-D) WT SGs begin to turn and elongate during embryonic stage 12 (arrows in A, C), whereas rib mutant SGs rarely turn and elongate (arrows in B, D). Rates of elongation failure are 4.8% in WT (n=105) and 90% in rib (n = 325; Kerman et al., 2008). (E-H) WT TR extend the apical domains of the dorsal trunk cells of one segment anteriorly to fuse with the apical domains from dorsal trunk cells of the neighboring anterior segment during embryonic stages 12 through 14 (arrowheads in E, G). The dorsal trunk cells of rib mutant TR extend their apical domains only very slightly (arrowheads in F, H) failing to establish connections with neighboring anterior dorsal trunk cells. Rates of dorsal trunk elongation failure in metamere 4 are 8.9% in WT (n=112) and 97.3% in rib (n=341; Kerman et al., 2008). An antibody to Sas marks SG and TR lumena in black (A, C, E, G), brown (B, D, F, H). All embryos are oriented with dorsal up and anterior to the left.
Live imaging of rib SGs reveals failure to bend and elongate the lumen
Standard confocal and two-photon microscopes failed to provide sufficient image quality for live imaging of weakly labeled tissues deep in the Drosophila embryo. Thus, we optimized a two-photon microscope with high sensitivity GaAsP detectors for this application (see Experimental Procedures). This advanced two-photon microscope permits the acquisition of high-resolution images while using low laser intensities to maintain cell viability. Importantly, WT embryos develop with the same dynamics under constant exposure to the laser illumination used for imaging as they do with minimal exposure. For analysis of both the SG and TR, we used tissue-specific expression of α-catenin-GFP to mark adherens junctions and cytoplasm and of DsRedN to mark cell nuclei. We examined tube morphogenesis using two null alleles of rib in trans (rib1/ribP7) to minimize the effects of background mutations (Kerman et al., 2008).
Live imaging revealed that the polarized epithelial SG primordia of WT and rib mutants invaginated to form tubes of nearly identical outer diameter and length (Fig. 2; Suppl. Tables 1, 2), although this process occurred more slowly in rib mutants (Suppl. Fig. 1, Suppl. Movies 1, 3). Because of the slower development in rib mutants, we aligned the WT and rib movies at the point where the distal portion of the SG made contact with the VM (denoted time 0′ in Fig. 2). Distal tip cells of both WT and rib SGs extended posteriorly-directed lamellipodia immediately after contact with the VM, while maintaining their organization as a polarized monolayer epithelium (Fig. 2A, WT and rib 15′ inset). In WT SGs, lamellipodial extension was followed by migration of the SG tube along the VM (Fig. 2A, WT 15′). In rib SGs, however, lamellipodia retracted after approximately fifteen minutes without significant SG migration (Fig. 2A, rib 15′). Lamellipodial re-extension was observed at later stages in some SGs (data not shown). WT SGs continued to migrate along the VM, elongating and reorienting the gland within approximately one hour (Fig. 2A, WT 15′-75′). In contrast, rib SGs failed to migrate along the VM and developed lumenal defects with only a small increase in length (Fig. 2A, rib 15′-75′). Interestingly, we observed cells with distally tilted apical-basal axes in both WT and rib SGs at early stages, but the degree of tilting in WT SGs was greatly reduced as the lumen elongated (Fig. 2A). Thus, rib SGs invaginate slowly to form a tube of initially normal dimensions but fail to maintain distal tip cell lamellipodia and migrate along the VM. In addition, the apical-basal axis tilt observed in TEMs of stage 12 rib SGs (Kerman et al., 2008) appears to result from failure to elongate the lumen.
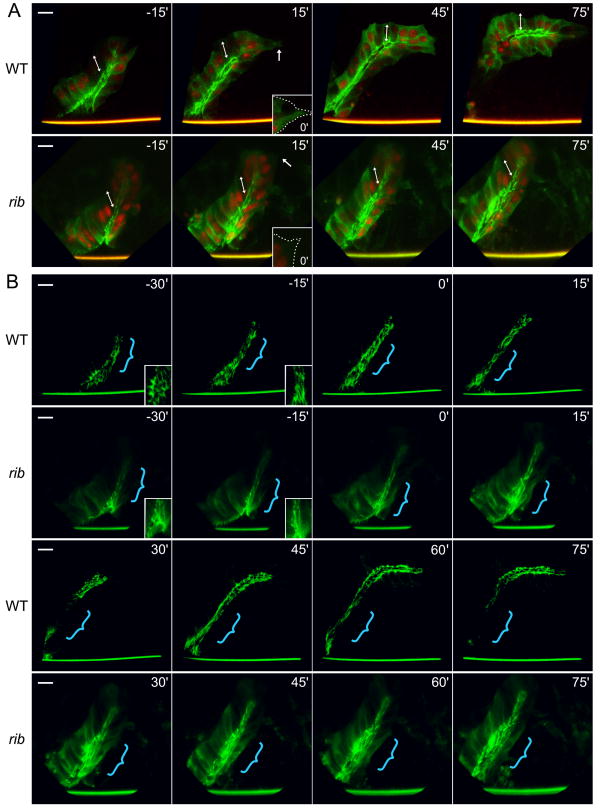
Fig. 2.
Live imaging of rib SGs reveals a failure to bend and elongate the lumen. Six WT and six rib null (rib1/ribP7) SGs were imaged and analyzed in detail. Movies were aligned at the initial point of contact with the VM (0′), and time in minutes from this point is denoted in the upper right. (A) α-catenin-GFP (green) marks adherens junctions and cytoplasm and DsRedN (red) marks cell nuclei. Arrows at 15′ indicate extending lamellipodium and distal tip cells in WT and retracting lamellipodium in rib, and inset with dotted line shows initial lamellipodial extension upon contact with the VM at 0′. Double-headed arrows indicate apical-basal cell axis, which remains distally-tilted in rib SGs. Note that the SG maintains its epithelial organization throughout embryogenesis in both WT and rib SGs. (B) Only the brightest pixels of the α-catenin-GFP signal are displayed to highlight lumenal dynamics. Insets in WT and rib -30′ and -15′ are magnified views of proximal-distal cell elongation. Stylized brackets indicate primary site of proximal-distal cell elongation in WT (-30′ to 75′) and rib (-30′ to 0′) as well as the region of lumen widening in rib (15′ to 75′). Note difference in length between WT and rib lumena. Scale bar is 10 μm.
We next displayed only the brightest pixels of the α-catenin-GFP signal to highlight apical cell shape changes (Fig. 2B, Suppl. Movies 2, 4). After invagination from the surface, both WT and rib SG cells narrowed their apices transversely and elongated along the proximal-distal axis (Fig. 2B, WT and rib, -30′ to 0′), indicating that rib cells retain both directional information and polarized force generation. As WT SG distal tip cells initiated migration along the VM, WT SG lumena bent posteriorly and elongated (Fig. 2B, WT 0′-45′). Despite lamellipodial projection in the rib distal tip cells, rib SG lumena failed to bend or elongate significantly, and continued cell internalization resulted in widening of the proximal end of the lumen (Fig. 2B, rib 0′-45′). Further cell internalization created a sharp bend in elongating WT SG lumena (Fig. 2B, WT 45′-75′) but resulted in further widening of the proximal lumen in rib SGs (Fig. 2B, rib 45′-75′). Thus, rib SG cells invaginate slowly to form a tube via normal mechanisms. However, the rib SG lumen subsequently fails to elongate sufficiently and instead widens as cells continue to internalize. Failure of lumenal elongation is unlikely to be a simple consequence of failed migration, since SGs missing integrin function fail to migrate on the VM yet still partially elongate their lumena (Bradley et al., 2003). Thus, live imaging reveals that rib mutant SGs exhibit an inherent failure in the rate and extent of lumenal elongation.
Live imaging of rib DTs reveals failure to elongate the lumen despite basal contact
rib mutants also exhibit profound defects in the TR (Bradley and Andrew, 2001; Shim et al., 2001; Kerman et al., 2008); these defects first become apparent during embryonic stage 12, when there is an apparent delay in migration of almost all primary branches (Fig. 1E, F). Although some branches will ultimately form relatively normal structures, the major anterior-posterior artery of the TR, the DT, is severely affected (Fig. 1G, H). Thus, we focused primarily on this branch for live imaging of WT and rib TR morphogenesis. As observed with the rib SG, all rib TR branches formed slowly (Fig. 3A, B, Suppl. Movies 5, 7); however, both WT and rib DTs maintained their epithelial organization throughout tube elongation. In addition, whereas all WT DTs established basal contact with the next anterior metamere (Fig. 3A, WT 20′), rib DTs established persistent basal contact with only relatively close metameres (Fig. 3A, rib 20′). WT DTs then elongated while increasing the degree of basal contact with neighboring metameres, ultimately fusing their apical domains to form a contiguous tube (Fig. 3A, WT 60′-140′). During this time, rib DTs failed to elongate significantly and exhibited repeated attempts to form stable basal contacts with more distant metameres (Fig. 3A, rib 60′-140′). Thus, rib DTs form slowly and fail to sufficiently elongate, limiting stable basal contacts to relatively close metameres.
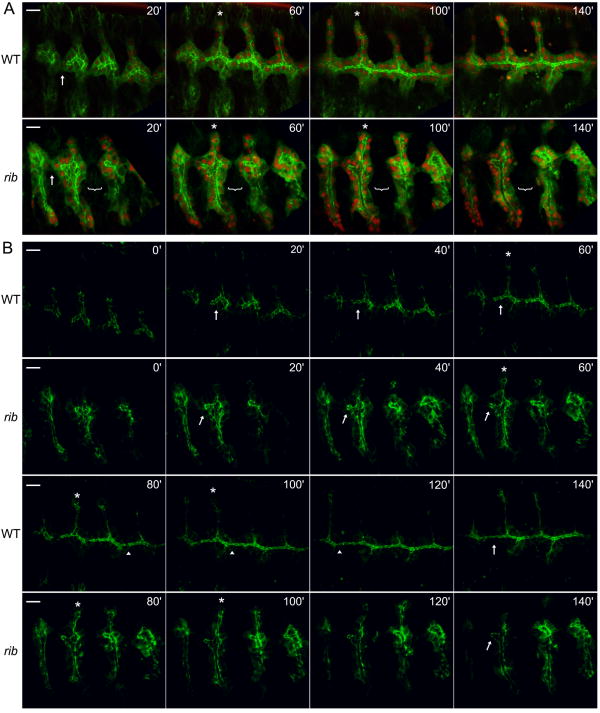
Fig. 3.
Live imaging of rib DTs reveals a failure to elongate the lumen despite basal contact. Four WT and four rib null (rib1/ribP7) DTs were imaged and analyzed in detail. Metameres 3-6 are shown, and time in minutes from the first image is denoted in the upper right. Note that in rib movies, metamere 6 enters the viewing area at 40′ and that the decreased curvature (of the entire embryo) results in ventral branches also being visible in the image stack. (A) α-catenin-GFP (green) marks adherens junctions and cytoplasm and DsRedN (red) marks cell nuclei. Arrow in WT and rib at 20′ indicates basal contact between metameres 3 and 4, and bracket in rib 20′-140′ indicates failure of stable basal contact between metameres 4 and the more distant metamere 5. Asterisks in WT and rib 60′ and 100′ indicate elongating dorsal branch of metamere 4. (B) Only brightest pixels of the α-catenin-GFP signal are shown to highlight DT lumenal dynamics. Arrows in WT and rib 20′ -60′ indicate TM4 DT lumen, which narrows and extends in WT but only narrows in rib. Asterisks in WT and rib 60′-100′ mark the dorsal branch elongating through cell rearrangements in both genotypes. Arrowheads in WT 80′ -120′ show sites of DT fusion, and arrows in WT and rib 140′ indicate failure of the rib DT lumen to elongate and fuse. Scale bar is 10 μm.
We examined DT lumenal morphogenesis in more detail by displaying only the brightest pixels of the α-catenin-GFP signal (Fig. 3B, Suppl. Movies 6, 8). Whereas WT DT lumena were narrow and elongated during primary branch formation, rib DT lumena were short and wide, reminiscent of an earlier stage in WT DT development (Fig. 3B, WT and rib 0′). WT DT lumena subsequently narrowed slightly and extended anteriorly, whereas rib DT lumena narrowed but failed to increase significantly in length (Fig. 3B, WT and rib 20′-60′). WT DT lumena continued to extend anteriorly with only a modest decrease in diameter, ultimately fusing with neighbors to form a contiguous lumen (Fig. 3B, WT 80′-140′). The morphology of the rib DT lumena did not change during this time (Fig. 3B, rib, compare 140′ to 60′). Interestingly, nuclear movement and junctional morphology characteristic of cell rearrangements (Jazwinska et al., 2003) were visible in the dorsal branches (DB) of both WT and rib (Fig. 3A, B, WT and rib 60′-100′). Thus, although stable basal contacts are established between close neighboring metameres, individual rib DTs fail to sufficiently elongate their apical surfaces to enable formation of a contiguous lumen. In contrast, rib DBs, which elongate via cell rearrangement, produce a lumen of relatively normal length (Fig. 1G-H, 3).
Increased apical resistance could explain failure in rib tube elongation
Live imaging reveals that rib SG and DT exhibit slowed and incomplete lumenal morphogenesis (Fig. 2, 3; Suppl. Movies 1-8), a finding consistent with the apically-localized molecular (decreased Crb and Rab11, increased Moe activity) and morphological (decreased vesicles, increased microvillar structure) defects identified in recent studies (Kerman et al., 2008). Live imaging also demonstrates that rib SG and DT leader cells extend lamellipodia with proper directionality and timing and that rib SG cells elongate along the proximal-distal axis, suggesting that guidance cues are intact and that rib cells are able to generate polarized force in response to these cues (Fig. 2, 3). Since morphogenesis is inherently a mechanical process, proceeding according to a local balance of force generation and tissue resistance, and since Moe activity correlates with cortical stiffness (Manno et al., 2005; Kunda et al., 2008), we hypothesized that increased apical resistance could explain much of the rib phenotype. To explore this hypothesis further, we first turned to a mechanical analysis of tube elongation. Live imaging enhances this analysis both by eliminating fixation artifacts, thus allowing accurate measurements of cell dimensions for computational modeling, and by yielding information on the rate of development for the subsequent analytical model.
During SG and DT morphogenesis, cells are likely subjected to multiple forward-directed forces as the tube elongates. At the same time, these cells may experience internal resistance to deformation (based on cellular material properties) or external resistance in the form of drag forces from adjacent tissues or lumenal content. The distal tilting of basal surfaces of cells that is observed during early stages of WT and rib SG invagination (Fig. 2) argues against significant basal drag from adjacent tissues and instead suggests relatively high resistance in the apical region of the cells. In accordance with this, the apical-basal axis becomes less tilted as the WT SG lumen elongates (Fig. 2), suggesting that deformation of the apical region is key to apical-basal alignment and tube elongation. At this stage, the SG and DT lumena do not contain electron-dense secretory products (Kerman et al., 2008; Seshaiah et al., 2001; Tsarouhas et al., 2007), suggesting a primarily aqueous lumen and thus relatively low lumenal resistance. Thus, it appears that internal resistance to deformation, particularly in the apical region, is a major component in the mechanics of tube elongation.
The deformation of a network occurs through the extension and rearrangement of its constituent elements. Correspondingly, the ability of F-actin gels to deform is significantly affected by the density and binding strength of F-actin cross-linkers (Wachsstock et al., 1994; Gardel et al., 2004). ERM family proteins are known cross-linkers of F-actin and membrane-associated proteins (Medina et al., 2002; Polesello et al., 2002), and activated Ezrin exhibits dramatically increased actin binding times in vertebrate cell microvilli (Coscoy et al., 2002). In addition, Moe activity has been shown to correlate with cortical stiffness in Drosophila S2 cells (Kunda et al., 2008), and increased activity of the ERM-related protein Band 4.1 has been shown to correlate with increased membrane stiffness in vertebrate erythrocytes (Manno et al., 2005).
rib SG and TR cells exhibit high apical levels of activated Moe and expression of constitutively active Moe in SG and TR mimics the rib phenotype (Kerman et al., 2008). In addition, TEM analysis indicates that rib cells have increased microvillar structure (Kerman et al., 2008), a phenotype consistent with increased linkage of membrane and cytoskeleton and in agreement with ERM family function in Drosophila photoreceptors and murine enterocytes, retinal microvilli and cultured hepatic cells (Karagiosis and Ready, 2004; Saotome et al., 2004; Bonilha et al., 2006; Lan et al., 2006). Thus, the elevated apical Moe activity observed in rib cells appears to be a major effector of the rib phenotype. Furthermore, based on observations in other systems, this increased apical Moe activity suggests increased cross-linking of the apical region and thus increased apical resistance to deformation. Diminished levels of Crb and Rab11-coincident apical vesicles (Kerman et al., 2008) may also serve to increase resistance to deformation by limiting apical membrane growth and/or rearrangement, a mechanical role hinted at by studies of cell membrane reservoirs and tethers (Sheetz et al., 2006). Of note, overexpression of Rib and dominant negative MoeT559A resulted in wider and more irregular SG lumena (Suppl. Fig. 2), consistent with a more deformable apical region.
Finally, the exact nature and distribution of the forces driving tube elongation in the SG and DT remain unclear, although a combination of migration, invagination and internally generated cell elongation forces may be required to produce full tube elongation. In support of this idea, SG integrin mutants fail to extend lamellipodia but still manage to partially elongate their lumena (Bradley et al., 2003), suggesting both that internal forces do play a significant role and that rib mutants suffer from increased resistance to elongation, regardless of the origin of the applied force. In the computational elastic model below (Fig. 4, Suppl. Fig. 3, 4), we simplify the mechanics and examine only a small group of cells at the distal tip of the SG deforming in response to a small distally-directed pulling force applied to leader cells. Although this pulling force is an overt simplification of the combination of forces that drive tube elongation, these simulations allow us to examine the hypothesis that Rib could achieve its effects by modulating the stiffness in only the thin apical region. In the subsequent analytical model (Fig. 5), we examine SG lumen growth as a viscoelastic process and discuss the mechanical implications of the growth rate observed through live imaging of WT and rib SGs.
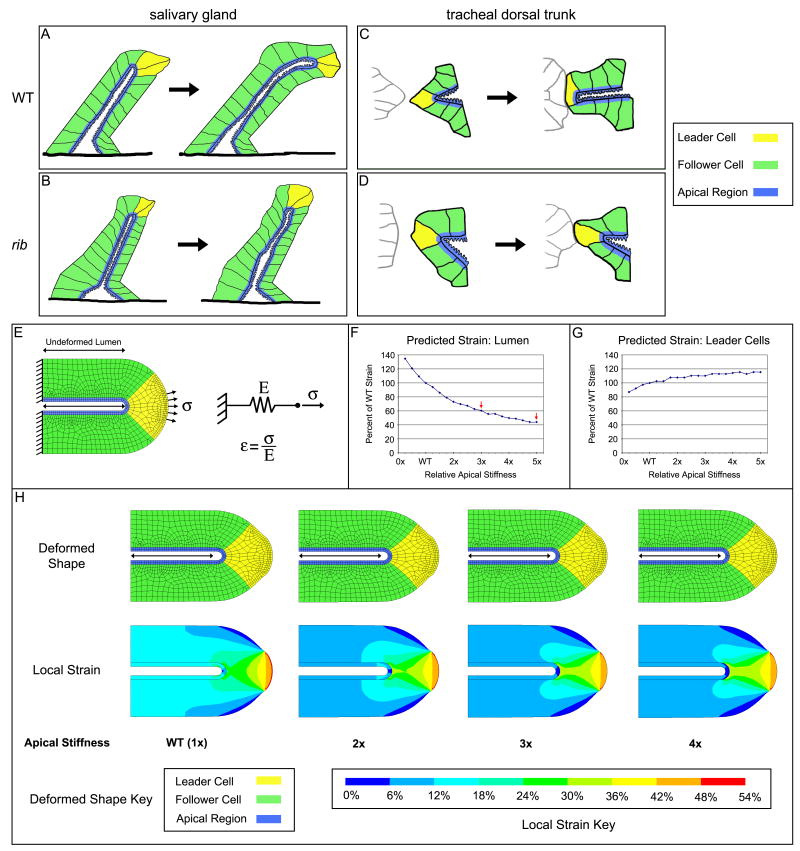
Fig. 4.
Mechanical analysis suggests rib cells have increased apical resistance. (A-D) Tissue level models of WT and rib mutant SG (A, B) and TR (C,D) during tube morphogenesis. In WT, both SG and TR lumena elongate either as the SG moves along the VM (A) or as the TR establishes basal contact with neighboring segments (C). Although rib mutant SGs send out basal projections and the leader cells elongate, the lumen fails to extend (B). Similarly, although basal contact is established between some neighboring TR metameres of rib mutants, the lumena fail to extend (D). For subsequent modeling, tubular organs are divided into two classes of cells: leader cells (yellow) that directly experience distally-directed pulling force and follower cells (green) that extend in response to these forces. As shown in Kerman et al., 2008, the molecular and cellular changes in rib mutants compared with WT are confined to the apical membrane region (blue). (E-H) Detailed descriptions of the elastic finite element model for tube elongation (E) are given in the text. The model predicts a significant dependence of lumenal strain (F) and a lesser dependence of leader cell strain (G) on apical stiffness. Predicted deformed shape and strain distribution for selected apical stiffness values are shown with keys (H), and the undeformed lumen length is indicated with a double-headed arrow. The full range of tested values from 0.25x to 5x can be found in Suppl. Fig. 3 and 4.
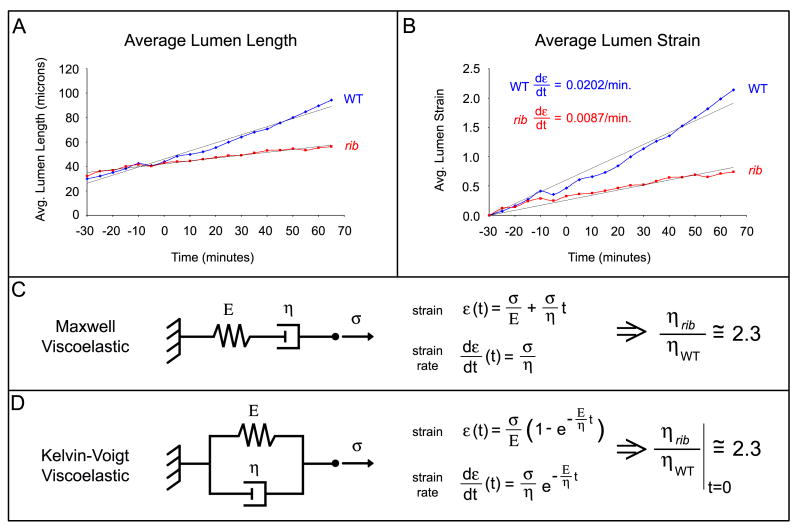
Fig. 5.
Viscoelastic analysis of lumen growth rate. The average lumen length over time is plotted for WT (blue) and rib mutant (red) SGs (A). Lumenal strain increases linearly in both WT and rib SGs (B). Both Maxwell (C) and Kelvin-Voigt (D) models predict 2.3x increased effective apical viscosity at early time points. See text for details and Supp. Fig. 1 for lumen length data for individual WT and rib SGs.
Elastic model of increased apical stiffness replicates elements of rib phenotype
To test the effects of increased apical stiffness on tube elongation, we formulated a model of tube elongation using a simple elastic material model (Fig. 4; see Experimental Procedures for details). In this model, the amount of strain (ε, change in length divided by original length) is equal to the applied stress (σ) divided by the stiffness (E). This simple material model allowed us to computationally simulate the complex geometry of a tube with different material properties in the cell bodies and apical region. This analysis is limited to the distal tube tip, comprises migratory leader cells (yellow) and three adjacent follower cells (green), and assumes no interactions with lumenal content or adjacent tissues. Tube geometry is based on measurements from live imaging of WT and rib SGs demonstrating nearly identical outer and similar lumenal diameters (n=3; Suppl. Tables 1, 2) and initial cell height is assumed to be uniform. Since direct measurement of cell-generated forces and mechanical moduli remains extremely difficult in this system due to other tissues surrounding the SG as well as limited access to the SG lumena, we assumed values for the forces and material properties based on the literature. In addition, since we rely on assumed values for force and moduli, our conclusions are based solely on the relative amounts of deformation as we vary a single parameter, the apical stiffness. Based on the literature, a migratory stress of 2.5 nN/μm2 (2500 Pa) was applied along the central basal surface of the leader cells (total force of 22.5 nN; Tan et al., 2003; du Roure et al., 2005; Prass et al., 2006). Since tissues generally have larger mechanical moduli than individual cells, the cell bodies (yellow, green) were assigned Young's moduli of 9 kPa (Davidson et al., 1999; Caille et al., 2002; Dahl et al., 2005; Rabinovich et al., 2005; Costa et al., 2006) and the dense actin-rich apical region (blue) was assigned a Young's modulus of 2.5x the cell body value (Yamada et al., 2000). The proximal (left) edge is free to narrow, and the lumen is free to elongate but not to narrow or buckle, consistent with the fixed lumenal diameter observed during early stages of SG elongation (Fig. 2). The elastic model predicts a slight narrowing and elongation of the tube in response to the pulling force; the deformed shape and local strains are shown (Fig. 4H, Suppl. Fig. 3, 4). This model predicts that increased apical stiffness results in decreased strain both along the tube lumen (Fig. 4F, H) and in the adjacent cell bodies (Fig. 4H), suggesting that the thin apical region can indeed limit elongation of the entire tube. Additionally, this model predicts that strain in leader cells should increase slightly with increasing apical stiffness (Fig. 4G, H), in agreement with the longer leader cells in rib SGs observed by TEM (Kerman et al., 2008) and in preliminary measurements of fixed confocal images (WT 8.46 ± 1.20 μm, n=3 versus rib 10.20 ± 1.00 μm, n=5; Supp. Table 3). Finally, this model predicts a mild decrease in lamellipodial leading edge strain with increasing apical stiffness, most clearly seen over the lower end of the simulation range (Suppl. Fig. 3). Live imaging of WT and rib SGs demonstrated that rib SG lumena achieve 60% of the WT length (Suppl. Fig. 1). If one assumes that this deformation occurs uniformly over the length of the SG and that the final SG length is determined entirely by strain, this model predicts that rib cells have approximately 3-fold increased apical stiffness (Fig. 4F). Alternatively, if one assumes that SGs undergo strain only after forming a tube of some length, the predictions become contingent on the assumed starting length. For example, with a starting length of 30 μm, the model predicts that rib cells have approximately 5-fold increased apical stiffness (Fig. 4F). A more accurate prediction awaits a better understanding of individual cell shape changes and movements during early SG development.
Viscoelastic analysis suggests that rib SGs have increased viscosity or decreased growth
Surprisingly, despite the presence of several concurrent morphogenic processes contributing to tube elongation, WT and rib SG lumen length and strain were found to increase linearly over time (Fig. 5A, Suppl. Fig. 1), with a strain rate of approximately 0.0202/minute in WT SGs and 0.0087/minute in rib SGs (Fig. 5B). We analyzed the SG lumen growth rate observed by live imaging using two common viscoelastic models for biological tissues. The first model, the Maxwell viscoelastic model, can be represented by a spring and dashpot in series (Fig. 5C). In response to an applied stress, this model predicts an initial jump based on the stiffness of the spring followed by infinite extension at a rate dependent upon the viscosity of the dashpot. The second model, the Kelvin-Voigt viscoelastic model, can be represented by a spring and dashpot in parallel (Fig. 5D). In response to an applied stress, this model predicts initial extension at a rate largely dependent upon the viscosity of the dashpot. Extension then slows and ceases as the spring reaches its equilibrium length.
In the Kelvin-Voigt model, increased viscosity and stiffness would lead to slower elongation and a shorter equilibrium length, consistent with the observed morphogenic defects in rib SG and DT. However, the lumenal strain rate fails to decrease in either genotype (Fig. 5B). Thus, it seems either that the later phase Maxwell (or viscous) model is a better fit, predicting a linear strain rate over some developmental time window, or that our live imaging data covers an insufficient interval of time to detect the decreasing strain rate predicted by the Kelvin-Voigt model. Longer-term live imaging would distinguish between these models. However, at early time points, the models behave similarly and predict that the effective apical viscosity in rib mutants is approximately 2.3x that of WT (Fig. 5C, D). Additionally, since relaxation times for embryonic tissues are on the order of tens of seconds (Forgacs et al., 1998) and SG and TR morphogenesis occurs over tens of minutes, the differences in effective apical viscosity identified here may actually reflect a growth process defect (e.g. membrane dynamics). A role for membrane growth is also supported by the relatively large strain observed in live WT SGs (Fig. 5B). Thus, we conclude that viscoelastic analysis also supports the idea of increased resistance in the rib apical region as a mechanism for failed tube morphogenesis, through a decreased lumenal growth rate stemming from increased apical viscosity and/or perturbed apical membrane dynamics.
Discussion
In this study, we optimized a two-photon microscope for deep tissue live imaging of the Drosophila embryo to directly examine tube dynamics in WT and rib mutant SG and TR. The primary defect observed in rib mutants by live imaging was delayed and incomplete tube elongation, a defect linked to the rate and extent of lumenal expansion and consistent with the decrease in Crb expression and increase in Moe activity recently documented in rib mutant SG and TR (Kerman et al., 2008). Based on these findings and the known roles of Crb and Moe, we hypothesized that the apical domains of rib mutant cells are more resistant to deformation than those of wild type. We tested this hypothesis by first using live cell dimensions to formulate a computational model to simulate complex tube geometry and determine the effects on the entire tube of changes in stiffness in only the thin apical domain. We then used the lumenal growth rate of WT and rib mutant SGs, determined through live imaging, to estimate relative differences in effective apical viscosity using two common viscoelastic models for biological tissues. The elastic material model predicts a shorter lumen and tube as well as a slight increase in leader cell length, both of which are observed in rib mutant SG and TR. Based on final SG lumen length and lumen growth rate, these analyses are consistent with a three- to five-fold increased apical stiffness and two-fold increased effective apical viscosity (likely reflecting apical membrane dynamics) in rib mutants relative to WT. From this and our earlier work (Kerman et al., 2008), we suggest that Rib and Lolal, members of the conserved but poorly understood BTB domain transcription factor family, cooperate to decrease mechanical resistance of the apical membrane region during epithelial tube elongation. Correspondingly, the differential effects on DT versus DB elongation in rib mutants can be largely explained by the mechanisms through which these tubes normally elongate (Fig. 3). The DB elongates primarily through well-described cell rearrangements, which have also been described for other branches minimally affected by loss of rib (Jazwinska et al., 2003). Tube elongation through cell rearrangement should not require extensive membrane growth or significant changes in membrane stiffness. On the contrary, elongation of the DT occurs by a mechanism very similar to elongation of the salivary gland, wherein the entire tube migrates and elongates towards its destination while maintaining its epithelial organization.
A new appreciation is emerging for the effects of the mechanical microenvironment on cell morphology and behavior (Discher et al., 2005; Nelson et al., 2005; Orr et al., 2006; Vogel and Sheetz, 2006). In particular, morphogenesis requires proper force generation and cell deformation to construct a tissue of the proper shape and position. Since the degree of cell deformation under a given force is contingent on its material properties, studies illuminating the transcriptional control of molecular mechanics are of significant interest to those who study morphogenesis and related diseases of organ form and function. During tube morphogenesis, epithelia undergo stereotypical cell shape changes resulting in invagination, elongation, migration, and lumen dilatation; many of these processes are driven or limited by apical properties. It will be interesting to uncover further details on how the molecular, morphological, and kinetic changes described for rib mutants lead to altered mechanical properties on a physical level. For instance, increased Crb levels during tube elongation may allow cells to expand their apical membrane surface area, redistribute apical membrane during cell elongation, and/or increase membrane fluidity. The post-invagination decrease in apical Moe activity in WT tubes may release apical membrane reservoirs from microvilli and/or increase cortical actin fluidity. The molecular defects of perturbed Crb and Moe, the morphological defects of diminished Rab11-coincident apical vesicles and increased microvillar structure, and the kinetic defects of slowed lumenal morphogenesis could well serve as a foundation for such future studies. In particular, these findings suggest that proper regulation of apical membrane dynamics and membrane-cytoskeleton interactions are critical in enabling the cell shape changes of tube morphogenesis.
Our studies examining the transcriptional modulation of apical mechanics during epithelial morphogenesis are likely to be widely applicable to other systems. Of note, both rib and lolal mutants are defective in dorsal closure, a process in which an epidermal sheet expands to cover a dorsal embryonic hole, a process similar to wound healing (Jack and Myette, 1997; Bradley and Andrew, 2001; Faucheux et al., 2003). An elegant series of studies has examined the morphogenic forces involved in dorsal closure and has highlighted the balance of contractile forces in the amnioserosa and epidermal leading edge with resistance due to stretching of the lateral epidermis (Hutson et al., 2003; Peralta et al., 2007). We propose that WT embryos must upregulate crb expression and downregulate apical Moe activity in the lateral epidermis to decrease this resistance, and thus predict that rib embryos fail in dorsal closure due to increased epidermal resistance and not from defects in force generation. Investigators will no doubt continue to unravel the role of tissue mechanics during in vivo morphogenesis, and high-resolution live imaging, with its accurate cellular dimensions and kinetic information, will very likely expedite these discoveries and their interpretations through modeling.
Experimental Procedures
Fly Strains and Genetics
Flies were grown and kept on standard cornmeal/agar medium. The fly lines used are described in Flybase unless otherwise indicated: Oregon R (wild-type), rib1, UAS-rib (Bradley and Andrew, 2001), ribP7, (Shim et al., 2001) UAS-MoeT559A (Speck et al., 2003), btl-Gal4 (Shiga et al., 1996), fkh-Gal4 (Henderson and Andrew, 2000) and UAS-αCatenin-GFP (Oda and Tsukita, 1999). The Gal4-UAS expression system (Brand and Perrimon, 1993) was used for tissue-specific expression. UAS-DsRedN contains an enhanced version of the DsRed cDNA (Bevis and Glick, 2002) cloned into the pUAST expression vector (Brand and Perrimon, 1993) with two nuclear localization signals. Germline transformation was performed as described (Rubin and Spradling, 1983).
Immunohistochemistry
Embryo fixation and staining were performed as described previously (Reuter et al., 1990). α-Sas antibody (a kind gift from E. Organ and D. Cavener) was used at a dilution of 1:500, α-βGal antibody (Promega; Madison, WI) was used at a dilution of 1:10,000. Appropriate secondary antibodies conjugated to biotin (Vector Labs; Burlingame, CA) were used at a 1:500 dilution. Mutant embryos were identified by the absence of βgal expression from the lacZ-containing balancer chromosome in fixed samples and by the absence of GFP fluorescence from the balancer chromosome in living embryos. Fixed embryos were visualized by Nomarski optics using a Zeiss Axiophot microscope.
Live Imaging
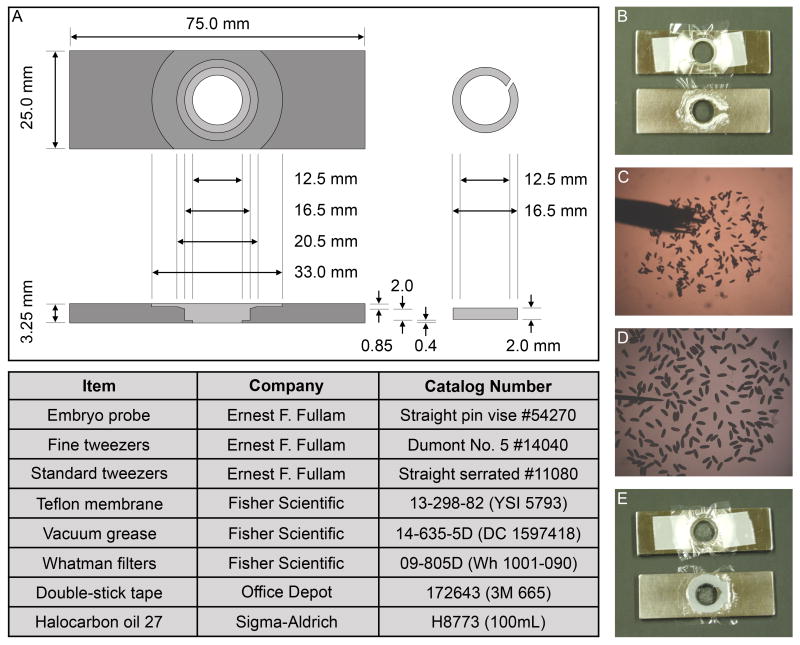
Live imaging protocols were adapted from previous work by D. Kiehart and J. Minden, wherein the embryos were maintained in halocarbon oil between a coverslip and Teflon membrane in a machined stainless steel live imaging slide (Kiehart et al., 1994). The slide dimensions were modified to decrease the size of the viewing window, allowing us to increase coverslip support and limit membrane flexion, and to increase the chamber depth to avoid embryo compression and any effects due to altered mechanical microenvironment (Fig. 6A). To increase adherence of the embryos, coverslips were coated with embryo glue (double-stick tape glue dissolved in heptane) and allowed to dry. The oxygen-permeable Teflon membrane was clamped in place using a split O-ring. Vacuum grease was placed around the viewing chamber, with the formation of a small canal to enable air escape during assembly (Fig. 6B). Halocarbon oil was then added to the chamber. Embryos were placed on the glue-coated coverslip using a small paintbrush, positioned and oriented using a small probe, and subsequently covered with halocarbon oil (Fig. 6C, D). Finally, the coverslip with adhered embryos and oil was inverted and lowered onto the imaging slide from left to right to allow air escape via the canal (Fig. 6E).
Fig. 6.
Live imaging slide design and assembly. (A) Drawing of the machined live imaging slide with top and side views. The most critical dimensions are the overall length and width that enable fit in a standard slide holder, and of the split ring outer diameter and split ring slide chamber, to securely clamp the membrane in place. (B-E) Assembly of the live imaging slide. Teflon membrane is clamped in place, and vacuum grease and halocarbon oil are added (B). Embryos are placed onto a glue-coated coverslip with a paintbrush (C). Embryos are oriented with a small probe (D). Coverslip with embryos is mounted onto live imaging slide (E).
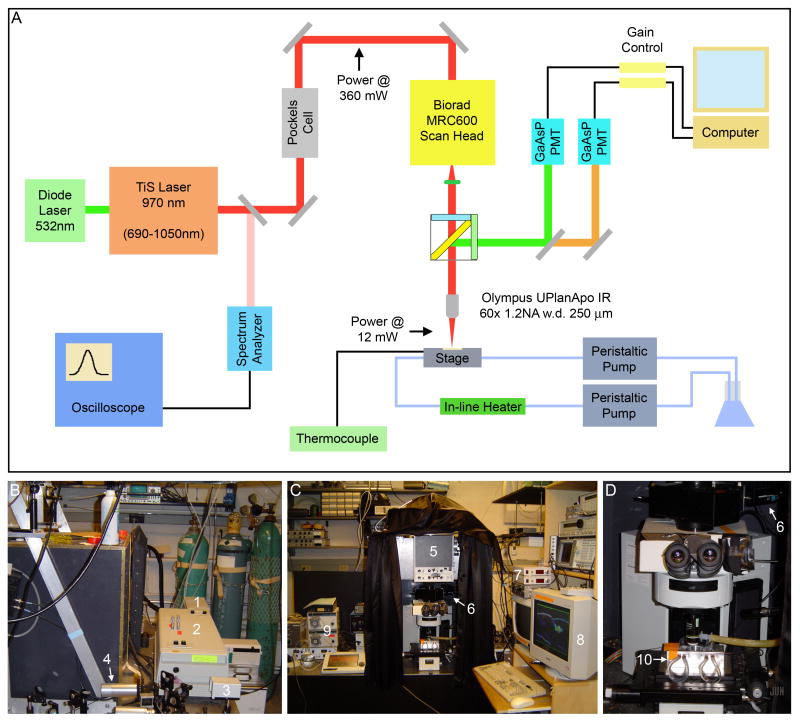
Time-lapse sequences were acquired using a custom-built multiphoton microscope equipped with a Tsunami Ti-Sapphire oscillator pumped by a 10W Millennia DPSS CW laser (Spectra-Physics; Mountain View, CA). This setup is similar to that of a modern commercial multiphoton microscope, except that individual components are more readily accessed and modified (Fig. 7). Continuous wave 532 nm light from a Spectra-Physics Millenia Xs 10.5W diode laser (1) was used to drive a Spectra-Physics Tsunami Ti:Sapphire oscillator (2) with a tunable range of 690 to 1020 nm. For our imaging, we maintained the Ti:Sapphire emission at 970 nm, as this wavelength resulted in robust excitation of both GFP and DsRed with minimal tissue interaction. A small fraction of the emitted light was diverted (using a near-infrared antireflective-coated slide) to a Ist-rees laser spectrum analyzer (3) connected to an LG OS-9020A 20 MHz oscilloscope, and laser cavity dimensions were manually adjusted to achieve the desired wavelength and mode locking. Laser power was modulated via a Pockels cell (4) and was measured post-Pockels cell and post-objective using a Newport Model 841-PE power meter. A Biorad MRC600 scan head (5) on an Olympus Provis microscope body was used for raster scanning, and emitted fluorescence was directed into two Hamamatsu H7422-P40MOD GaAsP PMTs (6) after passage through 500-550 nm bandpass and 575 nm longpass filters for green and red wavelengths, respectively. Gain was modulated (7) to avoid pixel saturation and damage to the sensitive GaAsP detectors, and images were acquired using custom software (8). Stage Z translation was controlled with a piezoelectric motor via the software. Embryos were maintained at 25 ± 1°C using heat transfer from warm water circulating through the stage (10) via copper piping coated with thermal grease. Water flow rate was controlled with two Masterflex peristaltic pumps (9), and temperature was modulated using a Warner TC-344A heater controller with inline heater and sensor taped to the imaging slide. As embryonic developmental rate is highly temperature-dependent (Powsner, 1935), temperature control allowed us to quantitatively compare the rate of morphogenesis between wild type and rib mutant embryos.
Fig. 7.
Configuration of custom multiphoton microscope. (A) Schematic of multiphoton microscope. A 10.5 W continuous wave diode laser (1) pumps a Ti:Sapphire oscillator (2) emitting at 970 nm. Light is sampled using a spectrum analyzer (3) connected to an oscilloscope, and laser power is modulated using a Pockels cell (4). A Biorad MRC600 scan head (5) is used for raster scanning, and light is collected using two GaAsP PMTs (6). Images are acquired using custom software on a computer (7), and gain is modulated (8) to avoid pixel saturation and PMT damage. Temperature is controlled using heated water pumped by peristaltic pumps (9) flowing through copper pipes in the stage (10). (B-D) Pictures of multiphoton microscope, labeled as above. (B) Back view showing laser light generation and modulation via Pockels cell. (C) Front view showing scan head, position of GaAsP PMTs, microscope stage, and peristaltic pumps. (D) Close-up view of microscope stage with temperature control using heated water.
For our imaging, we used an Olympus 60x UPlanApoIR water immersion lens with a relatively high N.A. of 1.2, a relatively long working distance of 250 μm, and good long wavelength transmissivity. Although oil lenses with a slightly higher N.A. resulted in slightly improved image quality, the water lens offered the practical advantage of decreased viscous drag on the coverslip during stage translation. Another advantage of this objective was the presence of a correction collar to adjust for spherical aberrations produced by embryonic pigment and the vitelline membrane. Adjustment of this correction collar from 0.17 (the standard setting for a number 1.5 coverslip) to 0.20 resulted in steadily increasing image brightness and contrast during salivary gland migration (Suppl. Fig. 5). Further adjustment in this direction maintained image brightness but slightly degraded resolution of apical cell shapes (Suppl. Fig. 5G-I), and adjustment in the opposite direction significantly degraded image quality (Suppl. Fig. 5J). The increased contrast between the SG and background and improved resolution of apical cell shapes can most clearly be seen in two successive images of SG cells at settings of 0.17 and 0.20 (Suppl. Fig. 5K).
Mechanical modeling
Live SG outer tube and lumenal diameters were measured in three embryos for each genotype at three locations per gland in two adjacent timepoints (n=18) using Volocity (Improvision). Values were averaged and compared in Excel (Microsoft; Redmond, WA). For simplicity, we treated the original 3D problem as a 2D one and constructed a finite element model in ANSYS ED (node-limited educational version; ANSYS Inc.; Canonsburg, PA) using 8-node plane elements. The mesh was generated in ANSYS, with higher resolution in the apical region and in a one micron thick region along the basal edge of the leader cells. The proximal (left) edge of the model was constrained to zero displacement in x only (free to narrow but not to move forward) and the lumenal surface was zero displacement in y only (free to extend but not to narrow or buckle). Values for force (Tan et al., 2003; du Roure et al., 2005; Prass et al., 2006) and cell moduli (Davidson et al., 1999; Caille et al., 2002; Dahl et al., 2005; Rabinovich et al., 2005; Costa et al., 2006) were taken from the literature, with an additional assumed 2.5x increase in the actin-dense apical region (Yamada et al., 2000). A Hookean linear elastic material model proved limiting for deformation of elements near the point of force application, so the tube model was constructed using an isotropic and incompressible hyperelastic Neo-Hookean material model. For incompressible materials with relatively small deformations, the Neo-Hookean model reduces to a Hookean model and the Neo-Hookean shear modulus equals one-third of the Hookean Young's modulus (Caille et al., 2002). Plots of simulated deformation and total strain intensity were generated in ANSYS ED (ANSYS Inc.). Simulated lumen and leader cell lengths were measured using Image J (NIH), and calculations and plots were performed in Excel (Microsoft; Redmond, WA). Experimental lumen length was measured in Volocity (Improvision) and was plotted and fit with a linear trendline in Excel (Microsoft; Redmond, WA). For preliminary leader cell length measurements, SGs were stained with SAS and α-spectrin and imaged on an LSM510 confocal microscope (Zeiss). The distance between apical and basal surfaces along the midline of the two leader cells was measured in Image J (NIH) and averaged in Excel (Microsoft).
Supplementary Material
Acknowledgments
We would like to thank members of the Andrew lab, Scot Kuo, and Douglas Robinson for helpful discussions; DRBIO Resource staff for live imaging assistance; Douglas Cavener, Benjamin Glick, Shigeo Hayashi, Mark Krasnow, Hiroki Oda, Donald Ready, Bloomington, DSHB, and Flybase for fly stocks and reagents. This work was funded by a Whitaker Foundation Graduate Fellowship to A.M.C., NIH NIDCR Grants R01 DE12873 and R01 DE13899 to D.J.A., and by NIH NIBIB Grant P41 EB01976 to W.R.Z.
References
- Bevis BJ, Glick BS. Rapidly maturing variants of the Discosma red fluorescent protein (DsRed) Nat Biotechnol. 2002;1:83–87. doi: 10.1038/nbt0102-83. [DOI] [PubMed] [Google Scholar]
- Bonilha VL, Rayborn ME, Saotome I, McClatchey AI, Hollyfield JG. Microvilli defects in retinas of ezrin knockout mice. Exp Eye Res. 2006;82:720–729. doi: 10.1016/j.exer.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Bradley PL, Andrew DJ. ribbon encodes a novel BTB/POZ protein required for directed cell migration in Drosophila melanogaster. Development. 2001;128:3001–3015. doi: 10.1242/dev.128.15.3001. [DOI] [PubMed] [Google Scholar]
- Bradley PL, Myat MM, Comeaux CA, Andrew DJ. Posterior migration of the salivary gland requires an intact visceral mesoderm and integrin function. Dev Biol. 2003;257:249–262. doi: 10.1016/s0012-1606(03)00103-9. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Caille N, Thoumine O, Tardy Y, Meister JJ. Contribution of the nucleus to the mechanical properties of endothelial cel. Journal of Biomechanics. 2002;35:177–187. doi: 10.1016/s0021-9290(01)00201-9. [DOI] [PubMed] [Google Scholar]
- Coscoy S, Waharte F, Gautreau A, Martin M, Louvard D, Mangeat P, Arpin M, Amblard F. Molecular analysis of microscopic ezrin dynamics by two-photon FRAP. Proc Natl Acad Sci U S A. 2002;99:12813–12818. doi: 10.1073/pnas.192084599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa KD, Sim AJ, Yin FC. Non-Hertzian approach to analyzing mechanical properties of endothelial cells probed by atomic force microscopy. J Biomech Eng. 2006;128:176–184. doi: 10.1115/1.2165690. [DOI] [PubMed] [Google Scholar]
- Cresawn KO, Potter BA, Oztan A, Guerriero CJ, Ihrke G, Goldenring JR, Apodaca G, Weisz OA. Differential involvement of endocytic compartments in the biosynthetic traffic of apical proteins. Embo J. 2007;26:3737–3748. doi: 10.1038/sj.emboj.7601813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl KN, Engler AJ, Pajerowski JD, Discher DE. Power-law rheology of isolated nuclei with deformation mapping of nuclear substructures. Biophys J. 2005;89:2855–2864. doi: 10.1529/biophysj.105.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson LA, Oster GF, Keller RE, Koehl MAR. Measurements of mechanical properties of the blastula wall reveal which hypothesized mechanisms of primary invagination are physically plausible in the sea urchin Strongylocentrotus purpuratus. Dev Biol. 1999;209:221–238. doi: 10.1006/dbio.1999.9249. [DOI] [PubMed] [Google Scholar]
- Discher DE. New insights into erythrocyte membrane organization and microelasticity. Curr Opin Hematol. 2000;7:117–122. doi: 10.1097/00062752-200003000-00008. [DOI] [PubMed] [Google Scholar]
- Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- du Roure O, Saez A, Buguin A, Austin RH, Chavrier P, Silberzan P, Ladoux B. Force mapping in epithelial cell migration. Proc Natl Acad Sci U S A. 2005;102:2390–2395. doi: 10.1073/pnas.0408482102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faucheux M, Roignant JY, Netter S, Charollais J, Antoniewski C, Theodore L. batman interacts with polycomb and trithorax group genes and encodes a BTB/POZ protein that is included in a complex containing GAGA factor. Mol Cell Biol. 2003;23:1181–1195. doi: 10.1128/MCB.23.4.1181-1195.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fievet B, Louvard D, Arpin M. ERM proteins in epithelial cell organization and functions. Biochim Biophys Acta. 2007;1773:653–660. doi: 10.1016/j.bbamcr.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Forgacs G, Foty RA, Shafrir Y, Steinberg MS. Viscoelastic properties of living embryonic tissues: a quantitative study. Biophys J. 1998;74:2227–2234. doi: 10.1016/S0006-3495(98)77932-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardel ML, Shin JH, MacKintosh FC, Mahadevan L, Matsudaira P, Weitz DA. Elastic behavior of cross-linked and bundled actin networks. Science. 2004;304:1301–1305. doi: 10.1126/science.1095087. [DOI] [PubMed] [Google Scholar]
- Henderson KD, Andrew DJ. Regulation and function of Scr, exd, and hth in the Drosophila salivary gland. Dev Biol. 2000;217:362–374. doi: 10.1006/dbio.1999.9560. [DOI] [PubMed] [Google Scholar]
- Hoekstra D, Tyteca D, van I SC. The subapical compartment: a traffic center in membrane polarity development. J Cell Sci. 2004;117:2183–2192. doi: 10.1242/jcs.01217. [DOI] [PubMed] [Google Scholar]
- Hutson MS, Tokutake Y, Chang MS, Bloor JW, Venakides S, Kiehart DP, Edwards GS. Forces for morphogenesis investigated with laser microsurgery and quantitative modeling. Science. 2003;300:145–149. doi: 10.1126/science.1079552. [DOI] [PubMed] [Google Scholar]
- Jack J, Myette G. The genes raw and ribbon are required for proper shape of tubular epithelial tissues in Drosophila. Genetics. 1997;147:243–253. doi: 10.1093/genetics/147.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazwinska A, Ribeiro C, Affolter M. Epithelial tube morphogenesis during Drosophila tracheal development requires Piopio, a luminal ZP protein. Nat Cell Biol. 2003;5:895–901. doi: 10.1038/ncb1049. [DOI] [PubMed] [Google Scholar]
- Karagiosis SA, Ready DF. Moesin contributes an essential structural role in Drosophila photoreceptor morphogenesis. Development. 2004;131:725–732. doi: 10.1242/dev.00976. [DOI] [PubMed] [Google Scholar]
- Kerman BE, Cheshire AM, Myat MM, Andrew DJ. Ribbon Modulates Apical Membrane during Tube Elongation through Crumbs and Moesin. Dev Biol. 2008 doi: 10.1016/j.ydbio.2008.05.541. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerman BE, Cheshire AM, Andrew DJ. From fate to function: the Drosophila trachea and salivary gland as models for tubulogenesis. Differentiation. 2006;74:326–348. doi: 10.1111/j.1432-0436.2006.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehart DP, Montague RA, Rickoll WL, Foard D, Thomas GH. High-resolution microscopic methods for the analysis of cellular movements in Drosophila embryos. Methods Cell Biol. 1994;44:507–532. doi: 10.1016/s0091-679x(08)60929-2. [DOI] [PubMed] [Google Scholar]
- Kunda P, Pelling AE, Liu T, Baum B. Moesin controls cortical rigidity, cell rounding, and spindle morphogenesis during mitosis. Curr Biol. 2008;18:91–101. doi: 10.1016/j.cub.2007.12.051. [DOI] [PubMed] [Google Scholar]
- Lan M, Kojima T, Murata M, Osanai M, Takano K, Chiba H, Sawada N. Phosphorylation of ezrin enhances microvillus length via a p38 MAP-kinase pathway in an immortalized mouse hepatic cell line. Exp Cell Res. 2006;312:111–120. doi: 10.1016/j.yexcr.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Laprise P, Beronja S, Silva-Gagliardi NF, Pellikka M, Jensen AM, McGlade CJ, Tepass U. The FERM protein Yurt is a negative regulatory component of the Crumbs complex that controls epithelial polarity and apical membrane size. Dev Cell. 2006;11:363–374. doi: 10.1016/j.devcel.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li BL, Satoh AK, Ready DF. Myosin V, Rab11, and dRip11 direct apical secretion and cellular morphogenesis in developing Drosophila photoreceptors. Journal of Cell Biology. 2007;177:659–669. doi: 10.1083/jcb.200610157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno S, Takakuwa Y, Mohandas N. Modulation of erythrocyte membrane mechanical function by protein 4.1 phosphorylation. J Biol Chem. 2005;280:7581–7587. doi: 10.1074/jbc.M410650200. [DOI] [PubMed] [Google Scholar]
- Medina E, Lemmers C, Lane-Guermonprez L, Le Bivic A. Role of the Crumbs complex in the regulation of junction formation in Drosophila and mammalian epithelial cells. Biol Cell. 2002;94:305–313. doi: 10.1016/s0248-4900(02)00004-7. [DOI] [PubMed] [Google Scholar]
- Myat MM, Andrew DJ. Organ shape in the Drosophila salivary gland is controlled by regulated, sequential internalization of the primordia. Development. 2000;127:679–691. doi: 10.1242/dev.127.4.679. [DOI] [PubMed] [Google Scholar]
- Myat MM, Andrew DJ. Epithelial tube morphology is determined by the polarized growth and delivery of apical membrane. Cell. 2002;111:879–891. doi: 10.1016/s0092-8674(02)01140-6. [DOI] [PubMed] [Google Scholar]
- Nelson CM, Jean RP, Tan JL, Liu WF, Sniadecki NJ, Spector AA, Chen CS. Emergent patterns of growth controlled by multicellular form and mechanics. Proc Natl Acad Sci U S A. 2005;102:11594–11599. doi: 10.1073/pnas.0502575102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nüsslein-Volhard C, Wieschaus E, Kluding H. Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster. I. Zygotic loci on the second chromosome. Roux's Arch Dev Biol. 1984;193:267–282. doi: 10.1007/BF00848156. [DOI] [PubMed] [Google Scholar]
- Oda H, Tsukita S. Dynamic features of adherens junctions during Drosophila embryonic epithelial morphogenesis revealed by a Dα-catenin-GFP fusion protein. Dev Genes Evol. 1999;209:218–225. doi: 10.1007/s004270050246. [DOI] [PubMed] [Google Scholar]
- Orr AW, Helmke BP, Blackman BR, Schwartz MA. Mechanisms of mechanotransduction. Dev Cell. 2006;10:11–20. doi: 10.1016/j.devcel.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Pellikka M, Tanentzapf G, Pinto M, Smith C, McGlade CJ, Ready DF, Tepass U. Crumbs, the Drosophila homologue of human CRB1/RP12, is essential for photoreceptor morphogenesis. Nature. 2002;416:143–149. doi: 10.1038/nature721. [DOI] [PubMed] [Google Scholar]
- Peralta XG, Toyama Y, Hutson MS, Montague R, Venakides S, Kiehart DP, Edwards GS. Upregulation of forces and morphogenic asymmetries in dorsal closure during Drosophila development. Biophys J. 2007;92:2583–2596. doi: 10.1529/biophysj.106.094110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polesello C, Delon I, Valenti P, Ferrer P, Payre F. Dmoesin controls actin-based cell shape and polarity during Drosophila melanogaster oogenesis. Nat Cell Biol. 2002;4:782–789. doi: 10.1038/ncb856. [DOI] [PubMed] [Google Scholar]
- Polesello C, Payre F. Small is beautiful: what flies tell us about ERM protein function in development. Trends Cell Biol. 2004;14:294–302. doi: 10.1016/j.tcb.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Powsner L. The effects of temperature on the durations of the developmental stages of Drosophila melanogaster. Physiol Zool. 1935;8:474–520. [Google Scholar]
- Prass M, Jacobson K, Mogilner A, Radmacher M. Direct measurement of the lamellipodial protrusive force in a migrating cell. J Cell Biol. 2006;174:767–772. doi: 10.1083/jcb.200601159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovich Y, Esayanur M, Daosukho S, Byer K, El-Shall H, Khan S. Atomic force microscopy measurement of the elastic properties of the kidney epithelial cells. Journal of Colloid and Interface Science. 2005;285:125–135. doi: 10.1016/j.jcis.2004.11.041. [DOI] [PubMed] [Google Scholar]
- Reuter R, Panganiban GEF, Hoffmann FM, Scott MP. Homeotic genes regulate the spatial expression of putative growth factors in the visceral mesoderm of Drosophila embryos. Development. 1990;110:1031–1040. doi: 10.1242/dev.110.4.1031. [DOI] [PubMed] [Google Scholar]
- Rubin GM, Spradling AC. Vectors for P element-mediated gene transfer in Drosophila. Nucleic Acids Res. 1983;11:6341–6351. doi: 10.1093/nar/11.18.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saotome I, Curto M, McClatchey AI. Ezrin is essential for epithelial organization and villus morphogenesis in the developing intestine. Dev Cell. 2004;6:855–864. doi: 10.1016/j.devcel.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Seshaiah P, Miller B, Myat MM, Andrew DJ. pasilla, the Drosophila homologue of the human Nova-1 and Nova-2 proteins, is required for normal secretion in the salivary gland. Dev Biol. 2001;239:309–322. doi: 10.1006/dbio.2001.0429. [DOI] [PubMed] [Google Scholar]
- Sheetz M. Cell control by membrane-cytoskeleton adhesion. Nature Rev Mol Cell Bio. 2001;2:392–396. doi: 10.1038/35073095. [DOI] [PubMed] [Google Scholar]
- Sheetz MP, Sable JE, Dobereiner HG. Continuous membrane-cytoskeleton adhesion requires continuous accomodation to lipid and cytoskeletal dynamics. Annu Rev Biophys Biomol Struct. 2006;35:417–434. doi: 10.1146/annurev.biophys.35.040405.102017. [DOI] [PubMed] [Google Scholar]
- Shiga Y, Tanaka-Matakatsu M, Hayashi S. A nuclear GFP/β-galactosidase fusion protein as a marker for morphogenesis in living Drosophila. Develop Growth Differ. 1996;38:99–106. [Google Scholar]
- Shim K, Blake KJ, Jack J, Krasnow MA. The Drosophila ribbon gene encodes a nuclear BTB domain protein that promotes epithelial migration and morphogenesis. Development. 2001;128:4923–4933. doi: 10.1242/dev.128.23.4923. [DOI] [PubMed] [Google Scholar]
- Speck O, Hughes SC, Noren NK, Kulikauskas RM, Fehon RG. Moesin functions antagonistically to the Rho pathway to maintain epithelial integrity. Nature. 2003;421:83–87. doi: 10.1038/nature01295. [DOI] [PubMed] [Google Scholar]
- Tan JL, Tien J, Pirone DM, Gray DS, Bhadriraju K, Chen CS. Cells lying on a bed of microneedles: An approach to isolate mechanical force. Proc Natl Acad Sci U S A. 2003;100:1484–1489. doi: 10.1073/pnas.0235407100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsarouhas V, Senti KA, Jayaram SA, Tiklova K, Hemphala J, Adler J, Samakovlis C. Sequential pulses of apical epithelial secretion and endocytosis drive airway maturation in Drosophila. Dev Cell. 2007;13:214–225. doi: 10.1016/j.devcel.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Vining MS, Bradley PL, Comeaux CA, Andrew DJ. Organ positioning in Drosophila requires complex tissue-tissue interactions. Dev Biol. 2005;287:19–34. doi: 10.1016/j.ydbio.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol. 2006;7:265–275. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- Wachsstock DH, Schwarz WH, Pollard TD. Cross-linker dynamics determine the mechanical properties of actin gels. Biophys J. 1994;66:801–809. doi: 10.1016/s0006-3495(94)80856-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Wirtz D, Kuo SC. Mechanics of living cells measured by laser tracking microrheology. Biophys J. 2000;78:1736–1747. doi: 10.1016/S0006-3495(00)76725-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.